Abstract
Androgen levels are lower in obese men as compared to normal weight individuals. However, there are no safety data regarding the chronic use of androgen supplements in middle-aged men. The present study was undertaken to determine the cardiovascular and metabolic effects of chronic (10 weeks) testosterone treatment in male obese Zucker rats (OZR), starting at 22 weeks of age, when testosterone levels were significantly decreased. Testosterone supplements increased plasma levels, 10 fold in both OZR and lean Zucker rats (LZR). In OZR, testosterone supplements reduced body weight, plasma insulin and cholesterol levels and improved oral glucose tolerance test. None of these parameters were affected in LZR. Mean arterial pressure was significantly increased in OZR, but not LZR. Testosterone supplements increased proteinuria and accelerated renal injury in LZR only. Thus treatment of obese men with chronic testosterone supplements should be done with careful monitoring of blood pressure.
Keywords: androgens, obesity, insulin resistance, metabolic syndrome, hypertension
Introduction
Obesity and the development of metabolic syndrome is a growing epidemic in Westernized cultures around the world 1. Obesity and metabolic syndrome are important factors in the increased prevalence of cardiovascular disease. One of the hallmarks of obesity in men is a reduction in serum testosterone levels 2, 3. Biswas and colleagues recently reported that 45 and 61% of men with type II diabetes had reductions in total and free serum testosterone levels, respectively 4. The mechanisms responsible for the reduction in androgens with obesity have not been elucidated. It is possible that increases in aromatase activity in the adipose tissue could cause conversion of testosterone to estradiol and thus reduce testosterone levels. In support of this contention, Tamler and colleagues recently reported that bariatric surgery, and subsequent weight loss, increased serum testosterone levels in obese men 5.
Many investigators have heralded the reduction in androgens in obese men as being a major causative factor for increased cardiovascular disease with consequences including endothelial dysfunction, erectile dysfunction, and hypertension 6–8. As a consequence, if androgen levels are decreased, obese men are often treated with androgen supplements. While studies in older men show that testosterone supplements improve libido, protect against osteoporosis, and improve overall feelings of well-being 9,10, no long term studies have carefully documented the cardiovascular consequences of chronic androgen treatment in men with metabolic syndrome. One concern is that serum testosterone levels are not always well controlled in androgen-treated individuals compared with age-matched men, and studies have not been performed to determine what “adequate” testosterone levels should be targeted since it is not clear whether chronically increasing androgens in obese men will protect them against cardiovascular disease.
In non-obese male rats that are prone to hypertension, androgens promote increases in blood pressure since castration often reduces their blood pressure 11. How androgens increase blood pressure in non-obese males has not been entirely elucidated. The fact that androgens contribute to increased blood pressure in male rats calls into question whether androgen supplements in obese men will further increase blood pressure and promote cardiovascular disease or will actually protect against further development of cardiovascular disease and reduce or attenuate the onset of hypertension.
Just as in obese men, obese male Zucker rats (OZR) have a reduction in serum testosterone levels as compared to lean male Zucker rats (LZR)12. The present study tests the hypothesis that chronic testosterone supplements in OZR will promote, rather than protect against, cardiovascular disease and hypertension.
Methods
All protocols complied with the Guidelines for the Care and Use of Laboratory Animals by the National Institutes of Health, and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Rat model
Obese Zucker rats (OZR) and their lean counterparts (LZR) were obtained at 12 weeks of age from the vendor (Harlan, Indianapolis, IN), and allowed to age to 22 weeks of age. We have preliminary data that BP in male OZR and LZR were not different between 12 and 20 weeks of age13. Thus at 22 weeks of age, rats were divided into two groups, those implanted with testosterone pellets (LZR, n = 13; OZR, n= 13) and controls (LZR, n=12; OZR, n=12). Rats were implanted subcutaneously on the back of the neck with testosterone-filled or empty silastic tubes (length, filled with testosterone propionate (Sigma Chemicals, St Louis, MO)) that were changed every 3 weeks for 10 weeks. Throughout the testosterone treatment period, food and water intake were measured daily for one week a month. Data are presented only for the end of the study. All studies were performed in rats, aged 32 weeks, as described below, after 10 weeks testosterone treatment.
Testosterone supplements and Plasma testosterone levels
Silastic implants (10 mm, id: 0.062 in, od: 0.125 in (Dow Chemical Co, Midland, MI)) were packed with testosterone decanoate, and sealed with silastic glue. Pellets were soaked overnight in 70% ethanol prior to implantation. Pellets were changed every 3 wks throughout the experiment. Testosterone levels were measured using a commercially available radioimmunoassay kit (Coat-A-Count testosterone kit; Diagnostic Products Corporation, Los Angeles, CA), as we have previously described 13.
Measurement of blood pressure (BP)
At 29–30 weeks of age, radiotelemeters were implanted as we have previously described 14. Following a 2 week recovery period, BP was measured for 5 days and recorded.
Urinary protein excretion
Rats (n=6–7/grp) were placed in plastic metabolic cages and urine was collected for 24 hrs. Urinary protein excretion was measured using the Bradford method using a commercially available reagent (BioRad, Richmond, CA).
Glomerular sclerosis
Kidney sections from rats were examined by a pathologist who was not aware of the identity of the groups. Kidneys were embedded in paraffin and cut into 5-μm sections. The sections were stained with periodic acid–Schiff reagent. Three hundred glomeruli from each kidney were examined, and each was graded for injury as follows: <25% of the glomerulus damaged; 25% to 50% damaged; 50% to 75% damaged; >75% damaged; and global sclerosis. The data from all rats in a group were averaged and expressed as a percentage of glomeruli from each kidney exhibiting the 5 levels of injury.
Measurement of metabolic parameters
Plasma leptin, insulin, and cholesterol were measured by commercially available kits (insulin, leptin: Linco Research, St. Charles, MO; cholesterol: Wako Pure Chemical Industries, Ltd., Richmond, VA). Oral glucose tolerance test was performed after 18 hrs fasting on all rats. Glucose (D-(+)-glucose in water; Sigma, St. Louis, MO; 2 g/kg body weight; total volume =500 μl) was given by oral gavage and then blood glucose levels were measured from a drop of tail blood using a glucometer (Accu-check Advantage; Roche) at times: 0, 30, 60, 90, 120 min afterwards and plotted. Data are presented as area under the curves for all groups. Visceral fat (as perirenal fat) was measured in all groups at the time of sacrifice and factored for body weight.
Statistical analyses
All data are expressed as mean ± S.E.M. Statistical differences were determined by analyses of variance (ANOVA), performed with SigmaStat software package version 3.1 (Systat software Inc., San Jose, CA). Differences were considered statistically significant at p<0.05.
Results
Plasma testosterone levels
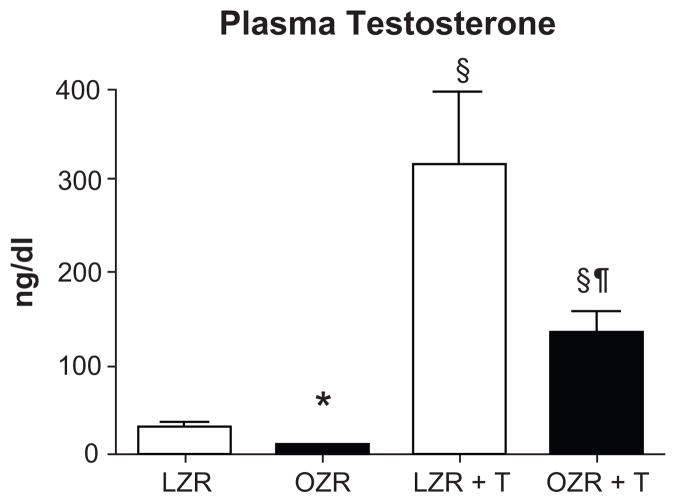
At 32 wks of age, plasma testosterone levels were 70% lower in control OZR compared to LZR, and testosterone pellets increased plasma testosterone by 10–12 fold in both groups (Figure 1).
Figure 1. Plasma testosterone levels in testosterone treated OZR (OZR +T) and LZR (LZR +T) and controls (OZR and LZR).
Testosterone supplements were begun at 22 weeks of age and continued for 10 weeks (n=13/grp). *, p< 0.05 compared with LZR control (L); §, p<0.05 compared with untreated rats of same strain; ¶, p<0.05 compared with testosterone treated LZR.
Body weights and kidney weights
At 22 weeks of age, body weights were higher in OZR than LZR (LZR: 472.6 ± 8 vs OZR: 765.5 ± 17 g; P<0.0001). At 32 wks of age, control OZR (OC) weighed considerably more than control LZR (LC) (see Table 1). Body weight in testosterone-treated LZR was not different compared to control LZR, but body weight was reduced by 21% in testosterone-treated OZR (Table 1).
Table 1.
Body weights, kidney weights, and kidney/body weight ratios for LZR and OZR controls and those treated with testosterone supplements (T).
| Rats | BW (g) | KW (g) | KW/BW × 10−3 |
|---|---|---|---|
| LZR Control (LC) (n=6) | 522 ± 14 | 3.33 ± 0.15 | 6.31 ± 0.25 |
| LZR + T (LT) (n=6) | 474 ± 14 | 3.52 ± 0.04 | 7.44 ± 0.17 |
| OZR Control (OC) (n=7) | 858 ± 28 | 4.45 ± 0.07 | 5.2 ± 0.17 |
| OZR + T (OT) (n=7) | 677 ± 19 | 4.82 ± 0.16 | 7.12 ± 0.18 |
| p values | |||
| LC vs OC | <0.05 | <0.05 | <0.05 |
| LT vs OT | <0.05 | <0.05 | NS |
| LC vs LT | NS | NS | <0.001 |
| OC vs OT | <0.05 | NS (=0.6) | <0.05 |
Abbreviations: BW, body weight; KW, kidney weight; KW/BW, kidney weight to body weight ratio.
Kidney weights were higher in control OZR than LZR, and were slightly increased with testosterone treatment in both groups (Table 1). However, kidney weight to body weight ratios (KW/BW) were significantly higher in LZR controls than OZR controls, and testosterone treatment increased KW/BW ratios by approximately 20% and 40% in LZR and OZR, respectively (Table 1).
Blood pressure and renal injury
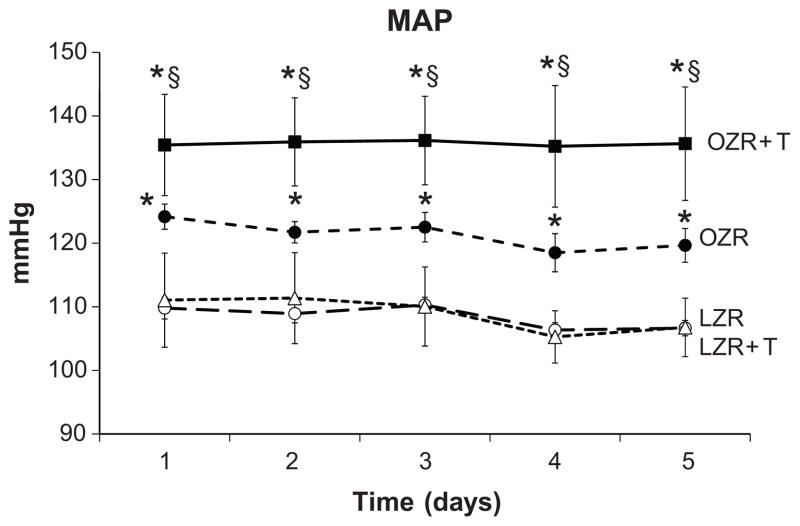
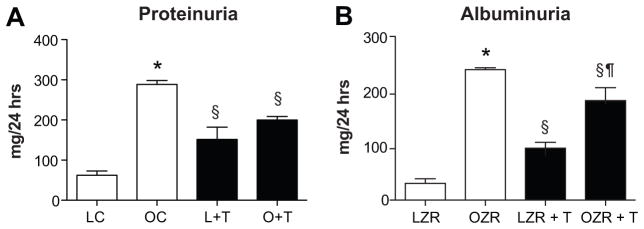
At 32 weeks of age, mean arterial pressure, as measured for 5 days by radiotelemetry was significantly higher in OZR than LZR (Figure 2). Testosterone treatment had no effect on MAP in LZR, but increased MAP by approximately 10 mm Hg in OZR. Proteinuria and albuminuria levels were increased in OZR compared to LZR, and testosterone treatment increased proteinuria and albuminuria in LZR, but reduced both in OZR (Figure 3A and B). Morphology showed that OZR had greater glomerular injury at 32 weeks than did LZR (Figure 4A vs 4B), but there was no difference in glomerular injury with testosterone treatment in OZR (Figure 4B). In contrast, testosterone treatment increased glomerular injury in LZR.
Figure 2. Mean arterial pressure measured 24 hrs per day by telemetry beginning at 31 weeks of age for 5 days.
*, p< 0.05 compared with LZR control (L) or LZR + T; §, p<0.05 compared with control OZR (n=6–7/grp).
Figure 3. Urinary protein (Panel A) and albumin (Panel B) excretion at 32 weeks of age after 10 weeks of testosterone or placebo (n=6–7/grp).
*, p< 0.05 compared with LZR control (L); §, p<0.05 compared with untreated rats of same strain; ¶, p<0.05 compared with testosterone treated LZR.
Figure 4. Glomerular sclerosis index in control (LC) and testosterone-treated (LT) LZR (Panel A) and control (OC) and testosterone-treated (OT) OZR (Panel B) at 32 wks of age (n=6–7/grp).
**, p< 0.05 compared with untreated controls.
Metabolic parameters
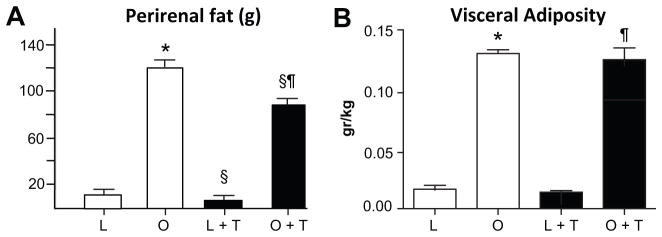
Perirenal fat was significantly greater in control OZR than LZR (Figure 5A). Testosterone treatment reduced perirenal fat weight by 40% in LZR, and by 25% in OZR. Visceral adiposity, measured as the mass of perirenal fat tissue factored for body weight, was also significantly higher in control OZR than LZR (Figure 5B), but testosterone treatment had no effect in either group. As expected, plasma leptin was considerably higher in control OZR than LZR, and testosterone treatment reduced plasma leptin by 30% in OZR but not LZR (Figure 6A). Plasma insulin was significantly higher in control OZR than LZR (Figure 6B). Testosterone treatment had no effect on insulin levels in LZR, but reduced insulin in OZR by 80%, similar to levels in testosterone treated LZR. Fasted blood glucose was higher in both control OZR than LZR (LC: 70 ± 4 vs OC: 123 ± 8 mg/dL; p<0.001), and testosterone–treated OZR than LZR (LT: 62 ± 4 vs OT: 91 ± 10, P<0.001). Testosterone treatment significantly reduced fasting glucose in OZR (OT) compared to control OZR (OC) (P<0.001), but not LZR (LC vs LT). Insulin resistance, measured as the area under the curve for oral glucose tolerance test, was significantly greater in control OZR than LZR (Figure 6C). Testosterone treatment had no effect in LZR, but reduced insulin resistance in OZR by 40%. Plasma cholesterol was 2 fold higher in control OZR than LZR, and was reduced significantly by testosterone treatment in OZR, but not LZR (Figure 6D).
Figure 5. Perirenal fat and adiposity (fat weight/body weight) in testosterone-treated and control LZR and OZR (n=6–7/grp).
*, p< 0.05 compared with LZR control (L); §, p<0.05 compared with untreated rats of same strain; ¶, p<0.05 compared with testosterone treated LZR.
Figure 6. Plasma leptin, insulin, cholesterol and area under the curve for oral glucose tolerance test in control and testosterone-treated OZR and LZR (n=6–7/grp).
Metabolic parameters were measured in rats at 32 weeks of age after 10 weeks testosterone or placebo. *, p< 0.05 compared with LZR control; §, p<0.05 compared with untreated rats of same strain; ¶, p<0.05 compared with testosterone treated LZR.
Discussion
Obesity in men is associated with reductions in plasma testosterone levels 3. In the present study we found similar reductions in plasma testosterone in a model of obesity and metabolic syndrome, the obese Zucker rat (OZR). In the present study we gave testosterone supplements to OZR that exhibited 80% reductions in testosterone levels compared to their lean counterparts, and increased plasma testosterone by 10 fold (approximately 4 fold higher than in untreated lean rats, LZR). We found that testosterone supplements in OZR significantly reduced their body weight, but had no effect on visceral adiposity when factored for body weight. We also found that testosterone supplements improved characteristics of the metabolic syndrome in OZR, such as reducing plasma insulin, cholesterol, leptin and improving OGTT. However, despite the positive metabolic syndrome effects, testosterone supplements significantly increased blood pressure in OZR.
Whether androgen supplements increase blood pressure in men is not clear. There have been no trials in which androgen supplements have been given for long periods of time and blood pressure followed throughout. There have been studies in female to male transsexuals who have been given testosterone supplements, and the data suggest that there is an increase in blood pressure with testosterone supplements in them 15. Basaria and colleagues reported that testosterone supplements in aging men caused a significant increase in adverse cardiac events compared to the placebo group 16. Blood pressure was not reported in this study.
Many studies in rodents have shown that androgens promote hypertension and renal disease, including age-related renal disease. For example, aged male Sprague Dawley rats exhibit accelerated renal aging and injury compared to females, and castration of males attenuates the injury, whereas ovariectomy of females does not worsen the renal injury 17,18. These data suggest that androgens promote renal injury. Young adult male spontaneously hypertensive rats (SHR) exhibit higher blood pressure than do females, and castration attenuates the hypertension, whereas ovariectomy of females has no effect on blood pressure 19,20. These data suggest that androgens promote the hypertension in male SHR rather than that estrogens protect the female SHR from higher blood pressure. We also found that blood pressure is higher in male Dahl salt sensitive rats than in females regardless of high or low salt diet 21. High salt diet does reduce serum testosterone levels in male DS rats (unpublished data, Yanes and Reckelhoff). Thus based on these data, it is possible that the naturally occurring reduction in androgens in OZR with progression of obesity and metabolic syndrome, may be protective against the development of further cardiovascular disease.
Men with low levels of androgens are given androgen supplements despite the presence of obesity and metabolic syndrome, both harbingers of future cardiovascular disease 22,23. Based on our data, testosterone supplements did improve insulin resistance, cholesterol and leptin levels, despite the fact that the OZR is a leptin-receptor deficient rat. These changes may have been due to the effect of testosterone to reduce body weight in the OZR. Wu and colleagues also reported improvement in metabolic syndrome characteristics in young obese men given testosterone supplements 24. In the present study absolute levels of perirenal fat were also reduced; however, the level of visceral adiposity was similar despite testosterone treatment due to concomitant loss of fat with reductions in body weight. However, there was no increase in glomerular injury in OZR with testosterone as determined by morphology. There was an increase in proteinuria with testosterone treatment in OZR but that was likely due to the increases in blood pressure.
In LZR, raising plasma testosterone by 10 fold had no effect on their blood pressure but increased urinary protein and albumin excretion and also caused glomerular injury, albeit minimal. These data suggest that with continued use of supraphysiological levels of androgens, testosterone would eventually increase blood pressure in LZR due to the progressive renal damage. Individuals with chronic kidney disease have reductions in testosterone levels 25,26. Future studies are necessary to determine whether long term testosterone supplements in individuals with chronic kidney disease would exacerbate their renal injury and lead to further nephron functional loss.
Why testosterone improved the characteristics of the metabolic syndrome but significantly increased blood pressure is not clear. Quan and colleagues reported that testosterone supplements in normotensive rats increased proximal sodium reabsorption 27, which would cause an increase in blood pressure. Obesity is associated with an increase in tubular reabsorption of sodium. Indeed, we found a significant increase in the blood pressure with testosterone treatment only in the obese rats in this study. Alternatively, testosterone supplements may increase vasoconstrictors such as angiotensin II and endothelin. Testosterone supplementation has been shown to increase plasma endothelin levels in female to male transsexuals 28. Renin and angiotensinogen expression are both upregulated in kidneys of male rats compared to their castrated counterparts 29,30. Schwartzman and colleagues have shown the androgens upregulate the synthesis of cytochrome P450 4A omega-hydroxylases in the vasculature of the kidney that would increase synthesis of pro-hypertensive 20-HETE 31,32. Another possibility is that the reduction in body weight and adipose tissue with androgen supplementation in OZR, could have reduced the amount of estrogens produced by the adipose tissue in the males. Estrogens are known to cause vasodilation via their effect on nitric oxide synthesis 33, and thus a reduced in adipose-produced estrogens could have contributed to the increase in blood pressure in the OZR treated with testosterone. Unfortunately, we did not measure plasma estrogen in this study. Thus future studies will be necessary to determine whether any of these mechanisms contribute to the hypertension in testosterone treated OZR.
Perspectives
In this study, chronic supplementation of male OZR with androgens reduced insulin resistance and dyslipidemia, and improved glucose mobilization, but significantly increased blood pressure. These data suggest that androgen supplements may have similar beneficial effects on metabolic syndrome in obese men, but that it is incumbent on physicians to monitor their blood pressure closely when prescribing androgen supplements. Furthermore, in addition to hypertension, high doses of androgens in men could promote prostate cancer 34.
Acknowledgments
Sources of Funding
These studies were supported by funds from National Institutes of Health, National Heart, Lung and Blood Institute, HL 69194 and HL66072.
Footnotes
Author Disclosures
Deborah D. Davis, M.S. – research technician – No disclosures
Arnaldo Lopez Ruiz, M.D. – research associate – No disclosures
Licy L. Yanes, M.D., resident in internal medicine – No disclosures
Radu Iliescu, M.D., Ph.D. – Associate professor – No disclosures
Kuichang Yuan, Ph.D. – Assistant professor – No disclosures
Mohadetheh Moulana, Ph.D. – Postdoctoral fellow – AHA postdoctoral fellowship grant
Jane F. Reckelhoff, Ph.D., Professor – NIH RO1s HL 69194 and HL66072, and PO1 HL 051971
References
- 1.Gluckman P, Hanson M, Zimmet P, Forrester T. Losing the war against obesity: The need for a developmental perspective. Sci Transl Med. 2011;3:93–99. doi: 10.1126/scitranslmed.3002554. [DOI] [PubMed] [Google Scholar]
- 2.Akishita M, Fukai S, Hashimoto M, Kameyama Y, Nomura K, Nakamura T, Ogawa S, IiJima K, Eto M, Ouchi Y. Association of low testosterone with metabolic syndrome and its components in middle-aged japanese men. Hypertens Res. 2010;33:587–591. doi: 10.1038/hr.2010.43. [DOI] [PubMed] [Google Scholar]
- 3.Allan C, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:224–232. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 4.Biswas M, Hampton D, Turkes A, Newcombe RG, Aled Rees D. Reduced total testosterone concentrations in young healthy South Asian men are partly explained by increased insulin resistance but not by altered adiposity. Clin Endocrinol (Oxf) 2010;73:457–462. doi: 10.1111/j.1365-2265.2010.03824.x. [DOI] [PubMed] [Google Scholar]
- 5.Tamler R. Diabetes, obesity and erectile dysfunction. Gender Medicine. 2009;6:4–16. doi: 10.1016/j.genm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Traish AM, Kypreos KE. Testosterone and cardiovascular disease: An old idea with modern clinical implications. Atherosclerosis. 2011;214:244–248. doi: 10.1016/j.atherosclerosis.2010.08.078. [DOI] [PubMed] [Google Scholar]
- 7.Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010;96:1821–1825. doi: 10.1136/hrt.2010.195412. [DOI] [PubMed] [Google Scholar]
- 8.Shabsigh R, Arver K, Channer KS, Eardley I, Fabbri A, Gooren LJ, Heufelder A, Haider J, Jones H, Meryn S, Zitzman M. The triad of erectile dysfunction, hypogonadism and the metabolic syndrome. Int J Clin Prac. 2008;62:791–798. doi: 10.1111/j.1742-1241.2008.01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Death A, Handelsman D. Androgens and cardiovascular disease. Endocrinol Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 11.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 12.Whitaker EM, Shaw MA, Hervey GR. Plasma oestradiol-17 beta and testosterone concentrations as possible causes of the infertility of congenitally obese zucker rats. J Endocrinol. 1983;99:485–490. doi: 10.1677/joe.0.0990485. [DOI] [PubMed] [Google Scholar]
- 13.Iliescu R, Chade A. Progressive renal renal vascular proliferation and injury in obese zucker rats. Microcirc. 2010;17:250–256. doi: 10.1111/j.1549-8719.2010.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in angiotensin II-induced hypertension: Impact of high sodium intake. Hypertension. 2008;51:1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 15.Mueller A, Haeberle L, Zollver H, Claassen T, Kronawitter D, Oppelt P, Cupisti S, Beckmann M, Dittrich R. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals. J Sex Med. 2010;7:3190–3198. doi: 10.1111/j.1743-6109.2010.01912.x. [DOI] [PubMed] [Google Scholar]
- 16.Basaria S, Coviello A, Travison T, Storer T, Farwell W, Jette A, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman K, Mazer N, Miciek R, Krasnoff J, Elmi A, Knapp P, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur M, Fiore D, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baylis C. Age-dependent glomerular damage in the rat. Dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest. 1994;94:1823–1829. doi: 10.1172/JCI117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baylis C. Changes in renal hemodynamics and structure in the aging kidney; sexual dimorphism and the nitric oxide system. Exp Gerontol. 2005;40:271–278. doi: 10.1016/j.exger.2005.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31:435–439. doi: 10.1161/01.hyp.31.1.435. [DOI] [PubMed] [Google Scholar]
- 20.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in the development of hypertension in shr: Role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 21.Yanes LL, Iliescu R, Sartori-Valinotti JC, Romero DG, Reckelhoff JF. Testosterone dependent hypertension and upregulation of intrarenal angiotensinogen in dahl salt sensitive rats. Am J Physiol Reg Integr Comp Physiol. 2009;296:F771–F779. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C, Jackson G, Jones T, Matsumoto A, Nehra A, Perelman M, Swerdloff R, Traish A, Zitzmann M, Cunningham G. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34:1669–1675. doi: 10.2337/dc10-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salazar M, Carbajal H, Espeche W, Dulbecco C, Aizpurúa M, Marillet A, Echeverría R, Reaven G. Relationships among insulin resistance, obesity, diagnosis of the metabolic syndrome and cardio-metabolic risk. Diab Vasc Dis Res. 2011;8:109–116. doi: 10.1177/1479164111403170. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Mao J, Zhang Q, Shi YF. Testosterone replacement therapy improves insulin sensitivity and decreases high sensitivity c-reactive protein levels in hypogonadotropic hypogonadal young male patients. Chin Med J. 2009;122:2846–2850. [PubMed] [Google Scholar]
- 25.Schmidt A, Luger A, Horl W. Sexual hormone abnormalities in male patients with renal failure. Nephrol Dial Transplant. 2002;17:368–371. doi: 10.1093/ndt/17.3.368. [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz M, Sonmez A, Qureshi A, Saglam M, Stenvinkel P, Yaman H, Eyileten T, Caglar K, Oguz Y, Taslipinar A, Vural A, Gok M, Unal H, Yenicesu M, Carrero J. Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1617–1625. doi: 10.2215/CJN.10681210. [DOI] [PubMed] [Google Scholar]
- 27.Quan A, Chakravarty S, Chen J-K, Chen J-C, Loleh S, Saini N, Harris R, Capdevila J, Quigley R. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol. 2004;287:F452–F459. doi: 10.1152/ajprenal.00188.2003. [DOI] [PubMed] [Google Scholar]
- 28.van Kesteren PJ, Kooistra T, Lansink M, Kamp GJv, Asscheman H, Gooren LJ, Emeis JJ, Vischer UM, Stehouwer CD. The effects of sex steroids on plasma levels of marker proteins of endothelial cell functioning. Thromb Haemost. 1998;79:1029–1033. [PubMed] [Google Scholar]
- 29.Chen Y-F, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger rna expression in hypertensive rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 30.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger rna expression. J Clin Invest. 1989;83:1941–1945. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C-C, Cheng J, Zhang FF, Gottlinger KH, Kelkar M, Zhang Y, Jat JL, Falck JR, Schwartzman ML. Androgen-dependent hypertension is mediated by 20-hete-induced vascular dysfunction: Role of inhibitor kappaB kinase. Hypertension. 2011;57:788–794. doi: 10.1161/HYPERTENSIONAHA.110.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh H, Schwartzman ML. Renal vascular cytochrome P450-derived eicosanoids in androgen-induced hypertension. Pharmacol Rep. 2008;60:29–37. [PubMed] [Google Scholar]
- 33.Weiner CP, Lizasain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of Calcium Dependent NO Synthase by Sex Hormones. Proc Nat Acad Sci USA. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultheiss D, Machtens S, Jonas U. Review Article: Testosterone therapy in the ageing male: what about the prostate? Andrologia. 2004;36:355–365. doi: 10.1111/j.1439-0272.2004.00630.x. [DOI] [PubMed] [Google Scholar]