Abstract
Layer-by-layer (LBL) assembly of alternate osmium redox polymers and glucose oxidase, at anode, and laccase, at cathode, using graphite electrodes form a membrane-less glucose/O2 enzymatic fuel cell providing a power density of 103 μW cm−2 at pH 5.5.
Enzymes can be used as catalysts in fuel cells, oxidizing the fuel at the anode and reducing the oxidant at the cathode, to convert chemical energy to electrical power.1–4 In particular, research has focused on glucose/O2 enzymatic fuel cells (EFC), utilizing glucose oxidase (GOx) for glucose oxidation at the anode and laccase for O2 reduction at the cathode, with potential application to powering implanted or portable electronic devices.5–9 Harnessing enzyme electron transfer activity can be achieved by direct electron transfer (DET) between the enzyme active site, typically embedded in an insulating protein shell, and the electrode. Though feasible for some enzymes,10 this approach can limit the current and power density in EFCs mainly due to low, sub-monolayer, coverage and inappropriate orientation of enzyme at the electrode surface.11 In general, mediated electron transfer (MET) to/from enzyme active sites, for example using redox polymers, permits assembly of 3-dimensional, electrically-contacted films capable of producing current and power densities several orders of magnitude higher than that of their DET type counterparts,12–15 although use of nanostructured surfaces can help promote DET to/from enzyme active sites for EFC applications.16 Immobilization of redox polymer and enzyme at electrodes can ensure high EFC current and power densities, whilst preventing short circuiting of current-flow.14,15 Redox polymers and enzymes have been immobilized on electrodes using covalent linkage17 drop-coating18 hydrogel formation19 and layer-by-layer adsorption20 approaches. Alternate layer-by-layer (LBL) electrostatic adsorption of charged polymers and enzymes is a simple method to build 3D electrocatalytic structures providing spatial distribution of redox polymer and enzymes.20
Most LBL assemblies are developed at gold electrodes although using graphite instead of gold electrodes eliminates the need for thiol modification to facilitate adsorption of the primary cationic layer.21–23 We report here the first study of a fully assembled membrane-less EFC in which both the anode and the cathode use redox polymer-enzyme LBL assembly at graphite electrodes. Previously we reported on an EFC based on crosslinked GOx or laccases with osmium redox polymer films at graphite5 and glassy carbon electrodes.24 The redox polymers [Os(4,4′-dimethoxy-2,2′-bipyridine)2(polyvinylimidazole) 10Cl]+,25,26 Eo′ = −0.05 V vs. Ag/AgCl (polymer I) and [Os(4,4′-dichloro-2,2′-bipyridine)2(polyvinylimidazole) 10Cl]+,13,26 Eo′ = +0.35 V vs. Ag/AgCl (polymer II) are selected to provide a voltage output in a glucose/O2 EFC assembly, whilst facilitating a thermodynamically favourable transfer of electrons from the GOx active site (Eo′ = −0.35 V vs. Ag/AgCl)25 to the T1 Cu site of a Trametes hirsuta laccase (ThLacc, Eo′ = +0.57 V vs. Ag/AgCl).27 Biocatalytic anodes and cathodes are assembled by contacting basal plane graphite electrodes alternately to redox polymer and enzyme (GOx 1500 U/ml or ThLacc 390 U/ml) solutions with a schematic of a self assembled (polymer I/GOx)2 film shown in Fig. 1(a). The isoelectric point of native GOx is 4.05,28 whilst that for ThLacc is 4.2,29 ensuring negatively charged enzyme in the deposition solutions of pH 7.4 and 5.0 respectively.20
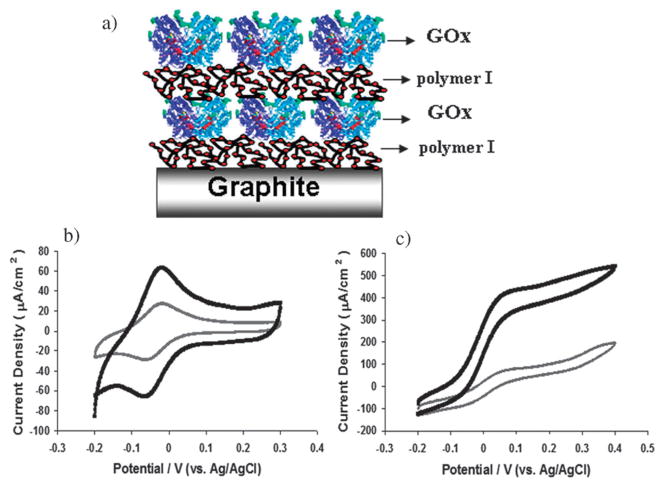
Fig. 1.
(a) Diagram showing layer by layer assembly of (polymer I/GOx)2 films at graphite electrode. CVs of (polymer I/GOx)1 (grey) and (polymer I/GOx)2 (black) films in 0.1 M potassium phosphate buffer containing 0.15 M NaCl at 37 °C, pH 7.4 in (b) absence and (c) presence of 0.1 M glucose. Scan rate 5 mVs−1.
Cyclic voltammetry in the absence of glucose for polymer I/GOx films, Fig. 1(b), displays Os(II/III) redox transition at −0.05 V vs. Ag/AgCl.25 Osmium surface coverages of 7.8 × 10−9 mol cm−2 and 1.6 × 10−8 mol cm−2 for films of (polymer I/GOx)1 and (polymer I/GOx)2, respectively, compare well to that of 8.3 × 10−11 mol cm−2 reported for LbL assembly of an (osmium redox polymer/GOx)2 at a modified gold surface. 20 In the presence of 0.1 M glucose at pH 7.4 sigmoidal signals indicative of bioelectrocatalytic glucose oxidation, Fig. 1(c), yield current densities of 190 and 540 μA cm−2 for (polymer I/GOx)1 and (polymer I/GOx)2 films, respectively. The 3 fold increase in current density for the (polymer I/GOx)2 film over the (polymer I/GOx)1 film, whilst only recording a 2 fold increase in osmium surface coverage could be due to a larger proportion of GOx molecules adsorbed in the second bilayer, further supported by monitoring of mass adsorbed for such systems at treated gold electrodes (Fig. S1 supplemental information).30 Current densities for glucose oxidation by (polymer I/GOx)2 films are comparable to the 600 μA cm−2 observed for a crosslinked system at miniaturised carbon fibre electrodes.26
A LbL approach used for (polymer II/ThLacc)2 films yields osmium surface coverage of 6.9 × 10−9 mol cm−2, with lower surface coverage due to incomplete dispersion/dissolution of polymer II compared to polymer I in the coating solutions, as observed previously.24 The osmium surface coverages are nonetheless higher than the picomoles/cm2 coverages reported for LBL assemblies of osmium redox polymer/laccase at modified gold surfaces.31
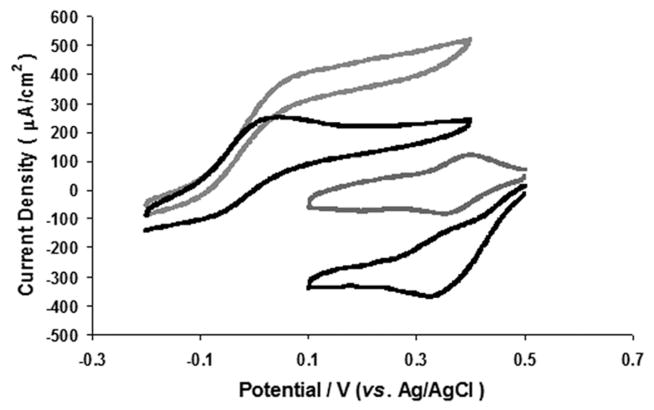
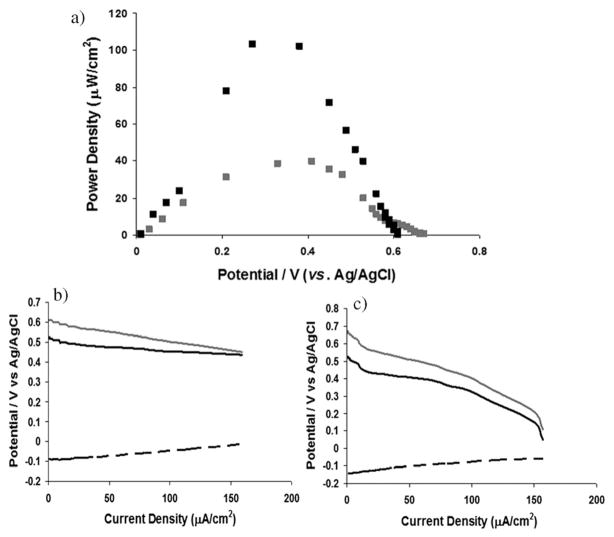
A membrane-less EFC with (polymer I/GOx)2 as anode demonstrates steady state glucose oxidation current density of 510 μA cm−2 and 240 μA cm−2 at pH of 7.4 and 5.5, respectively, confirming higher enzyme activity at neutral pH (Fig. 2).32 In the same solution the (polymer II/ThLacc)2 cathode provides oxygen reduction current densities of 60 μA cm−2 and 330 μA cm−2 at pH of 7.4 and 5.5, respectively, reflecting the lower activity of ThLacc at neutral pH.5,6 The oxygen reduction current densities compare well to current densities of ~40 μA cm−2 for the crosslinked films on glassy carbon at pH 4.513 and current densities of 280 μA cm−2 for crosslinked films on graphite at pH 4.7.5 In addition films of polymer II cross-linked with bilirubin oxidase on carbon cloth yield oxygen reduction current densities of ~300 μA cm−2 when rotated at 100 rpm.33 The membrane-less EFC was polarized under variable load conditions in non-stirred oxygenated buffer in 0.1 M glucose, 0.15 M NaCl, at 37 °C to yield maximum power density of 103 μW cm−2 (at 0.35 V), at a current density of 380 μA cm−2 in pH 5.5 buffer (Fig. 3a). A maximum power density of 40 μW cm−2 (at 0.42 V), at a current density of 150 μA cm−2 was observed when the membrane-less EFC operated in pH 7.4 buffer. Consistent with other studies,8,18,19 an open circuit voltage of 0.6 V observed for EFCs is greater than the difference between the redox polymer mediator potentials (0.4 V), postulated to occur because of a DET for oxygen reduction at the T1 copper redox potential at the ThLacc cathode.34 The presence of catalytic currents for oxygen reduction at potentials more positive than the Os(II/III) transition for polymer II in Fig. 2 provides further evidence that DET can contribute to the oxygen reduction current. The relatively low coverage of osmium for the (polymer II/ThLacc)2 films may permit adsorption of ThLacc onto the underlying graphite for DET to occur. As expected, independent cell polarization studies reveal that higher cathode current densities are sustained at pH 5.5 compared to pH 7.4 (Fig. 3b, c).
Fig. 2.
Catalytic CVs of assembled EFC of (polymer I/GOx)2 (left) and (polymer II/ThLacc)2 (right) films in oxygen-saturated 0.1 M potassium phosphate buffer containing 0.15 M NaCl at 37 °C, in the presence of 0.1 M glucose. Scan rate 5 mVs−1 (electrodes dried for 12 h). Grey pH 7.4 and black pH 5.5.
Fig. 3.
(a) Power density curve of an EFC composed of (polymer I/GOx)2 and (polymer II/ThLacc)2 films in oxygen-saturated 0.1 M potassium phosphate buffer containing 0.15 M NaCl at 37 °C, in the presence of 0.1 M glucose, at pH 5.5 (black square) and pH 7.4 (grey square). Cell behaviour during polarization of EFC at pH 5.5 (b) and pH 7.4 (c). (Grey line—Cell potential, Black line-Cathode potential, Dashed line—Anode potential).
The power density is an improvement on the 40 μW cm−2 at pH 5.5, and 16 μWcm−2 at pH 7.4, obtained using crosslinked redox hydrogels of GOx and laccase at graphite electrodes,5 and the 12.6 μWcm−2 for an EFC based on LBL self assembly of enzymes and gold nanoparticles on planar gold electrodes reported by Deng et al.35 The results also compare well with that obtained for an EFC using films of polymer I with glucose oxidase and polymer II with bilirubin oxidase on carbon fibre electrodes, which yielded 244 μW cm−2 at 0.36 V under physiological conditions.26
In conclusion we demonstrate that LbL assembly provides a facile method for production of redox polymer-based EFCs that can provide maximum power densities of 40 μW cm−2 at pH 7.4 or 103 μW cm−2 at pH 5.5. Improvements may be achieved by using LBL assembly to form thicker films (μ2 bilayers) and by use of alternate enzymes and redox polymers.
Supplementary Material
Footnotes
Electronic supplementary information (ESI) available: Experimental details, results of quartz crystal microbalance measurements of film assembly at modified gold electrodes and power density versus current density curves at pH 7.4 and 5.5. See DOI: 10.1039/c1cc15002b
Notes and references
- 1.Cracknell JA, Vincent KA, Armstrong FA. Chem Rev. 2008;108:2439. doi: 10.1021/cr0680639. [DOI] [PubMed] [Google Scholar]
- 2.Barton SC, Gallaway J, Atanassov P. Chem Rev. 2004;104:4867. doi: 10.1021/cr020719k. [DOI] [PubMed] [Google Scholar]
- 3.Davis F, Higson SPJ. Biosens Bioelectron. 2007;22:1224. doi: 10.1016/j.bios.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Kendall K. Nat Mater. 2002;1:211. doi: 10.1038/nmat782. [DOI] [PubMed] [Google Scholar]
- 5.Barriére F, Kavanagh P, Leech D. Electrochim Acta. 2006;51:5187. [Google Scholar]
- 6.Barriére F, Ferry Y, Rochefort D, Leech D. Electrochem Commun. 2004;6:237. [Google Scholar]
- 7.Yan Y, Zheng W, Su L, Mao L. Adv Mater. 2006;18:2639. [Google Scholar]
- 8.Chen T, Barton SC, Binyamin G, Gao Z, Zhang Y, Kim HH, Heller A. J Am Chem Soc. 2001;123:8630. doi: 10.1021/ja0163164. [DOI] [PubMed] [Google Scholar]
- 9.Mano N. Chem Commun. 2008:2221. doi: 10.1039/b801786g. [DOI] [PubMed] [Google Scholar]
- 10.Coman V, Ludwig R, Harreither W, Haltrich D, Gorton L, Ruzgas T, Shleev S. Fuel Cells. 2010:9. doi: 10.1039/b808859d. [DOI] [PubMed] [Google Scholar]
- 11.Ivnitski D, Branch B, Atanassov P, Apblett C. Electrochem Commun. 2006;8:1204. [Google Scholar]
- 12.Barton SC, Kim HH, Binyamin G, Zhang Y, Heller A. J Phys Chem. 2001;5:11917. [Google Scholar]
- 13.Jenkins P, Boland S, Kavanagh P, Leech D. Bioelectrochemistry. 2009;76:162. doi: 10.1016/j.bioelechem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Heller A. Phys Chem Chem Phys. 2004;6:209. [Google Scholar]
- 15.Palmore GTR, Kim HH. J Electroanal Chem. 1999;464:110. [Google Scholar]
- 16.Zebda A, Gondran C, Le Goff A, Holzinger M, Cinquin P, Cosnier S. Nat Commun. 2011;2:370. doi: 10.1038/ncomms1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battaglini F, Bartlett PN, Wang JH. Anal Chem. 2000;72:502. doi: 10.1021/ac990321x. [DOI] [PubMed] [Google Scholar]
- 18.Kavanagh P, Jenkins P, Leech D. Electrochem Commun. 2008;10:970. [Google Scholar]
- 19.Mano N, Mao F, Heller A. J Am Chem Soc. 2002;124:12962. doi: 10.1021/ja028514g. [DOI] [PubMed] [Google Scholar]
- 20.Calvo EJ, Etchenique R, Pietrasanta L, Wolosiuk A. Anal Chem. 2001;73:1161. doi: 10.1021/ac0011686. [DOI] [PubMed] [Google Scholar]
- 21.Gao Q, Yang X. Chem Commun. 2004:30. doi: 10.1039/b311535f. [DOI] [PubMed] [Google Scholar]
- 22.Zheng H, Okada H, Nojima S, Suye SI, Hori T. Sci Technol Adv Mater. 2004;5:371. [Google Scholar]
- 23.Spricigo R, Dronov R, Rajagopalan KV, Lisdat F, Leimkuehler S, Wollenberger U. Soft Matter. 2008;4:972. doi: 10.1039/b717694e. [DOI] [PubMed] [Google Scholar]
- 24.Kavanagh P, Boland S, Jenkins P, Leech D. Fuel Cells. 2009:79. [Google Scholar]
- 25.Taylor C, Kenausis G, Katakis I, Heller A. J Electroanal Chem. 1995;396:511. [Google Scholar]
- 26.Mano N, Heller A. J Electrochem Soc. 2003;150:A1136. [Google Scholar]
- 27.Shleev S, Jarosz-Wilkolazka A, Khalunina A, Morozova A, Yaropolov O, Ruzgas T, Gorton L. Bioelectrochemistry. 2005;67:115. doi: 10.1016/j.bioelechem.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Voet JG, Coe J, Epstein J, Matossian V, Shipley T. Biochemistry. 1981;20:7182. doi: 10.1021/bi00528a020. [DOI] [PubMed] [Google Scholar]
- 29.Shleev SV, Morozova OV, Nikitina OV, Gorshina ES, Rusinova TV, Serezhenkov VA, Burbaev DS, Gazaryan IG, Yaropolov AI. Biochimie. 2004;86:693. doi: 10.1016/j.biochi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Rusling JF. Anal Chem. 2001;73:4780. doi: 10.1021/ac0105639. [DOI] [PubMed] [Google Scholar]
- 31.Scodeller P, Carballo R, Szamocki R, Levin L, Forchiassin F, Calvo EJ. J Am Chem Soc. 2010;132:11132. doi: 10.1021/ja1020487. [DOI] [PubMed] [Google Scholar]
- 32.Wiebel M, Bright H. J Biol Chem. 1971;246:2734. [PubMed] [Google Scholar]
- 33.Shin H, Cho S, Heller A, Kang C. J Electrochem Soc. 2009;156:F87. [Google Scholar]
- 34.Ramírez P, Mano N, Andreu R, Ruzgas T, Heller A, Gorton L, Shleev S. Biochim Biophys Acta, Bioenerg. 2008;1777:1364. doi: 10.1016/j.bbabio.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Deng L, Wang F, Chen H, Shang L, Wang L, Wang T, Dong S. Biosens Bioelectron. 2008;24:329. doi: 10.1016/j.bios.2008.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.