Abstract
We aimed to estimate the association of BMI and risk of systemic hypertension in African-American females aged 65 years and older. In this retrospective, cross-sectional study, medical charts were randomly reviewed after obtaining institutional review board approval and data collection was conducted for height, weight, BMI, age, ethnicity, gender, and hypertension. A multivariable logistic regression analysis was performed. The mean BMI was significantly higher in hypertensive subjects than normotensives (30.3 vs. 29 kg/m2; P = 0.003). A higher proportion of hypertensive subjects had a BMI >23 kg/m2 as compared to normotensives (88.9% vs. 83.5%; P = 0.023). When the log odds of having a history of hypertension was plotted against BMI as a continuous variable, we found that the odds showed an increasing trend with increasing BMI and a steep increase after a BMI of 23 kg/m2. When BMI was analyzed as a categorical variable, a BMI of 23–30 kg/m2 was found to have an odds ratio of 1.43 (95% confidence interval 1.01–2.13; P = 0.05) and a BMI of >30 kg/m2 had an odds ratio of 1.76 (95% confidence interval 1.17–2.65; P = 0.007) when compared to a BMI of <23 kg/m2. This association remained significant in both univariate and multivariate analysis. We conclude that BMI is an independent predictor of hypertension in elderly African-American females. Our results indicate that the risk of hypertension increased significantly at BMI of >23 kg/m2 in this ethnic group. Weight reduction to a greater extent than previously indicated could play an integral role in prevention and control of high blood pressure in this particular population.
INTRODUCTION
Obesity is designated as a major risk factor for the development of various cardiovascular (CV) diseases by the American Heart Association (1). According to the 2007–2008 National Health and Nutritional Health Examination Survey (NHANES), 68% of US adults are overweight or obese (2). The percentage of obesity among adults 20–74 years of age has more than doubled from 1976–1980 to 2007–2008, increasing from 15 to 33.8% (2,3). Obesity accounts for 2–6% of total health-care costs in several developed countries; some estimates put the figure as high as 7%. The true costs are undoubtedly much greater as not all obesity-related conditions are included in these calculations. In the United States, $147 billions are spent on obesity each year (4).
Various anthropometric indexes such as BMI, waist or hip circumference, and waist-to-hip ratio have been implicated to estimate the CV risks (5–11). Obesity significantly raises risks for many fatal and morbid diseases including but not limited to hypertension, diabetes mellitus, coronary artery disease, depression, dyslipidemias, and various cancers. CV disease is the leading cause of mortality in the elderly population (12), with comparatively higher incidence and prevalence observed in overweight and/or obese African-American population (13). The age-adjusted death rate in African Americans exceeds the white people for ischemic heart disease and stroke, which continues to be the leading cause of death among the African-American population (14). About 50% of the African-American females >60 year of age are obese (2). Studies have demonstrated an increased CV risk in people with higher BMI, especially in elderly populations (15,16) Obesity has been shown to be associated with increased arterial stiffness, which contributes to systemic hypertension and increases the incidence and prevalence of this disease in the general population.
The World Health Organization (WHO) defines overweight and obesity as a BMI range of 25–29.9 and ≥30 kg/m2, respectively. These values were obtained from the BMI and mortality associations in European populations, which were J-shaped with the nadir of the curve between 18.5 and 25 kg/m2 (16–19). Similar finding were observed in Asian populations (20,21). These cutoffs have since been used as a standard in different populations and ethnic groups with the assumption that different ethnic groups have similar mortality and morbidity risk at these BMI cutoffs. However, controversy regarding the optimal BMI range in various ethnic populations still exists. It is translating into a much bigger problem in elderly population complicated by the phenomenon of sarcopenic obesity (22). Wannamethee et al. concluded that the current BMI cutoffs were appropriate when used to predict CV risk in 4,232 elderly men across 24 British towns (23). In contrast, certain recent studies conducted in Asian populations showed that Asians have a higher prevalence of CV disease, increased risk for diabetes mellitus, systemic hypertension, dyslipidemias, and albuminuria at comparatively lower BMI values (21,24,25). These phenomena could be explained, in part, by the findings demonstrating that Asian people have lower BMI values but higher body fat percentage than the white or other ethnic groups (26). For this reason, a WHO international task force has recommended overweight status for these populations at BMI of ≥23 kg/m2 (27). However, these studies and recommendations are limited to the Asian population.
Since the change in stratification parameters to define overweight and/or obesity in the Asian population, application of standardized cutoffs in other ethnicities are at risk of being rendered obsolete and requires further investigation. The higher prevalence of obesity in the elderly African-American female population and a higher CV mortality in this population led us to evaluate the relationship in this ethnic group. The primary objective of our study was to examine the association of BMI and hypertension in African-American females ≥65 years of age. The secondary end point was to determine specific BMI cutoff values as a predictor of hypertension in this ethnic population. Our study is unique as it evaluated the relationship between BMI and hypertension in an elderly African-American female population.
METHODS AND PROCEDURES
Study design
This is a retrospective, cross-sectional study. The St Luke’s Roosevelt Hospital Center institutional review board approved the study.
Subject selection
The study population consisted of 962 African-American females of age 65 years or above. These patients were selected by reviewing consecutive medical records from the New York Obesity Research Center.
Data collection
The data for this study were collected from the New York Obesity Research Center at St Luke’s Roosevelt Hospital Center, affiliated with Columbia University (New York, NY). Medical charts were reviewed after obtaining institutional review board approval and data pertaining to height, weight, BMI, age, ethnicity, gender, and hypertension for the period of January 1998–July 2008 were collected. After screening based on ethnicity (i.e., African-American female population), 631 patients were found to have high blood pressure (diagnosed systemic hypertension and/or use of antihypertensive medications) and 331 patients were without high blood pressure.
Definitions
Height of the patient was measured with a standard stadiometer, while patient was wearing a thin pair of socks and the weight was measured with a standard electric beam scale. BMI was calculated as weight in kg divided by height in m2 (kg/m2). WHO criteria was used for definitions pertaining to body weight ranges. Normal weight was defined as BMI of 20–24.9 kg/m2, overweight as BMI of 25–29.9 kg/m2, obesity as BMI of ≥30 kg/m2, and morbid obesity as BMI ≥40 kg/m2.
Hypertension was defined as (i) previous diagnosis of hypertension, (ii) blood pressure ≥140 mm Hg systolic or 90 mm Hg diastolic on at least two occasions, or (iii) use of antihypertensive pharmacological therapy. The data for defining a patient as a hypertensive or not was collected after careful review of the patient charts and examination reports.
History of diabetes mellitus was defined as (i) previous diagnosis of type 2 diabetes mellitus and/or (ii) use of antidiabetic medications.
Active smoking was defined as smoking within the previous 1 month. In our study, there were only a few subjects who were active smokers (23 hypertensive and 9 normotensive subjects).
Corticosteroid use was defined as daily use of corticosteroid therapy for any medical condition for a duration >3 weeks during the last 6 months.
Statistical analysis
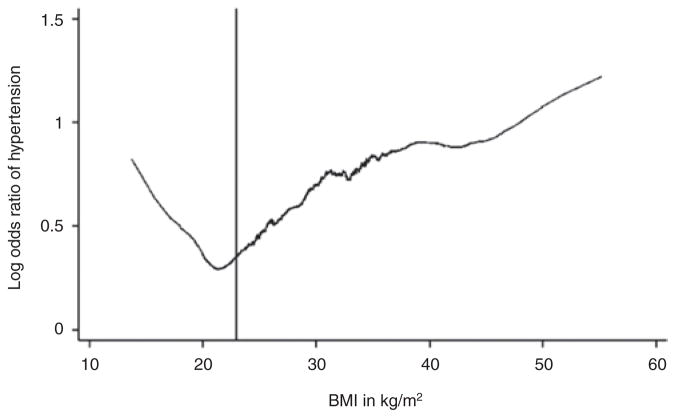
BMI was analyzed both as a continuous and categorical variable. Differences in the mean BMI between hypertensive and nonhypertensive participants were analyzed using the Student t-test. For differences in the proportions in each BMI category between the two groups, the χ2-test was used. Odds ratios and 95% confidence intervals for the associations between explanatory variables and a history of hypertension were estimated using multivariable logistic regression models. P values <0.05 were considered to be statistically significant. The association of hypertension and BMI was also graphed using the odds of hypertension and BMI as continuous variables and specific cutpoints were derived from the graph. Estimates for the association between the primary explanatory variable of interest (BMI) and a history of hypertension were adjusted in multivariate models that included potential confounding covariates that were statistically significant in univariate logistic regression or which were significant components of the confounding pathway. The inflection point for the incremental increase in odds for hypertension was derived from the figure and then different cutpoints were analyzed until the starting point for the increased risk was estimated. BMI as a continuous and as a categorical variable was included in different models. Thus the categories were designated to be <23, 23–30, and >30 kg/m2 and the odds were found to be 1.43 for 23–30 kg/m2 and 1.76, respectively when compared to a BMI of <23 kg/m2 which was statistically significant (Figure 1). These confounders included a history of diabetes mellitus, history of smoking, history of corticosteroid use. The association of hypertension and interactions between the confounders were also assessed. Statistical analysis was performed using STATA version 10 software package (STATA, College Station, TX).
Figure 1.
Relationship between log odds ratio of hypertension with BMI.
RESULTS
Out of a total of 962 subjects, 631 (65%) had a history of hypertension, while 331 (35%) did not. The baseline characteristics of the subjects are shown in Table 1. Subjects with a history of hypertension were found to have a higher prevalence of diabetes mellitus (25.8% vs. 12.5%; P < 0.001) as compared to those without a history of hypertension.
Table 1.
Baseline characteristics of hypertensive and normotensive subjects (N = 962)
| Subjects with a history of hypertension (n = 631) | Subjects without a history of hypertension (n = 331) | P | |
|---|---|---|---|
| Age, mean (s.d.) | 75 (6.7) | 77 (41.3) | 0.1292 |
| History of diabetes, n (%) | 163 (25.8) | 42 (12.5) | <0.001 |
| History of smoking, n (%) | 23 (3.7) | 12 (3.6) | 0.960 |
| History of corticosteroid use, n (%) | 14 (2.2) | 4 (1.2) | 0.262 |
| History of oophorectomy, n (%) | 92 (14.6) | 44 (13.1) | 0.539 |
Out of the 631 hypertensive patients, 219 (34.7%), 50 (7.9%), 38 (6%), and 33 (5.2%) were on angiotensin-converting enzyme inhibitor (ACE-I), diuretic, β-blocker (BB) and calcium channel blocker (CCB) monotherapy, respectively. Dual therapy included 34 (5.4%) on an ACE-I and diuretic, 24 (3.8%) on an ACE-I and BB, 24 (3.8%) on a BB and diuretic, 129 (20.4%) on ACE-I and CCB, 7 (1.1%) on CCB and diuretic, and 6 (0.9%) on a BB and CCB combination therapy. A total of 62 (9%) were on three antihypertensive agents of which 50 (7.9%) were on a CCB: ACE-I: diuretic regimen and 12 (1.9%) were on a BB: ACE-I: diuretic regimen. Only five (0.8%) were on other anti-hypertensive agents. The mean BMI was not significantly higher in patients on BB (n = 104) when compared to those on other antihypertensive medications (31 vs. 30 kg/m2, P = 0.16).
The distribution of the BMI of subjects is shown in Table 2. Subjects with a history of hypertension had higher mean BMI as compared to those without (30.3 kg/m2 vs. 29 kg/m2; P = 0.003). The proportion of subjects in the overweight and obese categories was also significantly higher in hypertensive subjects compared to nonhypertensive subjects (45% vs. 37%; P = 0.023).
Table 2.
Distribution of BMI among hypertensive and normotensive subjects (N = 962)
| BMIa | Subjects with a history of hypertension (n = 631) | Subjects without a history of hypertension (n = 331) | P |
|---|---|---|---|
| Mean (s.d.) | 30.3 | 29 | 0.003 |
| ≤23 | 70 (11.09) | 55 (16.42) | 0.023 |
| 23–30 | 277 (43.90) | 153 (45.67) | 0.04 |
| >30 | 284 (45.01) | 127 (37.91) | 0.023 |
BMI in kg/m2.
When a multivariable logistic regression analysis was done with hypertension as the independent variable, we see that the odds ratio for hypertension increases exponentially for every 1 kg/m2 increase after a BMI of 23 kg/m2, when BMI was analyzed as a continuous variable (Table 3 and Figure 1). The association remained constant even after adjusting for confounding factors including smoking, diabetes, and corticosteroid use. An interesting observation was a steep increase in the odds for hypertension after a BMI of 23 kg/m2. Though we observed an increase in odds of hypertension with BMI ≤18 kg/m2, this was not statistically significant (P = 0.85), as shown in Figure 1.
Table 3.
Univariate and multivariate analysis of hypertension and BMI
| Univariate analysis
|
Multivariate analysis
|
|||
|---|---|---|---|---|
| Odds ratio of hypertension (95% CI) | P | Odds ratio of hypertension (95% CI) | P | |
| BMI | 1.03 (1.01–1.05)a | 0.004 | 1.03 (1.01–1.05)a | 0.023 |
| History of diabetes | 2.43 (1.70–3.52) | <0.001 | 2.33 (1.60–3.38) | <0.001 |
| History of smoking | 1.02 (0.50–2.07) | 0.960 | 0.92 (0.44–1.91) | NS |
| History of corticosteroid use | 1.88 (0.61–5.75) | 0.270 | 1.96 (0.63–6.07) | NS |
| BMI ≤23 | 1 (Ref) | — | 1 (Ref) | — |
| BMI 23–30 | 1.43 (1.01–2.13) | 0.05 | 1.41 (1.01–2.12) | 0.05 |
| BMI >30 | 1.76 (1.17–2.65) | 0.007 | 1.59 (1.05–2.42) | 0.028 |
CI, confidence interval.
Rise in odds ratio with rise in BMI of 1 kg/m2.
When BMI was analyzed as a categorical variable (Table 3), a BMI of 23–30 kg/m2 was found to have an odds ratio of 1.43 (95% confidence interval 1.01–2.13; P = 0.05) and a BMI of >30 kg/m2 had an odds ratio of 1.76 (95% confidence interval 1.17–2.65; P = 0.007) when compared to a BMI of <23 kg/m2. This association remained significant even after adjusting for confounding factors.
DISCUSSION
Our study is unique in evaluating the relationship between BMI and hypertension in an elderly African-American female population in the United States. The prevalence of hypertension increases with increase in BMI, which corresponds with previous studies pointing to a positive relationship between BMI and hypertension in various ethnic populations (13). The higher prevalence of hypertension may be secondary to the fact that African Americans have been shown to have a high blood pressure compared to other ethnic populations, with the additive effect of the postmenopausal status in this specific cohort. The relative risk of being hypertensive as assessed by odds ratio was significantly higher with a higher BMI and concomitant diabetes mellitus, which was constant in the multivariate analysis.
We observed a novel yet robust finding when the curves were plotted for the log odds of hypertension with BMI in this African-American female population (Figure 1). It displayed a positive association with log odds ratio of hypertension. The steep rise in slope from a threshold BMI ≥23 kg/m2 demonstrates the significant growth of hypertension risk after this BMI value (P = 0.001). The results of our study suggest that in elderly African-American females a BMI <23 kg/m2, which falls into the normal weight category per WHO classification, should be considered as target BMI to avoid hypertension. The results from our study conflict with the recommendations of the US Department of Agriculture that higher weights are accepted and preferred in the elderly (28). It has already been debated that definitions of ideal body weight in terms of CV risks should be segregated according to age (29). A recent Danish study by Thinggaard et al. comprised of a patient population with an age range of 70–95 years has shown increasing mortality with higher BMI in the female population, which supports our hypothesis (30). Similarly, a 23-year follow-up of 1,723 patients in the Framingham Heart Study showed increased mortality with increasing BMI in the elderly (31).
The pathophysiology of the relationship between obesity and hypertension is multifactorial. Obesity is associated with increased arterial stiffness that contributes to hypertension and increases the incidence and prevalence of this disease in the general population. Arterial stiffness is strongly associated with obesity at any given age and increased stiffness augments the risk of CV disease in various populations (32–35). Age is an independent and powerful determinant of large artery stiffness, so the risk of CV events may increase in the elderly population secondary to increased obesity-related arterial stiffness. However, there is limited data related to obesity-associated risk of CV disorders in the elderly. Other noteworthy explanations of this association include more fat mass in females as compared to men, an increase in the percent body fat with age in the elderly population (36), disproportionate distribution of fat and lean body mass in African Americans (37) and sedentary lifestyle (38). Furthermore, there is a difference in the attitudes and cultural beliefs toward body weight in addition to the fact that elderly African-American females have a comparatively lower exercise capacity (39,40).
Our study results strongly suggest a higher risk of systemic hypertension at a BMI of ≥23 kg/m2, which as per WHO classification is rendered as normal BMI. This cutoff for BMI incidentally is the same as that recommended by the WHO committee for the Asian population. Of note, we are reporting the association between BMI and hypertension in this population but the cross-sectional nature of our study does not allow mortality and morbidity evaluation. In the Nurses Health Study, it has been shown that obesity and progressive weight gain with age are associated with increased risks for CV diseases and premature mortality (41). Similarly, maintaining lower BMI values in elderly AfricanAmericans may reduce the development of systemic hypertension, which is a critical risk factor for cerebrovascular accidents and coronary artery disease leading to higher morbidity and mortality (29). Previously, studies have shown that there exists an “obesity paradox” wherein people with a higher BMI have comparatively better CV prognosis as compared to normal weight individuals. However, these studies put an emphasis on “purposeful” weight reduction that can be achieved by loss of more fat mass as opposed to lean muscle mass, which lowers mortality. Weight loss also helps in decreasing the risk for CV disease risk factors (1,42). Our study strikingly demonstrated a higher incidence of hypertension at a BMI <18.5 kg/m2, Though not statistically significant, the trend is consistent with previous studies that malnourishment is associated with increased CV mortality (31,43).
However, randomized studies are required to prove whether achieving a BMI <23 kg/m2 would translate into a decreased CV mortality in elderly African-American women.
This study further emphasizes the need of ethnic specific BMI cutoff modifications in various populations as shown by the proposed changes in the recommendations by WHO Task Force for the Asian population (27).
Study limitations
There are some limitations in our study, including its cross-sectional nature and lack of follow-up data. We could not assess the physical activity and dietary habits, including salt intake, which can influence BMI and hypertension and act as potential confounders. The data on other anthropometric variables were not available for all the study population and therefore could not be compared in this study. The population included in our study was urban females and thus the results may not extrapolate to the general population.
Acknowledgments
We are thankful to the entire research team of the New York Obesity Research Center (NYORC) for their untiring efforts in the completion of this study. This research project was supported by NYORC National Institute of Health (NIH) research grants.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Poirier P, Giles TD, Bray GA, et al. American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 4.Sherry B, Blanck G, Pan L, Dietz WH, Balluz L. Vital Signs: State-Specific Obesity Prevalence Among Adults—United States. Centers for Disease Control and Prevention; 2009. < http://www.cdc.gov/mmwr/preview/mmwrhtml/mm59e0803a1.htm?s_cid=mm59e0803a1_e%0D%0A> (2010) [PubMed] [Google Scholar]
- 5.Panagiotakos DB, Chrysohoou C, Pitsavos C, et al. Hierarchical analysis of anthropometric indices in the prediction of 5-year incidence of hypertension in apparently healthy adults: the ATTICA study. Atherosclerosis. 2009;206:314–320. doi: 10.1016/j.atherosclerosis.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 7.Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 8.Allison DB, Zhu SK, Plankey M, Faith MS, Heo M. Differential associations of body mass index and adiposity with all-cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int J Obes Relat Metab Disord. 2002;26:410–416. doi: 10.1038/sj.ijo.0801925. [DOI] [PubMed] [Google Scholar]
- 9.Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 10.Unal B, Critchley JA, Capewell S. Modelling the decline in coronary heart disease deaths in England and Wales, 1981–2000: comparing contributions from primary prevention and secondary prevention. BMJ. 2005;331:614. doi: 10.1136/bmj.38561.633345.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart Disease and Stroke Statistics-2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 13.Crawford AG, Cote C, Couto J, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE Centricity Electronic Medical Record database. Popul Health Manag. 2010;13:151–161. doi: 10.1089/pop.2009.0039. [DOI] [PubMed] [Google Scholar]
- 14.Towfighi A, Ovbiagele B, Saver JL. Therapeutic milestone: stroke declines from the second to the third leading organ- and disease-specific cause of death in the united states. Stroke. 2010;41:499–503. doi: 10.1161/STROKEAHA.109.571828. [DOI] [PubMed] [Google Scholar]
- 15.He Y, Jiang B, Wang J, et al. BMI versus the metabolic syndrome in relation to cardiovascular risk in elderly chinese individuals. Diabetes Care. 2007;30:2128–2134. doi: 10.2337/dc06-2402. [DOI] [PubMed] [Google Scholar]
- 16.Song Y, Manson JE, Meigs JB, et al. Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. Am J Cardiol. 2007;100:1654–1658. doi: 10.1016/j.amjcard.2007.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Report of a WHO Consultation. WHO Technical Report Series No 894. Obesity: Preventing and Managing the Global Epidemic. [PubMed] [Google Scholar]
- 18.Report of a WHO Expert Committee. WHO technical report series no 854. World Health Organization; Geneva, Switzerland: 1995. Physical Status: The Use and Interpretation of Anthropometry. [PubMed] [Google Scholar]
- 19.Deurenberg P, Deurenberg Yap M, Wang J, Lin FP, Schmidt G. The impact of body build on the relationship between body mass index and percent body fat. Int J Obes Relat Metab Disord. 1999;23:537–542. doi: 10.1038/sj.ijo.0800868. [DOI] [PubMed] [Google Scholar]
- 20.Ko GT, Chan JC, Cockram CS, Woo J. Prediction of hypertension, diabetes, dyslipidaemia or albuminuria using simple anthropometric indexes in Hong Kong Chinese. Int J Obes Relat Metab Disord. 1999;23:1136–1142. doi: 10.1038/sj.ijo.0801043. [DOI] [PubMed] [Google Scholar]
- 21.The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. International Diabetes Institute; Victoria, Australia: 2000. < http://www.diabetes.com.au/research/report_obesity.htm>. [Google Scholar]
- 22.Stephen WC, Janssen I. Sarcopenic-obesity and cardiovascular disease risk in the elderly. J Nutr Health Aging. 2009;13:460–466. doi: 10.1007/s12603-009-0084-z. [DOI] [PubMed] [Google Scholar]
- 23.Goya Wannamethee S, Gerald Shaper A, Whincup PH, Walker M. Overweight and obesity and the burden of disease and disability in elderly men. Int J Obes Relat Metab Disord. 2004;28:1374–1382. doi: 10.1038/sj.ijo.0802775. [DOI] [PubMed] [Google Scholar]
- 24.Deurenberg-Yap M, Chew SK, Deurenberg P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes Rev. 2002;3:209–215. doi: 10.1046/j.1467-789x.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- 25.Thomas GN, Ho SY, Lam KS, et al. Hong Kong Cardiovascular Risk Factor Prevalence Study Steering Committee. Impact of obesity and body fat distribution on cardiovascular risk factors in Hong Kong Chinese. Obes Res. 2004;12:1805–1813. doi: 10.1038/oby.2004.224. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Thornton JC, Russell M, et al. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr. 1994;60:23–28. doi: 10.1093/ajcn/60.1.23. [DOI] [PubMed] [Google Scholar]
- 27.The Asia-Pacific Perspective: Redefining Obesity and Its treatment. International Diabetes Institute; Victoria, Australia: 2000. < http://www.diabetes.com.au/research/report_obesity.htm>. [Google Scholar]
- 28.The Report of the Dietary Guidelines Advisory Committee on Dietary Guidelines for Americans. 2005 < http://www.health.gov/dietaryguidelines/dga2005/report>.
- 29.Krauss RM, Eckel RH, Howard B, et al. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 30.Thinggaard M, Jacobsen R, Jeune B, Martinussen T, Christensen K. Is the relationship between bmi and mortality increasingly u-shaped with advancing age? A 10-year follow-up of persons aged 70–95 years. J Gerontol A Biol Sci Med Sci. 2010;65A:526–531. doi: 10.1093/gerona/glp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris T, Cook EF, Garrison R, et al. Body mass index and mortality among nonsmoking older persons. The Framingham Heart Study. JAMA. 1988;259:1520–1524. [PubMed] [Google Scholar]
- 32.Zebekakis PE, Nawrot T, Thijs L, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens. 2005;23:1839–1846. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 33.Iannuzzi A, Licenziati MR, Acampora C, et al. Carotid artery stiffness in obese children with the metabolic syndrome. Am J Cardiol. 2006;97:528–531. doi: 10.1016/j.amjcard.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 34.Safar ME, Czernichow S, Blacher J. Obesity, arterial stiffness, and cardiovascular Risk. J Am Soc Nephrol. 2006;17:109–111. doi: 10.1681/ASN.2005121321. [DOI] [PubMed] [Google Scholar]
- 35.Kumanyika SK, Landis JR, Matthews-Cook YL, Almy SL, Boehmer SJ. Systolic blood pressure trends in US adults between 1960 and 1980: influence of antihypertensive drug therapy. Am J Epidemiol. 1998;148:528–538. doi: 10.1093/oxfordjournals.aje.a009678. [DOI] [PubMed] [Google Scholar]
- 36.Ding J, Kritchevsky SB, Newman AB, et al. Harris for the Health ABC Study. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am J Clin Nutr. 2007;85:405–410. doi: 10.1093/ajcn/85.2.405. [DOI] [PubMed] [Google Scholar]
- 37.Aleman Mateo H, Lee SY, Javed F, et al. Elderly Mexicans have less muscle and greater total and truncal fat compared to African-Americans and Caucasians with the same BMI. J Nutr Health Aging. 2009;13:919–923. doi: 10.1007/s12603-009-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeno SA, Kim-Dorner SJ, Deuster PA, et al. Cardiovascular fitness and risk factors of healthy African Americans and Caucasians. J Natl Med Assoc. 2010;102:28–35. doi: 10.1016/s0027-9684(15)30472-7. [DOI] [PubMed] [Google Scholar]
- 39.Millstein RA, Carlson SA, Fulton JE, et al. Relationships between body size satisfaction and weight control practices among US adults. Medscape J Med. 2008;10:119. [PMC free article] [PubMed] [Google Scholar]
- 40.Lavie CJ, Kuruvanka T, Milani RV, Prasad A, Ventura HO. Exercise capacity in adult African-Americans referred for exercise stress testing: is fitness affected by race? Chest. 2004;126:1962–1968. doi: 10.1378/chest.126.6.1962. [DOI] [PubMed] [Google Scholar]
- 41.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA. 1995;273:461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 42.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Bopp MM, Roberson PK, Sullivan DH. Undernutrition and risk of mortality in elderly patients within 1 year of hospital discharge. J Gerontol A Biol Sci Med Sci. 2002;57:M741–M746. doi: 10.1093/gerona/57.11.m741. [DOI] [PubMed] [Google Scholar]