Abstract
The influence of non-additive genetic influences on personality traits has been increasingly reported in adult populations. Less is known, however, with respect to younger samples. In this study, we examine additive and non-additive genetic contributions to the personality trait of extraversion in 1,689 Dutch twin pairs, 1,505 mothers and 1,637 fathers of the twins. The twins were on average 15.5 years (range 12–18 years). To increase statistical power to detect non-additive genetic influences, data on extraversion were also collected in parents and simultaneously analyzed. Genetic modeling procedures incorporating age as a potential modifier of heritability showed significant influences of additive (20–23%) and non-additive genetic factors (31–33%) in addition to unshared environment (46–48%) for adolescents and for their parents. The additive genetic component was slightly and positively related to age. No significant sex differences were found for either extraversion means or for the magnitude of the genetic and environmental influences. There was no evidence of non-random mating for extraversion in the parental generation. Results show that in addition to additive genetic influences, extraversion in adolescents is influenced by non-additive genetic factors.
Keywords: Personality, Temperament, Adolescence, Extraversion, Genetic, Environment
Introduction
Extraversion refers to a tendency towards being outgoing, energetic, and social. Over time, this trait has been confirmed as one of the major higher order personality dimensions according to a Big Three (Eysenck and Eysenck 1975) or Big Five taxonomy (Costa and McCrae 1992). Extraversion has links to other partially overlapping dimensions such as surgency (Rothbart et al. 2001), novelty seeking (Cloninger et al. 1994), and positive emotionality (Tellegen 1985). Components of extraversion often include aspects such as warmth, sociability, assertiveness, and ease to experience emotions such as happiness and joy. In addition, there can be a tendency towards impulsivity, aggressiveness, and extravagance.
Findings from adult twin studies have confirmed moderate genetic influence of extraversion similar to most other major personality dimensions (Johnson et al. 2004; Loehlin 1992; Riemann et al. 1997). One large study of approximately 15,000 Finnish twins aged 18–59 found evidence for decreasing heritability with age from 52 to 41% (Viken et al. 1994). No significant sex differences in the genetic and environmental parameters were found. Heath and coworkers found evidence for very large additive genetic effects (73%) in their study of 826 adult female twins (Heath et al. 1992).
One limitation of classical twin studies has been a lack of power to detect possible non-additive genetic effects (Eaves 1972; Martin et al. 1978; Posthuma and Boomsma 2000). Hur found evidence for non-additive genetic effects for hostility and for most of the Eysenck personality scales with the exception of neuroticism and extraversion (Hur 2006, 2007). However, these studies were not powered to distinguish between additive and non-additive components. Some researchers have employed extended twin designs that include data of siblings, parents, or other relatives to increase their statistical power (Posthuma and Boomsma 2000). The results of many of these efforts have indeed been the finding of significant additive and non-additive genetic influences across several major personality dimensions (Eaves et al. 1998; Keller and Coventry 2005; Keller et al. 2005). For extraversion, the Keller et al. study reported a significant estimate of non-additive genetic variance in a large sample of adult Australian twins and their siblings (Keller et al. 2005). Evidence for non-additive genetic effects has also been found for other personality traits such as propensity towards anger and “type A” personality (Rebollo and Boomsma 2006a, b).
There is substantially less information on the etiology of individual differences in extraversion in children and adolescents. While not measuring extraversion per se, early studies by Buss and Plomin (1984) found evidence for substantial genetic effects and possible non-additive genetic variance for activity level and sociability as indicated by markedly higher correlations for monozygotic (MZ) compared to dizygotic (DZ) twins (the latter were occasionally negative). Studies in children, however, have had to rely on informants who rate the behavior of both twins. In this design, non-additive genetic influences are difficult to distinguish from rater contrast effect, in which an informant magnifies differences in DZ twins to a larger extent than in MZ twins (Rietveld et al. 2003). Many studies of infants and toddlers show heritability estimates for extraversion and its correlates from approximately 35–47% across twin and adoption designs (Dilalla and Jones 2000). From the MacArthur Longitudinal Twin Study, the trait of positive affect and extraversion was found to have a broad heritability (i.e., the added effects of additive and non-additive genetic influence) of 24–35% for young children aged 14–24 months (Saudino and Cherny 2001). Another twin study of Norwegian youths between 7 and 17 years of age using the parent-report EAS scale (Buss and Plomin 1984) found high heritabilities for both sociability and activity level, although their study could not differentiate between additive and non-additive effects (Gjone and Stevenson 1997).
The one age group that has been relatively neglected in genetic studies of personality is adolescence. One exception was a self-report study of 540 Australian adolescent twins aged 12–16 years (Gillespie et al. 2004). The authors found that additive genetic effects explained between 41 and 47% of the variance in extraversion. No sex differences were found with the genetic and environmental parameters and most of the genetic influence at age 12 continued to exert an effect at age 14 and 16. This study, however, was not sufficiently powered to fully estimate non-additive genetic or shared environmental effects.
Most twin studies have generally failed to find gender differences in the magnitude of the genetic and environmental influences underlying extraversion. Tests for age and gender effects on extraversion means in these studies show that, from early to middle adulthood, males have higher extraversion scores, with both males and females showing decreasing levels over time (Viken et al. 1994). Non-twin studies spanning multiple cultures also show mean decreases in extraversion from adolescence into middle age (McCrae et al. 2000). Other investigators, however, have found few gender differences in young adulthood and no decrease over many years (McGue et al. 1993). Keller and colleagues found slightly higher means in women that decreased with age (Keller et al. 2005). Gillespie et al. found higher extraversion means in males but only at age 12. Gjone and Stevenson in their child and adolescent sample found decreasing activity and sociability scores over a 2 year interval and lower sociability scores in boys (Gjone and Stevenson 1997).
We present self-report data from a sample of 3,314 twins (aged 12–18 years) from the Netherlands Twin Registry to study the genetic architecture of extraversion as a function of age and sex. The use of a self-report instrument solves the problem of having to differentiate between non-additive genetic effects and potential contrast effects. Because of the addition of parental data, this study is equipped to examine the effects of potentially confounding factors such as assortative mating and has sufficient power to differentiate additive from non-additive genetic effects (Boomsma et al. 2002a, 2003). To our knowledge, this is the largest twin study on adolescent extraversion to date.
Method
Participants
This study was developed from the Netherlands Twin Register—a large ongoing twin-family study of health, lifestyle and personality. The details of this study have been presented elsewhere (Boomsma et al. 2000, 2002b; Koopmans and Boomsma 1996). Briefly, families were recruited by asking city councils in the Netherlands for the addresses of twins aged 12–22. In total, 252 city councils provided approximately 4,000 addresses in the first wave of data in 1991. A second wave of questionnaires was sent in 1993 to twins who both did and did not respond the first wave of questionnaires and 1987 new addresses in additional Dutch cities. The present study focuses on adolescent twins between the ages of 12 and 18 and their parents who were assessed through these two waves of mailed surveys. For those subjects who completed questionnaires at both time points, we used their data from the first occasion in order to obtain a younger sample.
The final adolescent sample included 3,314 twin individuals (1,625 twin pairs with complete data and 64 pairs with incomplete data). Extraversion scores were also obtained from their parents (1,637 mothers and 1,505 fathers). The twin sample was composed of 291 MZ male twin pairs, 403 MZ female twin pairs, 244 DZ male twin pairs, 261 DZ female twin pairs, and 490 DZ opposite sex twin pairs. The average age of the twins was 15.48 years (SD = 1.41 years). The average age of mothers and fathers was 43.53 (SD = 4.27) and 45.75 (SD = 4.78), respectively.
Measures
Extraversion was assessed using the Amsterdamse Biografische Vragenlijst (ABV), which is a 107 item self-report personality instrument similar in content to the Eysenck Personality Questionnaire (Eysenck and Eysenck 1975). The scale has demonstrated good reliability and external validity (Wilde 1970). The extraversion scale comprises 21 statements (Cronbach’s α = 0.84) to which the respondent answers along a 3-point scale (no, do not know, yes). If more than three items are missing or given multiple responses, the score is not computed. If there are three or less missing items, those items are converted into the “don’t know” response. The final extraversion score is then calculated as a weighted sum of the 21 items and can vary from 11 to 88. The items of the extraversion scale, translated in English, are shown in Table 1. In addition to extraversion, the ABV contains scales for neuroticism, somatic complaints, and test attitude. The neuroticism data have been presented elsewhere (Rettew et al. 2006).
Table 1.
Extraversion Items from the Amsterdamse Biografische Vragenlijst (ABV), translated from Dutch
| 1. Do you prefer to keep your contact with other people limited to a few very good friends and acquaintances? (R) |
| 3. Do you almost always have an answer ready if someone makes some comment to you? |
| 6. Are you usually quick and certain in your actions? |
| 13. Do you gladly seek company and do you like contact with other people? |
| 22. Do you often have a wonderful time at parties and that sort of thing? |
| 30. Does it usually come easy to you to make new acquaintances? |
| 38. Can you easily let yourself go at a merry party and enjoy it tremendously? |
| 42. When you are in company, do you have the tendency to behave inconspicuously? (R) |
| 45. Do you think you are a talkative person? |
| 47. Would you feel very unhappy if it was impossible for you to get in contact with many other people? |
| 49. Do you enjoy occasions most when there needs to be quick action? |
| 53. Do other people think that you are a lively person? |
| 59. When you do something with a group of people, do you usually prefer to be in charge? |
| 60. Are you usually unconcerned about the next day? |
| 64. Do you think that you are a lively person? |
| 70. Do you enjoy having a lot of appointments and associate with a lot of other people? |
| 73. Do you find it difficult to let yourself go, even at a cheerful party? (R) |
| 77. Do you like work which demands a lot of accuracy, even in the small details? |
| 79. Do you prefer to stay in the background, when you are in company? (R) |
| 84. Does it initiate with you when you make new friends and acquaintances? |
| 85. Do you enjoy participating when people let themselves go in jubilant mood? |
R = reversed
Zygosity
Zygosity assignment in the same-sex twin pairs was based on DNA typing for 338 twin pairs. In the remaining pairs, zygosity was assessed using a questionnaire that asked about the degree of similarity between the twins and confusion by family, friends, and strangers. The level of agreement was 97% between the two methods (Rietveld et al. 2000; Willemsen et al. 2005). If there was a disagreement between methods, DNA zygosity was used.
Genetic analyses
In genetic analyses, models are tested that ascribe variation in a variable of interest into several components (Plomin et al. 2001). Additive genetic influence (A) describes the effect of multiple genes that exert influence in a linear or additive fashion. Non-additive genetic factors (NA), by contrast, describe interactive effects of different alleles and include genetic dominance (within locus interaction) and epistasis (across locus interaction). In most twin studies, non-additive effects are modeled as genetic dominance (D). Common environmental factors (C) represent environmental effects that tend to make members of the same family more similar whereas unique environmental factors (E) influence siblings in the same family to be different from each other. This last term also includes measurement error. Genetic modeling takes advantage of the varying degrees of genetic relatedness among different types of twins. MZ twin share all of their additive and non-additive genetic effects while DZ twins share 50% of their additive and 25% of the non-additive genetic effects, on average, in the absence of assortative mating. As shared environmental effects and non-additive genetic effects are confounded in a twin model, only one of them can be estimated. The choice between C and D is based on the pattern of twin correlations and/or results from previous literature. The addition of data from parents of twins increases the statistical power to estimate genetic parameters and to assess the extent to which there is assortative mating and cultural transmission. Parents and offspring also share A, but not D. The correlation between additive genetic values of parents and offspring is 0.5, in the absence of assortative mating.
We fitted a series of theoretical models to the twin-parent data using structural equation modeling with maximum likelihood estimation of parameters. Nested models were evaluated to arrive at the best fitting model, starting with a saturated model in which all parameters (means, variances, and covariances between relatives) were allowed to vary freely. Subsequent nested models that attempt to constrain and simplify the model were compared by subtracting differences in the log-likelihood (−2LL), obtaining a chi-square statistic. A non-significant P-value indicates that the subsequent model can be retained without a significant loss of fit and is then used as the basis of comparison for additional nested models. Because of the large sample sizes, an alpha level of 0.01 was chosen. Due to the sensitivity of the Chi-square to the sample size, we also used a descriptive measure of the overall fit of the model, the root mean square error of approximation (RMSEA). Values under 0.05 can be considered as a good fit, values between 0.05 and 0.08 as an adequate fit, and values between 0.08 and 0.10 as a mediocre fit, whereas values above 0.10 are not acceptable (Schermelleh-Engle et al. 2003).
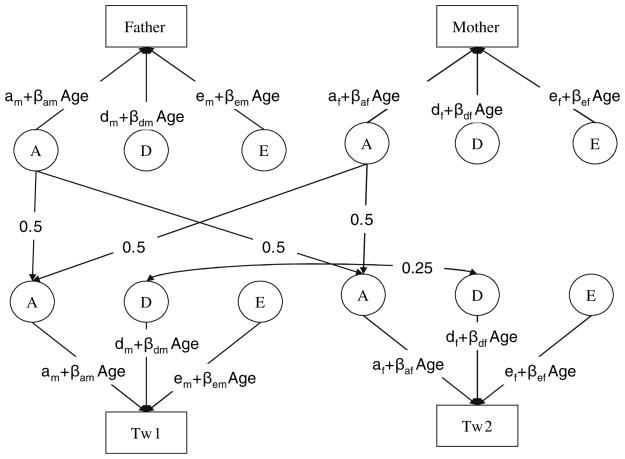
All model fitting utilized the structural equation software package Mx (Neale et al. 2004). Before proceeding to the primary models of interest, several assumptions were tested, including whether or not the mean, variances, and covariances can be assumed to be equal between and among twins and parents. With non-additive genetic influence, the covariance between twins is not expected to be equal to the covariance between twins and parents. The results of these tests inform our genetic modeling. In the genetic models, we estimated the influence of A, D, and E on extraversion and allowed these estimates to be a function of measured moderator variables age and sex (Purcell 2002). The full model is depicted in Fig. 1 for an opposite sex twin pair. The estimates for the genetic and environmental parameters are initially allowed to differ between males and females. Additionally, each of the a, d, and e parameters can be moderated by age. For example, the additive genetic parameter for a male twin includes the mean component for males (am) plus the age modifying coefficient for males βam multiplied by the subject’s age. The additive genetic variance is obtained by squaring the entire term, i.e., (am+ βamAge)2. The models we used assume equilibrium across generations, that is, that the parameter estimates for the variance components are equal for both parents and offspring. Note, however, that evidence for a moderator effect of age will lead to differences in variances and heritabilities between parents and offspring. No constraints across generations were put on extraversion means. The models also assume no assortative mating by extraversion which could increase correlations between DZ twins. This particular assumption was first tested within this dataset by computing the Pearson correlation between extraversion scores of mothers and fathers of twins. As the raw extraversion scores approximated a normal distribution, no transformation was applied. Age and sex differences on the means were tested in the saturated model. None were significant and thus are not included in the genetic model.
Fig. 1.
Full model for an opposite sex pair where the first born is a male and the second born is a female. Tw1 and Tw2 represent the phenotype for first and second born twins, and the boxes above represent the phenotypes of the parents. The total variance of extraversion is explained by A-Additive genetic effects, D-non-additive genetic effects, and E-non-shared environmental effects. Each parent shares with each of the twins an average of 50% of the additive genetic variation. This turns into an average correlation between the additive genetic effects of the OS twins of 0.5. DZ twins share on average 25% of the non-additive genetic variation. The phenotypes of the parents are uncorrelated, according to the results of the saturated model. Different parameters are estimated in the full model for males and females, represented by the subscripts m-males and f-females. Age is included in the model as a moderator, modeling the effects of each source of variance as a linear function of age. Therefore, a, d and e are the unmoderated components of the variance, and βa*Age, βd*Age and βe*Age are the moderated components. It is an assumption of the model that no generational effects exist, and the same decomposition of the variance applies to the parental and offspring generations
Results
Tests of assumptions
Extraversion means, standard deviations and correlations are presented in Tables 2. Table 3 shows the variance-covariance and correlation matrices by zygosity group, as estimated by the full saturated model. In the saturated model, the means and variances were constrained to be equal for both members of a twin pair, and equal for mother and father across zygosity groups. The parent-offspring correlations are also constrained to be equal across zygosity groups. In this saturated model, a number of assumptions regarding the means, variances, and covariances between groups were tested using Mx. Extraversion means did not differ between MZ and DZ twins and between male and female twins (submodels 3 and 4). Significant differences were found, however, between parents and children with adolescents having higher extraversion scores than their parents (submodel 5). Constraining the age regression effect on the means at zero did not worsen the fit of the model (submodel 2). Thus, the differences in extraversion scores between parents and offspring may not be directly attributed to age, but may reflect cohort effects. Tests for homogeneity of the variance across sex, generation, and zygosity (submodels 6–8) found no differences with the exception of larger variances in parents compared to their offspring.
Table 2.
Extraversion means and standard deviations for offspring (twins) and their parents
| N | Mean | SD | |
|---|---|---|---|
| Male twins | 1,529 | 58.51 | 15.11 |
| Female twins | 1,785 | 58.71 | 15.05 |
| Fathers | 1,505 | 53.27 | 17.41 |
| Mothers | 1,637 | 53.64 | 16.44 |
SD = standard deviation
Table 3.
Variance–covariance matrix (lower triangle) and correlation matrix (upper triangle) in extraversion scores for twins and their parents by zygosity as estimated under the saturated model in Table 4
| Twin 1 | Twin 2 | Father | Mother | |
|---|---|---|---|---|
| MZM | ||||
| Twin 1 | 232.63 | 0.51 | 0.16 | 0.14 |
| Twin 2 | 120.34 | 232.63 | 0.16 | 0.14 |
| Father | 39.71 | 39.71 | 285.92 | 0.04 |
| Mother | 37.51 | 37.51 | 14.27 | 285.92 |
| DZM | ||||
| Twin 1 | 229.94 | 0.14 | 0.16 | 0.14 |
| Twin 2 | 32.35 | 229.94 | 0.16 | 0.14 |
| Father | 39.71 | 39.71 | 285.92 | 0.04 |
| Mother | 37.51 | 37.51 | 14.27 | 285.92 |
| MZF | ||||
| Twin 1 | 229.88 | 0.56 | 0.19 | 0.10 |
| Twin 2 | 129.71 | 229.88 | 0.19 | 0.10 |
| Father | 47.88 | 47.88 | 285.92 | 0.04 |
| Mother | 24.95 | 24.95 | 14.27 | 285.92 |
| DZF | ||||
| Twin 1 | 214.12 | 0.21 | 0.19 | 0.10 |
| Twin 2 | 45.79 | 214.12 | 0.19 | 0.10 |
| Father | 47.88 | 47.88 | 285.92 | 0.04 |
| Mother | 24.95 | 24.95 | 14.27 | 285.92 |
| DZMF | ||||
| Twin 1 | 213.87 | 0.06 | 0.16 | 0.14 |
| Twin 2 | 13.45 | 213.87 | 0.19 | 0.10 |
| Father | 39.71 | 47.88 | 285.92 | 0.04 |
| Mother | 37.51 | 24.95 | 14.27 | 285.92 |
| DZFM | ||||
| Twin 1 | 242.73 | 0.01 | 0.19 | 0.10 |
| Twin 2 | 4.17 | 242.73 | 0.16 | 0.14 |
| Father | 47.88 | 39.71 | 285.92 | 0.04 |
| Mother | 24.95 | 37.51 | 14.27 | 285.92 |
MZM = monozygotic twins, males; DZM = dizygotic twins, males; MZF = monozygotic twins, females; DZF = dizygotic twins, females; DOS = opposite sex twins
In submodels 9.1–9.4, covariances could be constrained to be equal between adolescent males and females for each zygosity group and between same sex and opposite sex DZ twins. This last step indicates an absence of sex-specific genes influencing the trait (Eaves et al. 1998). Finally, in submodels 10–12, no significant differences were found in the covariances between parents and offspring by sex (e.g., in the correlations between mothers and daughters compared to mothers and sons). Given the constraints allowed in the best fitting model (12), correlations were recalculated in Mx along with 95% confidence intervals. The MZ correlation across males and females was 0.54 (95% CI 0.49–0.59) and the DZ correlation incorporating male, female, and opposite sex twins of either birth order was .11 (95% CI 0.05–0.17). The correlation between parents and twins was very similar to the DZ correlation with a point estimate of 0.15 (95% CI 0.12–0.17). The overall pattern of correlations suggests that in addition to additive genetic influences, non-additive effects will also play a role.
Test for assortative mating
Assortative mating refers to the phenotypic similarity between parents. The presence of assortative mating can increase the genetic variance in the offspring generation and will increase the dizygotic twin and parent-offspring correlations and thus either lower heritability estimates (in the classical twin design) or increase heritability estimates based on parent-offspring data (Agrawal et al. 2006; Plomin et al. 2001; Van Leeuwen et al. in press). In the current study, the correlation between mothers and fathers was obtained from the best fitting model and found to be non significant (point estimate 0.05; 95% CI −0.00 to 0.10).
Genetic modeling of extraversion scores
Our primary genetic models tested the significance of a, d, and e parameters and included age as modifier for the variance components. The full genetic model was compared to the saturated model number 7, as the genetic model is not nested under the most restricted model 12. As shown in Table 5, the first model (1) tested against the saturated model allowed the magnitude of the parameters and the age modifier coefficients (Betas) to differ between males and females. The fit of this model differed significantly from that of the saturated model, however the RMSEA provided an acceptable value under 0.10, and thus the model was retained. Model 2 attempted to constrain the age regression components of the genetic and environment parameters to be equal between males and females and was also retained (P > 0.01). The next steps attempted to fix each of the age regression coefficients for the three (a, d, e) parameters to zero. This resulted in no deterioration of fit for βd and βe but was rejected for βa. Model 4 showed the best fit and, according to the RMSEA below 0.05, it also provided a good fit to the data. Thus, the best fitting model was an ADE model that constrained estimates for parameters in males and females to be equal and one in which the additive genetic component increased slightly with age. The overall variance in the parental generation was larger than in the offspring generation because the additive genetic variance increased as a function of age. The standardized estimate for A across the parent age range was 29.2% at age 30 and rose to 34.4% at age 40 and 39.3% by age 50. As the age coefficients for D and E could be constrained to 0, the unstandardized estimates remained the same and thus, their standardized estimates decreased as A increased. Thus, broad-sense heritability (%a + %d) in adults across the age range of most parents rose from 57.9% at age 30 to 63.9% at age 50.
Table 5.
Primary model-fitting results for extraversion scores
| −2LL | DF | CT | X2 (df difference) | P | RMSEA | |
|---|---|---|---|---|---|---|
| 1 ADE M and F, βa, βd & βe M and F | 53720.65 | 6,442 | SAT* | 10.34 (1) | <0.01 | 0.086 |
| 2 ADE βa, βd & βe M = F | 53735.92 | 6,448 | 1 | 15.27 (6) | 0.01 | 0.043 |
| 3 ADE βa, βd βe = 0 | 53736.12 | 6,449 | 2 | 0.20 (1) | 0.65 | 0.039 |
| 4 ADE βa | 53736.43 | 6,450 | 3 | 0.31 (1) | 0.58 | 0.036 |
| βd = 0 | SAT* | 29.26 (9) | <0.01 | |||
| 5 ADE βa = 0 | 53776.75 | 6,451 | 4 | 40.32 (1) | <0.001 | 0.059 |
A = additive genetics; D = non-additive genetics, E = unshared environment; −2LL = −2 Log likelihood; DF = Degrees of freedom; CT = Compared to model number; βa = modifier age on A, βd = modifier age on D, βe = modifier age on E. AIC = Aikieke Information Criteria; RMSEA = root mean square error of approximation; M = male; F = female; Bold print indicates best fitting model.
This model is compared to model 7 in Table 4. See text for details
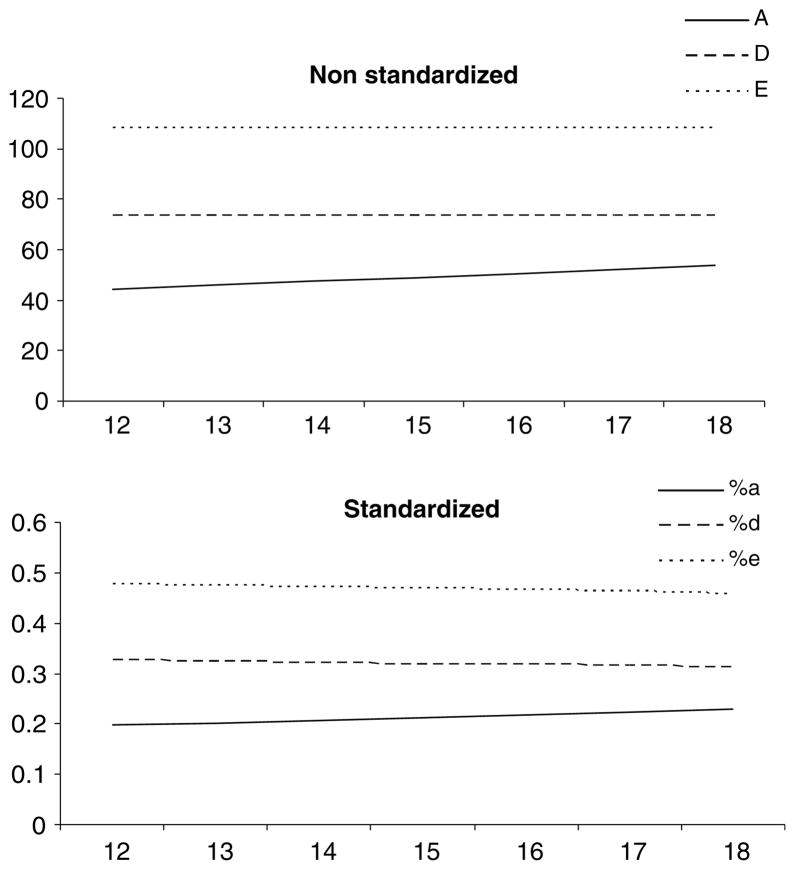
For the adolescent twins, Table 6 displays the percentage of variance attributable to each of the parameters by age. As shown, non-additive genetics explained approximately 31–33% of the variance across all ages while unshared environmental factors contributed between 46% and 48%. Between 20% and 23% of the variance was explained by additive genetics, and this amount increased slightly with age, as depicted graphically in Fig. 2. The unstandardized beta for the regression coefficient for additive genetics was calculated at 0.11.
Table 6.
Extraversion raw and standardized variance parameters attributed to additive genetics, non-additive genetics, and unshared environment Adolescent Twins
| Age | A | D | E | Total | %a | %d | %e |
|---|---|---|---|---|---|---|---|
| 12 | 44.49 | 73.62 | 107.95 | 226.06 | 0.20 | 0.33 | 0.48 |
| 13 | 45.97 | 73.62 | 107.95 | 227.54 | 0.20 | 0.32 | 0.47 |
| 14 | 47.47 | 73.62 | 107.95 | 229.04 | 0.21 | 0.32 | 0.47 |
| 15 | 49.00 | 73.62 | 107.95 | 230.57 | 0.21 | 0.32 | 0.47 |
| 16 | 50.55 | 73.62 | 107.95 | 232.12 | 0.22 | 0.32 | 0.46 |
| 17 | 52.13 | 73.62 | 107.95 | 233.70 | 0.22 | 0.31 | 0.46 |
| 18 | 53.73 | 73.62 | 107.95 | 235.30 | 0.23 | 0.31 | 0.46 |
A = additive genetic variance; D = non-additive genetic variance; E = unshared environment variance
Fig. 2.
Extraversion raw and standardized variance components across adolescence. Model fitting analyses demonstrate no significant changes across adolescence with regard to the magnitude of non-additive genetic or unshared environmental effects and a small increase in additive genetic influences as a function of age. a = additive genetics; d = non-additive genetics, e = unshared environment
Discussion
This study tested the relative contribution of genetic and environmental influences to the variance of extraversion in a large sample of adolescent Dutch twins and their parents. The best fitting model to explain the variance was a model which incorporated additive and non-additive genetic factors in addition to unshared environment and in which there was no assortative mating. No significant differences in the magnitude of the genetic and environmental components across sex were found. Few age effects were found with the exception of slightly increasing additive genetic effects with age. The standardized estimates for A started at 20% at age 12 and slowly increased to 23% by age 18. No significant age effects for the non-additive genetic and environmental parameters were found. Therefore, the absolute amount of variance attributed to these effects was constant across adolescence. The proportion of variance explained by D and E thus reflected the increase in A. In this sample, D decreased from 33% to 31% while E decreased from 48% to 46%.
It might be argued that the interaction effect found for the additive genetic component can be an artifact of scaling. If the mean of the phenotype changes across age, and the changes in the mean are associated with changes in the variance, that could spuriously produce a G × E pattern. To test that this was not the case we regressed extraversion on age, and correlated the residuals again with age. If there was a mean variance relationship, the dispersion of the scores would increase with age and with it the residuals. However, the correlations obtained were 0.04 for twins and −0.01 for parents. This suggests that the G × E pattern is real.
These results stand in some contrast to the adolescent study by Gillespie and coworkers which found more evidence for additive genetic effects (Gillespie et al. 2004). Since shared environmental and non-additive genetic effects compete towards making DZ correlations more or less than half the MZ correlations, it is possible that, if both effects are present, the effect of one was masked by the other. Non-additive genetic effects were suspected in the study by Viken et al who assessed a sample ranging in age from 18 to 53 years (Viken et al. 1994). Our broad-sense heritability (additive plus non-additive) estimate of 52–54% through adolescence also fits closely with their heritability estimate of their youngest cohort of 52%.
Our results were also quite similar to those obtained by Keller in colleagues (Keller et al. 2005) in an Australian sample whose average age was 35 years old. They found evidence for robust non-additive effects of 24% compared to our estimates of 27–29%. Another shared finding between studies was the lack of evidence that different genes underlie extraversion in males and females. In their study additive genetic factors explained 23% of the variance which was slightly lower than the 29–34% estimated for the same age group in our study. However, in the current study, the additive genetic parameter was the one dimension that was significantly related to age. An additional difference between studies was found in extraversion means. In their sample, slightly higher means were found in females with no significant interaction between age and gender. By contrast, we found no gender differences in extraversion means. The present study used 21 items to measure extraversion in contrast to fewer items in many previous reports. In addition, this is one of the few twin studies to examine self-report personality in a non-adult sample. While the use of self-report measures may be subject to some forms of bias, it is less likely to be distorted by contrast effects which are possible when one parent rates more than one offspring.
The non-additive genetic effects found in this study were modeled as interactions of genetic effects within the same loci, i.e., dominance. However, other non-additive genetic effects may also be present, such as epistasis which refers to interactions between genes at different loci (Coventry and Keller 2005; Eaves 1988). However the estimation of epistatic effects is practically impossible with the current extended twins design. Power analyses showed that a sample of more than 71,000 families would be necessary to detect a proportion of 0.10 of the variance explained by epistatic non-additive genetic effects.
Gene-environment interactions may play a role in adolescent personality development (Reiss et al. 2000). One study with extraversion of identical twins reared apart, for example, found that twins reared in less controlling families were more likely to become extraverted regardless of their genetic liability, whereas only twins with high genetic loading were extroverted in highly controlling families (Bergeman et al. 1988). Any such effects of shared family environment (G × C interaction) will contribute to the genetic variance in our study.
The absence of main effects of shared family environment is bolstered by the very low correlation between extraversion scores of spouses. Previous work has not supported mate selection being strongly influenced by similar personalities, although stronger effects have been observed when measuring constructs such as social or political attitudes (Plomin et al. 2001). Extraversion correlations between spouses have been found to be generally small and positive, on the order of 0.05–0.12 (Ahern et al. 1982; Loehlin et al. 1985). These effects are thought not to be sufficient to violate the random mating assumption in twin study analyses (Eaves et al. 1999). The present study similarly did not find evidence of assortative mating by extraversion, with the correlation between mothers and fathers being statistically non-significant.
We found few age and gender effects on extraversion means. One exception to this was that extraversion means were significantly higher in the adolescent twins in comparison to their parents. The amount of difference was about 1/3 of a standard deviation. In this study, we could not conclude that this difference was directly related to age, due in part to having little data on individuals aged between adolescence and early adulthood: a time when the greatest reductions in extraversion levels have been observed (Costa and McCrae 1992; Viken et al. 1994; Zuckerman 1979). Generational effects on means might also be present such that individuals born in the 1970s are on the average more extraverted than their parents who were mostly born around the 1940s.
We did not find significant gender differences in extraversion means for either the twins or their parents, in contrast to other studies which have shown gender differences in both directions. Interestingly, gender differences in personality have been found to be strongest in American and European cultures in comparison to African and Asian cultures, although these differences tend to be small in magnitude, i.e., less than a half of a standard deviation (Costa et al. 2001). As alluded to earlier, some of the inconsistency with finding gender differences in extraversion may be due to the fact that the scale combines multiple facets, some of which are more traditionally masculine and others more traditionally feminine. The ABV is not broken into labeled components, although inspection of the items reveals many questions relating to disinhibition and gregariousness as opposed to items loading onto warmth or assertiveness which have been found to show larger differences between men and women.
Strengths and limitations
Principal strengths of this study include the large sample size and extended twin design. These elements provide for increased statistical power and the ability to test for potential confounds such as assortative mating. The use of parental data reduces the parameter bias inherent in the classic twin design (Coventry et al. 2005), providing more robust estimates of the additive genetic component, and the variance due to dominant genetic interactions. However, some parameter bias might still remain in the final estimates if non-allelic genetic interactions make a significant contribution to the phenotypic variance, as it has been suggested by other authors (Eaves 1988; Mather 1974). The similar size of the DZ correlation and the parent-offspring correlation suggests that additive by additive epistatic interactions might indeed contribute to the variance in extraversion. If this were the case, the estimate of the additive genetic variance might be overestimated in this study. Higher order interactions between dominance or epistasis with dominance components would lead to an overestimation of the variance assigned to dominance interactions in the ADE model. However, the presence of such interactions would increase the difference in resemblance between parents and offspring, and the DZ twins. Furthermore, the presence of assortative mating or cultural transmission might also produce an overestimation of the additive genetic component. The results of the present study combined with previous work show that mating is at random with respect to extraversion, similar to other personality variables (Eaves et al. 1999). Eaves et al. (1999) showed as well, using an extended pedigrees design, that cultural transmission and other shared environmental factors have negligible effects in the variance of extraversion.
As previously mentioned, the use of a self-report instrument also has the advantage of being less prone to contrast effects. Some aspects of personality may also be less apparent to parents of adolescents as their offspring disclose less to them and spend more time with peers. On the other hand, bias relating to social desirability may be more present in self-report measures. This sample comes from a relatively homogenous population with regard to ethnic background and results, therefore, may not generalize to other groups. Genetic analyses of adolescent extraversion using other informants such as parents or peers are scarce. While there is some evidence to suggest differences in mean ratings, with peers rating individuals as more extraverted compared to self or spousal report (McCrae 1991), this would not necessarily translate into changes in the genetic and environmental parameters. Indeed, a previously mentioned study of adult female twin pairs found that while extraverted individuals tend to underestimate the level of extraversion in their co-twin, significant genetic effects continued to be supported in their model testing (Heath et al. 1992).
This study represents one of the largest studies to date of adolescent twins and their parents to investigate the genetic and environmental contributions to the personality trait of extraversion. Results support previous work in other age groups which has found influence of both additive genetic and non-additive genetic factors. Strong unshared environmental factors were also found and were consistent across age. Similar to most previous reports, no shared environmental influence was found. Additional research is indicated to explore more fully the possibility of varying genetic and environmental parameters across the various components of extraversion which may reflect various continuities and discontinuities in these facets’ underlying neurophysiology.
Table 4.
Model-fitting results for extraversion scores testing basic assumptions of means, variances, and covariances
| −2LL | N Par | DF | CT | χ2 (df difference) | P | |
|---|---|---|---|---|---|---|
| 1. Saturated | 53702.238 | 26 | 6,430 | |||
| Tests on means | ||||||
| 2. Age β = 0 | 53703.275 | 25 | 6,431 | 1 | 1.03 (1) | 0.31 |
| 3. MZ = DZ&DOS | 53706.873 | 21 | 6,435 | 2 | 3.6 (4) | 0.46 |
| 4. Male = Female | 53707.177 | 20 | 6,436 | 3 | 0.30 (1) | 0.58 |
| 5. Twins = Parents | 53893.732 | 19 | 6,437 | 4 | 186.56 (1) | <0.01 |
| Tests on Variances (departing from the best fitting model) | ||||||
| 6. Mz = Dz&OS | 53710.198 | 16 | 6,440 | 4 | 3.02 (4) | 0.55 |
| 7. Male = Female | 53710.307 | 15 | 6,441 | 6 | 0.12 (1) | 0.73 |
| 8. Twins = Parents | 53751.590 | 14 | 6,442 | 7 | 41.28 (1) | <0.01 |
| Tests on Covariances (departing from the best fitting model) | ||||||
| 9.1. OSmf = OSfm | 53710.516 | 14 | 6,442 | 7 | 0.2 (1) | 0.65 |
| 9.2. MZM = MZF | 53711.578 | 13 | 6,443 | 9.1 | 1.07 (1) | 0.30 |
| 9.3. DZM = DZF | 53712.555 | 12 | 6,444 | 9.2 | 0.97 (1) | 0.32 |
| 9.4. DZ = DOS | 53717.828 | 11 | 6,445 | 9.3 | 5.28 (1) | 0.02 |
| 10. Father-male twin equal father-female twin covariances | 53718.614 | 10 | 6,446 | 9.4 | 0.78 (1) | 0.38 |
| 11. Mother-male twin equal mother-female twin covariances | 53720.501 | 9 | 6,447 | 10 | 1.89 (1) | 0.17 |
| 12. Father-twin equal mother-twin covariance | 53723.523 | 8 | 6,448 | 11 | 3.02 (1) | 0.08 |
−2LL = −2 Log likelihood; Npar = Number of free parameters estimated; DF = Degrees of freedom; CT = Compared to model number; AgeB = Age regression coefficient, M = Means, Var = Variance, Tw1 = first born, Ttw2 = second born; MZ = monozygotic; MZM = monozygotic male; MZF = monozygotic female; DZ = dizygotic; DZM = dizygotic male; DZF = dizygotic female; DOS = diyzygotic opposite sex; OSmf = opposite sex, male born first; OSfm = opposite sex, female born first; Bold print indicates best fitting model.
Note: each subsequent model incorporates the restrictions of the previous model to which is it compared
Acknowledgments
This work was supported by the Netherlands Organization for Scientific Research (NWO 985-10-002, NWO 900-562-137, and NWO-MagW 480-04-004), Spinozapremie NWO/SPI 56-464-14192, and by grants K08 MH069562, MH58799 and MH52813 from the National Institute of Mental Health, Rockville, Maryland, USA.
Contributor Information
David C. Rettew, Email: david.rettew@uvm.edu, Department of Psychiatry, University of Vermont College of Medicine, 1 South Prospect Street, Arnold 3, Burlington, VT 05401, USA
Irene Rebollo-Mesa, Department of Biological Psychology, VU University, Amsterdam, The Netherlands.
James J. Hudziak, Department of Psychiatry, University of Vermont College of Medicine, 1 South Prospect Street, Arnold 3, Burlington, VT 05401, USA
Gonneke Willemsen, Department of Biological Psychology, VU University, Amsterdam, The Netherlands.
Dorret I. Boomsma, Department of Biological Psychology, VU University, Amsterdam, The Netherlands
References
- Agrawal A, Heath AC, Grant JD, Pergadia ML, Statham DJ, Bucholz KK, Martin NG, Madden PA. Assortative mating for cigarette smoking and for alcohol consumption in female Australian twins and their spouses. Behav Genet. 2006;36:553–566. doi: 10.1007/s10519-006-9081-8. [DOI] [PubMed] [Google Scholar]
- Ahern FM, Johnson RC, Wilson JR, McClearn GE, Vandenberg SG. Family resemblances in personality. Behav Genet. 1982;12:261–280. doi: 10.1007/BF01067847. [DOI] [PubMed] [Google Scholar]
- Bergeman CSR, Plomin R, McClearn E, Pedersen NL, Friber LT. Genotype-environment interaction in personality development. Psychol Aging. 1988;3:399–406. doi: 10.1037//0882-7974.3.4.399. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002a;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Boomsma I, Beem AL, van den Berg M, Dolan CV, Koopmans JR, Vink JM, de Geus EJ, Slagboom PE. Netherlands twin family study of anxious depression (NETSAD) Twin Res. 2000;3:323–334. doi: 10.1375/136905200320565300. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Princen HM, Frants RR, Gevers Leuven JA, Kempen HJ. Genetic analysis of indicators of cholesterol synthesis and absorption: lathosterol and phytosterols in Dutch twins and their parents. Twin Res. 2003;6:307–314. doi: 10.1375/136905203322296674. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Vink JM, van Beijsterveldt TC, de Geus EJ, Beem AL, Mulder EJ, Derks EM, Riese H, Willemsen GA, Bartels M, van den Berg M, Kupper NH, Polderman TJ, Posthuma D, Rietveld MJ, Stubbe JH, Knol LI, Stroet T, van Baal GC. Netherlands Twin Register: a focus on longitudinal research. Twin Res. 2002b;5:401–406. doi: 10.1375/136905202320906174. [DOI] [PubMed] [Google Scholar]
- Buss AH, Plomin R. Temperament: early developing personality traits. Erlbaum; Hillsdale, NJ: 1984. [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The temperament and character inventory (TCI): a guide to its development and use. Center for Psychobiology of Personality, Washington University; St. Louis, MO: 1994. [Google Scholar]
- Costa PT, McCrae RR. Revised NEO personality inventory and NEO five-factor inventory: professional manual. Psychological Assessment Resources, Inc; Odessa, FL: 1992. [Google Scholar]
- Costa PT, Terracciano A, McCrae RR. Gender differences in personality traits across cultures: robust and surprising findings. J Pers Soc Psychol. 2001;81:322–331. doi: 10.1037/0022-3514.81.2.322. [DOI] [PubMed] [Google Scholar]
- Coventry WL, Keller MC. Estimating the extent of parameter bias in the classical twin design: A comparison of parameter estimates from extended twin-family and classical twin designs. Twin Res Hum Genet. 2005;8:214–223. doi: 10.1375/1832427054253121. [DOI] [PubMed] [Google Scholar]
- DiLalla LF, Jones S. Genetic and environmental influences on temperament in preschoolers. In: Molfese VJ, Molfese DL, editors. Temperament and personality development across the life span. Lawrence Erlbaum Associates; Mahway, NJ: 2000. pp. 33–55. [Google Scholar]
- Eaves LJ. Computer simulation of sample size and experimental design in human psychogenetics. Psychol Bull. 1972;77:144–152. doi: 10.1037/h0032182. [DOI] [PubMed] [Google Scholar]
- Eaves LJ. Dominance alone is not enough. Behav Genet. 1988;18:27–33. doi: 10.1007/BF01067073. [DOI] [PubMed] [Google Scholar]
- Eaves L, Heath A, Martin N, Maes H, Neale M, Kendler K, Kirk K, Corey L. Comparing the biological and cultural inheritance of personality and social attitudes in the Virginia 30,000 study of twins and their relatives. Twin Res. 1999;2:62–80. doi: 10.1375/136905299320565933. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Heath AC, Neale MC, Hewitt JK, Martin NG. Sex differences and non-additivity in the effects of genes on personality. Twin Res. 1998;1:131–137. doi: 10.1375/136905298320566267. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual for the Eysenck personality inventory (adult and junior) Digits; San Diego, CA: 1975. [Google Scholar]
- Gillespie NA, Evans DE, Wright MM, Martin NG. Genetic simplex modeling of Eysenck’s dimensions of personality in a sample of young Australian twins. Twin Res. 2004;7:637–648. doi: 10.1375/1369052042663814. [DOI] [PubMed] [Google Scholar]
- Gjone H, Stevenson J. A longitudinal twin study of temperament and behavior problems: common genetic or environmental influences? J Am Acad Child Adolesc Psychiatry. 1997;36:1448–1456. doi: 10.1097/00004583-199710000-00028. [DOI] [PubMed] [Google Scholar]
- Heath AC, Neale MC, Kessler RC, Eaves LJ, Kendler KS. Evidence for genetic influences on personality from self-report and informant ratings. J Pers Soc Psychol. 1992;63:85–96. doi: 10.1037//0022-3514.63.1.85. [DOI] [PubMed] [Google Scholar]
- Hur YM. Evidence for nonadditive genetic effects on Eysenck Personality Scales in South Korean twins. Twin Res Hum Genet. 2007;10:373–378. doi: 10.1375/twin.10.2.373. [DOI] [PubMed] [Google Scholar]
- Hur YM. Nonadditive genetic effects on hostility in South Korean adolescent and young adult twins. Twin Res Hum Genet. 2006;9:637–641. doi: 10.1375/183242706778553408. [DOI] [PubMed] [Google Scholar]
- Johnson AM, Vernon PA, Harris JA, Jang KL. A behavior genetic investigation of the relationship between leadership and personality. Twin Res. 2004;7:27–32. doi: 10.1375/13690520460741417. [DOI] [PubMed] [Google Scholar]
- Keller MC, Coventry WL. Quantifying and addressing parameter indeterminancy in the classical twin design. Twin Res Hum Genet. 2005;8:201–213. doi: 10.1375/1832427054253068. [DOI] [PubMed] [Google Scholar]
- Keller MC, Coventry WL, Heath AC, Martin NG. Widespread evidence for non-additive genetic variation in Cloninger’s and Eysenck’s personality dimensions using a twin plus sibling design. Behav Genet. 2005;35:707–721. doi: 10.1007/s10519-005-6041-7. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Boomsma DI. Familial resemblances in alcohol use: genetic or cultural transmission? J Stud Alcohol. 1996;57:19–28. doi: 10.15288/jsa.1996.57.19. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. Genes and environment in personality development. Sage; Newbury Park, CA: 1992. [Google Scholar]
- Loehlin JC, Willerman L, Horn JM. Personality resemblances in adoptive families when the children are late-adolescent or adult. J Pers Soc Psychol. 1985;48:376–392. doi: 10.1037//0022-3514.48.2.376. [DOI] [PubMed] [Google Scholar]
- Martin NG, Eaves LJ, Kearsey MJ, Davies P. Power of classical twin study. Heredity. 1978;40:97–116. doi: 10.1038/hdy.1978.10. [DOI] [PubMed] [Google Scholar]
- Mather K. Non-allelic interaction in continuous variation of randomly breeding populations. Heredity. 1974;32:414–419. doi: 10.1038/hdy.1974.53. [DOI] [PubMed] [Google Scholar]
- McCrae RR. The counterpoint of personality assessment: self-reports and ratings. Paper presented at the meeting of the American Psychological Association; San Francisco. 1991. [Google Scholar]
- McCrae RR, Costa PT, Ostendorf F, Angleitner A, Hrebickova M, Avia MD, Sanz J, Sanchez-Bernardos ML, Kusdil ME, Wood-field R, Saunders PR, Smith PB. Nature over nurture: temperament, personality, and life span development. J Pers Soc Psychol. 2000;78:173–186. doi: 10.1037//0022-3514.78.1.173. [DOI] [PubMed] [Google Scholar]
- McGue M, Bacon S, Lykken DT. Personality stability and change in early adulthood: a behavioral genetic approach. Dev Psychol. 1993;29:96–109. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling. Department of Psychiatry; Richmond, VA: 2004. [Google Scholar]
- Plomin R, DeFries JC, MCClearn GE, McGuffin P. Behavior genetics. Worth Publishers; New York: 2001. [Google Scholar]
- Posthuma D, Boomsma DI. A note on the statistical power in extended twin designs. Behav Genet. 2000;30:147–158. doi: 10.1023/a:1001959306025. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rebollo I, Boomsma DI. Genetic analysis of anger: genetic dominance or competitive sibling interaction. Behav Genet. 2006a;36:216–228. doi: 10.1007/s10519-005-9025-8. [DOI] [PubMed] [Google Scholar]
- Rebollo I, Boomsma DI. Genetic and environmental influences on type A behavior pattern: evidence from twins and their parents in the Netherlands Twin Register. Psychosom Med. 2006b;68:437–442. doi: 10.1097/01.psy.0000204631.76684.28. [DOI] [PubMed] [Google Scholar]
- Reiss D, Neiderhiser JM, Hetheringon EM, Plomin R. The relationship code: deciphering genetic and social influences on adolescent development. Harvard University Press; Cambridge, MA: 2000. [Google Scholar]
- Rettew DC, Vink J, Willemsen G, Doyle A, Hudziak JJ, Boomsma DI. The genetic architecture of neuroticism in 3301 Dutch adolescent twins as a function of age and sex: a study from the Dutch Twin Register. Twin Res Hum Genet. 2006;9:24–29. doi: 10.1375/183242706776403028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann R, Angleitner A, Strelau J. Genetic and environmental influences on personality: a study of twin reared together using the self- and peer report NEO-FFI scales. J Person. 1997;65:449–476. [Google Scholar]
- Rietveld MJ, Hudziak JJ, Bartels M, Van Beijsterveldt CE, Boomsma DI. Heritability of attention problems in children: I. cross-sectional results from a study of twins, age 3–12 years. Am J Med Genet. 2003;117B:102–113. doi: 10.1002/ajmg.b.10024. [DOI] [PubMed] [Google Scholar]
- Rietveld MJ, van Der Valk JC, Bongers IL, Stroet TM, Slagboom PE, Boomsma DI. Zygosity diagnosis in young twins by parental report. Twin Res. 2000;3:134–141. doi: 10.1375/136905200320565409. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey K, Fisher P. Investigations of temperament at 3–7 years: the children’s behavior questionnaire. Child Dev. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Saudino KJ, Cherny SS. Sources of continuity and change in observed temperament. In: Emde RN, Hewitt JK, editors. Infancy to early childhood: genetic and environmental influences on developmental change. Oxford University Press; New York: 2001. pp. 89–110. [Google Scholar]
- Schermelleh-Engle K, Moosbrugger H, Muller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness of fit measures. Meth Psychol Res Online. 2003;8:23–74. [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuma AH, Maser JD, editors. Anxiety and the anxiety disorders. Erlbaum; Hillsdale, NJ: 1985. pp. 681–706. [Google Scholar]
- Van Leeuwen M, van den Berg SM, Boomsma DI. Learn Indiv Diff. A twin-family study of general IQ. in press. [Google Scholar]
- Viken RJ, Rose RJ, Kaprio J, Kosenvuo M. A developmental genetic analysis of adult personality: extraversion and neuroticism from 18 to 59 years of age. J Pers Soc Psychol. 1994;66:722–730. doi: 10.1037//0022-3514.66.4.722. [DOI] [PubMed] [Google Scholar]
- Wilde GJS. Neurotische labiliteit gemeten volgens de vragenlijstmethode (The questionnaire method as a means of measuring neurotic instability) Van Rossen; Amsterdam: 1970. [Google Scholar]
- Willemsen G, Posthuma D, Boomsma DI. Environmental factors determine where the Dutch live: results from the Netherlands twin register. Twin Res Hum Genet. 2005;8:312–317. doi: 10.1375/1832427054936655. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: beyond the optimal level of arousal. Lawrence Erlbaum Associates; Hillsdale, NJ: 1979. [Google Scholar]