SUMMARY
In mammalian embryonic stem cells, the acquisition of pluripotency is dependent upon Nanog, but the in vivo analysis of Nanog has been hampered by its requirement for early mouse development. In an effort to examine the role of Nanog in vivo, we identified a zebrafish Nanog ortholog, and found that its knockdown impaired endoderm formation. Genome-wide transcription analysis revealed that nanog-like morphants fail to develop the extra-embryonic yolk syncytial layer (YSL), which produces Nodal required for endoderm induction. We examined the genes that were regulated by Nanog-like, and identified the homeobox gene mxtx2, which is both necessary and sufficient for YSL induction. Chromatin immunoprecipitation assays and genetic studies indicated that Nanog-like directly activates mxtx2, which in turn specifies the YSL lineage by directly activating YSL genes. Our study identifies a Nanog-like-Mxtx2-Nodal pathway and establishes a role for Nanog-like in regulating the formation of the extra-embryonic tissue required for endoderm induction.
INTRODUCTION
Pluripotency is defined as the capacity of a cell to give rise to all three germ layers. The homeobox gene Nanog was discovered on the basis of its ability to drive Leukemia inhibitory factor (LIF) independent self-renewal of pluripotent mouse embryonic stem (ES) cells (Chambers et al., 2003; Mitsui et al., 2003). In ES cells, Nanog is a central player in the transcriptional regulatory circuitry of pluripotency (Boyer et al., 2005; Cole et al., 2008; Loh et al., 2006; Wang et al., 2006). In the early mouse embryo, Nanog expression marks the pluripotent epiblast and is essential for its establishment (Mitsui et al., 2003; Silva et al., 2009). A recent study has shown that activation of Nanog is required for acquisition of the pluripotent ground state in both embryonic development and somatic cell reprogramming (Silva et al., 2009). These studies raised the question of what function Nanog plays during embryonic development, and prompted us to investigate this issue in the zebrafish model system.
The zebrafish blastula stage embryo is composed of three distinct lineages: the extra-embryonic enveloping layer (EVL), the extra-embryonic yolk syncytial layer (YSL), and the intermediate deep cells, which form the entire embryo later. The YSL is unique to teleosts, and has been thought to share a common evolutionary origin with the mouse extra-embryonic primitive endoderm. Primitive endoderm markers such as hex, gata6, gata4, pdgfra, hnf4a, and foxa3 are also expressed in the YSL (Brown et al., 2010; Fan et al., 2007; Ho et al., 1999; Sprague et al., 2006), and both tissues function as the signaling center to pattern the head mesoderm and endoderm (Chen and Kimelman, 2000; Fan et al., 2007; Ober and Schulte-Merker, 1999; Rodaway et al., 1999; Varlet et al., 1997). Between the 512 and 1K-cell stages, the collapse of marginal cells into the yolk cell results in the formation of the YSL precursor (Kimmel and Law, 1985b). Each YSL nucleus (YSN) undergoes usually three, but sometimes four or five, metasynchronous nuclear divisions, and becomes post-mitotic, just before the onset of epiboly (Kane et al., 1992; Kimmel and Law, 1985a). The YSL plays a critical role in producing the force to drive epiboly and inducing the ventrolateral mesoderm and endoderm (Chen and Kimelman, 2000; Mizuno et al., 1996; Ober and Schulte-Merker, 1999; Rodaway et al., 1999; Solnica-Krezel and Driever, 1994; Strähle and Jesuthasan, 1993).
During the mid-blastula stage, induction of endoderm requires the secreted Nodal proteins Ndr1 and Ndr2, which are expressed in the YSL and marginal blastomeres (Chen and Kimelman, 2000; Feldman et al., 1998; Ober and Schulte-Merker, 1999; Rodaway et al., 1999). In response, the transcription of the endoderm transcription factor gata5/faust, mixer/bon, mezzo, sox32/cas is induced in marginal blastomere cells (Dickmeis et al., 2001; Kikuchi et al., 2001; Kikuchi et al., 2000; Poulain and Lepage, 2002; Reiter et al., 1999). Among them, sox32/cas plays a central role in endoderm induction, as its expression can autonomously induce the endodermal differentiation markers sox17 and foxA2 in the absence of Nodal signaling (Dickmeis et al., 2001; Kikuchi et al., 2001). sox32/cas requires pou5f1, a ubiquitously expressed transcription factor, to maintain its expression and to activate sox17 (Lunde et al., 2004; Reim et al., 2004).
In an effort to understand the role of Nanog in early zebrafish development, we identified the zebrafish nanog ortholog and found that it is required for YSL development. Knockdown of nanog-like eliminates ventrolateral Nodal signaling, resulting in an absence of ventrolateral endoderm formation. By microarray analysis, we found that nanog-like mophants do not express genes specific to the YSL, where Nodal signals are produced for endoderm induction. Genetic studies and chromatin immunoprecipitation based sequencing (ChIP-Seq) analysis revealed that Nanog-like directly activates the transcription of the homeodomain factor Mxtx2, which in turn specifies the YSL and promotes ventrolateral endoderm formation. Our study illustrates a critical role for Nanog-like in regulating endoderm formation through the Mxtx2-Nodal pathway.
RESULTS
nanog-like is required for early gastrulation
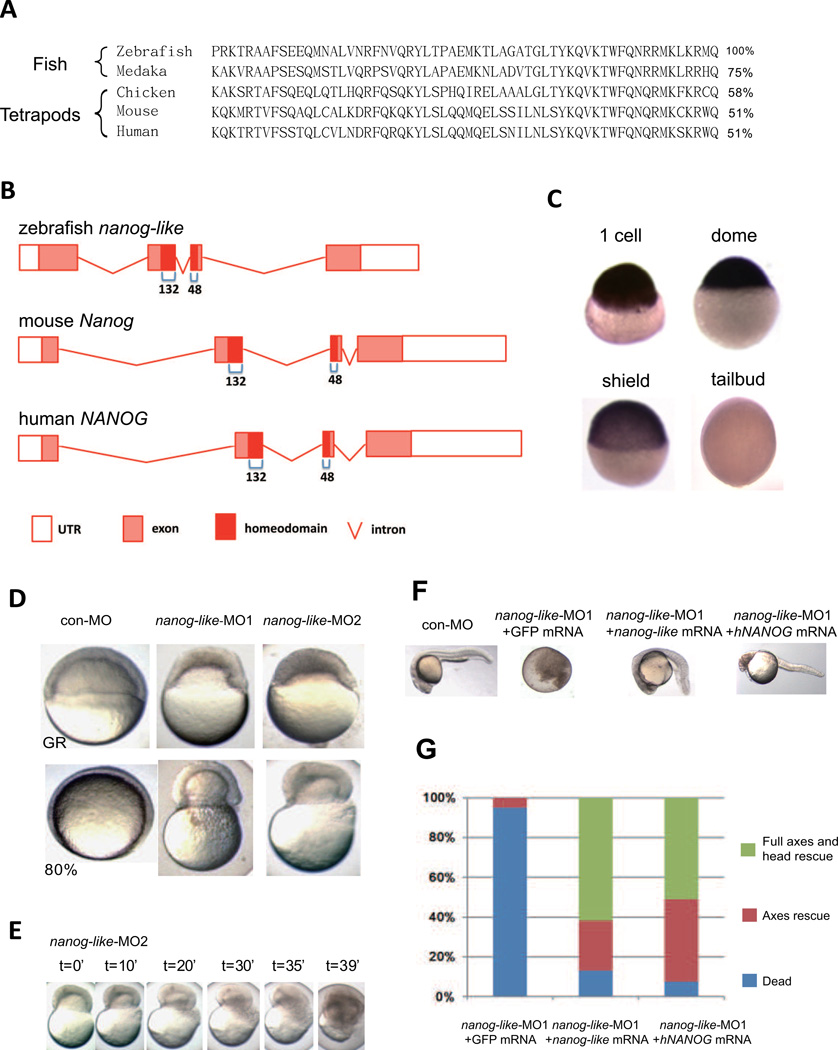
To identify the zebrafish ortholog, the mouse Nanog protein sequence was used for a BLAST search. This approach resulted in the identification of Zgc:193933 as a potential zebrafish Nanog ortholog. The homology between mouse Nanog and zebrafish Zgc:193933 is evident in the homeodomain. Alignment of human, mouse, chicken, zebrafish and medaka Nanog homeodomains showed high sequence conservation (Figure 1A). Furthermore, zebrafish, mouse and human Nanog show a conserved intron/exon structure (Figure 1B). Significantly, in both fish and mammalian Nanog, the 60aa homeodomain is encoded by the last 132bp of the second exon and the first 48bp of the third exon (Figure 1B). Such an intron/exon structure is not found in other closely related homeobox genes (Figure 1B and S1A). No chromosomal synteny was present between the zebrafish and mammalian Nanog loci.
Figure 1. Identification of the zebrafish nanog-like gene.
(A) Alignment of the homeodomain sequence of fish and tetrapod Nanog proteins. The percentage similarity of each to zebrafish Nanog-like is shown at the right.
(B) Intron/exon structure of the zebrafish, mouse and human Nanog genes. The 60aa homeodomain is encoded by the last 132bp of the second exon and the first 48bp of the third exon in all three organisms.
(C) Whole mount in situ hybridization of nanog-like identified high maternal (1-cell) and blastula (dome) expression, and rapidly diminishing expression during gastrulation (shield and tailbud stages).
(D) Blastomeres of nanog-like-knockdown embryos, injected with one of two distinct morpholinos targeting the translational start site, failed to thin at the germ ring stage, and constricted to squeeze out the yolk cell by the 80% epiboly stage.
(E) Time-lapse microscopy of the nanog-like-MO2-injected embryo showing the yolk burst process.
(F) Rescue of the nanog-like-MO1-injected embryos by co-injection of 100pg zebrafish or human NANOG mRNA. Embryos shown are at 24 hpf.
(G) Statistics for the rescue of nanog-like-MO1-injected embryos by zebrafish or human Nanog mRNA. 100 GFP mRNA injected, 115 nanog-like mRNA injected, and 209 human NANOG mRNA injected embryos were analyzed. Phenotype classes were counted at 24 hpf and are presented as a percentage of the whole.
The zebrafish nanog-like cDNA was cloned from a blastula-stage embryo library, and was used to examine gene expression by whole mount in situ hybridization. nanog-like transcripts are maternally deposited (1-cell stage), expressed during the blastula stage (dome stage), and diminish rapidly during gastrulation (shield stage) (Figure 1C). At the end of gastrulation (tailbud stage), the transcripts can no longer be detected (Figure 1C).
To examine the role for nanog-like in early embryogenesis, we knocked down the Nanog-like protein level using two distinct morpholinos targeting the nanog-like translational start site. Embryos injected with 10ng of nanog-like-MO1 or 10ng of nanog-like-MO2 at the 1-cell stage exhibited similar gastrulation defects. The nanog-like knockdown blastomere showed slowed epibolic movement, resulting in the accumulation of blastomere cells at the animal pole at later stages (Figure 1D). When control morpholino-injected embryos reached the 80% epiboly stage, the majority of the nanog-like morphants (95/100) exhibited dramatic constriction of the marginal cells, causing the yolk cell to burst (Figure 1D). As shown by time-lapse microscopy of the nanog-like-MO2 injected embryo, the yolk burst process happened within 40 minutes (Figure 1E). To confirm that the gastrulation defects were specifically due to the knockdown of nanog-like, we injected 100pg of nanog-like mRNA into the nanog-like morphant. The sequences at the nanog-like transcriptional start site were mutated to prevent hybridization with morpholinos. Phenotypes induced by both nanog-like-MO1 and nanog-like-MO2 were rescued by nanog-like mRNA (Figure 1F, G and S1B). Notably, mRNA encoding human NANOG was also able to rescue the defects at a similar efficiency comparable to zebrafish nanog-like mRNA, confirming the evolutionary conservation between mammalian Nanog and zebrafish Nanog-like (Figure 1F, G and S1B).
To test if Nanog-like functions in mouse ES cells, we examined whether mouse ES cells overexpressing nanog-like could self-renew in the absence of LIF. Previous studies have found that dimerization is required for Nanog to convey self-renewal in ES cells (Dixon et al., 2010; Wang et al., 2008). Examining the sequence outside of the homeodomain, we were unable to find the sequence for dimerization. To enable dimerization, Nanog-like was fused to the WR domain of mouse Nanog, which is required for dimerization (Figure S1D). By overexpressing engineered nanog-like, we were not able to rescue LIF dependence in ES cells (Figure S1E and S1F), despite findings that axolotl Nanog successfully rescues LIF dependence (Dixon et al., 2010). It seems that the zebrafish Nanog ortholog lacks the well-known self-renewal function that has been described in tetrapods. We therefore decided to name it Nanog-like.
Nanog-like regulates endoderm formation by activating Nodal
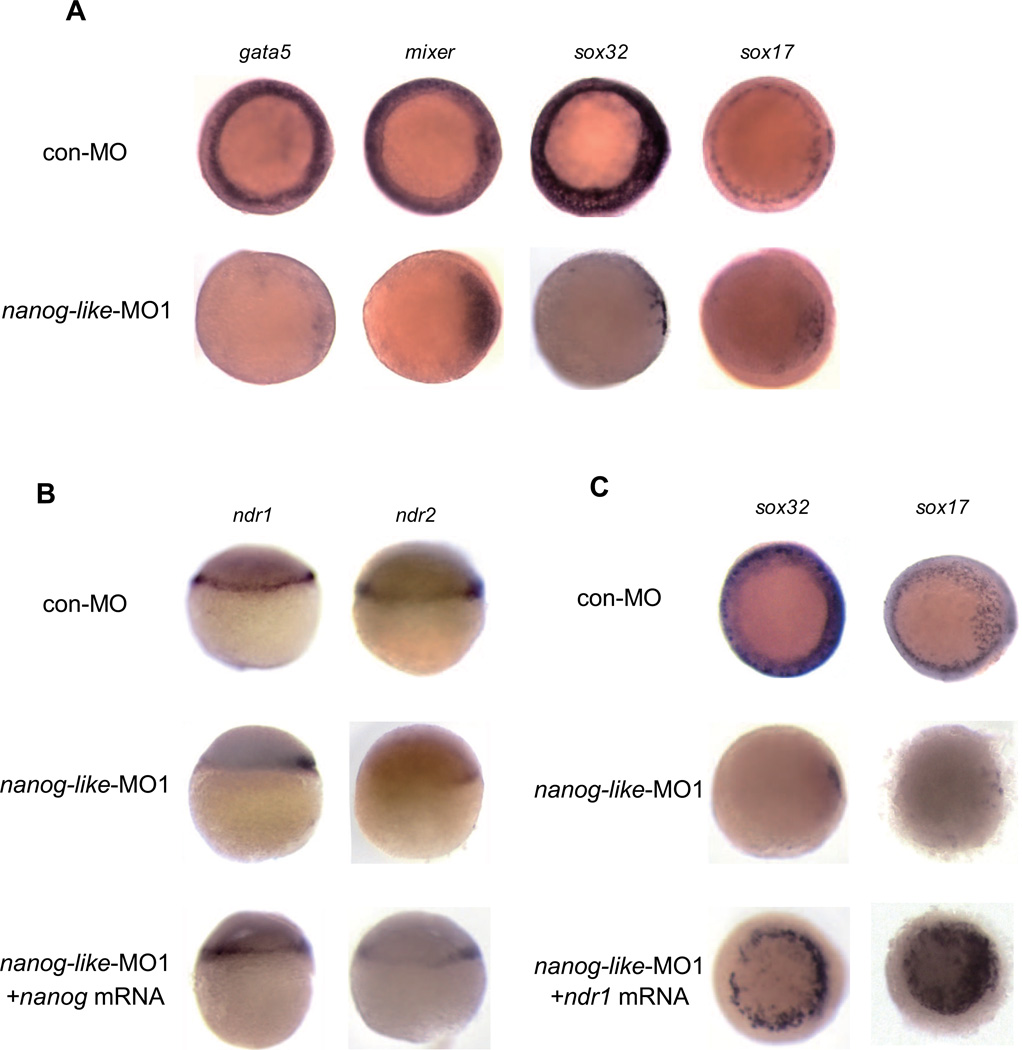
The most prominent defect observed in the nanog-like morphant is the impaired endoderm formation at the ventrolateral margin. The expression of endoderm genes gata5, mixer, sox32, and sox17 is mostly restricted to the dorsal area in nanog-like morphants (Figure 2A). In zebrafish, endoderm formation is induced by high levels of Nodal, which activate gata5/faust, mixer/bon, and mezzo (Dickmeis et al., 2001; Feldman et al., 1998; Gritsman et al., 1999; Kikuchi et al., 2001; Kikuchi et al., 2000; Poulain and Lepage, 2002; Reiter et al., 1999; Schier et al., 1997). These three transcription factors in turn control the expression of sox32. Sox32 cooperates with Pou5f1 to activate sox17, thereby specifying the endoderm lineage (Lunde et al., 2004; Reim et al., 2004). To determine if the lack of endoderm cells at the lateral and ventral margin was due to reduced Nodal signaling, we examined the expression of Nodal gene ndr1 and ndr2. At the sphere stage, ndr1 and ndr2 expression was normal at the dorsal margin in the nanog-like morphant (data not shown), but failed to spread to the ventrolateral area at the dome stage (Figure 2B). This defect is specifically due to the knockdown of nanog-like, as ventrolateral ndr1 and ndr2 expression can be rescued by restoring nanog-like mRNA (Figure 2B). Ventrolateral ndr1 and mixer expression was evident in some nanog-like morphants at the 50% epiboly stage (data not shown). To confirm that the ventrolateral endoderm defect was due to a lack of local Nodal signaling, we injected ndr1 mRNA into the vegetal yolk of the 4-cell stage embryo. This approach allowed the targeting of the YSL and marginal cells with ndr1 mRNA (Figure S2A and S2B). As expected, ndr1 expression restored the ventrolateral endoderm markers in the nanog-like morphant (Figure 2C).
Figure 2. Nanog-like regulates endoderm formation by activating Nodal.
(A) nanog-like knockdown impairs ventrolateral endoderm formation. The endoderm genes mixer, gata5, sox32, and sox17 are absent at the ventrolateral margin in nanog-like-MO1-injected embryos. All views are animal with the dorsal side to the right.
(B) ndr1 and ndr2 expression is restricted to the dorsal area in the nanog-like-MO1-injected embryo; ventrolateral expression of both genes can be restored by co-injection of nanog-like mRNA. All views are lateral with the presumptive dorsal side to the right.
(C) Ventrolateral sox32 and sox17 expression can be rescued by ndr1 mRNA. All views are animal with the presumptive dorsal side to the right.
Since the ventrolateral expression of ndr1 and ndr2 is dependent on activated Nodal signaling by an autoregulatory feedback, the absence of ventrolateral Nodal expression in nanog-like morphants might be due to the inability of cells to induce ndr1 and ndr2 expression upon receiving Nodal signals. To test this possibility, we activated the Nodal pathway by expressing the constitutively active Nodal receptor Tar*. ndr1 and ndr2 expression can be induced in Tar* expressing nanog-like morphants, indicating that the lack of ventrolateral Nodal expression is not due to a modulated competence for Nodal autoregulation (Figure S2C).
Nanog-like regulates YSL transcription
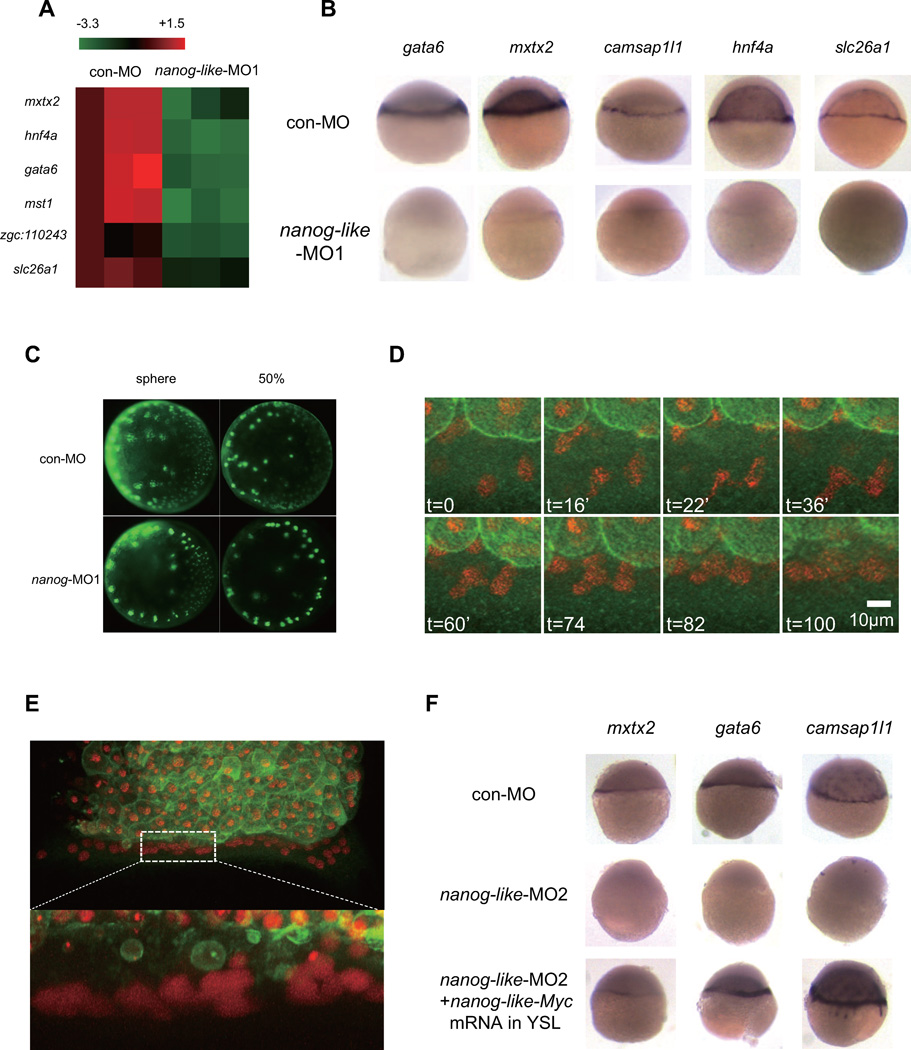
The yolk burst phenotype seen in the nanog-like morphant is unlikely due to the endoderm defect, as endoderm mutants undergo epiboly without constricting the yolk (Dickmeis et al., 2001; Feldman et al., 1998; Gritsman et al., 1999; Kikuchi et al., 2001; Schier et al., 1997). To globally analyze which target genes Nanog-like regulates during early embryogenesis, we performed a genome-wide transcription analysis using sphere stage (4 hpf) nanog-like morphants. We identified Nanog-like regulated genes by a q-value smaller than 0.01 in three separate experiments. Nanog-like regulates a discrete set of genes (609 in total, 362 upregulated and 247 downregulated). Genome-wide ontology analysis revealed that both upregulated and downregulated genes are enriched for the role of Nanog in mammalian embryonic stem cell pluripotency (Figure S3A and S3B). Among the top downregulated genes, we noticed a group of genes that are expressed exclusively in the YSL, suggesting a YSL defect in nanog-like morphants (Figure 3A). We further verified the decreased YSL gene expression in sphere and 50% epiboly stage embryos by RT-PCR (Figure S3C) and in situ hybridization (Figure 3B and Figure S4A).
Figure 3. Lack of YSL transcription in nanog-like morphants leads to epiboly and endoderm defects.
(A) Microarray analysis of gene expression in nanog-like morphants revealed an absence of YSL gene transcription. YSL genes downregulated in three separate experiments are displayed as a heat map.
(B) The expression of gata6, mxtx2, camsap1l1, hnf4a, and slc26a1 is absent in the YSL of nanog-like morphants. All views are lateral with the presumptive dorsal side to the right.
(C) The YSN of the wild-type and nanog-like knockdown embryos at the sphere stage and the 50% epiboly stage labeled with SYTOX green. All views are animal with the dorsal side to the right.
(D) Time-lapse microscopy of the YSNs of a nanog-like-MO2-injected embryo revealed the YSN aggregation process. 3 YSNs undergo the 12th division at t=16’. At t=22’, the upper left YSNs separate normally, while the lower YSNs fail to completely separate. At t=36’, the resulting 6 daughter YSNs start to aggregate.
(E) Higher magnitude confocal microscopy revealed that the aggregated YSNs in Figure 3D remained separated.
(F) Nanog-like is required autonomously for YSL transcription. To achieve YSL-specific expression, 300pg nanog-like-Myc mRNA was injected into the yolk at 2.5 hpf. The expression of mxtx2, gata6, and camsap1l1 was rescued by YSL-specific nanog-like-Myc expression.
The YSL forms at 2.5 hpf, when the marginal cells collapse into the yolk cell to form a multi-nucleated syncytium. To examine if the YSL formed correctly in the nanog-like morphant, we injected the vital dye SYTOX into the yolk cell of 1K-cell stage embryos to visualize the YSN. YSN in nanog-like morphants were present at the ring area indicating that the syncytial structure formed correctly (Figure 3C). However, at the 50% epiboly stage, we observed decreased YSN number in the nanog-like morphant (Figure 3C). To visualize the division and movement of YSNs, we performed time-lapse confocal microscopy (Supplemental Movie 1 and 2). Both wild-type and morphant embryos undergo the 12th division, but some YSNs in the morphant fail to separate completely (Figure 3D and Supplemental Movie 2). During and after the 12th division, aggregation of YSNs is evident in the morphant (Figure 3D and Supplemental Movie 2). Imaging of the aggregated YSNs under higher resolution shows that they stack together with clear nuclear boundaries, suggesting that the decrease in YSN number is due only to the aggregation of YSNs (Figure 3E). Fusion of YSNs was not observed in the time-lapse movies (Supplemental Movie S1 and S2).
Active remodeling of cytoskeletal filaments at the YSL-EVL junction provides force for vegetal movement of the blastoderm. Within the YSL is an F-actin band, which is considered critical for epiboly movement (Cheng et al., 2004; Köppen et al., 2006). Rhodamine-labeled phalloidin staining showed that no detectable F-actin band formed in nanog-like morphants (Figure S4B). We postulate that the absence of F-actin band might be due to the defective YSL transcription in nanog-like morphants.
Lack of YSL transcription leads to the epiboly and endoderm defects
To examine the effect of defective YSL transcription, we injected RNase into the yolk cell of the wild-type 1K-cell stage embryo. Previous studies showed that this approach ablates the RNA transcripts specifically in the YSL without affecting protein stability (Chen and Kimelman, 2000). Consistent with the previous report (Chen and Kimelman, 2000), we noticed that injected embryos showed slowed epiboly movements followed by a yolk burst at the shield stage, phenocopying the defects seen in nanog-like morphants (data not shown). Previous study showed that the YSL transcripts are required for ventrolateral mesoendoderm induction (Chen and Kimelman, 2000). We re-examined the Nodal signals and endoderm lineage, and found that the Nodal genes ndr1 and ndr2, and the endoderm gene mixer were absent in the ventrollateral area (data not shown). All of these data suggested that the lack of YSL gene expression in the nanog-like morphant was responsible for the epiboly and endoderm defects.
To confirm that the epiboly defect in nanog-like morphants was due to defective YSL formation, we generated chimeric embryos by exchanging blastoderm and YSL tissues between wild-type and nanog-like knockdown embryos at the 1K-cell stage. Chimeric embryos with a wild-type YSL and a nanog-like morphant blastoderm were able to fully undergo epiboly and survived to 3 dpf (data not shown). In contrast, chimeric embryos with a nanog-like knockdown YSL and a wild-type blastoderm failed to initiate epiboly, resulting in the accumulation of wild-type blastomere cells at the animal pole (data not shown). As a complementary approach to address the YSL-autonomous requirement for Nanog-like, we knocked down Nanog-like in the whole embryo and restored its YSL expression by injecting nanog-like-Myc mRNA into the yolk after YSL formation, a method employed to achieve YSL-specific expression (Figure S4C). Expression of mxtx2, gata6, camsap1l1, and hnf4a was rescued by YSL-specific expression of Nanog-like-Myc (Figure 3E and data not shown), suggesting that Nanog-like regulates YSL transcription in a cell-autonomous manner. These results indicate that the YSL is critical for epiboly movements and that Nanog-like regulates transcription in the YSL to promote both epiboly and endoderm development.
Nanog-like controls the expression of mxtx2, a homeodomain factor required for and sufficient for YSL induction
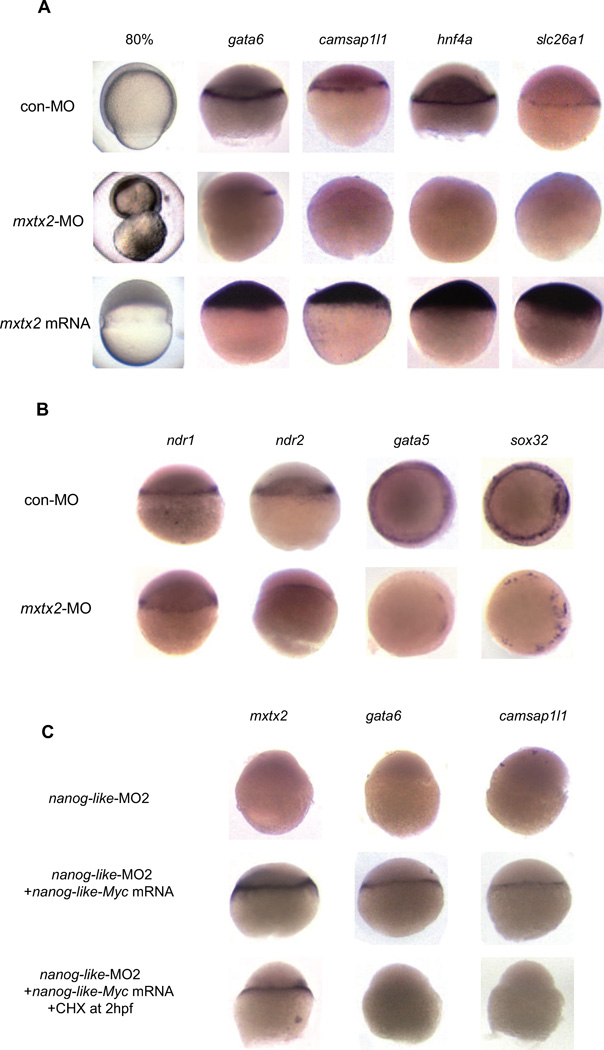
As Nanog-like alone cannot induce YSL, we were interested in identifying the key mediators downstream of Nanog-like that regulate the proper formation and function of YSL. One candidate downregulated in the nanog-like morphants is mxtx2, a homeobox gene that is among the earliest genes known to be expressed in the YSL (Bruce et al., 2005; Wilkins et al., 2008), mxtx2 transcription is induced by stabilized β-catenin shortly after the mid-blastula transition in the dorsal area and can be detected in the marginal blastoderm and the YSL at later stages. Knockdown of mxtx2 leads to a yolk burst phenotype similar to that observed in the nanog-like morphant (Figure 4A) and (Wilkins et al., 2008). In nanog-like morphants, mxtx2 expression is initiated in the dorsal area but fails to spread to ventrolateral regions suggesting that mxtx2 expression consists of Nanog-like-dependent ventrolateral and Nanog-like-independent dorsal expression (Figure 3B and data not shown).
Figure 4. Mxtx2 is required for and sufficient for YSL induction.
(A) Lateral view of embryos injected with 500pg control morpholino (con-MO), 500pg mxtx2 morpholino (mxtx2-MO), or 100pg mxtx2 mRNA at the 1-cell stage. At the 80% epiboly stage, mxtx2 morphants develop a yolk burst phenotype, and the mxtx2 mRNA-injected embryo failed to undergo epiboly. The YSL markers gata6, camsap1l1, hnf4a, and slc26a1 were absent in the mxtx2 morphant. YSL genes were ectopically expressed in the embryos injected with mxtx2 mRNA.
(B) Defective Nodal signaling and endoderm formation in the mxtx2 morphant. ndr1 and ndr2 is shown as a lateral view with the presumptive dorsal side to the right. gata5, and sox32 staining is shown as an animal view with the dorsal side to the right.
(C) By overexpressing Nanog-like-Myc, expression of mxtx2, gata6, and camsap1l1 in nanog-like morphants can be rescued. Cycloheximide (CHX) was added at 2hpf to allow for the translation of injected nanog-like-Myc mRNA, but to prevent translation of the earliest zygotic transcripts. When CHX is added at 2hpf, expression of mxtx2, but not gata6 or camsap1l1, can still be rescued, indicating that mxtx2 is the direct target of Nanog-like.
To further characterize the role of Mxtx2 in YSL development, we examined the expression of YSL genes in mxtx2 morphants. Notably, all YSL genes examined were absent in mxtx2 morphants (Figure 4A). Conversely, embryos overexpressing mxtx2 in blastomeres showed severe gastrulation defects and ectopic expression of YSL genes (Figure 4A). We also found that Nodal signaling and endoderm formation were compromised in the mxtx2 morphant. ndr1 is expressed in the ventrolateral margin with delayed onset (Figure 4B and data not shown). Expression of ndr2 and endodermal genes was impaired at the ventrolateral margin (Figure 4B). Individual gata5 and sox32 positive cells were sparsely present at the ventrolateral margin (Figure 4B and data not shown), which were presumably induced by residual Ndr1. A recent study by Hong et al also found that Mxtx2 directly activates ndr2 in the YSL (Hong et al., 2011). Our study reveals a role for Mxtx2 in the induction of YSL gene transcription beyond ndr2.
To determine if mxtx2 is the direct target of Nanog-like, we used cycloheximide (CHX) at 2hpf to block translation of the earliest zygotic transcripts, but allow the translation of injected nanog-like-Myc mRNA. When CHX is added at 2hpf, expression of mxtx2, but not gata6, camsap1l1, or hnf4a, can be rescued in nanog-like morphants, indicating that mxtx2 is the direct target of Nanog-like (Figure 4C and data not shown).
Genome-wide association of Nanog-like binding with expression profile of the nanog-like morphant
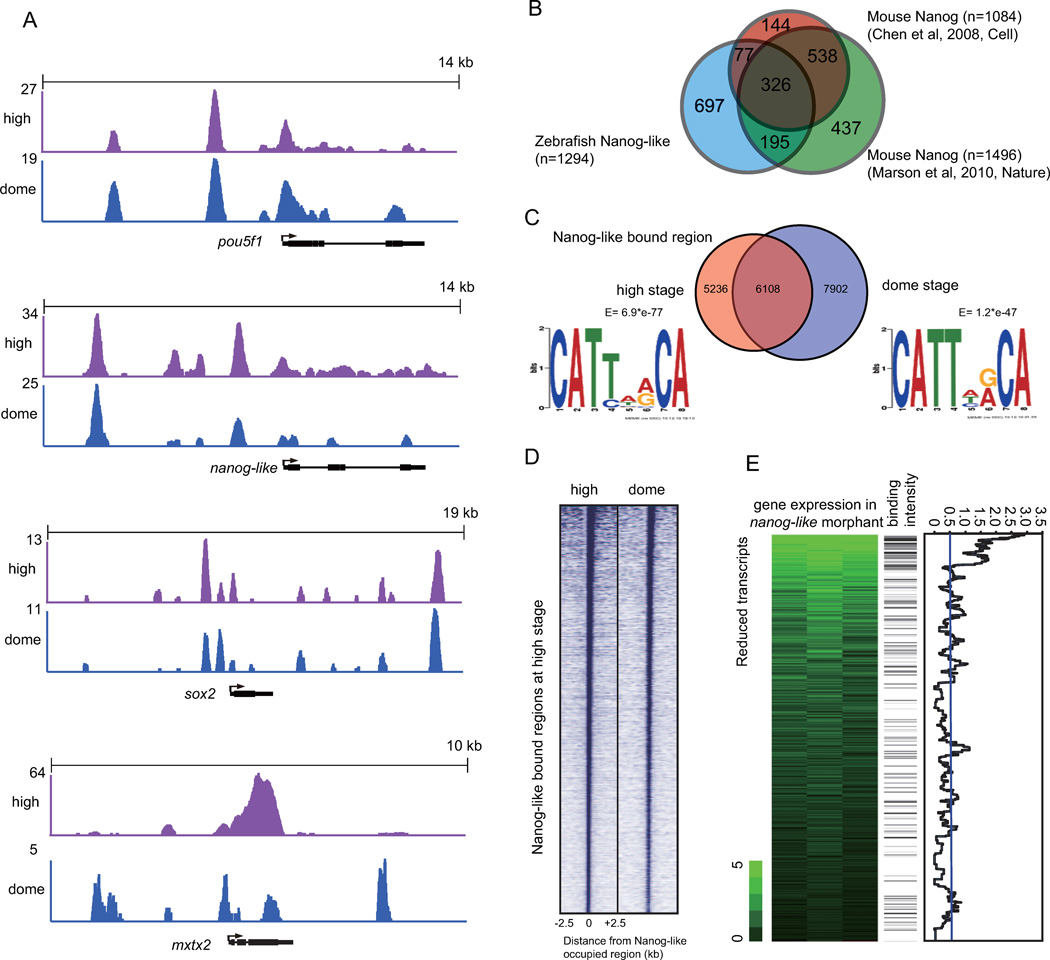
To determine the downstream targets of Nanog-like and Mxtx2, we performed ChIP-Seq analysis on blastula embryos. The 3’ end of the nanog-like coding sequence was fused to a Myc tag sequence. nanog-like-Myc mRNA rescued nanog-like morphants at a similar efficiency as nanog-like mRNA (Figure S1C). Wild-type embryos were injected with nanog-like-Myc mRNA at a dosage (25pg/embryo) that did not cause any developmental abnormality, and were collected for ChIP-Seq analysis at the high (3.3 hpf) or dome stage (4.3 hpf). We found that Nanog-like bound to many known mouse Nanog targets like pou5f1, nanog-like, and sox2 (Figure 5A). In an effort to determine if zebrafish and mice share similar Nanog targets, we compared our targets with two previous studies in mouse ES cells, and found that zebrafish Nanog-like targets are highly enriched in mouse targets from both studies, suggesting evolutionary conservation of the transcription network (Figure 5B)(Chen et al., 2008; Marson et al., 2008).
Figure 5. Genome-wide association of Nanog-like binding with the expression profile of nanog-like morphants.
(A) Nanog-like binding profiles at pou5f1, nanog-like, sox2, mxtx2 loci. ChIP-Seq data are shown in reads per million with the y-axis floor set to 2 reads per million.
(B) We generated a list with 4736 genes that can be identified in both zebrafish and mice. We restricted our comparison of Nanog targets to these genes. The Venn diagram shows the overlap among zebrafish Nanog-like targets and two previously reported mouse Nanog targets (Chen et al., 2008; Marson et al., 2008). By the hyper-geometric test, we found that zebrafish Nanog-like targets are enriched in both Chen’s (P-value=3e-16) and Marson’s (P-value=3e-15) mouse Nanog targets.
(C) Venn diagram indicating the number of sites bound by Nanog-like at the high or dome stage (P<e−15). For each occupancy dataset, the de novo binding motif is indicated.
(D) Region map showing that Nanog-like binding sites at the high and dome stages co-localize. For each Nanog-like occupied region at the high stage, the occupancy of dome-stage Nanog-like is indicated within a 5-kb window centered on the high-stage Nanog-like region.
(E) Left: Transcripts reduced in nanog-like morphants detected in triplicate samples by a ratio relative to controls. Middle: Nanog-like binding frequency within 100kb of the corresponding genes, indicated by intensity of black shading. Binding is observed more frequently near loci with reduced expression, suggesting direct regulation of many top downregulated targets. Right: moving average of the Nanog-like binding intensity. The average binding intensity of 0.52 is derived from the ratio of the sum of Nanog-like binding frequency over the total number of genes interrogated (vertical blue line).
Overall, we identified 11,344 regions bound at the high stage and 14,010 regions bound at the dome stage. This correlated to 3577 genes bound at the high stage, and 4595 genes bound at the dome stage. Nanog-like binding profiles between the two stages were similar, indicated by a large number of co-occupied regions (6108) and co-localization heat map analysis (Figure 5C and 5D). For each developmental stage, we performed de novo motif analysis, and found similar motifs to the previously reported mouse Nanog binding motifs (Figure 5C) (Chen et al., 2008; Loh et al., 2006). To associate the binding data with the microarray data, we examined the downregulated genes from microarray analysis and their binding frequency, and found that the top 100 downregulated genes are highly enriched for Nanog-like binding (Figure 5E).
To analyze how Nanog-like regulates mxtx2 expression, we examined Nanog-like’s binding profile at the mxtx2 locus. The binding of Nanog-like at the mxtx2 locus is dynamic. At the high stage, Nanog-like binding covers the entire coding region. Notably, as indicated by the total target counts, the binding at the mxtx2 locus is the strongest across the entire genome, suggesting a particularly available locus for selective binding by Nanog-like. At the dome stage, binding is distributed to distinct peaks at the promoter, in the gene, and downstream regions (Figure 5A), and the peak height is lower than the one at the high stage.
Mxtx2 directly binds genes expressed in YSL
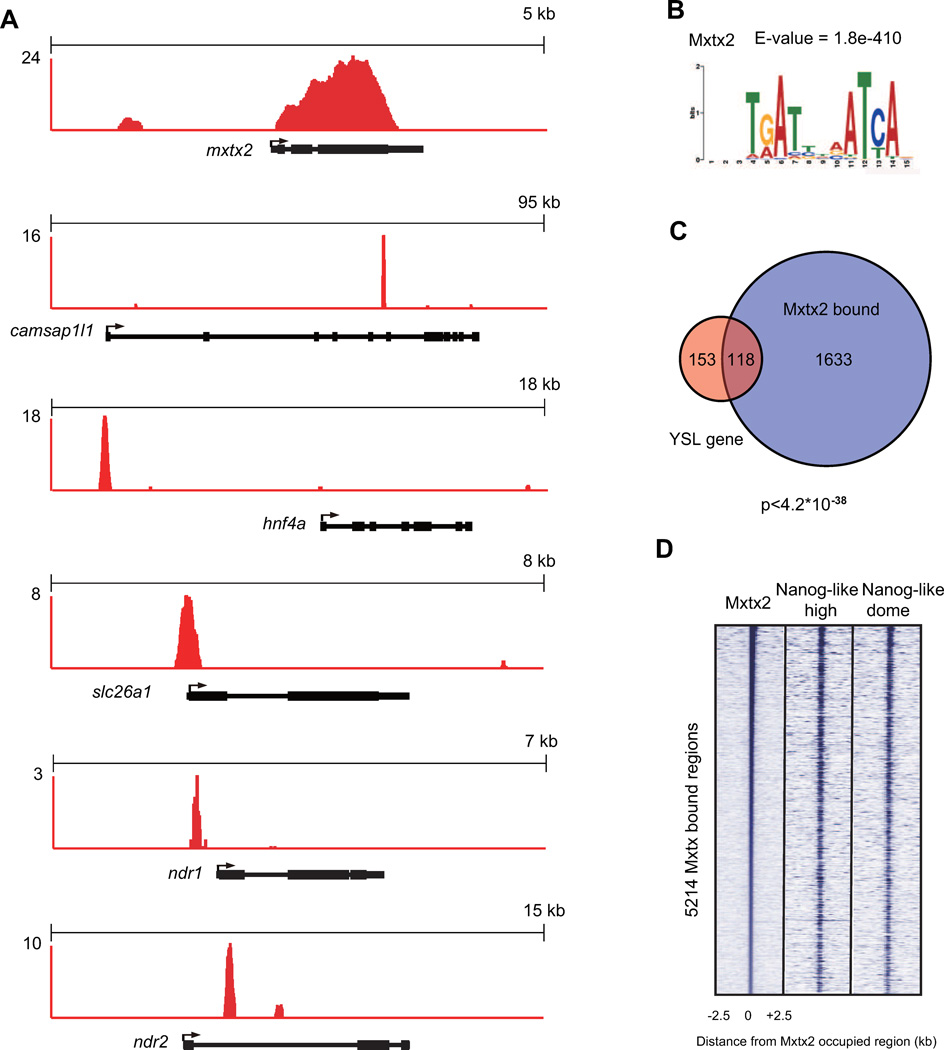
To determine whether YSL genes are direct Mxtx2 targets, we performed ChIP-Seq analysis on mxtx2-Myc mRNA-injected embryos at the dome stage. The 3’ end of the mxtx2 coding sequence was fused to a Myc tag repeat sequence. To determine if the fused Mxtx2-Myc protein was physiologically functional, we injected the mxtx2-Myc mRNA into 1-cell stage embryos, and found that it was capable of inducing ectopic YSL gene expression (Figure S5A). We examined all of the 12 YSL genes published by Hong et al (Hong et al., 2010), and found that 11 out of the 12 were bound by Mxtx2 (Figure 6A and Figure S5B). We also found that ndr1 and ndr2 were bound by Mxtx2, suggesting a direct activation of Nodal genes by Mxtx2 (Figure 6A). We expanded this analysis further by generating a YSL gene list containing all 271 genes having expression in the YSL during gastrulation (Table S3B) based on expression patterns in ZFIN (Sprague et al., 2006). We found 11.3% of the genes (1751 out of all annotated 15500 zebrafish genes) and 43.6% of the YSL genes (118 out of 271 genes expressed in the YSL) were bound by Mxtx2 (Figure 6C). The bound genes are highly enriched for YSL genes, as indicated by the hyper-geometric test (p<4.2*10−38). We performed de novo motif analysis, and identified a palindromic Mxtx2 binding motif, which suggested that dimerization of Mxtx2 may be required for DNA binding (Figure 6B). We noticed that many of the Mxtx2 binding loci were also occupied by Nanog-like (Figure S5C). This includes the presence of Nanog-like and Mxtx2 binding at the nanog-like and mxtx2 loci, potentially allowing for cross-regulation and auto-regulation of these genes.
Figure 6. Mxtx2 directly binds genes expressed in YSL.
(A) Mxtx2 binding profiles at mxtx2, camsap1l1, hnf4a, slc26a1, ndr1, and ndr2 loci. ChIP-Seq data are shown in reads per million with the y-axis floor set to 2 reads per million.
(B) de novo prediction of the Mxtx2 binding motif, utilizing sequences of the top 1000 Mxtx2 binding regions.
(C) Venn diagram showing the overlap of high-confidence (P<10−9) Mxtx2-occupied sites with genes expressed in the YSL. The hyper-geometric test suggests that the Mxtx2-bound genes are highly enriched for known YSL genes (p<4.2*10−38).
(D) Region map showing that Mxtx2 and Nanog-like co-localize at many Mxtx2-bound sites. For each Mxtx2-occupied region, the occupancy of Nanog-like is indicated within a 5-kb window centered on the Mxtx2-bound region.
Mxtx2 rescues defects in the nanog-like morphant
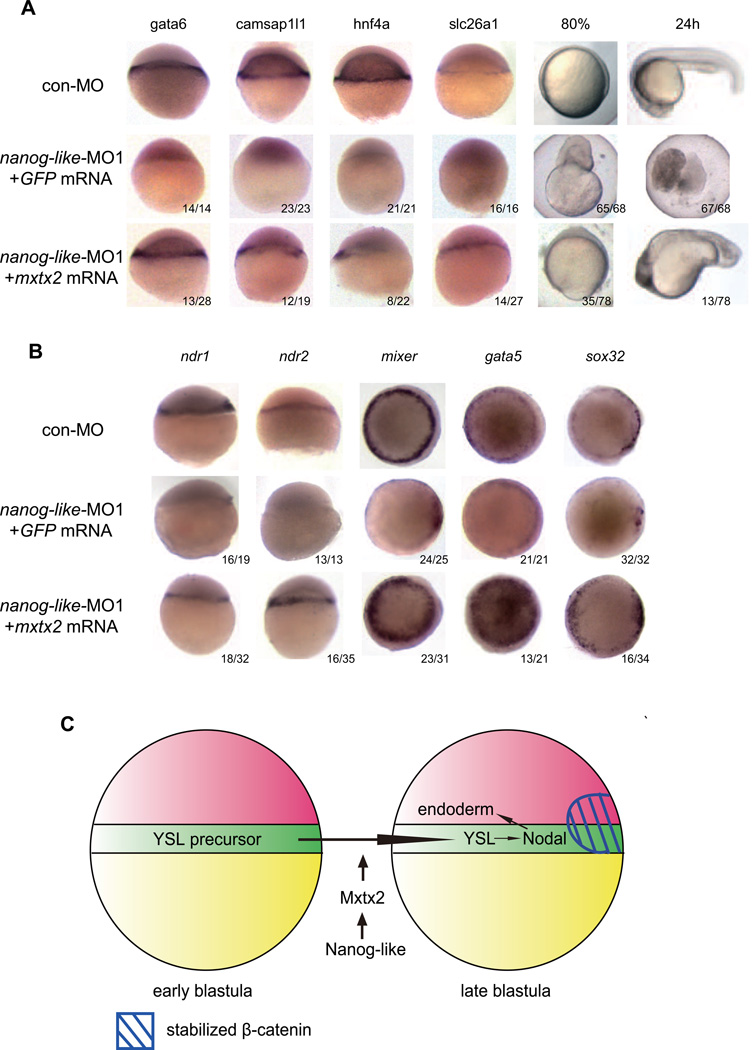
To test if the nanog-like morphant phenotype could be rescued by restoring Mxtx2, we co-injected mxtx2 mRNA and GFP mRNA into the yolk of the 4-cell stage nanog-like morphant and collected embryos with GFP expression in the YSL for further analysis. We examined different YSL markers, and found that Mxtx2 restored the YSL lineage in the nanog-like morphant (Figure 7A). We further examined whether gastrulation defects could be rescued by Mxtx2. Among the 78 mxtx2-injected nanog-like morphants, 35 exhibited normal epiboly at the 80% epiboly stage, suggesting that the yolk burst phenotype was, at least in part, due to a lack of mxtx2 expression (Figure 7A). At 24 hpf, 13 of 78 embryos were rescued from yolk lysis. However, all of the rescued embryos showed severe morphological defects (Figure 7A). To investigate if the ventrolateral endoderm could also be rescued by Mxtx2, we examined the expression patterns of Nodal genes and endoderm genes, and found that the expression patterns of both Nodals and endoderm genes were restored by mxtx2 in nanog-like morphants (Figure 7B). In addition, YSL and endoderm defects in mxtx2 morphants cannot be rescued by overexpressing nanog-like (data not shown). Together, these results suggest that Nanog-like induces the expression of mxtx2, which acts downstream of Nanog-like to establish the proper function of the YSL required for endoderm induction.
Figure 7. Mxtx2 expression rescues the nanog-like morphant.
(A) The YSL lineage was restored by Mxtx2 expression in the nanog-like morphant. Lateral view of embryos injected with 10ng control morpholino (con-MO), or 10ng nanog-like morpholino (nanog-like-MO1). nanog-like-MO1-injected embryos were injected with either 100pg GFP or 100pg mxtx2 mRNA and 25pg GFP at the 4-cell stage. About one third of injected embryos had GFP expression in the YSL and were collected for further analysis. gata6 expression was rescued in 46% (13/28) of mxtx2-injected embryos; camsap1l1 expression was rescued in 63% (12/19) of mxtx2-injected embryos, hnf4a expression was rescued in 36% (8/22) of the mxtx2-injected embryos, and slc26a expression was rescued in 52% (14/27) of mxtx2-injected embryos. Mxtx2 expression also rescue the yolk burst phenotype in the nanog-like morphant. At the 80% epiboly stage, 96% (65/68) GFP-injected nanog-like morphants showed the yolk burst phenotype, 45% (35/78) nanog-like morphants having mxtx2 and GFP expression in the YSL showed normal epiboly progress. At 24hpf, 99% (67/68) of GFP-injected nanog-like morphants were dead, and 17% (13/78) of nanog-like morphants having mxtx2 and GFP expression in the YSL showed axes rescue with defective head formation and body elongation.
(B) Mxtx2 expression rescued the endoderm defect in the nanog-like morphant. Lateral view of embryos with dorsal side to the right. Ventrolateral ndr1 expression was rescued in 56% (18/32) of the embryos; ndr2 expression was rescued in 45% (16/35) of the embryos; mixer expression was rescued in 74% (23/31) of the embryos, gata5 expression was rescued in 62% (13/21) of the embryos, sox32 expression was rescued in 47% (16/34) of the embryos.
(C) Model for endoderm and YSL induction. During early blastula development, the YSL precursor is formed by the collapse of marginal cells into the yolk cell. Acquisition of the YSL fate is mediated by Nanog-like through activation of the YSL master regulator Mxtx2. Stabilized β-catenin activates Nodal signals for endoderm induction in the dorsal region. The YSL and marginal blastomeres produce Nodal signals to induce the ventrolateral endoderm formation
DISCUSSION
Our studies have established a role for Nanog-like during zebrafish YSL development. We propose a model in which Nanog-like regulates endoderm formation through an Mxtx2-Nodal pathway. During the early blastula stage (2.5 hpf), marginal blastomere cells collapse to form the YSL precursor. In the YSL precursor, Nanog-like directly activates Mxtx2, which in turn specifies the YSL lineage by directly activating the expression of YSL genes. During the late blastula stage, the YSL functions as a signaling center producing Nodal molecules for ventrolateral endoderm induction. The dorsal endoderm is induced by stabilized β-catenin-activated Nodal independent of Nanog-like (Figure 7C).
The pluripotency network described in embryonic stem cells has not been studied in the developmental ICM or in the epiblast. Zebrafish produce a large number of embryos, providing us with an opportunity to access the in vivo network. One important element in succeeding in a ChIP-Seq experiment is having a reliable antibody for the transcription factor of interest. To overcome the lack of antibodies in zebrafish studies, we developed a technique allowing us to perform ChIP-Seq analysis utilizing embryos expressing Myc tagged transcription factors. Using this technique, we examined the Nanog-like and Mxtx2 gene regulatory network in blastula stage zebrafish embryos. We found that Nanog-like binds to known pluripotency genes like pou5f1, sox2, and nanog-like. We also found that Nanog-like bound to genes involved in extraembryonic lineage differentiation, like gata3 and krt4 for EVL differentiation, and mxtx2 and slc26a1 for YSL differentiation, mesoderm specification like ntl and tbx3, cell movement like wnt11 and cxcr4b, and signaling genes like ndr1, bmp2b, fgf8a and wnt8a. The binding profile suggests that Nanog-like may play a versatile role involving many developmental processes. Another intriguing finding is that Nanog-like and Mxtx2 bound to nanog-like and mxtx2 loci, suggesting potential cross-regulation and auto-regulation loops between these two genes. This observation prompted us to test if Nanog-like could rescue mxtx2 morphants. By overexpressing Nanog-like, we were not able to rescue the YSL and endoderm defects in mxtx2 morphants (data not shown). This is consistent with our findings that Mxtx2 functions downstream of Nanog-like.
Mxtx2 is critical for YSL induction. The expression of mxtx2 is highly regulated, suggested by the fact that both the high stage Nanog-like and Mxtx2 binding sites at the mxtx2 locus are the strongest across the entire genome. Nanog-like is ubiquitously expressed in all blastomere cells and its overexpression does not induce ectopic mxtx2 expression, suggesting that it is not responsible for the spatial expression of mxtx2. The spatial restriction of mxtx2 expression to the YSL may be a result of the formation of the YSL structure through collapsing of marginal cells into the yolk. One possibility is that one signaling pathway is activated in the YSL by this event, which in turn activates the expression of mxtx2 in the YSL.
The YSL is unique to teleosts, and has been considered an equivalent of the mouse primitive endoderm. Interestingly, Nanog is required for primitive endoderm formation, but with a distinct mechanism. Recent studies found that Nanog regulates primitive endoderm formation through a non-cell autonomous mechanism (Frankenberg et al., 2011; Messerschmidt and Kemler, 2010). Our study suggests that in zebrafish Nanog-like regulates YSL formation through a cell-autonomous mechanism. While the YSL and primitive endoderm share similar gene expression and both function as the signaling center that patterns the head mesoderm and endoderm, the regulation mechanism involving their formation is different. The FGF signal that is required for mouse primitive endoderm induction seems not to be involved in YSL formation (Chazaud et al., 2006; Yamanaka et al., 2010). Moreover, Gata4 and Gata6, key transcription factors in regulating primitive endoderm formation, are dispensable for YSL formation (Holtzinger and Evans, 2007; Koutsourakis et al., 1999; Peterkin et al., 2007).
Compelling evidence suggests that pluripotency factors have distinct roles in mammals and teleosts. In zebrafish, the acquisition of pluripotency by deep cells (the mouse epiblast equivalent) is independent of Nanog-like, as nanog-like-deficient deep cells can readily differentiate into the three germ layers (Figure 2A and data not shown). It should be noted that maternally deposited Nanog-like protein cannot be eliminated by the morpholino knockdown approach. A similar observation was made in the maternal-zygotic (MZ) pou5f1 mutant. Pou5f1 is required for the formation of pluripotent ICM in the mouse blastocyst (Nichols et al., 1998), but the zebrafish MZ pou5f1 mutant, lacking both maternal and zygotic expression, was still capable of differentiating into three germ layers (Lunde et al., 2004; Reim et al., 2004). Despite Schuff et al recently showed that zebrafish Nanog-like prevents murine embryoid body (EB) differentiation (Schuff et al., 2011), our data reveals that overexpression of zebrafish Nanog-like does not rescue LIF dependence in murine ES cells (Figure S1D, S1E, and S1F). Certain structural domains may be responsible, as zebrafish pou5f1 does not rescue mouse Pou5f1 mutant ES cells (Morrison and Brickman, 2006). The conservation of Nanog-like and Pou5f1 is supported by the fact that both zebrafish mutants can be rescued by their mammalian counterparts (Figure 1F and 1G)(Onichtchouk et al., 2010). However, Nanog-like’s role in YSL differentiation and Pou5f1’s role in endoderm differentiation do not seem to be conserved in mammals (Figure 3A and 3B)(Lunde et al., 2004; Reim et al., 2004). We speculate that the ancestors of pluripotency regulators adopted distinct functions during teleost evolution.
Our work reveals a role for Nanog-like in regulating the formation of the extra-embryonic lineage, which later secretes Nodal signals to break down the pluripotency of the deep cell. Our studies suggest that the pluripotency network may have distinct roles on germ layer formation in vivo.
EXPERIMENTAL PROCEDURES
Zebrafish maintenance
Zebrafish were maintained in accordance with Animal Research Guidelines at Children’s Hospital Boston. The wild-type embryos were collected by natural spawning from the TU strain and staged as described (Westerfield, 1995).
Morpholino and mRNA injection
Morpholinos were obtained from Gene Tools, LLC, (nanog-like-MO1: 5'-CTGGCATCTTCCAGTCCGCCATTTC-3'; nanog-like-MO2: 5'-AGTCCGCCATTTCGCCGTTAGATAA-3'; mxtx2-MO: 5'-CATTGAGTATTTTGCAGCTCTCTTG-3'; control-MO: 5'-CCTCTTACCTCAGTTACAATTTATA-3') and injected into one-cell stage embryos. Capped mRNA was generated using mMessage mMachine (Ambion) and injected into 1-cell stage embryos if not specified.
Microarray analysis
RNA was extracted by Trizol reagent (Invitrogen). Microarray hybridization was performed at Roche NimbeGen (Reykjavi´k, Iceland) using the NimbleGen zebrafish expression array. The goldenspike package in Bioconductor/R was used to process CEL files and to identify genes with relative changes in mRNA levels between wild-type and nanog-like morphants. A log2 mean fold change was calculated along with a q-value. Heat map was generated by Arraystar (DNASTAR).
RNase and rhodamine dextran injection
Ribonuclease A (Sigma-Aldrich) was diluted in water, for a final concentration of 10 µg/ml, and co-injected with 4mg/ml rhodamine dextran (Invitrogen) in the yolk of 1K-cell stage (3 hpf) embryos. 1nl was injected per embryo.
Blastoderm transplants
Blastoderm transplantation was performed as described previously (Holloway et al., 2009; Yamaha et al., 2001). Briefly, 1K-cell stage embryos were dechorinated in agarose-coated petri dishes. Wild-type and morphant embryos were transferred to an agarose-coated petri dish with in 1X Ringers/1.6% whipped egg white. A pulled glass capillary knife and a polished glass capillary probe were used to separate the blastoderm from the yolk. Donor blastoderms were positioned onto host yolks under gentle pressure until adherent. After 5–10 minutes of healing, chimeric embryos were transferred to 1/3 Ringers solution for further development.
ChIP-Seq
3’ ends of nanog-like and mxtx2 coding sequences were tagged with a repeated Myc-epitope-coding sequence using the tol2kit (Harbison et al., 2004; Kwan et al., 2007). 1nl of 25ng/µl capped mRNA was injected per embryo. For each CHIP experiment, we used 2000 injected embryos.
Chromatin immunoprecipitation experiments were performed as previously described (Lee et al., 2006) with modifications at the crosslinking step. Injected embryos were staged and dechorionated by pronase (Roche), and washed twice with E3 embryo water. Embryos were transferred to 15ml falcon tubes in E3 and crosslinked by the addition of 37% formaldehyde to a final concentration of 1% for 5 minutes at room temperature with shaking. The crosslinking was quenched by the addition of 1/20 volume 2.5M Glycine for 3 minutes at room temperature with shaking. Embryos were washed twice with ice-cold PBS and the pellet was flash-frozen in liquid nitrogen. Embryos were kept at −80°C until the experiments were performed. The Myc tag antibody (Abcam, ab9132) was used in the immunoprecipitation step. Detailed ChIP methods are described in the Extended Experimental Procedure.
The ChIP DNA samples were prepared with the Illumina/Solexa Genomic DNA kit (Illumina- IP-102-1001), and sequenced on the Illumina Genome Analyzer 1G. Detailed analysis methods are described in the Extended Experimental Procedure.
Highlights.
Nanog-like knockdown impairs endoderm formation due to decreased Nodal signaling
Nanog-like promotes the formation of extraembryonic tissue (YSL) that secretes Nodal
Nanog-like’s target Mxtx2 activates YSL by binding directly to YSL genes
Supplementary Material
Acknowledgements
Supported by National Institutes of Health (NIH) grants 5R01HL048801-18 and 5U01HL10001-02 (L.I.Z.), R01 HG002668 (R.A.Y.), and HL056182 (T.E.). We thank I. Swinburne, J. Mullor and R. Zhao for providing reagents, Y. Zhou, A. Chen, and L. Lawson for technical assistance, and O. Tamplin for critical appraisal of the work. L.I.Z. and G.Q.D. are Howard Hughes Medical Institute (HHMI) investigators. L.I.Z. is a founder and stock holder of Fate, Inc. and a scientific advisor for Stemgent.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers
Microarray (GSE34682) and ChIP-seq (GSE34683) data were deposited in GEO (http://www.ncbi.nlm.nih.gov/geo/) under the accession numbers indicated.
REFERENCES
- Boyer LA, Lee TI, Cole M, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Legros S, Artus J, Doss MX, Khanin R, Hadjantonakis AK, Foley A. A comparative analysis of extra-embryonic endoderm cell lines. PLOS One. 2010;5:e12016. doi: 10.1371/journal.pone.0012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AEE, Howley C, Fox MD, Ho RK. T-Box gene eomesodermin and the Homeobox containing Mix/Bix gene mtx2 regulate epiboly movements in the zebrafish. Developmental Dynamics. 2005:105–114. doi: 10.1002/dvdy.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Developmental Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Chen S-r, Kimelman D. The role of the yolk syncytial layer in germ layer patterning in zebrafish. Development. 2000;127:4681–4689. doi: 10.1242/dev.127.21.4681. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Cheng JC, Miller AL, Webb SE. Organization and function of microfilaments during late epiboly in zebrafish embryos. Dev Dyn. 2004;231:313–323. doi: 10.1002/dvdy.20144. [DOI] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmeis T, Mourrain P, Saint-Etienne L, Fischer N, Aanstad P, Clark M, Strähle U, Rosa F. A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev. 2001;15:1487–1492. doi: 10.1101/gad.196901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JE, Allegrucci C, Redwood C, Kump K, Bian Y, Chatfield J, Chen YH, Sottile V, Voss SR, Alberio R, et al. Axolotl Nanog activity in mouse embryonic stem cells demonstrates that ground state pluripotency is conserved from urodele amphibians to mammals. Development. 2010;137:2973–2980. doi: 10.1242/dev.049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Hagosa EG, Xu B, Sias C, Kawakami K, Burdine RD, Dougan ST. Nodal signals mediate interactions between the extra-embryonic and embryonic tissues in zebrafish. 2007 doi: 10.1016/j.ydbio.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Frankenberg S, Gerbe F, Bessonnard S, Belville C, Pouchin P, Bardot O, Chazaud C. Primitive Endoderm Differentiates via a Three-Step Mechanism Involving Nanog and RTK Signaling. Developmental Cell. 2011;21:1005–1013. doi: 10.1016/j.devcel.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Harbison CT, Gordon DB, Lee TI, Rinaldi NJ, Macisaac KD, Danford TW, Hannett NM, Tagne JB, Reynolds DB, Yoo J, et al. Transcriptional regulatory code of a eukaryotic genome. Nature. 2004;431:99–104. doi: 10.1038/nature02800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Houart C, Wilson SW, Stainier DY. A role for the extraembryonic yolk syncytial layer in patterning the zebrafish embryo suggested by properties of the hex gene. Current biology. 1999;9:1131–1134. doi: 10.1016/s0960-9822(99)80485-0. [DOI] [PubMed] [Google Scholar]
- Holloway BA, Gomez dlTCS, Ye Y, Slusarski DC, Freisinger CM, Dosch R, Chou MM, Wagner DS, Mullins MC. A novel role for MAPKAPK2 in morphogenesis during zebrafish development. PLOS Genetics. 2009;5:e1000413. doi: 10.1371/journal.pgen.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzinger A, Evans T. Gata5 and Gata6 are functionally redundant in zebrafish for specification of cardiomyocytes. Developmental biology. 2007;312:613–622. doi: 10.1016/j.ydbio.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jang M, Brown J, McBride A, Feldman B. Embryonic mesoderm and endoderm induction requires the actions of non-embryonic Nodal-related ligands and Mxtx2. Development. 2011;138:787–795. doi: 10.1242/dev.058974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SK, Levin CS, Brown JL, Wan H, Sherman BT, Huang dW, Lempicki RA, Feldman B. Pre-gastrula expression of zebrafish extraembryonic genes. BMC Dev Biol. 2010;10 doi: 10.1186/1471-213X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppen M, Fernández BG, Carvalho L, Jacinto A, Heisenberg CP. Coordinated cell-shape changes control epithelial movement in zebrafish and Drosophila. Development. 2006;133:2671–2681. doi: 10.1242/dev.02439. [DOI] [PubMed] [Google Scholar]
- Kane DA, Warga RM, Kimmel CB. Mitotic domains in the early embryo of the zebrafish. Nature. 1992;360:735–737. doi: 10.1038/360735a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Agathon A, Alexander J, Thisse C, Waldron S, Yelon D, Thisse B, Stainier DY. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001;15:1493–1505. doi: 10.1101/gad.892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Trinh LA, Reiter JF, Alexander J, Yelon D, Stainier DY. The zebrafish bonnie and clyde gene encodes a Mix family homeodomain protein that regulates the generation of endodermal precursors. Genes Dev. 2000;14:1279–1289. [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Law RD. Cell lineage of zebrafish blastomeres. I. Cleavage pattern and cytoplasmic bridges between cells. Developmental biology. 1985a;108:78–85. doi: 10.1016/0012-1606(85)90010-7. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Law RD. Cell lineage of zebrafish blastomeres. II. Formation of the yolk syncytial layer. Developmental biology. 1985b;108:86–93. doi: 10.1016/0012-1606(85)90011-9. [DOI] [PubMed] [Google Scholar]
- Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Developmental Dynamics. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Lee TI, Johnstone SE, Young RA. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat Protoc. 2006;1:729–748. doi: 10.1038/nprot.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nature Genetics. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Lunde K, Belting HG, Driever W. Zebrafish pou5f1/pou2, homolog of mammalian Oct4, functions in the endoderm specification cascade. Current biology. 2004;14:48–55. doi: 10.1016/j.cub.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmidt DM, Kemler R. Nanog is required for primitive endoderm formation through a non-cell autonomous mechanism. Developmental biology. 2010;344:129–137. doi: 10.1016/j.ydbio.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Yamaha E, Wakahara M, Kuroiwa A, Takeda H. Mesoderm induction in zebrafish. Nature. 1996;383:131–132. [Google Scholar]
- Morrison GM, Brickman JM. Conserved roles for Oct4 homologues in maintaining multipotency during early vertebrate development. Development. 2006;133:2011–2022. doi: 10.1242/dev.02362. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Ober EA, Schulte-Merker S. Signals from the Yolk Cell Induce Mesoderm, Neuroectoderm, the Trunk Organizer, and the Notochord in Zebrafish. Developmental biology. 1999;215:167–181. doi: 10.1006/dbio.1999.9455. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Geier F, Polok B, Messerschmidt DM, Mössner R, Wendik B, Song S, Taylor V, Timmer J, Driever W. Zebrafish Pou5f1-dependent transcriptional networks in temporal control of early development. Mol Syst Biol. 2010;6:365. doi: 10.1038/msb.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkin T, Gibson A, Patient R. Redundancy and evolution of GATA factor requirements in development of the myocardium. Developmental biology. 2007;311:623–635. doi: 10.1016/j.ydbio.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain M, Lepage T. Mezzo, a paired-like homeobox protein is an immediate target of Nodal signalling and regulates endoderm specification in zebrafish. Development. 2002;129:4901–4914. doi: 10.1242/dev.129.21.4901. [DOI] [PubMed] [Google Scholar]
- Reim G, Mizoguchi T, Stainier DY, Kikuchi Y, Brand M. The POU domain protein spg (pou2/Oct4) is essential for endoderm formation in cooperation with the HMG domain protein casanova. Developmental Cell. 2004;6:91–101. doi: 10.1016/s1534-5807(03)00396-4. [DOI] [PubMed] [Google Scholar]
- Reiter JF, Alexander J, Rodaway A, Yelon D, Patient R, Holder N, Stainier DY. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13:2983–2995. doi: 10.1101/gad.13.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaway A, Takeda H, Koshida S, Broadbent J, Price B, Smith JC, Patient R, Holder N. Induction of the mesendoderm in the zebrafish germ ring by yolk cell-derived TGF-beta family signals and discrimination of mesoderm and endoderm by FGF. Development. 1999;126:3067–3078. doi: 10.1242/dev.126.14.3067. [DOI] [PubMed] [Google Scholar]
- Schier A, Neuhauss S, Helde K, Talbot W, Driever W. The one-eyedpinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Schuff M, Siegel D, Philipp M, Bundschu K, Heymann N, Donow C, Knöchel W. Characterization of Danio rerio Nanog and Functional Comparison to Xenopus Vents. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnica-Krezel L, Driever W. Microtubule arrays of the zebrafish yolk cell: organization and function during epiboly. Development. 1994;120:2443–2455. doi: 10.1242/dev.120.9.2443. [DOI] [PubMed] [Google Scholar]
- Sprague J, Bayraktaroglu L, Clements D, Conlin T, Fashena D, Frazer K, Haendel M, Howe DG, Mani P, Ramachandran S, et al. The Zebrafish Information Network: the zebrafish model organism database. Nucl. Acids Res. 2006;34:D581–D585. doi: 10.1093/nar/gkj086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U, Jesuthasan S. Ultraviolet irradiation impairs epiboly in zebrafish embryos: evidence for a microtubule-dependent mechanism of epiboly. Development. 1993;119:909–919. doi: 10.1242/dev.119.3.909. [DOI] [PubMed] [Google Scholar]
- Varlet I, Collignon J, Robertson EJ. nodal expression in the primitive endoderm is required for specification of the anterior axis during mouse gastrulation. Development. 1997;124:1033–1044. doi: 10.1242/dev.124.5.1033. [DOI] [PubMed] [Google Scholar]
- Wang J, Levasseur DN, Orkin SH. Requirement of Nanog dimerization for stem cell self-renewal and pluripotency. Proc Natl Acad Sci U S A. 2008;105:6326–6331. doi: 10.1073/pnas.0802288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. University of Oregon Press, Eugene, USA. 1995 [Google Scholar]
- Wilkins SJ, Yoong S, Verkade H, Mizoguchi T, Plowman SJ, Hancock JF, Kikuchi Y, Heath JK, Perkins AC. Mtx2 directs zebrafish morphogenetic movements during epiboly by regulating microfilament formation. Developmental biology. 2008;314:12–22. doi: 10.1016/j.ydbio.2007.10.050. [DOI] [PubMed] [Google Scholar]
- Yamaha E, Kazama-Wakabayashi M, Otani S, Fujimoto T, Arai K. Germ-line chimera by lower-part blastoderm transplantation between diploid goldfish and triploid crucian carp. Genetica. 2001;111:227–236. doi: 10.1023/a:1013780423986. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Lanner F, Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development. 2010;137:715–724. doi: 10.1242/dev.043471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.