Abstract
The intestinal epithelium provides a barrier between a variety of luminal antigens and provides the components of intestinal innate and adaptive immunity. It is crucial that at this interface, the epithelial cell layer and the components of the intestinal immunity interact with dietary and bacterial antigens in a regulated way to maintain homeostasis. Failure to tightly control immune reactions can be detrimental and result in inflammation. In the current review, we described the regulatory mechanisms controlling host–immune homeostasis and the role of regulatory CD4+ T cells, with a special emphasis in the regulatory T-cell subsets (Tregs). Furthermore, the participation of innate cell cross-talk in the polarization of intestinal immune responses is also evaluated. Finally, the recent characterization of host responses to normal commensal flora, the role of bacteria and bacterial factors in the maintenance of immunomodulation, and the disruption of this balance by bacterial enteric pathogens is also summarized.
Keywords: bacteria, homeostasiss, immunomodulation, innate immune system, T cells
Intestinal homeostasis is maintained by multiple interactions between the microbiota, the intestinal epithelium and the host immune system [1]. Because the GI tract is heavily populated with more than 100 trillion microorganisms that maintain a symbiotic relationship, but which can also potentially display a pathogenic phenotype, it is important that the immune system establishes and maintains a strong presence at this mucosal boundary, exemplified by lymphocytes, macrophages and other cells that are involved in maintaining immune tolerance [2].
Despite the hostile environment of the GI tract (low pH, presence of digestive enzymes and the detergent activity of bile salts), this anatomical site remains as a major route of entry for numerous pathogens. Several components are required to maintain a stable environment, such as physical barriers, including epithelial cells (ECs) joined firmly by tight junction proteins, brush-border microvilli, and a dense layer of mucin [3]. Antimicrobial peptides, such as defensins produced by ECs and Paneth cells, provide another barrier for protection [4]. Finally, the presence of the gut-associated lymphoid tissue (GALT), which is comprised of several different organized lymphoid structures, protects the body from the invasion of pathogens [5]. GALT contains approximately 70% of the total lymphocytes in the body, and, based on location, the GALT is distributed in three basic populations: Peyer’s patches (PPs), lymphoid follicles (ILFs) and lamina propria (LP). The PPs with a predominance of B lymphocytes and a well-characterized site for the initiation of intestinal IgA responses [6]. The Isolated ILF and LP are also important in the induction of intestinal IgA responses [3]. As the primary draining lymph nodes for the intestinal tract, the mesenteric lymph nodes (MLN) are also an important location for the initiation of gut-associated immune responses.
An important component of the GI immune system is the antigen-sampling microfold or membranous cells (M cells), which are found in the follicle-associated epithelium, covering the PPs. The M cells endocytose a variety of protein and peptide antigens, allowing the selective and efficient transfer of antigens from the intestinal lumen into PPs, where they are taken up by the lamina propria CD103+ dendritic cells (DCs) and macrophages [7]. Thus, M cells are considered key players in antigen sampling and a gateway for the mucosal immune system. The DCs can also sample luminal antigens through extending dendrites between ECs [8], leading ultimately to the appearance of IgA-secreting plasma cells in the mucosa and secretory IgA in the lumen, resulting in the interference of adhesion and invasion of bacteria [9]. The T cells exposed to antigen in the PPs also migrate to the lamina propria and the epithelium, where they mature to cytotoxic T cells, providing another mechanism for containing microbial assaults [10].
In addition to the GALT discussed above, several other processes play key roles in the regulation of bacterial communities in the intestine and defining the repertoire of gut microbiota, which is closely linked to the proper functioning of the immune system. In this review article, the distinct events during intestinal homeostasis and pathogenic processes are summarized.
Regulatory mechanisms controlling host immune homeostasis
The GI tract is home to 400–1000 different species of microorganisms [11]. With such a vast microbial burden, it is inconceivable that the intestinal immune system generates an active response against all of the antigens encountered. The dialog between the microbiota and the intestinal immune system defines intestinal homeostasis, where shifts in the balance result in microbial infection, or aberrant activation of the intestinal immune system (Figure 1). Deregulated activation of the immune system often results in a chronic inflammatory state of the intestinal tract and in development of inflammatory bowel disease. As such, there are a variety of mechanisms involved in regulation of the host response to the commensal microbiota.
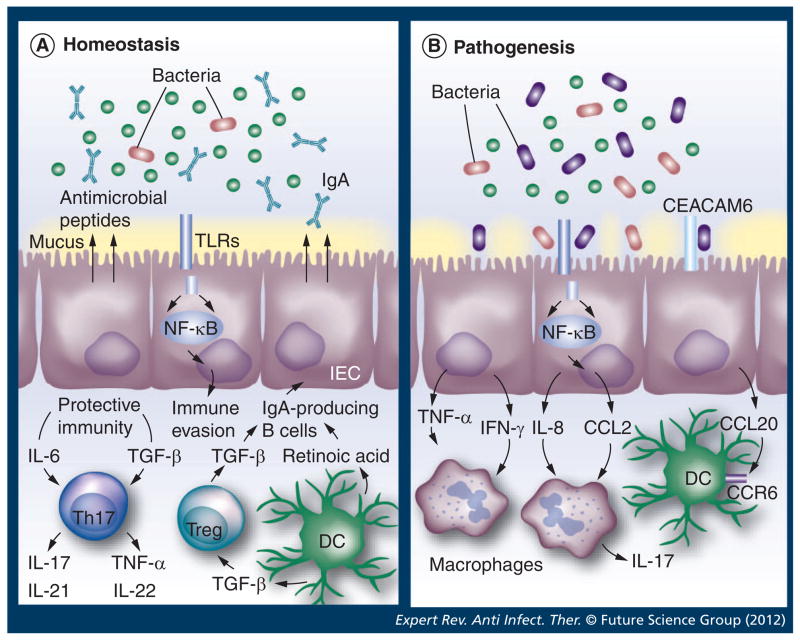
Figure 1. Intestinal homeostasis and its breakdown during pathogenic processes.
(A) Intestinal homeostasis requires the establishment of a balance between the microbiota, the intestinal epithelium and the host immune system. Diverse regulatory mechanisms and cross-talk between intestinal and immune cells help to maintain intestinal homeostasis, and a breakdown in these pathways may precipitate the inflammatory pathology observed during pathogenic processes (B). IECs and intestinal DCs sense distinct infectious agents, leading to the production of factors that direct different immune responses (black arrows). Regulatory T cell (Treg; blue) can suppress all types of inflammatory responses and enhance the production of protective secretory IgA antibodies. Th17 type (purple), together with other Th cell types, participate in the maintenance of the protective immunity. CD103+ DCs display the ability to drive the differentiation of Tregs and to inhibit Th1 and Th17 cell development. A bacterial pathogenic process causes the destruction of the intestinal epithelial integrity, resulting in the recruitment of immune cells to the site of infection. Inflammation may affect the differentiation of tolerogenic macrophages from recruited monocytes, leading to reduction in Treg differentiation and inability to control the activity of DCs. As a result, a pathogenic process is initiated.
DC: Dendritic cell; IEC: Intestinal epithelial cell; TLR: Toll-like receptor.
Mucus
Specialized ECs; goblet cells, secrete mucin glycoproteins that form a thick mucus layer which coats the intestinal epithelium. This mucus coating protects the apical epithelium from bacterial contact, whereas the outer layers of mucus contain large amounts of bacteria that are unable to penetrate, thereby limiting bacterial contact with the epithelium. The protection afforded by the mucus layer is clearly noticeable [12], and emphasized in mice lacking mucin-1 or mucin-2, which results in direct bacterial contact with the epithelium throughout the intestinal tract, and causing spontaneous intestinal inflammation [13].
Antimicrobial peptides
Intestinal epithelial Paneth cells secrete antimicrobial peptides that neutralize bacteria. These peptides come from diverse protein families, including defensins, cathelicidins, and C-type lectins, and are regulated by distinct mechanisms. In essence, most of the antimicrobial peptides function similarly to antibiotics, with bactericidal activity resulting in the disruption of the cell wall or inner membrane of bacteria. In general terms, all ECs secrete antimicrobial peptides and it is clear that they are produced to protect the epithelial layer [14,15]. Most of the secreted peptides are retained in the mucus layer; therefore it appears that this layer provides a concentrated bactericidal barrier for the intestinal epithelium.
IgA
Approximately 80% of all plasma cells reside within the intestinal tract [16], and the majority of them secrete IgA. As the most abundant antibody isotype in our body, IgA is secreted across mucosal membranes. Secreted IgA has been shown to play a role both in the host response to infections and in the maintenance of a balance between the host and its residential microbiota [17]. Both neonates and germ-free mice have very low levels of IgA in the intestinal tract, however, upon colonization, pathogen-free mice have abundant levels of IgA [18]. Mucosal IgA comprises antibodies that recognize specific antigens with high-affinity, as well as low-affinity antibodies that target conserved bacterial motifs. In general, first, high-affinity IgA neutralizes microbial toxins and invasive pathogens [19] and, second, low-affinity IgA binds to conserved bacterial motifs in order to limit bacterial adherence to the epithelium and thereby preventing bacterial infiltration and immune activation from intestinal antigens [20,21]. The variability of the IgA repertoire is also constantly stimulated by microbial species through the induction of somatic hypermutations. In mice with deficiencies on activation-induced cytidine deaminase, a critical enzyme for somatic hypermutations, it was found that Yersinia enterocolotica was easier to recover intragastrically than from wild-type mice, suggesting an impairment on the mucosal barrier, probably due to the hyperplasia of germinal centers observed in PPs [19].
Role of Th17 & Treg responses
Large numbers of CD4+ T cells roam between the intestinal mucosa and surrounding lymph nodes and lymphoid follicles. Upon presentation of their cognate antigen by antigen-presenting cells, CD4+ T cells differentiate into various subsets based on the cytokine cues in the environment. Two specialized T helper subsets, Th17 and Treg cells, which are found dominantly in the intestinal tract, and are dichotomous in protecting the intestine from infection (Figure 1), are discussed further.
In mice, Th17 cells are generated in the presence of IL-6 and TGF-β. Upon induction of the transcription factor ROR-γt, T cells commit to the Th17 lineage. After differentiation, they can secrete their signature cytokines IL-17A, IL-17F, IL-21 and IL-22, in addition to other proinflammatory cytokines including TNF-α and GM-CSF, while IL-23 is required to maintain Th17 populations [22]. Through their inflammatory cytokine panel, Th17 cells are viewed as critical in controlling extracellular infections. Therefore the highest populations of Th17 cells are found in the airway and the intestinal mucosa, the two surfaces that are exposed to the external environment and thus, come into contact with a large number of microbes. However, in the absence of microbiota, germ-free mice have very limited numbers of Th17 cells [23], indicating a major role for microbiota in the generation of Th17 cells. Particularly, monoassociation of germ-free mice with segmented filamentous bacteria directly results in the induction of ROR-γt and Th17 cells [24,25]. Both IL-17A and IL-17F have potent proinflammatory properties that act upon a wide range of cells inducing the expression of proinflammatory cytokines, chemokines, and metalloproteinases. Notably, IL-17A and IL-17F are very effective in generating responses from neutrophils, through recruitment, activation and subsequent migration [26]. Due to the potency of the Th17 response, it has been implicated in the clearance of many bacterial pathogens, as both IL-17A and IL-17 receptor-deficient mice are greatly susceptible to Klebsiella pneumoniae infection, specifically characterized by a deficiency in neutrophil migration [27,28]. Such deficiencies in IL-17 also lead to susceptibility to fungal infections by Candida albicans [29] and Aspergillus fumigatus [30]. With regards to the intestinal tract, Th17 cells also play a vital role in protective immunity. IL-17A is a strong inducer of β-defensin-2, a potent antimicrobial peptide that is constitutively produced by intestinal ECs that is key for maintaining homeostasis [15]. Moreover, IL-22 from Th17 cells induces small intestinal ECs to produce RegIIIγ, a C-type lectin that targets Gram-positive bacteria, which is important in reducing bacterial contact with the epithelium [31,32]. In the case of infection by Shigella flexneri, Th17 cells are generated by the initial infection. While clearance of the primary infection is independent of IL-17, memory Shigella-specific Th17 cells confer protective immunity against a reinfection [33].
As the master regulators of the immune system, regulatory T cells (Treg) are generated in the thymus, and are generally referred to as natural Tregs. Additionally, inducible Tregs are generated in the periphery from antigen presentation, in the presence of TGF-β. Upon expression of the transcription factor forkhead box P3 (FOXP3), Tregs suppress the function of other immune cells primarily through production of TGF-β and IL-10, two universally-pleiotropic suppressive cytokines. The function of Tregs is critical in the regulation of the immune system, as mice with deficiencies in TGF-β or FOXP3 die within 3 weeks of birth from extensive multiorgan inflammation caused by hyperproliferation of CD4+ T cells and high levels of inflammatory cytokines [34–37]. Mice deficient in IL-10 also develop severe spontaneous inflammation in the large intestine [38]. FOXP3+ Treg and IL-10-producing Tr1 cells thereby act to regulate the proliferation and function of both innate and adaptive immune cells, but particularly in the intestinal tract, where they are required for reinforcing intestinal homeostasis and restricting damage to host tissues from the inflammatory response (Figure 1). When CD45RBhigh naive effector T cells are transferred into immunodeficient RAG-deficient or SCID mice, the effector T-cell population expands rapidly in the intestinal tract into Th1 and Th17 and secretes high levels of proinflammatory cytokines. The expansion and corresponding intestinal inflammation does not occur when CD45RBhigh T cells are transferred into germ-free mice, thereby indicating that the inflammatory response of transferred effector T cells is driven by stimulation from commensal antigens [39]. Effector T-cell-mediated inflammation is abrogated when Tregs are cotransferred with CD45RBhigh cells, indicating that Tregs functions to primarily limit a hyperactive immune response to commensal antigens that do not pose a threat.
While Tregs contribute to intestinal homeostasis mainly by immunosuppression, they can also play an important role in neutralization of bacterial antigens and preventing them from ever activating the intestinal immune system. This occurs indirectly through induction of large quantities of IgA on the intestine. After an antigen encounter and T-cell interaction, B cells undergo a class-switch recombination to produce a single Ig subtype, depending on cytokine cues from the environment. Of note, TGF-β is the key cytokine that drives differentiation to IgA-producing B cells. Through this, Tregs are a major helper for inducing a sufficient IgA response. In support of this notion, it has been shown that Tregs are a major cellular source of TGF-β for IgA class switching. Depletion of CD25+ Treg strongly decreased intestinal IgA production (total and antigen-specific) as well as total numbers of IgA+ B cells. Furthermore, repletion of Tregs restored intestinal IgA production, particularly in a TGF-β-dependent manner [17]. Tregs also directly induced high IgA production from naive B cells in vitro [17]. Plasticity from Tregs has also been demonstrated within the PPs, where Tregs have been shown to acquire characteristics of CD4+ follicular helper T cells (TFH) [40]. Therefore, it becomes clear that the colonization from microbes, and expression of TGF-β from Tregs both utilize IgA in order to maintain a mutualistic relationship between the host and commensal flora [41].
Role of innate immune cells cross-talk in polarization of the intestinal epithelial response
The evolution of additional regulatory mechanisms in the intestinal tract attests to the differences in microbial load between the systemic and mucosal immune systems. As discussed above, CD4+ T cells can drive or suppress inflammation through the secretion of different cytokines. Due to this delicate balance between pro-inflammatory or regulatory CD4+ T cells, innate immune cells, functioning as antigen-presenting cells, have a key role in establishing the cytokine milieu in the intestinal environment, thereby directing CD4+ T-cell differentiation. The mechanisms directing innate cell responses to the microbiota are discussed below.
Dendritic cells
DCs are key antigen-presenting cells which program immune responses by priming naive T cells and influence their differentiation through their cytokine expression. DCs constantly patrol the intestinal tract, from the mucosa to the mesenteric lymph node. CD11c+ DCs expressing CX3CR1 have the unique ability to extend pseudopods through the intestinal epithelium [42] to ‘sample’ luminal antigens and present them to T cells in the MLN [8], preferentially inducing Th1 and Th17 cells [43,44]. However, DCs at the mucosal surfaces tend to be more inclined to have regulatory functions during healthy, steady-state conditions (Figure 1). Retinoic acid educates DCs arising from the bone marrow to migrate to the intestine, where they show increased capability of inducing Tregs [45] and IgA-producing B cells [46]. The CD103+ DCs are abundant in the intestinal tract and secrete retinoic acid and TGF-β, and drive Treg development, indicating a high capacity for immune regulation [47]. Production of retinoic acid by CD103+ DCs requires MyD88 signaling, further indicating a role for microbiota in the regulation of the intestinal environment [48]. However, dietary factors also contribute to retinoic acid production, as vitamin A deficiency significantly reduces the capacity of CD103+ DCs to metabolize vitamin A into retinoic acid [49]. In addition to inducing Treg and IgA expression, production of retinoic acid induces lymphocytes to migrate from the periphery to the intestinal tract [46,50,51]. Furthermore, analysis of human intestinal DCs indicate that they express lower levels of TLR2 and TLR4 than peripheral DCs [52].
Macrophages
The macrophages are among the most abundant phagocytic cells in the intestine, contributing to the clearance of any microorganisms that cross through the epithelium. However, in relation to their peripheral counterparts, human intestinal macrophages are more tolerogenic, and generally do not respond to antigens in an inflammatory manner. Intestinal macrophages respond poorly to lipopolysaccharide stimulation, lacking production of IL-1, IL-6, TNF-α, IL-12 or IL-8, that are characteristically seen in peripheral macrophages. However, they still maintain their phagocytic and bactericidal capabilities [53]. Further, microarray analyses of intestinal macrophages revealed upregulation of a number of immunoregulatory molecules, including IL-10, TGF-β and PD-L1 [54]. Production of these cytokines results in the differential capability of intestinal macrophages to inhibit Th1 differentiation, whereas splenic macrophages normally promote Th1 differentiation. Intestinal macrophages also exhibit substantial affinity for inducing Treg differentiation through IL-10 and TGF-β production. By promoting IL-10 secretion, intestinal macrophages not only promote Tregs but at the same time limit their own secretion of IL-6, preventing a switch to a Th17 lineage. These characteristics identify macrophages as key players in cleaning up bacteria that has breached the epithelium, without stimulating an inflammatory response. The intestinal macrophages also express low levels of MHC class II [55]. Macrophages continue having anti-inflammatory roles even after an immune response. Upon T-cell expansion and subsequent clearance of the pathogen, the majority of the T cells undergo apoptosis. Phagocytosis of apoptotic T cells triggers TGF-β production from macrophages thereby providing a microenvironment that is favorable for inducing Tregs [56]. This provides for a rapid transition back to a homeostatic environment that is devoid of inflammation, as well as pathogens.
Epithelial cells
The single layer of intestinal ECs (IECs) provides the only structural barrier separating the bacteria from the underlying mucosa. With the constant movement of luminal contents, IECs slough off and the entire epithelium is renewed every 5 days in humans as intestinal stem cells in the crypts of the villi proliferate and differentiate into IECs [57]. In order to protect the stem cells that reside inside the crypts, specialized Paneth cells lie at the base and secrete high levels of bactericidal enzymes, namely α-defensins and lysozyme. Additionally, a thick mucus layer secreted by goblet cells also protects the crypts from bacterial penetration. The lectin RegIIIγ also contributes to the segregation of bacterial populations and the gut barrier (IECs), since it is expressed in response to Gram-positive bacteria, limiting the numbers of IEC-associated bacteria [11]. The IECs also contribute to barrier integrity through the formation of tight junctions. Mice deficient in MyD88 display an impaired immunity to infection, and a deficiency in TLR5 often develops into spontaneous colitis [58–60]. This is a direct result of bacteria coming into closer association with the colonic epithelium, whereas the immune system is unable to control the microflora. This has also been reported in inflammatory bowel disease patients, where NOD2 polymorphisms have consistently been associated with reduced antimicrobial peptide production, and impaired innate immunity [61,62]. IECs also play a significant role in preventing immune activation. Studies in TLR-transgenic mice illustrated a striking association with TLR activation in IECs, B-cell recruitment and IgA production, as a result of increased production of the chemokines CCL20 and CCL28 to induce B-cell homing to the intestine, as well as APRIL and BAFF to regulate IgA class-switching [63–65]. Remarkably, colonic IECs express high amounts of TGF-β [66] and IL-25 [67], which promote IL-10 production from innate immune cells.
Role of commensal bacteria in regulation of intestinal immunity
The human gut is colonized not only by commensal bacteria (microbiota) but also by certain pathogenic bacteria. How commensal bacterial populations modulate the activation of intestinal immunity, in order to avoid harmful responses that disrupt homeostasis, or how responses elicited by microbiota ameliorate inflammatory effects triggered by enteric bacterial pathogens is currently an extensive field of investigation. In this section we will review current findings focused on how bacterial components, or products of their metabolism, contribute to immune modulation either maintaining the homeostasis with commensals, or suppressing harmful responses for enteric pathogens. Additionally, how commensal bacteria interact with the intestinal epithelium, an area of constant investigation, is also discussed.
Intestinal epithelial cell interaction with commensal bacteria
The intestinal epithelium works as the place where initial contact with the lumen content occurs. Upon contact with ECs, microbiota or pathogenic bacteria can influence innate or adaptive immunity via cytokines or chemokines secreted by IECs [68]. Several studies have been focused on differential effects elicited on the intestinal epithelia by commensal bacteria and pathogenic bacteria. While several proinflammatory molecules are secreted upon pathogenic bacteria challenge, such as the chemokines IL-8 and CCL2 (hallmarks of inflammatory responses in the intestine and responsible for neutrophil recruitment), commensal bacteria such as Lactobacillus can modulate the secretion of these mediators in IECs. The reduction in chemokines is commensal-specific because, for example, Lactobacillus plantarum, but not Lactobacillus acidophilus, is able to reduce CCL2 via a NF-κB dependent pathway [69]. Other Lactobacillus species, such as Lactobacillus casei, can also modulate intestinal homeostasis by reducing the expression of IL-8 and CCL2 after stimulation with Salmonella lipopolysaccharide [70]. Interestingly, the induction of CCL20 secretion occurred with pathogenic bacteria such as Salmonella enterica serovar Typhimurium or Salmonella Typhimurium flagellin, and commensal bacteria, such as Bifidobacterium infantis and Lactobacillus salivaris, where they are not only able to reduce CCL20 to baseline levels but also caused a reduction in CCL20 secretion after treatment with Salmonella Typhimurium [71]. CCL20 has been implicated in gut and lung immunity, its action through the specific receptor CCR6, induces recruitment of macrophages and DCs to sites of infection [72]. Complete shutdown of CCL20 could be detrimental for certain bacterial pathogen infections; therefore, commensal bacteria modulate the inflammatory responses. Further, animal studies suggest that the effect of microbiota on the intestinal epithelium is critical during in vivo tolerance [73]. These findings suggest that one of the mechanisms by which commensal bacteria modulate intestinal immunity involves regulation of recruitment of innate and adaptive immune cells via interaction with the intestinal epithelium.
Commensal bacterial factors that mediate immunomodulation
Although it has been described that commensal bacteria can modulate host immune responses, the bacterial factors that mediate such effects remain to be identified until recently.
Bacteroides fragilis polysaccharide A
Commensal bacteria can influence adaptive immunity through modulation of innate immunity components. For example, Bacteroides fragilis polysaccharide A (PSA) induces Treg at the expense of Th17 responses, which suggest a clear difference in the way commensal bacteria can influence innate response compared with enteric pathogens. B. fragilis trigger responses that favor symbiotic colonization via TLR signaling but not inflammatory responses [74,75]. Due to these properties, the B. fragilis PSA has recently been classified as a ‘symbiont-associated molecular patterns’.
Lactobacillus lipoteichoic acid
The lipoteichoic acid (LTA) from Lactobacillus species can also be important in shaping the intestinal microenvironment. In the absence of L. acidophilus LTA, Treg cells frequencies are increased in mice colon following an oral challenge with a LTA-deficient L. acidophilus mutant. Furthermore, this increase in Tregs is in accordance with the finding that the LTA-deficient Lactobacillus caused amelioration of symptoms on dextran sulfate sodium-induced colitis mice [76]. The finding is consistent with previous reports where modification of the charge of Lactobacillus LTA was able to ameliorate colitis symptoms [77]. The mechanisms by which Lactobacillus LTA induces such modulation is still largely unknown; however, LTA could act through recognition by DCs TLR2. Evidence suggests that a change on Lactobacillus could modulate Treg responses in the gut.
Lactobacillus reuteri cyclopropane fatty acids
Some strains of L. reuteri are capable of suppressing the secretion of TNF-α. Further, L. reuteri defective in cyclopropane fatty acid (CFA) production (CFA synthase mutants) is less efficient in suppressing TNF-α than the wild type. However, there is no direct effect on suppression of TNF-α by purified CFAs. It is thus possible that CFA action occurs through changes on the membrane composition leading to a reduced secretion of immunomodulatory compounds [78].
As discussed above, members of the microbiota could modulate immune responses in the gut, depending on specific bacterial components, and at the same time regulation will differ depending on the component of intestinal immunity that is targeted (i.e., IECs, resident DCs and so on), exerting different effects over innate or adaptive responses. A recent analysis of the transcriptome of commensal bacteria support the hypothesis that they can differentially regulate intestinal immunity, depending on the bacterial strain evaluated. Further, TLR signaling pathways are differentially regulated by different commensal strains, and for example, L. salivarius Ls33 is rather anti-inflammatory while L. acidophilus is proinflammatory [79]. The differences are due to their differential regulation of the adaptor molecules MyD88, TRIF, TRAF6 and IRAK1.
Disruption of gut immunomodulation by bacterial enteric pathogens
The host response against enteric pathogens includes an important inflammatory component and therefore, the pathogens need to modulate this response or suppress it to assure their survival. Some reports have focused on specific gut surveillance cells, such as intestinal DCs and how they are regulated by enteric pathogens [80]. This section will focus on two well established enteric pathogens, Salmonella and Vibrio cholerae, and a Crohn’s disease-associated adherent-invasive E. coli (AIEC), a bacteria linked to a chronic inflammatory condition [81].
Vibrio cholerae
Products of bacterial metabolism can contribute to immunomodulation of the host response by V. cholerae, where the product of glucose metabolism, 2,3-butanediol, modulates the activation of host response by suppressing the secretion of IL-8 and TNF-α in an NF-κB dependent fashion on intestinal ECs [82]. The metabolite is specific for V. cholerae El Tor strains, which have been shown a competitive advantage over V. cholerae classical strains [83]. Of note, V. cholerae stimulates a potent proinflammatory response; therefore, the evolutionary success of El Tor strains could be in part attributed to their ability to down regulate IL-8 and TNF-α. Further investigation is required regarding the role of this metabolite in vivo, using animal models of infection.
Salmonella enterica
Salmonella enterica induces intestinal epithelial damage by poly-morphonuclear cells influx and an increase in IL-17A, IL-17F as well as arrangement of chemokines [84]. IL-17 has been indicated as a major player in Salmonella Typhimurium induced diarrhea, as it is strongly induced in the infected gut. However, the mechanism of induction of an inflammatory response could be redundant as demonstrated recently, in that IL-17 receptor deficiency in the cecum tissue of Salmonella Typhimurium-infected mice did not affect the cytokine cascade known to be triggered by IL-17 [85], an indication that an inflammatory response in the mice cecum by Salmonella Typhimurium can also be activated by multiple signals, such as IL-22 or IFN-γ, in addition to IL-10. This is true at least during the acute phase of Salmonella enterocolitis. How type III secretion systems effectors encoded in Salmonella pathogenicity islands 1 and 2 activate IL-17 responses, or alternatively IL-22 or IFN-γ, needs to be further investigated.
In order to disseminate throughout the host, Salmonella Typhi, the causative agent of typhoid fever, have to cross the intestinal epithelial barrier, and at the same time they must avoid the intestinal innate immune response. Bacterial components, such as flagellin, can be potent activators of innate immune responses via TLR5 recognition on IECs. However, Salmonella Typhi can avoid the sentinel function of the epithelium via the flagellin repressor TviA [86]. Furthermore, this repressor does not only inhibit flagellin expression after the bacteria has already crossed the ileal mucosal but also stimulates the expression of the Vi capsular antigen, which prevents the detection of Salmonella by TLR4 [87]. Together, these actions allow Salmonella Typhi systemic dissemination by impairing innate responses in the epithelium, such as the reduction of the chemokines CCL20 and CXCL1.
Salmonella can also alter the initiation of adaptive immune responses by promoting ubiquitination of MHC class II in infected DCs, resulting in the decrease of antigen presentation capability to CD4+ T cells. The effector responsible for the ubiq-uitination of MHC class II is encoded by the ssaV gene located in the pathogenicity island 2 [88].
Adherent invasive AIEC
AIEC are increased in the ileal mucosa of patients with CD, which represent isolates that do not have the classical virulence factors found in other E. coli pathotypes. Recently, efforts have been devoted to identify whether AIEC contributes to initiation or persistence of CD. An important finding has been that AIEC can target PPs [89]. PPs are covered by M cells, specialized for antigen transport and adherence. In order to cross the intestinal epithelial barrier and surviving the host responses, several enteric pathogens, such as Yersinia and Shigella, target M cells. One of the mechanisms by which AIEC target PPs is similar to that used by Salmonella where expression of the long polar fimbriae participate in invasion of PPs in an ex vivo experiment, whereas the long polar fimbriae-deficient strain displayed a reduced invasive phenotype. Additionally, AIEC can upregulate the expression of CEACAM6 on ileal ECs, which serves as a receptor for AIEC type 1 pili (Figure 1). CEACAM6 expression can also be upregulated by IFN-γ and TNF-α [90]. Once gaining access to the intestinal epithelial cell layer, AIEC stimulate more production of proinflammatory cytokines such as IFN-γ and TNF-α; therefore, it has been suggested that such AIEC stimulation contributes to the persistence of the bacteria and the chronic inflammatory response.
Expert commentary & five-year view
As summarized above, significant progress has been made in understanding the complex diversity of physiological functions of innate immune responses in the intestinal epithelial barrier. Elucidation of the regulatory mechanisms associated with maintenance of a homeostatic stage and potential disruption by pathogenic organisms has allowed a better understanding of the cross talk between intestinal epithelia, immune cells and commensal microflora. However, many questions remain to be answered, including the use of therapeutic approaches to help maintaining the intestinal immune balance and, therefore, preventing disease. For example, interesting advances have been achieved on the therapeutic use of peptides, particularly vasoactive intestinal peptide, on immune cell activation and function, and the potential use as anti-inflammatory compounds [86]. However, the challenge remains to be the delivery of the peptide for therapeutic use because these compounds tend to be degraded quickly. Therefore, there is a need to develop and optimize a delivery system that protects the peptide from degradation, while maintaining its biochemical properties.
Another area that requires significant attention is the current use of antibiotics on high-risk patients that can develop chronic inflammatory responses. How different classes of antibiotic treatments have an effect on commensal populations and the subsequent consequences on infections should be investigated. One key area of current development is the use of high-throughput sequencing, which allows a nonbiased global analysis of commensal populations, thereby identifying the bacterial species that are critical to maintain in healthy individuals. Furthermore, it is evident that the interactions between the commensal flora and the host immune system are bidirectional, with important ramifications for intestinal homeostasis. Therefore, there is a need to increase the number of studies correlating intestinal and peripheral immune responses with commensal bacteria, which should provide novel methods not only to impact the commensal flora, but also to regulate immune responses through the manipulation of commensal populations.
A third area of opportunity for development includes the signaling events that participate in the maintenance of the homeostatic environment. Although several studies have shown the participation of TLRs (i.e., TLR2) and NOD-like receptors in the modulation of inflammatory responses in the gut, cumulative evidence indicate that TLR signals are dispensable for the induction and regulation of chronic intestinal pathology. Therefore, future studies should focus on alternative pattern recognition receptor pathways that may play a nonredundant role in inflammatory bowel disease pathogenesis.
Last but definitely not least, it is now clear that microbiota control gut colonization by pathogens, and once the GI tract is colonized, they actively regulate microbiota compositions as well as colonization dynamics. Recent evidence further demonstraes that some viruses require help from microbiota to establish infection by ‘borrowing’ lipopolysaccharide from microbiota which in turn stimulate IL-10 production via binding TLR4 by innate immune cells. Thus, investigating the interplay between microbiota and enteric pathogens, and how such interplay regulates the host response to pathogens will provide great insights into understanding intestinal infections and development of therapeutics for such infections.
In conclusion, the gut microbiota is the next frontier in understanding human health, because full characterization of the factors and players controlling homeostasis are a step forward for the development of specific therapeutics. By using probiotics, antibiotics and other compounds, investigators can modulate the composition of the gut to improve the health of the host. Further optimization of methods to characterize the microbiota still remain to be elucidated; however, full understanding of the microbiota will be beneficial not only for the field of biomedical research but also for healthcare, because establishment of biomarkers of a given microbiota can serve as indicators of disease.
Key issues.
Microbiota regulate innate pathways that activate microbiota antigen-specific T-cell responses for the maintenance of intestinal homeostasis.
The host immune system regulates microbiota for the maintenance of intestinal homeostasis.
Intestinal pathogens regulate the host immune system for infection.
Microbiota regulate colonization of intestinal pathogens.
Acknowledgments
The authors are grateful to Katie Johnston for creating the figure.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Disclaimer
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID or NIH.
Financial & competing interests disclosure
The work in AG Torres’ laboratory (Department of Microbiology and Immunology, University of Texas Medical Branch [UTMB]) was supported by NIH/NIAID grants AI079154 and AI09956001. The work in Y Cong’s laboratory was supported by NIH DK079918 and AI083484 grants (Department of Microbiology and Immunology, UTMB). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1••.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. Excellent overview of the factors affecting the balance between homeostasis and intestinal inflammation. [DOI] [PubMed] [Google Scholar]
- 2.Jarchum I, Pamer EG. Regulation of innate and adaptive immunity by the commensal microbiota. Curr Opin Immunol. 2011;23:353–360. doi: 10.1016/j.coi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.07.003. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 4.Peters BM, Shirtliff ME, Jabra-Rizk MA. Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog. 2010;6:e1001067. doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 6.Kunisawa J, Nochi T, Kiyono H. Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol. 2008;29:505–513. doi: 10.1016/j.it.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Schulz O, Jaensson E, Persson EK, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. First identification of myeloid-derived mucosal dendritic cells populating the lamina propria of the small intestine. [DOI] [PubMed] [Google Scholar]
- 9.Milling S, Yrlid UV, Cerovic V, Macpherson G. Subsets of migrating intestinal dendritic cells. Immunol Rev. 2001;234:259–267. doi: 10.1111/j.0105-2896.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Kawamoto S, Maruya M, Fagarasan S. GALT: organization and dynamics leading to IgA synthesis. Adv Immunol. 2010;107:153–185. doi: 10.1016/B978-0-12-381300-8.00006-X. [DOI] [PubMed] [Google Scholar]
- 11••.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. Discusses intestinal immune system adaptations maintaining homeostatic interactions with resident microbiota. [DOI] [PubMed] [Google Scholar]
- 12.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Der Sluis M, De Koning BA, De Bruijn AC, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Ramasundara M, Leach ST, Lemberg DA, Day AS. Defensins and inflammation: the role of defensins in inflammatory bowel disease. J Gastroenterol Hepatol. 2009;24:202–208. doi: 10.1111/j.1440-1746.2008.05772.x. [DOI] [PubMed] [Google Scholar]
- 15.Wehkamp J, Koslowski M, Wang G, Stange EF. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn’s disease. Mucosal Immunol. 2008;1:S67–S74. doi: 10.1038/mi.2008.48. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Ha SA, Tsuji M, Fagarasan S. Intestinal IgA synthesis: a primitive form of adaptive immunity that regulates microbial communities in the gut. Semin Immunol. 2007;19:127–135. doi: 10.1016/j.smim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 17•.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci USA. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. Indicates that the role of the Treg IgA response is to maintain commensalisms with the microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hapfelmeier S, Lawson MA, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol. 2011;12:264–270. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
- 20.Murthy AK, Dubose CN, Banas JA, Coalson JJ, Arulanandam BP. Contribution of polymeric immunoglobulin receptor to regulation of intestinal inflammation in dextran sulfate sodium-induced colitis. J Gastroenterol Hepatol. 2006;21:1372–1380. doi: 10.1111/j.1440-1746.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- 21.Peterson DA, Mcnulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Mcgeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 23.Niess JH, Leithauser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 27.Aujla SJ, Chan YR, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 30.Werner JL, Gessner MA, Lilly LM, et al. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun. 2011;79:3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ismail AS, Severson KM, Vaishnava S, et al. γδ intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci USA. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 33•.Sellge G, Magalhaes JG, Konradt C, et al. Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity. J Immunol. 2010;184:2076–2085. doi: 10.4049/jimmunol.0900978. Supported the importance of pathogen-specific Th17 immunity. [DOI] [PubMed] [Google Scholar]
- 34.Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 35.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 36.Liston A, Farr AG, Chen Z, et al. Lack of FOXP3 function and expression in the thymic epithelium. J Exp Med. 2007;204:475–480. doi: 10.1084/jem.20062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor FOXP3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Matharu KS, Mizoguchi E, Cotoner CA, et al. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology. 2009;137:1380–1390. e1–e3. doi: 10.1053/j.gastro.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cahill RJ, Foltz CJ, Fox JG, Dangler CA, Powrie F, Schauer DB. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Tsuji M, Komatsu N, Kawamoto S, et al. Preferential generation of follicular B helper T cells from FOXP3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. Reports that in immunologically privileged areas of the gut, FOXP3+ acquire characteristics of follicular helper cells, contributing to the levels of IgA in the gut. [DOI] [PubMed] [Google Scholar]
- 41.Feng T, Elson CO, Cong Y. Treg cell-IgA axis in maintenance of host immune homeostasis with microbiota. Int Immunopharmacol. 2010;11:589–592. doi: 10.1016/j.intimp.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- 44.Medina-Contreras O, Geem D, Laur O, et al. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011;121(12):4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng T, Cong Y, Qin H, Benveniste EN, Elson CO. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. J Immunol. 2010;185:5915–5925. doi: 10.4049/jimmunol.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 47.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of FOXP3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villablanca EJ, Wang S, De Calisto J, et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology. 2011;141:176–185. doi: 10.1053/j.gastro.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agace WW, Persson EK. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol. 2011 doi: 10.1016/j.it.2011.10.001. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 50.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Hammerschmidt SI, Friedrichsen M, Boelter J, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121:3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ng SC, Kamm MA, Stagg AJ, Knight SC. Intestinal dendritic cells: their role in bacterial recognition, lymphocyte homing, and intestinal inflammation. Inflamm Bowel Dis. 2010;16:1787–1807. doi: 10.1002/ibd.21247. [DOI] [PubMed] [Google Scholar]
- 53.Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 55.Smythies LE, Shen R, Bimczok D, et al. Inflammation anergy in human intestinal macrophages is due to Smad-induced IκBα expression and NF-κB inactivation. J Biol Chem. 2010;285:19593–19604. doi: 10.1074/jbc.M109.069955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-β from phagocytes digesting apoptotic T cells. Nat Med. 2008;14:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 57.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Brandl K, Plitas G, Schnabl B, Dematteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII γ and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol. 2007;179:566–577. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- 61••.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. First report experimentally demonstrating that NOD2 gene product confers susceptibility to Crohn’s disease. [DOI] [PubMed] [Google Scholar]
- 62.Maeda S, Hsu LC, Liu H, et al. Nod2 mutation in Crohn’s disease potentiates NF-κB activity and IL-1β processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 63.Shang L, Fukata M, Thirunarayanan N, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He B, Xu W, Santini PA, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 65.Wells JM, Rossi O, Meijerink M, Van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA. 2011;108:4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor β. Gastroenterology. 1993;105:1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- 67.Caruso R, Sarra M, Stolfi C, et al. Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology. 2009;136:2270–2279. doi: 10.1053/j.gastro.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 68.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32:256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Wang B, Li J, Chen J, Huang Q, Li N. Effect of live Lactobacillus plantarum L2 on TNF-α-induced MCP-1 production in Caco-2 cells. Int J Food Microbiol. 2010;142:237–241. doi: 10.1016/j.ijfoodmicro.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 70.Fang HW, Fang SB, Chiang Chiau JS, et al. Inhibitory effects of Lactobacillus casei subsp rhamnosus on Salmonella lipopolysaccharide-induced inflammation and epithelial barrier dysfunction in a co-culture model using Caco-2/peripheral blood mononuclear cells. J Med Microbiol. 2010;59:573–579. doi: 10.1099/jmm.0.009662-0. [DOI] [PubMed] [Google Scholar]
- 71.Sibartie S, O’Hara AM, Ryan J, et al. Modulation of pathogen-induced CCL20 secretion from HT-29 human intestinal epithelial cells by commensal bacteria. BMC Immunol. 2009;10:54. doi: 10.1186/1471-2172-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ito T, Carson WFT, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317:613–619. doi: 10.1016/j.yexcr.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fink LN, Metzdorff SB, Zeuthen LH, et al. Establishment of tolerance to commensal bacteria requires a complex microbiota and is accompanied by decreased intestinal chemokine expression. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00428.2010. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 74•.Round JL, Lee SM, Li J, et al. The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. Experimental description of how commensal bacteria exploit the Toll-like receptor pathway to actively suppress immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Round JL, Mazmanian SK. Inducible FOXP3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohamadzadeh M, Pfeiler EA, Brown JB, et al. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA. 2011;108:4623–4630. doi: 10.1073/pnas.1005066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grangette C, Nutten S, Palumbo E, et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci USA. 2005;102:10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones SE, Whitehead K, Saulnier D, Thomas CM, Versalovic J, Britton RA. Cyclopropane fatty acid synthase mutants of probiotic human-derived Lactobacillus reuteri are defective in TNF inhibition. Gut Microbes. 2011;2:69–79. doi: 10.4161/gmic.2.2.15282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Putaala H, Barrangou R, Leyer GJ, et al. Analysis of the human intestinal epithelial cell transcriptional response to Lactobacillus acidophilus, Lactobacillus salivarius, Bifidobacterium lactis and Escherichia coli. Benef Microbes. 2010;1:283–295. doi: 10.3920/BM2010.0003. [DOI] [PubMed] [Google Scholar]
- 80.Bedoui S, Kupz A, Wijburg OL, Walduck AK, Rescigno M, Strugnell RA. Different bacterial pathogens, different strategies, yet the aim is the same: evasion of intestinal dendritic cell recognition. J Immunol. 2010;184:2237–2242. doi: 10.4049/jimmunol.0902871. [DOI] [PubMed] [Google Scholar]
- 81.Strober W. Adherent-invasive E. coli in Crohn disease: bacterial “agent provocateur”. J Clin Invest. 2011;121:841–844. doi: 10.1172/JCI46333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bari W, Song YJ, Yoon SS. Suppressed induction of proinflammatory cytokines by a unique metabolite produced by Vibrio cholerae O1 El Tor biotype in cultured host cells. Infect Immun. 2011;79:3149–3158. doi: 10.1128/IAI.01237-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pradhan S, Baidya AK, Ghosh A, Paul K, Chowdhury R. The El Tor biotype of Vibrio cholerae exhibits a growth advantage in the stationary phase in mixed cultures with the classical biotype. J Bacteriol. 2010;192:955–963. doi: 10.1128/JB.01180-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nunes JS, Lawhon SD, Rossetti CA, et al. Morphologic and cytokine profile characterization of Salmonella enterica serovar Typhimurium infection in calves with bovine leukocyte adhesion deficiency. Vet Pathol. 2010;47:322–333. doi: 10.1177/0300985809358037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Songhet P, Barthel M, Rohn TA, et al. IL-17A/F-signaling does not contribute to the initial phase of mucosal inflammation triggered by S. Typhimurium . PLoS One. 2010;5:e13804. doi: 10.1371/journal.pone.0013804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Winter SE, Winter MG, Godinez I, et al. A rapid change in virulence gene expression during the transition from the intestinal lumen into tissue promotes systemic dissemination of Salmonella. PLoS Pathog. 2010;6:e1001060. doi: 10.1371/journal.ppat.1001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tran QT, Gomez G, Khare S, et al. The Salmonella enterica serotype Typhi Vi capsular antigen is expressed after the bacterium enters the ileal mucosa. Infect Immun. 2010;78:527–535. doi: 10.1128/IAI.00972-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lapaque N, Hutchinson JL, Jones DC, et al. Salmonella regulates polyubiquitination and surface expression of MHC class II antigens. Proc Natl Acad Sci USA. 2009;106:14052–14057. doi: 10.1073/pnas.0906735106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chassaing B, Rolhion N, De Vallee A, et al. Crohn disease-associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J Clin Invest. 2011;121:966–975. doi: 10.1172/JCI44632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barnich N, Carvalho FA, Glasser AL, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]