SUMMARY
Chlamydia trachomatis is the most common cause of acute salpingitis worldwide. The socioeconomic impact of sexually transmitted infections (STI) caused by C. trachomatis is considerable. The purpose of this study was to investigate secretion of a unique chemokine, CXCL13, during the inflammatory process in human fallopian tube tissue in response to infection with C. trachomatis. We employed two models for our experiments: archived fallopian tube paraffin sections from known cases of salpingitis of unknown etiology and human fallopian tube organ culture established from fresh fallopian tube biopsies subsequently infected in vitro with C. trachomatis serovar E. We used immunohistochemistry, microarray analysis and cytometric bead array to study these specimens. In both models, we found that the fallopian tissue infected with C. trachomatis expressed CXCL13 and other characteristics of tertiary lymphoid tissue. In addition, we found that CXCL13 was expressed in multiple cell types, including endothelial cells, demonstrating a mechanism for the lymphoid aggregation seen in fallopian tube tissue during salpingitis and infection with C. trachomatis.
INTRODUCTION
Chlamydia trachomatis is an obligate intracellular, Gram-negative bacteria, and a common cause of acute salpingitis which is often asymptomatic (1). The socioeconomic impact of sexually transmitted infections (STI) caused by C. trachomatis is considerable, not only in the USA where 4 million new cases occur each year, but also worldwide where C. trachomatis continues to be a major cause of morbidity. Chlamydial infection is estimated by the World Health Organization to cause 90–500 million cases of STIs each year (2). Past studies have shown that infection of female reproductive tissue in vivo with chlamydiae causes the formation of lymphocytic aggregates that have well-formed germinal centers in situ (3). Subsequent examination has shown that infection of both murine (4, 5) and human (6) fallopian tube tissue induces expression of many chemokines and adhesion molecules and supports leukocyte migration suggesting an active local immune response.
The process of lymphoid aggregation, or lymphoid neogenesis, was originally described by Kratz et al. (7) and refers to the process by which structures resembling organized lymphoid tissues accumulate and organize during chronic inflammation. Characteristics of lymphoid neogenesis include: i) distinct organization of T-cell and B-cell populations; ii) expression of homeostatic chemokines (CXCL13, CCL21); and iii) expression of adhesion molecules such as CD106 found on high endothelial-like venules (8). Lymphoid neogenesis is initiated by the accumulation of CD4+ CXCR5+ inducer cells which activate stromal cells through their expression of the lymphotoxin (LT)-β receptor (R) with LTα1β2 (9). This results in the secretion of chemokines and adhesion molecules and causes organization of mononuclear cells within tissues. This process was first described to occur during development of secondary lymphoid tissue but recently has been reported to occur during certain chronic infectious processes (10–12) as well as several autoimmune diseases (13–16).
The accumulation of inducer cells expressing LT α1β2 requires secretion of the chemokine CXCL13. Chemokines are small chemoattractant proteins that deliver signals to cells expressing the corresponding seven-transmembrane G protein-coupled receptor(s) (17). Stimulation of LTβR activates the nuclear factor (NF) κB1 and NFκB2 pathways resulting in the secretion of a number of chemokines and organization of lymphoid tissue. Thus, the homeostatic chemokine CXCL13 is indispensable for attracting inducer cells expressing the only known receptor, CXCR5, and development of most lymph nodes (18, 19). We report here that salpingitis specimens contain organized lymphoid tissue and characteristics of lymphoid neogenesis. Additionally, we have found that C. trachomatis infection of fallopian tube tissue induces significant expression of CXCL13 and is expressed on endothelial cells. These findings support the hypothesis that chlamydial infection directly induces the accumulation of mononuclear cells by CXCL13 expression.
METHODS
Antibodies
Mouse antihuman antibodies purchased from Pharmingen (San Diego, CA, USA) included: anti-C. trachomatis lipopolysaccharide (LPS; clone CHL-888), anti-von Willebrand Factor (vWF; clone 2F2A9), negative control (clone MOP31-C) and anti-CD106 (clone 51-10C9). Mouse antihuman antibodies purchased from DAKO Co. (Santa Barbara, CA, USA) included: T cell (anti-CD3, clone UCHT1), B cell (anti-CD20, clone L26), cytokeratin-18 (clone CD10), vimentin (clone V9) and isotype-matched negative controls. Biotin-labeled goat antimouse antibody was purchased from Southern Biotech (Birmingham, AL, USA). Goat antihuman anti-CXCL13 (affinity purified) as well as goat serum were purchased from R&D Systems (Minneapolis, MN, USA). Other purchased antibodies included: biotin labeled rabbit antigoat antibody from Antibodies, Inc. (Davis, CA, USA), rabbit antihuman vWF polyclonal antibody from Chemicon (Temecula, CA, USA), which was used for both frozen sections and those prepared with paraffin, and biotin labeled goat antirabbit polyclonal antibody purchased from Biosource (Camarillo, CA, USA).
Immunohistochemistry
Archived hematoxylin and eosin stained slides were selected by pathologists (J.R. and S.N.) from the Tissue Procurement Core Laboratory (TPCL) in the Department of Pathology at University of California, Los Angeles (UCLA). A subset of these were chosen, and archived paraffin blocks from five patients diagnosed with salpingitis and five patients who had tubal ligations (normal tissue) were selected by pathologists (J.R. and S.N.) from the Tissue Procurement Core Laboratory (TPCL) in the Department of Pathology at UCLA. All of the tubal ligation specimens were from premenopausal females. Of the patients with salpingitis, one was premenopausal, three were perimenopausal and one was post-menopausal. Sections were made from the paraffin blocks and deparaffinized.
The TPCL used the following procedure to stain the sections. The sections were incubated with 70% hydrogen peroxide in methanol for 10 min and washed with phosphate buffered saline with Tween (PBST; 0.01 mol/L sodium phosphate, 0.15 mol/L NaCl and 0.05% Tween 20) for 5 min. The sections were then incubated for 2 h at room temperature with the primary antibody diluted in 2% bovine serum albumin (1:20) and washed with PBST. The specimens were incubated with Envision horseradish peroxidase polymer (DAKO Co., Santa Barbara, CA, USA), washed with PBST and incubated in diaminobenzidine for 10 min and washed in tap water. The sections were counterstained with Harris hematoxylin and dehydrated in 95% ethanol, 100% ethanol, and xylene. Positive and negative controls were prepared on tonsil and colon tissues obtained from TPLC. Negative controls for each stained section consisted of substitution of the primary antibody by a non-cross-reacting isotype-matched monoclonal antibody (MAB). Histopathologic slides were read and scored in a masked fashion. Immunohistochemical staining intensity was graded as positive or negative. Images were collected with a color video camera (Sony Electronics).
Fallopian tube segments (see next section) were snap-frozen into blocks using freezing medium (OCT; Fisher Scientific, Rockville, MD, USA) and 5 μm frozen sections were prepared from the blocks by the UCLA TPCL. The frozen sections were fixed in acetone, washed in PBS and incubated in methanol:H2O2 for 30 min. Tissue biotin sites were blocked with avidin and biotin, followed by goat AB-negative serum and 30 min of incubation in a humidified box at room temperature. The tissue was washed with PBS, primary antibody (see section above) was applied, incubated for 45 min at room temperature in a humidified box and rinsed with PBS. The secondary antibody (biotin-labeled goat antimouse or biotin labeled rabbit antigoat) was diluted in 10% goat serum, applied to the appropriate tissue and incubated for 45 min at room temperature in a humidified box. Neutra-avidin conjugated to horseradish peroxidase (Pierce Chemical; Rockford, IL, USA) was applied and the tissue incubated for 45 min at room temperature in a humidified box. The bound enzyme was visualized with metal-enhanced diaminobenzidine (DAB) substrate (Pierce Chemical) and immunoperoxidase counterstain and preserved with crystal mount (Fisher Scientific). Images were collected with a color video camera (Sony Electronics, Los Angeles, CA, USA).
Human fallopian tube organ culture model
Anatomically normal fallopian tubes were obtained shortly after harvest from the Gross Pathology Laboratory at UCLA from surgical cases of benign gynecological processes. The fallopian tubes were opened longitudinally and 4 mm punch biopsies were taken from the lumen. These tubal segments were placed in a 24-well tissue culture plate (Corning, Corning Plaza, NY, USA) and covered with OPTI-MEM media (Invitrogen, Carlsbad, CA, USA), along with 1 μg/mL of penicillin and streptomycin, and 25 μg/mL of vancomycin and gentamicin (Fisher Scientific, Rockville, MD, USA). After a 24-h incubation period at 37 °C in 5% carbon dioxide, the fallopian tube segments were washed free of the medium containing antibiotic and inoculated with 50 μL of 1 × 108 infection-forming units (IFU)/mL C. trachomatis serovar E grown in HeLa cells as described (6). Tissue from each specimen was also mock-infected with media but no bacteria.
Microarray analysis
Forty-eight hours after infection of human fallopian tube organ culture (HFTOC; see above), mRNA was isolated with RNA-Bee (Tel-test; Friendswood, TX, USA) and purified with an RNeasy column (Qiagen; Valencia, CA, USA). The isolated mRNA was quantified using a Ribogreen RNA Quantification Reagent Kit (Molecular Probes; Carlsbad, CA, USA). A pool consisting of 1 μg mRNA/specimen was made from infected and matched mock HFTOC controls as described above for a total of 5 μg of mRNA/pool. The mRNA was analyzed by GEArray Q series (SuperArray; Frederick, MD, USA) which hybridizes to mRNA from > 90 human cytokine and chemokine species and receptors. The resulting values were blanked against the signal from the DNA plasmid vector PUC18 and normalized to a housekeeping gene (GAPDH).
Cytometric bead array
Supernates were harvested from five fallopian tube biopsies 48 h after infection with C. trachomatis or mock control as described above. A 50 μL aliquot was subjected to BD™ cytometric bead array (BD Biosciences, San Jose, CA, USA). The cytokines interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-10, IL-1β and lymphotoxin (LT)-α were measured by the FACSCalibur (BD Biosciences) and analyzed on FCAP Array™ software as described by the manufacturer (BD Biosciences). Stimulated and control peripheral blood mononuclear cells (PBMC) were used as positive and negative controls, respectively. Select cytokine levels were confirmed by ELISA assay (BD Biosciences).
RESULTS
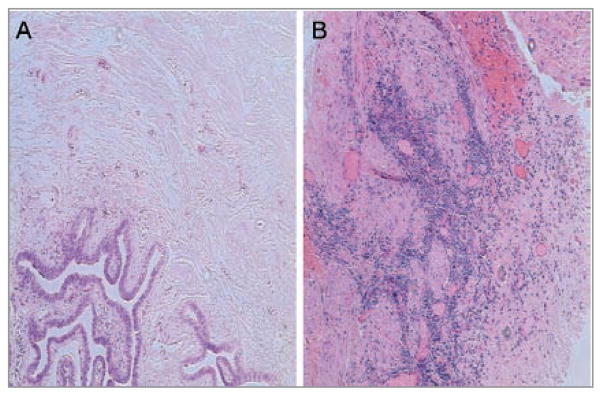
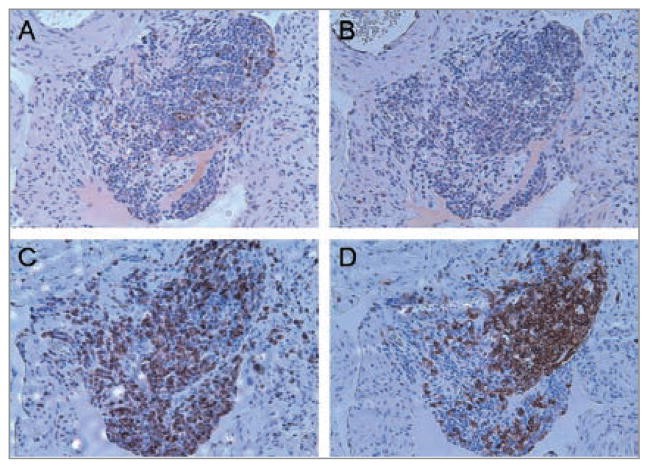
Normal human fallopian tissue is mostly devoid of lymphocytes (Fig. 1A), but those containing lymphoid aggregates are pathognomonic for salpingitis. Salpingitis consists of infection and inflammation of the upper genital tract (the oviduct and/or fallopian tubes) in females and is observed in hematoxylin and eosin (H&E) staining as a collection of mononuclear cells (Fig. 1B) (3). To characterize the organization of fallopian tube lymphoid aggregates, we examined the aggregates in the identified salpingitis cases for characteristics of tertiary lymphoid tissue, as previously described (8). Paraffin sections were stained by the UCLA Immunohistochemistry (IHC) Lab for CXCL13, distinct T-cell (CD3) and B-cell (CD20) populations and CD106, a representative adhesion molecule found on high endothelial-like venules. The staining revealed that the lymphoid aggregates found within the salpingitis cases expressed the attributes of tertiary lymphoid tissue formed during lymphoid neogenesis, as reported by others (10, 12–16). This can be appreciated in Figure 2 which shows discrete T-cell (C) and B-cell (D) populations, observed within a large lymphoid aggregate from a representative salpingitis specimen.
Figure 1.
Chronic inflammation is a hallmark of salpingitis. Paraffin sections from a tubal ligation and a salpingitis case were subjected to hematoxylin and eosin stain. A lymphocytic infiltrate was found on hematoxylin and eosin stained paraffin sections from salpingitis (B) but not tubal ligation (A) samples. Representative images from five salpingitis and five tubal ligation cases. Original magnification (original x100).
Figure 2.
Characteristics of lymphoid neogenesis expressed in lymphoid aggregates found in a salpingitis sample. Paraffin sections of a diagnosed case of salpingitis with large lymphoid aggregates were stained with various antibodies to characterize attributes defining lymphoid neogenesis. Notice the distinct expression of (A) CXCL13 and (C) T-cell (CD3) and (D) B-cell (CD20) subsets found within a large lymphoid aggregate identified in human fallopian tube tissue with known salpingitis. Negative control (B). Representative images from five salpingitis and five tubal ligation cases. (original 200x magnification).
We examined a total of five archived specimens from patients diagnosed with salpingitis. As expected, all of the cases we examined contained a lymphocytic infiltration (Table I). As a control, we compared these results to fallopian tube cross-sections from five individuals undergoing tubal ligation (normal tissue) and did not note any lymphoid aggregates. We further characterized the degree and size of lymphocytic infiltration seen in the salpingitis H&E specimens by scanning the entire section. All of the salpingitis samples studied expressed rather large lymphoid aggregates, and distinct subpopulations of lymphocytes were found within the aggregates in four out of five salpingitis samples (Table I). However, we noted heterogeneity of the number of aggregates seen. In two of the samples, greater than 50 lymphoid aggregates were identified. One sample contained a moderate number of aggregates (20–50 aggregates), while the remaining two samples showed only a few aggregates (Table I). These data demonstrate that the lymphocytes organize into lymphoid aggregates as opposed to a generalized lymphocytic infiltrate. Interestingly, the degree of organization varied among the individual specimens.
Table I.
Characterization of lymphoid aggregates in known salpingitis samples. The number and size of aggregates was determined from hematoxylin and eosin (H&E) stained paraffin sections examined under x100 and x250 magnification.
| Fallopian Tube Sample | Number of aggregates (H&E) | Size of aggregates (H&E) | Lymphocyte segregation (IHC) | Expression of endothelial cell marker CD106 (IHC) | Expression of CXCL13 (IHC) |
|---|---|---|---|---|---|
| SS-HCT06 | 1+ | 3+ | distinct | + | + |
| SS-HCT07 | 2+ | 3+ | distinct | + | 0 |
| SS-HCT09 | 3+ | 3+ | distinct | + | + |
| SS-HCT010 | 3+ | 3+ | distinct | + | + |
| SS-HCT11 | 1+ | 1+ | none | 0 | 0 |
The number and size of aggregates was determined as follows: number, 0 = none, 1+ = 1–20, 2+ = 21–50, 3+ = > 50; size, 0=none, 1+ = 5–20, 2+ = 20–50, 3+ = > 50. Lymphocyte segregation was determined from examination of CD3 (T cell) and CD20 (B cell) stained sections. Lymphocyte segregation was noted as none, diffuse, overlapping or distinct. Expression of the endothelial cell marker, CD106, was evaluated in sections using immunohistochemical staining and examined under x100, x250 and x400 magnification. “+” denotes that one or more of these markers is expressed and a “−“ denotes no expression.
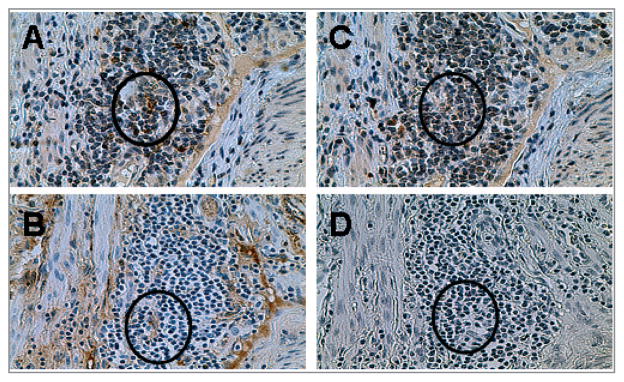
During lymph node development, CXCL13 is expressed in tissue stromal cells to attract formation of a lymph node at that site (9). We determined whether the homeostatic chemokine CXCL13 was expressed in salpingitis cases. Our IHC staining revealed that CXCL13 was expressed on endothelial cells within lymphoid aggregates (Fig. 2A). Parallel sections were stained with von Willebrand Factor (vWF), a known marker of endothelial cells to confirm endothelial cell staining pattern of CXCL13. We further examined other lymphoid aggregates and again observed CXCL13 staining (Fig. 3A) on vWF positive endothelial cells. Analysis of the five individual salpingitis cases revealed that three out of five salpingitis samples, but none of the tubal ligation samples, expressed CXCL13 (Table I). The expression of CXCL13 superimposed on organized lymphoid aggregates implies that CXCL13 participates in recruiting and organizing lymphocytes. To summarize, the lymphoid aggregates found in the majority of salpingitis specimens examined were highly organized and expressed CXCL13 on endothelial structures, placing CXCL13 in a position to recruit cells expressing CXCR5 and initiate lymphoid neogenesis within fallopian tube tissue.
Figure 3.
Expression of CXCL13 in a known case of salpingitis. Paraffin slides from a salpingitis specimen with lymphoid aggregates were stained with antibodies against CXCL13 (A) or an endothelial cell marker, von Willebrand factor (B). Panels C and D were stained with control antibodies. Circle indicates matched endothelial cells. Images are at x400 magnification (original) and are representative of five samples.
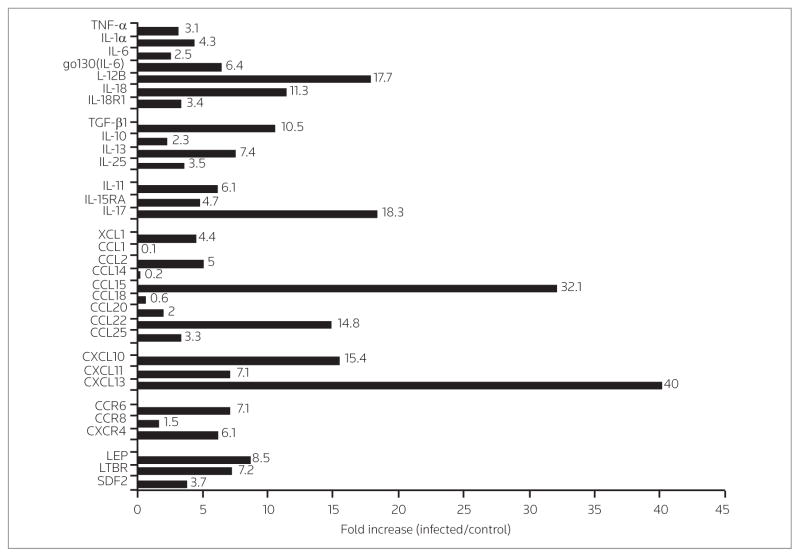
Salpingitis is a result of C. trachomatis infection (20) but also occurs following infections with other organisms such as Neisseria gonorrhea. However, salpingitis patients at UCLA are not evaluated for serum antibody against C. trachomatis. In order to determine whether C. trachomatis can induce CXCL13 we measured mRNA induction and expression of CXCL13 protein in an in vitro model of HFTOC, as previously described (6, 21). Five fallopian tube biopsies were infected with C. trachomatis serovar E and the mRNA was harvested and pooled from both infected and mock-infected controls (see HFTOC above). The pools of human fallopian tube mRNA were subjected to microarray analysis, and values obtained from mock-infected tissue were subtracted from those obtained from the samples infected with C. trachomatis. Figure 4 shows fold increases of the mRNA species induced following infection as compared to mock-infected controls. As predicted, infection of fallopian tubes with C. trachomatis caused the tissue to induce numerous inflammatory cytokines and receptors, among them TNF-α, IL-1α, IL-6, IL-12R, IL-18 and IL-18R, as has been demonstrated by others (6, 22, 23). These cytokines are associated with the T helper 1 (Th1) response necessary to eradicate C. trachomatis infection (24). We did not find an increase in interferon (IFN)-γ, suggesting that this cytokine may be present in cells recruited during infection rather than in tissue cells.
Figure 4.
Fallopian tube mRNA species elevated upon infection with C. trachomatis. The mRNA from five fallopian tube biopsies was pooled. The fold increase in chemokine and cytokine mRNA induced in fallopian tubes following in vitro infection with C. trachomatis serovar E was determined over that of matched mock-infected biopsy specimens. There was a marked increase of select chemokines compared to cytokines.
While increases were found in numerous chemokines and cytokines, the fold-induction was greater in chemokines as compared to cytokines. As shown in Figure 4, C. trachomatis induced a 40-fold increase in mRNA for CXCL13. Interestingly, a greater than fivefold increase was also noted in LTβR, which induces lymphoid aggregation and further amplification of CXCL13 by transcription via the NFκB alternative pathway (25). This leads to the hypothesis that the increase seen in CXCL13 mRNA represents an increase in functional CXCL13 protein. Interestingly, CXCL13 levels were increased over mock-infected controls but were below the limit of detection of the assay (data not shown). IHC staining revealed that CXCL13 was expressed on endothelial as well as other cell types within infected HFTOC following infection with C. trachomatis serovar E (data not shown).
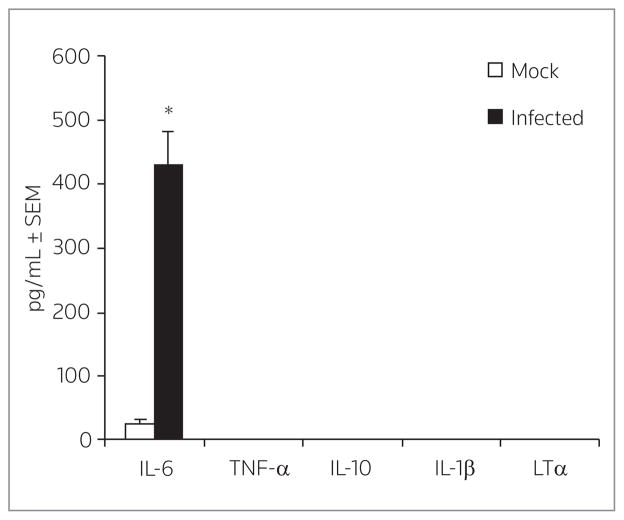
We have previously shown that IL-6 is secreted in this model following infection (6). We confirmed that the human fallopian tube samples were infected by producing high levels of IL-6 as measured by cytometric bead array, while mock-infected samples remained negative for IL-6 (Fig. 5). Although other inflammatory cytokines (TNF-α, IL-10, IL-1β and LT-α) were elevated 10-fold compared to mock-infected samples, all levels were below the limit of detection of the assay and were considered negative.
Figure 5.
Cytokine production following C. trachomatis infection of human fallopian tube organ culture (HFTOC). HFTOC (n = 5) were infected with C. trachomatis for 48 h and the supernatants collected for cytokine measurement with bead array analysis. A parallel set of HFTOC were treated with sucrose-phosphate-glutamate buffer as mock controls. Peripheral blood mononuclear cells were a positive control for all cytokines. *P < 0.009 by Student’s t-test.
DISCUSSION
Our results show that highly organized lymphoid aggregates are found in fallopian tube tissue in patients diagnosed with salpingitis. These structures express CXCL13 and also have characteristics of tertiary lymphoid tissue such as T–B-cell segregation and adhesion molecules, thereby defining this process as lymphoid neogenesis (7). Although infection with other organisms can result in salpingitis, we specifically focused on the organism C. trachomatis. Using an in vitro fallopian tube model, we have demonstrated that CXCL13 mRNA and protein are induced upon C. trachomatis infection. Lymphoid neogenesis has been found in a wide array of chronic diseases including infections with Helicobacter pylori (26), Bartonella henselae (27), Borrelia burgdorferi (28) and West Nile virus (29). Additionally, it has also been found in various autoimmune diseases such as rheumatoid arthritis (15), Sjögren’s disease (16), ulcerative colitis (13), autoimmune encephalomyelitis (11) and many others (30). Finally, lymphoid neogenesis has been shown to appear in dissimilar disease processes such as allograft rejections, allergic lung diseases, arteriosclerosis and cancer (30), suggesting that it is a common phenomenon in chronic inflammation.
The degree of CXCL13 expression varies with the development of tertiary lymphoid tissue, suggesting that lymphoid neogenesis possibly occurs in stages orchestrated by the chemokine CXCL13. CXCL13 production is necessary for initiation of lymphoid neogenesis or the aggregation of lymphocytes within tissue (14), but this process also requires other factors (14, 31) for proper development (32, 33). Although transgenic studies have demonstrated that CCL21 can also mediate lymphoid follicle formation through the LTβR pathway (31), acute infection of fallopian tissue in vitro did not induce mRNA for CCL21 (Fig. 4).
Preliminary data suggest that multiple cells types express CXCL13, including chlamydial-infected epithelial cells, suggesting that direct stimulation by the organism causes secretion of CXCL13. In support of this, stimulation of Toll-like receptor 4 (TLR4) has been shown to induce mRNA expression of CXCL13 in human monocytes/macrophages (34) and dendritic cells (35). It is important to note that endometrial stromal cells have been shown to express TLR4 (36) and fibroblasts are susceptible to infection with C. trachomatis. Thus, chlamydial infected cells could secrete CXCL13 through TLR4 ligation. Although CXCL13 participates in lymphoid aggregation, CXCL13 may have other roles. Recently, Yuvaraj et al. have reported that CXCL13 is responsible for stimulating receptor activator of NFκB ligand (RANKL) secretion a known inflammatory factor which activates osteoclast activity (37). RANKL is a member of the tumor necrosis family that can also stimulate LTβR and contribute to lymphoid aggregation.
Endothelial-bound chemokines are necessary to initiate cellular arrest and direct inflammatory cells to the site of infected tissue (38). We have found that CXCL13 is expressed on endothelial cells in infected fallopian tube tissue (Figs. 2 and 3), suggesting that CXCL13 may play a role in chronic inflammation of fallopian tubes in case of salpingitis. It is possible that human endothelial cells cannot secrete CXCL13 but rather acquire the protein from other cells, possibly via a transcytosis mechanism (39).
While it is clear that infection with replicating C. trachomatis organisms induces CXCL13 expression, it is possible that the presence of nonviable organisms also stimulates CXCL13 production. The presence of aberrant forms of C. trachomatis, isolation of chlamydial DNA and mRNA, plus repeated persistence of strains in the absence of convincing reinfection, add support that retention of C. trachomatis may occur in vivo (40). Induction of CXCL13 by viable and/or nonviable C. trachomatis will contribute to the involvement of a persistent immune response and chronic inflammation of the female upper genital tract. It is challenging to investigate the involvement of fallopian tube lymphoid aggregates in the fertility of infected women. Our findings suggest that the presence of lymphoid aggregates and CXCL13 secretion in salpingitis specimens suggest continual stimulation. Whether this is directly induced by microbes or microbial products or reflects aberrant CXCL13 secretion in certain individuals is currently not known. Regardless of the mechanism for CXCL13 secretion, formation and continued presence of lymphocytic aggregates in fallopian tubes may play a pivotal role in chlamydial pathogenesis.
In this regard and in light of the observation that the expression of CXCL13 and many other chemokines and cytokines present in the female genital tract are cyclically altered in expression during the menstrual cycle in a location-specific and programmed manner, it would be of interest to extend the hypothetical appraisal of the contribution of normal reproductive biologic processes on infection linked chronic and progressive pathology, with respect to the possible role of these processes on the location of sites of infection within and throughout the female genital tract (41). It is conceivable that the temporal clustering of the symptoms of salpingitis toward the end of menses or early in the menstrual cycle is caused by the chemokines and cytokines that functionally direct this phase of the cycle. Changes in the levels of these chemokines and cytokines may also alter the degree of lymphoid aggregate activation and the symptoms associated with this activation. Conceptually appreciating this interplay of endogenously programmed processes and exogenously induced responses, although difficult to integrate, is reflective of the diverse and seemingly unpredictable nature of the sequelae associated with infections of the female genital tract. It is critical to the design of effective intervention strategies that we understand the complex interactions between the immunoinflammatory components of human reproductive biology and the genital tract responses to infection, and, more importantly, that we develop and employ models that mimic these complexities. An approach would be to utilize the expertise of different laboratories to integrate human host factors, detected through genetic analysis, with environmental and bacterial factors in an in vivo murine model to achieve a greater understanding of chlamydial-induced reproductive dysfunction in females.
Acknowledgments
We gratefully acknowledge the expertise of the UCLA Immunohistochemistry Lab headed by I. Peter Shintaku, PhD, for providing consultation of the immunohistochemical staining of human fallopian tube tissues. We also thank Dr. Robert Strieter, MD (UCLA, Los Angeles, USA), and Dr. Martin Lipp (Max-Delbruck Center for Molecular Medicine, Berlin, Germany) for their fruitful discussions and helpful suggestions.
This research is supported by grants from NIH R21-AI148146, NIH R56-AI066200 and the UCLA Iris-Cantor Women’s Center for Excellence. The paper was presented in part at the American Society of Reproductive Immunologists, June 2006, Nashville, Tennessee, USA. M. King and H. Poya `buted equally to this work. This study was approved by the Institutional Review Board at University of California, Los Angeles, USA.
Footnotes
DISCLOSURE
The authors have nothing to disclose.
References
- 1.Mardh PA, Ripa T, Svensson L, Westrom L. Chlamydia trachomatis infection in patients with acute salpingitis. N Eng J Med. 1977;296:1377–9. doi: 10.1056/NEJM197706162962403. [DOI] [PubMed] [Google Scholar]
- 2.Morrison RP. New insights into a persistent problem –chlamydial infections. J Clin Invest. 2003;111:1647–9. doi: 10.1172/JCI18770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiviat NB, Wolner-Hanssen P, Eschenbach DA, et al. Endometrial histopathology in patients with culture-proved upper genital tract infection and laproscopically diagnosed acute salpingitis. Am J Surg Path. 1990;14:167–75. doi: 10.1097/00000478-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Kelly KA. Cellular immunity and Chlamydia genital infection: Induction, recruitment, and effector mechanisms. Int Rev Immunol. 2003;22:3–41. doi: 10.1080/08830180305229. [DOI] [PubMed] [Google Scholar]
- 5.Maxion HK, Kelly KA. Differential chemokine expression in distinct regions of the murine genital tract during Chlamydia trachomatis infection. Infect Immun. 2002;70:1538–46. doi: 10.1128/IAI.70.3.1538-1546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly KA, Natarajan S, Ruther P, Wisse A, Chang MH, Ault KA. Chlamydia trachomatis infection induces mucosal addressin cell adhesion molecule-1 and vascular cell adhesion molecule-1, providing an immunologic link between the fallopian tube and other mucosal tissues. J Infect Dis. 2001;184:885–91. doi: 10.1086/323341. [DOI] [PubMed] [Google Scholar]
- 7.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–72. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shomer NH, Fox JG, Juedes AE, Ruddle NH. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infect Immun. 2003;71:3572–7. doi: 10.1128/IAI.71.6.3572-3577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 10.Facchetti F, Vermi W, Mason D, Colonna M. The plasma-cytoid monocyte/interferon producing cells. Virchows Arch. 2003;443:703–17. doi: 10.1007/s00428-003-0918-8. [DOI] [PubMed] [Google Scholar]
- 11.Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:11–23. doi: 10.1016/j.jneuroim.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 12.Yssel H, Shanafelt Soderberg C, Schneider PV, Anzola J, Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991;174:593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlsen HS, Baekkevold ES, Johansen FE, Haraldsen G, Brandtzaeg P. B cell attracting chemokine 1 CXCL13. and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut. 2002;51:364–71. doi: 10.1136/gut.51.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–81. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 15.Shi K, Hayashida K, Kaneko M, et al. Lymphoid chemokine B cell-attracting chemokine-1 CXCL13. is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001;166:650–5. doi: 10.4049/jimmunol.166.1.650. [DOI] [PubMed] [Google Scholar]
- 16.Xanthou G, Polihronis M, Tzioufas AG, Paikos S, Sideras P, Moutsopoulos HM. “Lymphoid” chemokine messenger RNA expression by epithelial cells in the chronic inflammatory lesion of the salivary glands of Sjogren’s syndrome patients: Possible participation in lymphoid structure formation. Arthritis Rheum. 2001;44:408–18. doi: 10.1002/1529-0131(200102)44:2<408::AID-ANR60>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Zlotnik A, Yoshie O. Chemokines: A new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 18.Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 19.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–47. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 20.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 21.Cooper MD, Rapp J, Jeffery-Wiseman C, Barnes RC, Stephens DS. Chlamydia trachomatis infection of human fallopian tube organ cultures. J Gen Microbiol. 1990;136:1109–15. doi: 10.1099/00221287-136-6-1109. [DOI] [PubMed] [Google Scholar]
- 22.Ault KA, Tawfik OW, Smith-King MM, Gunter J, Terranova PT. Am J Obstet Gynecol. Vol. 175. Tumor necrosis factor-α response to infection with Chlamydia trachomatis in human fallopian tube organ culture; pp. 1242–5. [DOI] [PubMed] [Google Scholar]
- 23.Reddy BS, Rastogi S, Das B, Salhan S, Verma S, Mittal A. Cytokine expression pattern in the genital tract of Chlamydia trachomatis positive infertile women – implication for T-cell responses. Clin Exp Immunol. 2004;137:552–8. doi: 10.1111/j.1365-2249.2004.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–52. [PubMed] [Google Scholar]
- 25.Ware CF. Network communications: Lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 26.Mazzucchelli L, Blaser A, Kappeler A, Scharli P, Laissue JA, Baggiolini M, Uguccioni M. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest. 1999;104:R49–54. doi: 10.1172/JCI7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermi W, Facchetti F, Riboldi E, et al. Role of dendritic cell-derived CXCL13 in the pathogenesis of Bartonella henselae B-rich granuloma. Blood. 2006;107(2):454–62. doi: 10.1182/blood-2005-04-1342. [DOI] [PubMed] [Google Scholar]
- 28.Lakshminarayanan V, Beno DWA, Costa RH, Roebuck KA. Differential regulation of interleukin-8 and intercellular adhesion molecule-1 by H2O2 tumor necrosis factor-α in endothelial epithelial cells. J Biol Chem. 1997;272:32910–8. doi: 10.1074/jbc.272.52.32910. [DOI] [PubMed] [Google Scholar]
- 29.Kazuya S, Takashi K, Tetsuya M, Hiroaki K, Ikuo T. Different chemokine expression in lethal and non-lethal murine west nile virus infection. J Med Virol. 2004;74:507–13. doi: 10.1002/jmv.20205. [DOI] [PubMed] [Google Scholar]
- 30.Iglesias BM, Cerase J, Ceracchini C, Levi G, Aloisi F. Analysis of B7-1 and B7-2 costimulatory ligands in cultured mouse microglia: Upregulation by interferon-gamma and lipopolysaccharide and downregulation by interleukin-10, prostaglandin E2 and cyclic AMP-elevating agents. J Neuroimmunol. 1997;72:83–93. doi: 10.1016/s0165-5728(96)00155-5. [DOI] [PubMed] [Google Scholar]
- 31.Luther SA, Bidgol A, Hargreaves DC, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–33. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 32.Thaunat O, Field AC, Dai J, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci USA. 2005;102:14723–8. doi: 10.1073/pnas.0507223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takemura S, Braun A, Crowson C, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167:1072–80. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 34.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 B-cell-attracting chemokine 1. in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104:3021–7. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 35.Perrier P, Martinez FO, Locati M, et al. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: Induction of the B cell-activating chemokine, CXC chemokine ligand 13. J Immunol. 2004;172:7031–42. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]
- 36.Hirata T, Osuga Y, Hirota Y, et al. Evidence for the presence of Toll-like receptor 4 system in the human endometrium. J Clin Endocrinol Metab. 2005;90:548–56. doi: 10.1210/jc.2004-0241. [DOI] [PubMed] [Google Scholar]
- 37.Yuvaraj S, Griffin AC, Sundaram K, Kirkwood KL, Norris JS, Reddy SV. A novel function of CXCL13 to stimulate RANK ligand expression in oral squamous cell carcinoma cells. Mol Cancer Res. 2009;7:1399–407. doi: 10.1158/1541-7786.MCR-08-0589. [DOI] [PubMed] [Google Scholar]
- 38.Shamri R, Grabovsky V, Gauguet JM, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 39.Baekkevold ES, Yamanaka T, Palframan RT, et al. The CCR7 ligand ELC CCL19 is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. 2001;193:1105–12. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogan RJ, Mathews SA, Mukhopadhyay S, Summersgill JT, Timms P. Chlamydial persistence: beyond the biphasic paradigm. Infect Immun. 2004;72:1843–55. doi: 10.1128/IAI.72.4.1843-1855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyons JM. An integrated approach to the study of Chlamydia trachomatis infection of the female genital tract. Drugs Today (Barc) 2006;42(Suppl A):83–97. [PubMed] [Google Scholar]