Abstract
There are still no effective treatments to prevent, halt or reverse Alzheimer’s disease, but research advances over the past three decades could change this gloomy picture. Genetic studies demonstrate that the disease has multiple causes. Interdisciplinary approaches combining biochemistry, molecular and cell biology, and transgenic modeling have revealed some of its molecular mechanisms. Progress in chemistry, radiology, and systems biology is beginning to provide useful biomarkers and the emergence of personalized medicine is poised to transform pharmaceutical development and clinical trials. However, investigative and drug development efforts should be diversified to fully address the multifactoriality of the disease.
INTRODUCTION
Alzheimer’s disease (AD) is characterized by progressive loss of memory and other cognitive functions. Typically, a decade or so passes before the illness has taken its course and patients die in a completely helpless state. The long duration of AD and its attack on the fragile structures that harbor the very essence of who we are place an enormous emotional and financial burden on patients, their families and society. AD is estimated to have cost the world $604 billion in 2010 alone (Wimo and Prince, 2010). These costs are staggering, particularly in light of predictions that the worldwide number of AD cases, currently estimated at 36 million, will triple by 2050 (Wimo and Prince, 2010). Few health care systems will be able to cope with this development. This Review highlights some of the most informative developments in AD research and raises major unresolved issues.
SUBSTRATES OF COGNITIVE DECLINE
AD causes a large loss in brain weight and volume and affects some brain regions and neuronal populations more than others (Gomez-Isla et al., 1996). Although AD clearly causes loss of neurons in specific brain regions (e.g., of pyramidal cells in lamina II of the entorhinal cortex and in the CA1 region of the hippocampus), much of the overall loss of brain volume appears to be due to the shrinkage and loss of neuronal processes.
Progress in radiological imaging techniques has advanced morphometric measurements from postmortem tissues to live patients (Hampel et al., 2010). For example, progressive decreases in cortical thickness can be detected in multiple brain regions by magnetic resonance imaging (MRI) in AD patients, correlate with cognitive decline, and predict conversion from mild cognitive impairment (MCI) to AD (Frisoni et al., 2010; Putcha et al., 2011). Consequently, this measure is increasingly used in the early diagnosis of AD and as a biomarker in clinical trials.
Beyond such anatomical alterations, functional MRI (fMRI) has revealed alterations in neural network activities in patients with AD and people at risk for developing the disease. These include abnormal activity and connectivity in the so-called default mode network, which in healthy people is most active when they do not think about anything in particular, and hyperactivation of the hippocampus during the execution of memory tasks (Sperling et al., 2010), which correlates with decreased hippocampal volume and abnormal cortical thinning in AD-vulnerable brain regions (Putcha et al., 2011). Consistent with electrophysiological and biochemical data obtained in related transgenic mouse models (Palop and Mucke, 2010; Verret et al.), these observations suggest that AD does not simply silence neurons and neural networks, but rather causes aberrant network activity that might actively interfere with the intricate processes underlying learning, memory and other cognitive functions. In addition, overstimulation of specific neuronal populations could result in excitotoxicity, which likely contributes to neurodegeneration in AD and related conditions. It is interesting in this regard that AD is associated with an increased incidence of epileptic seizures, which is most evident in patients with early-onset forms of the disease (Palop and Mucke, 2009). Findings in transgenic mouse models suggest that these complications may be the tip of an iceberg, representing an escalation of more subtle alterations of neural network activity (Palop and Mucke, 2010; Verret et al.).
Much evidence suggests that synapses and dendrites, the specializations through which neurons send and receive signals, respectively, are particularly vulnerable to AD. Loss of synapses and dendritic spines correlates better with cognitive decline in AD than loss of neurons (Palop et al., 2006). Synaptodendritic rarefaction is also observed in neuronal cultures and in brains of transgenic mice exposed to factors suspected of causing AD. In these models, the degeneration is preceded by alterations in synaptic function and aberrant network activity (Marchetti and Marie, 2011; Palop and Mucke, 2010), suggesting that these types of dysfunction are early manifestations of the disease and, possibly, also contribute to its progression.
Recent advances in radiological imaging have also enabled the detection of pathological hallmarks of AD in live patients, improving diagnostic accuracy and patient selection for clinical trials. After injection into the blood stream, Pittsburg compound B (PIB) traverses the blood-brain barrier (BBB) and binds to deposits of fibrillar amyloid-β (Aβ) peptides (amyloid plaques), whose abnormal accumulation in brain is a requirement for the pathological diagnosis of AD. PIB binding to amyloid plaques can be detected by positron emission tomography (PET) or single-photon emission computed tomography (SPECT). A multitude of other radiopharmaceutical probes are being developed for the detection of plaques or neurofibrillary tangles (NFTs) (Kim et al., 2010), another pathological hallmark of AD.
While the detection of these pathological hallmarks in live patients clearly has diagnostic value, it is still uncertain if these hallmarks actually contribute to cognitive dysfunction in AD and if they represent useful outcome measures for clinical trials. Plaque loads determined histopathologically or radiologically do not correlate well with functional impairments, as illustrated most strikingly by people with substantial plaque burdens and normal cognition (Giannakopoulos et al., 2003). Recent studies have demonstrated an association between increased PIB binding and development of AD five years later (Morris et al., 2009), which has been interpreted as a likely cause-effect relationship. However, some cases with autosomal dominant AD show high levels of PIB binding in the basal ganglia but no greater propensity to develop extrapyramidal motor impairments, indicating dysfunction of these brain regions, than cases with sporadic AD, who have different distributions of PIB binding (Villemagne et al., 2009).
Furthermore, inheritance of the E693Δ mutation in Aβ, which inhibits formation of insoluble amyloid fibrils and promotes the formation of soluble Aβ oligomers, causes a syndrome that closely resembles AD clinically but does not appear to be associated with significant increases in cerebral PIB binding (Shimada et al., 2011). Additionally, the pattern of PIB accumulation differs between patients with sporadic late-onset AD and patients with APP locus duplication (Remes et al., 2008). These observations and a large body of data obtained in transgenic mouse models (Palop et al., 2006; Palop and Mucke, 2010) caution against the interpretation that plaques are the main cause and plaque-associated neuritic dystrophy the main substrate of cognitive decline in AD. Although NFTs correlate more closely with cognitive decline in AD than plaques (Giannakopoulos et al., 2003), results obtained in transgenic mouse models indicate that NFTs do not necessarily impair neuronal function and that the microtubule-associated protein tau, the main constituent of NFTs, can cause neuronal dysfunction independently of these structures, as reviewed in (Morris et al., 2011).
Taken together, these studies suggest that aberrant neural network activity, dysfunction and loss of synapses, and degeneration of specific neuronal populations are the main substrates of cognitive decline in AD. As outlined below, it is likely that these abnormalities are caused by co-pathogenic interactions among diverse factors and pathways (Figure 1).
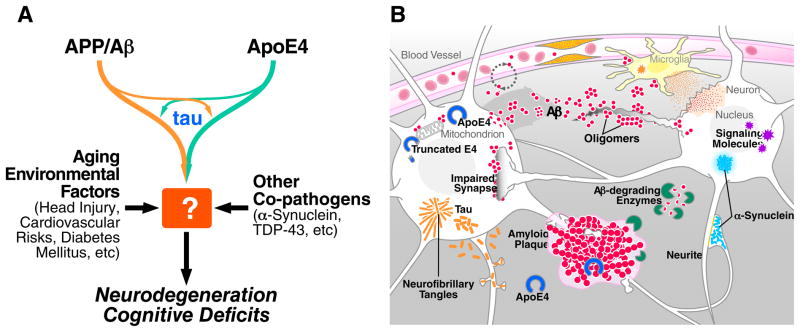
Figure 1. Multifactorial Basis of Alzheimer’s Disease Pathogenesis.
(A) Alzheimer’s disease (AD) is likely caused by co-pathogenic interactions among multiple factors, including APP/Aβ, apoE4, tau, α-synuclein, TDP-43, aging, and various co-morbidities. How exactly they conspire to impair neuronal functions and survival remains to be determined. (B) Aggregation and accumulation of Aβ in the brain may result from increased neuronal production of Aβ decreased degradation by Aβ-degrading enzymes, or reduced clearance across the blood-brain barrier. Aβ oligomers impair synaptic functions and related signaling pathways, changing neuronal activities, and trigger the release of neurotoxic mediators from glial cells. Fibrillar amyloid plaques displace and distort neuronal processes. The lipid transport protein apoE4 impairs Aβ clearance and promotes Aβ deposition. When expressed within stressed neurons, apoE4 is cleaved, to a much greater extent than apoE3, into neurotoxic fragments that disrupt the cytoskeleton and impair mitochondrial functions. Tau, which is normally most abundant in axons, becomes mislocalized to the neuronal soma and dendrites and forms inclusions called neurofibrillar tangles (NFTs). α-synuclein can also self-assemble into pathogenic oligomers and form larger aggregates (Lewy bodies). Both tau and α-synuclein can also be released into the extracellular space, where they may spread to other cells. Vascular abnormalities impair the supply of nutrients and removal of metabolic byproducts, cause microinfarcts, and promote the activation of glial cells.
MULTIFACTORIAL ETIOLOGY OF AD
Genetics, Epigenetics, and Epidemiology
AD is likely caused by complex interactions among multiple genetic, epigenetic and environmental factors. Mutations in three genes—amyloid protein precursor (APP), presenilin (PS)-1 and PS-2—cause early-onset (<60 years) autosomal dominant AD (Bertram et al., 2010), which probably accounts for less than 1% of AD cases (Campion et al., 1999). The mutations affect APP processing, leading to altered production of different Aβ peptides and, thus, their relative ratios (Bertram et al., 2010). Down’s syndrome patients carrying an extra copy of chromosome 21, on which the APP gene resides, develop early-onset dementia with pathological hallmarks of AD in their brains (Millan Sanchez et al., 2011), consistent with the idea that overexpression of APP causes early-onset AD. In strong support of this notion, duplication of the APP gene alone leads to early-onset AD (Rovelet-Lecrux et al., 2006). Moreover, increased APP gene expression caused by genetic variations in the promoter sequence may be a risk factor for late-onset AD, with levels of APP expression correlating inversely with age of disease onset (Brouwers et al., 2006).
Apolipoprotein (apo) E4 (used here to refer to either the APOE ε4 allele or the protein it encodes) has been genetically linked to late-onset (>60 years) familial and sporadic AD, which accounts for most AD cases; it has a gene-dose effect on increasing the risk and lowering the age of onset of the disease (Corder et al., 1993; Farrer et al., 1997). All well-conducted genome wide association studies (GWAS) on late-onset AD from different populations around the world identified apoE4 as the top late-onset AD gene with extremely high confidence (Bertram et al., 2010). Remarkably, the lifetime risk estimate of developing AD for individuals with two copies of the apoE4 allele (~2% of the population) is ~60% by the age of 85, and for those with one copy of the apoE4 allele (~25% of the population) ~30%. In comparison, the lifetime risk of AD for those with two copies of the apoE3 allele is ~10% by the age of 85. Thus, apoE4 should be considered a major gene, with semi-dominant inheritance, for late-onset AD (Genin et al., 2011). A recent GWAS also identified apoE4 as the only significant gene associated with age-related cognitive decline in humans (De Jager et al.), which is in line with a longitudinal study showing that age-related memory decline in non-demented apoE4 carriers diverges from that of non-demented noncarriers before the age of 60 years (Caselli et al., 2009). Thus, apoE4’s detrimental effect on cognition occurs before the typical signs of AD arise. In contrast, apoE2 may protect against AD in some populations (Farrer et al., 1997). GWAS also identified other genes that modulate the risk of late-onset AD, including CLU, CR1, PICALM, BIN1, SORL1, GAB2, ABCA7, MS4A4/MS4A6E, CD2AP, CD33, EPHA1 and HLA-DRB1/5 (Bertram et al., 2010; Logue et al., 2011). However, the relative contribution of these genes to AD is moderate as compared to apoE4.
Epigenetic mechanisms may also play a role in AD pathogenesis (Day and Sweatt, 2011). Studies on human postmortem brain samples and peripheral leukocytes, as well as transgenic animal models, have shown that aging and AD are associated with epigenetic dysregulation at various levels, including abnormal DNA methylation and histone modifications (Chouliaras et al., 2010). Although it is unclear whether the epigenetic changes observed in AD represent a cause or a consequence of the disease, twin studies support the notion that epigenetic mechanisms modulate AD risk (Chouliaras et al., 2010). Interestingly, pharmacologically inhibiting DNA methylation in the hippocampus after a learning task impaired memory consolidation in mice (Day and Sweatt, 2011), and promoting histone acetylation improved learning and memory in a mouse model of AD and increased learning-related gene expression in aged wildtype mice (Fischer et al., 2007; Peleg et al., 2010), suggesting epigenetic regulation of learning and memory in health and disease.
Aging is the most important known non-genetic risk factor for late-onset AD. Potential environmental risk factors for late-onset AD include head injury, low educational levels, hyperlipidemia, hypertension, homocysteinemia, diabetes mellitus, and obesity (Barnes and Yaffe, 2011; Rosendorff et al., 2007; Sharp and Gatz, 2011; Van Den Heuvel et al., 2007). However, several of these associations remain controversial (Daviglus et al., 2010). Combinations of apoE4 with one or more of these environmental risk factors may further increase the risks for late-onset AD and age-related cognitive decline (Caselli et al., 2011; Haan et al., 1999).
Aβ and Other APP Products
Aβ peptides, the main constituent of amyloid plaques, and various other metabolites are derived from APP by proteolytic cleavage (Citron, 2010; De Strooper et al., 2010). Diverse lines of evidence support the hypothesis that APP and Aβ contribute causally to the pathogenesis of AD (Figure 2). Overexpression of APP in humans through duplication of its gene or trisomy of chromosome 21 causes early-onset AD (see above), whereas partial trisomy 21 excluding the APP gene does not (Prasher et al., 1998). The catalytic subunit of the γ-secretase protein complex that releases Aβ peptides from its precursor is formed by PS1 or PS2. Autosomal dominant mutations in APP, PS1 or PS2 that alter APP processing and the production or self-aggregation of Aβ, promoting aggregation and accumulation of Aβ in brain, also cause early-onset AD (Bertram et al., 2010). Neuronal expression of mutant human APP (hAPP) either alone or in combination with mutant PS1 in transgenic rodents causes several AD-like alterations, as reviewed in (Ashe and Zahs, 2010; Jucker, 2010; Marchetti and Marie, 2011; Palop and Mucke, 2010; Querfurth and LaFerla, 2010) and described further below.
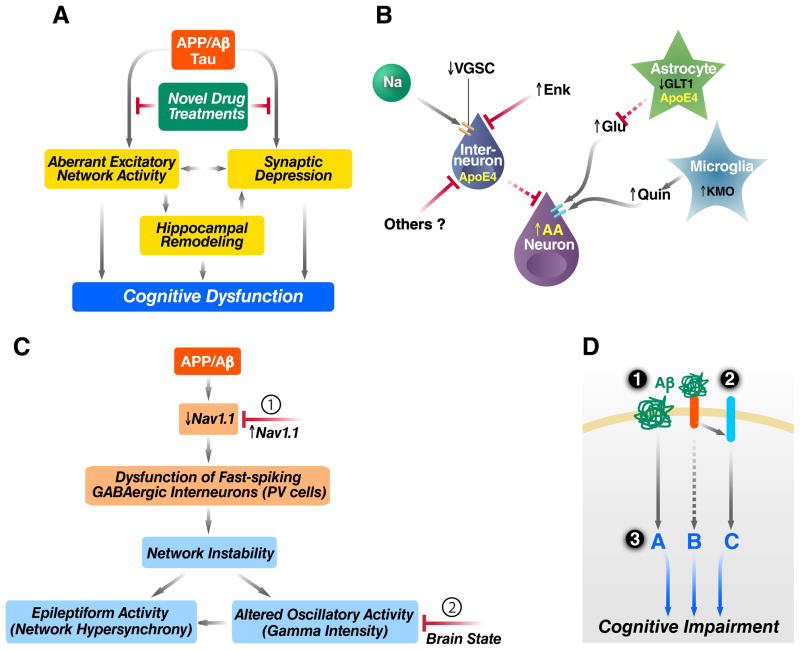
Figure 2. Aβ in AD Pathogenesis and Related Therapeutic Strategies.
(A) Pathogenic Aβ assemblies cause tau-dependent aberrant excitatory network activity and synaptic depression, which may lead directly, or indirectly through hippocampal remodeling, to cognitive dysfunction. Identification and blockade of the upstream mechanisms that trigger this cascade are important objectives. (B) Aβ-dependent aberrant network activity may be caused by impairments of inhibitory interneurons resulting from depletion of voltage-gated sodium channel subunits (VGSC), intracellular activities of neurotoxic apoE4 fragments, or elevated metenkephalin (Enk) levels. Additional mechanisms that may cause neuronal overexcitation in AD include impairment of glutamate transporters (e.g., GLT1), resulting in decreased clearance of glutamate (Glu), and increased neuronal or microglial production of factors that promote excitotoxicity, including arachidonic acid (AA) and quinolinic acid (Quin). (C) hAPP mice have depletions of the VGSC subunit Nav1.1 that impair the function of parvalbumin (PV)-positive inhibitory interneurons (PV cells), resulting in reduced intensity of gamma oscillations and network hypersynchrony. These network abnormalities can be prevented by increasing Nav1.1 levels in PV cells (1) and counteracted, at least temporarily, by behavioral interventions that enhance gamma activity (2). (D) Aβ may impair neuronal functions by receptor-mediated and receptor-independent mechanisms. Potential therapeutic strategies include (1) lowering Aβ production with secretase inhibitors or modulators and enhancing Aβ clearance with antibodies or activation of Aβ-degrading enzymes, (2) blocking the interaction of Aβ with cell surface receptors, and (3) modulating downstream pathways to make the brain more resistant against whatever Aβ cannot be removed effectively and safely.
Several lines of evidence suggest that Aβ regulates neuronal and synaptic activities and that accumulation of Aβ in the brain causes an intriguing combination of aberrant network activity and synaptic depression (Figure 2A and (Palop and Mucke, 2010)). Impairments of inhibitory interneurons and aberrant stimulation of glutamate receptors, which can result in excitotoxicity, appear to play important upstream roles in this pathogenic cascade (Figure 2B, 2C and (Meilandt et al., 2008; Palop and Mucke, 2010; Sanchez-Mejia et al., 2008; Verret et al.). Aberrant neuronal activity might trigger a vicious cycle by augmenting Aβ production, which is regulated, at least in part, by neuronal activity (Bero et al., 2011). The immediate early gene Arc, which directly binds to PS1 to regulate γ-secretase trafficking, is required for neuronal activity-dependent Aβ production (Wu et al., 2011).
Results obtained in diverse experimental models suggest that insoluble Aβ fibrils found in amyloid plaques and monomeric Aβ are less pathogenic than soluble, nonfibrillar assemblies of Aβ such as Aβ dimers, trimers and larger oligomers (Figure 1B). However, which Aβ assemblies are most pathogenic and how their accumulation in brain causes synaptic and neuronal dysfunction are unresolved topics of intense study and debate (Benilova et al.; Li et al., 2011; Palop and Mucke, 2010). These moieties may act extracellularly and intracellularly and engage proteins as well as lipids. Several cell surface molecules have been implicated in Aβ oligomer-induced toxicity (Figure 2D), including the receptor tyrosine kinase EphB2 (Cisse et al., 2011) and the receptor for advanced glycation end products (Srikanth et al., 2011). Potential downstream mechanisms include changes in the distribution or activity of neurotransmitter receptors and related signaling molecules (Jo et al., 2011; Li et al., 2011; Palop and Mucke, 2010; Renner et al., 2010; Ronicke et al., 2011), disruption of intracellular calcium homeostasis (Camandola and Mattson, 2011), and impairments of axonal transport and mitochondrial functions (Decker et al., 2010; Du et al., 2008; Querfurth and LaFerla, 2010; Sheng and Cai; Vossel et al., 2010).
A major current obstacle in the field is the lack of techniques to reliably quantify the abundance of soluble Aβ assemblies in brain tissues, particularly on neuronal membranes. This problem has precluded correlations of cognitive impairments in AD patients with levels of these assemblies in strategic sites. It has also made it impossible to know whether any of the Aβ-related drugs that underwent clinical trials actually lowered the levels of functionally relevant Aβ assemblies in AD-vulnerable brain regions. Other uncertainties about the value of Aβ-lowering drugs relate to potential physiological functions of APP and different APP metabolites, including Aβ (Duce et al., 2010; Giuffrida et al., 2009; Lee et al., 2010b; Puzzo et al., 2011; Weyer et al., 2011; Yang et al., 2009) and to the question of whether APP itself or APP metabolites other than Aβ (e.g., the C-terminal fragments C99, intracellular domain, or C31) also contribute to the pathogenesis of AD (Ghosal et al., 2009; Harris et al., 2010; Simon et al., 2009).
ApoE4
The major impact of apoE4 on AD risk is clearly at odds with the relatively small amount of attention it has received in the field, as compared to, for instance, APP, Aβ, tau or inflammation. To encourage improvement in this situation, we will review the pathobiology of apoE4 in greater depth here. Since apoE4 was identified as a genetic risk factor for AD, in vitro and in vivo studies have explored its structural properties and functions in neurobiology, its cellular source-dependent physiological and pathophysiological activities in brain, and its Aβ-dependent and independent roles in AD pathogenesis (Figure 3).
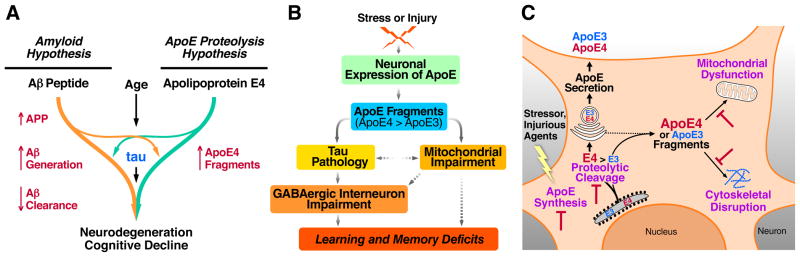
Figure 3. ApoE4 in AD Pathogenesis and Related Therapeutic Strategies.
(A) ApoE4 probably has both Aβ-dependent and Aβ-independent roles in AD pathogenesis. ApoE4 impairs Aβ clearance and promotes Aβ deposition (left). In addition, neuronal apoE4 undergoes proteolytic cleavage to generate neurotoxic fragments, contributing to AD pathogenesis independently of Aβ stresses or injuries, neuronal apoE expression is triggered to facilitate neuronal repair. However, neuronal apoE undergoes proteolytic cleavage, with apoE4 being more susceptible to the cleavage than apoE3, resulting in the formation of neurotoxic fragments. The apoE4 fragments enter the cytosol and cause tau pathology and mitochondrial impairment. Hilar GABAergic interneurons in the dentate gyrus are particularly vulnerable to apoE4 fragment toxicity and the resulting impairments contribute to learning and memory deficits. (C) Neuronal expression of apoE and apoE proteolysis-related neurotoxicity represent targets for the development of novel therapeutics. Inhibiting apoE4 expression in neurons should reduce the level of toxic apoE4 fragments and their downstream detrimental effects. Identification of the protease that cleaves apoE in neurons should enable the development of specific protease inhibitors to reduce the production of neurotoxic apoE fragments. It may also be possible to develop drugs to protect mitochondria and the cytoskeleton from attack by the toxic apoE fragments.
ApoE polymorphisms and functions in neurobiology
ApoE is a polymorphic protein with three common isoforms, apoE2, apoE3, and apoE4, in humans. The three isoforms differ from one another by single amino acid substitutions (Mahley et al., 2006). ApoE has important and diverse roles in neurobiology (Bu, 2009; Huang, 2006; Kim et al., 2009; Mahley et al., 2006). It has isoform-specific roles in neurite remodeling: apoE3 stimulates neurite outgrowth and apoE4 inhibits it (Huang, 2006, 2010; Mahley et al., 2006). ApoE also takes up lipids generated after neuronal degeneration and redistributes them to cells requiring lipids for proliferation, membrane repair, or remyelination of new axons (Huang, 2006; Mahley et al., 2006). Synaptic and dendritic alterations are observed in apoE-deficient mice (Masliah et al., 1995) and transgenic mice expressing apoE4 in neurons (Buttini et al., 1999). In addition, apoE modulates glutamate receptor function and synaptic plasticity by regulating apoE receptor recycling in neurons, with apoE3 stimulating and apoE4 inhibiting this process (Chen et al., 2010).
Interaction between the N- and C-terminal domains, is a unique biophysical property of apoE4 (Zhong and Weisgraber, 2009). Crystallographic and other biophysical studies revealed that this domain interaction is mediated by a salt bridge formation between Arg-61 and Glu-255 (Zhong and Weisgraber, 2009). Mutation of Arg-61 to threonine or of Glu-255 to alanine in apoE4 prevents domain interaction and makes apoE4 more apoE3-like (Zhong and Weisgraber, 2009). The domain interaction is responsible for impaired intraneuronal trafficking of apoE4 (Brodbeck et al., 2011), apoE4’s susceptibility to proteolysis (Huang, 2010; Mahley et al., 2006), apoE4-induced impairments in neurite outgrowth and mitochondrial functions (Brodbeck et al., 2011; Chen et al.), and apoE4-associated astrocytic dysfunction (Zhong and Weisgraber, 2009).
Cellular source-dependent roles of apoE4 in AD pathogenesis
ApoE derived from different cellular sources has distinct roles in both physiological and pathophysiological pathways (Huang, 2006, 2010; Mahley et al., 2006). Astrocytes have long been recognized as the primary source of apoE in brain, and expression of apoE in astrocytes is increased during aging and in response to estrogen and activation of liver X receptor or NF-kB (Huang, 2006, 2010; Mahley et al., 2006). In vitro and in vivo studies suggest that astrocyte-derived apoE has isoform-specific effects on Aβ clearance or deposition (Kim et al., 2009), neurite outgrowth (Holtzman et al., 1995), and behavioral performance (Hartman et al., 2001).
CNS neurons also express apoE, but mostly in response to stresses and injuries (Huang, 2006; Xu et al., 2006). Interestingly, astrocyte-conditioned medium upregulates apoE expression in neurons, and this regulation is controlled by intron-3 retention/splicing of the apoE gene through the extracellular signal–regulated kinase pathway (Harris et al., 2004; Xu et al., 2008). Neuron-derived apoE3 and apoE4 differ in their intracellular trafficking (Brodbeck et al., 2011), their susceptibility to proteolysis (Brecht et al., 2004), and their effects on mitochondrial function (Chang et al., 2005; Chen et al.), tau phosphorylation (Brecht et al., 2004), lysosomal (Ji et al., 2002) and neuronal integrity (Buttini et al., 1999), androgen receptor levels (Raber et al., 2002), and cognitive functions (Raber et al., 1998). Interestingly, when expressed in neurons, apoE3 is excitoprotective, whereas apoE4 is not; however, when expressed in astrocytes, apoE3 and apoE4 are equally excitoprotective (Buttini et al., 2010). Thus, the cellular source of apoE markedly affects its physiological and pathophysiological activities. One potential mechanism underlying these differential effects is apoE4 proteolysis, which generates neurotoxic fragments and occurs in neurons but not in astrocytes (Huang, 2010).
Aβ-dependent roles of apoE4 in AD pathogenesis
In vivo, apoE is associated with amyloid plaques and in vitro lipid-free apoE3 and apoE4 can form stable complexes with Aβ peptides, with apoE4 forming complexes more rapidly and effectively (Huang, 2006; Kim et al., 2009). Studies in apoE-deficient mice expressing mutant hAPP demonstrate that apoE is actually required for the formation of fibrillar amyloid plaques (Bales et al., 1999; Holtzman et al., 2000). However, when incubated with Aβ peptides, lipidated apoE3 and apoE4 yield different results. ApoE3 binds Aβ with a 20-fold greater affinity than apoE4, suggesting that lipidated apoE differs from lipid-free apoE in its ability to interact with Aβ peptides (Huang, 2006; Kim et al., 2009). Interestingly, decreasing apoE’s lipidation status by knocking out ABCA1 in mice expressing mutant hAPP significantly increases brain amyloid loads, whereas increasing apoE lipidation status by overexpressing ABCA1 decreases amyloid levels (Kim et al., 2009). Thus, altering apoE lipidation changes its ability to mediate Aβ clearance or deposition in brain.
In hAPP transgenic mice, human apoE stimulates Aβ clearance. ApoE2 and apoE3 clear more Aβ than apoE4 (Bales et al., 1999; Holtzman et al., 2000), which may be related to apoE isoform-dependent effects on astroglial degradation of Aβ deposits (Koistinaho et al., 2004). Measuring Aβ clearance rates by microdialysis in brains of hAPP transgenic mice expressing apoE3 or apoE4 revealed that apoE4 decreases Aβ clearance by about 40% compared to apoE3 (Castellano et al., 2011). A recent study demonstrated that C-terminally truncated apoE4, which is found in AD brains, inefficiently clears Aβ and acts in concert with Aβ to elicit neuronal and behavioral deficits in transgenic mice (Bien-Ly et al., 2011). In vitro and in vivo studies suggest that the low-density lipoprotein (LDL) receptor, the LDL receptor-related protein-1 (LRP1), and the very-low-density lipoprotein (VLDL) receptor are all involved in apoE-mediated Aβ clearance from brain (Bu, 2009; Deane et al., 2008; Kim et al., 2009).
While apoE4 clearly increases Aβ accumulation and amyloid plaque formation in both humans and transgenic mouse models (Figure 3A), it is still uncertain if this process actually contributes to cognitive deficits in AD. As indicated above, plaque loads determined histopathologically or radiologically do not correlate well with cognitive impairments in humans (Giannakopoulos et al., 2003). Furthermore, in the oldest old (>90 years of age), the presence of apoE2 is associated with a reduced risk of dementia but an increased amyloid burden, relative to apoE3 (Berlau et al., 2009).
Aβ-independent roles of apoE4 in AD pathogenesis
Several transgenic or gene-targeted mouse lines expressing human apoE3 or apoE4 have been established, without co-expression of mutant hAPP. Transgenic mice expressing apoE4 in neurons on a murine Apoe knockout background show age- and female gender-dependent spatial learning and memory deficits that are not observed in neuron-specific apoE3 mice (Raber et al., 1998; Raber et al., 2000). Morphological studies demonstrated that neuronal apoE3, but not apoE4, prevents the age-dependent neurodegeneration seen in apoE-null mice as well as excitotoxin-induced neurodegeneration (Buttini et al., 2010; Buttini et al., 1999). ApoE4 also impairs synaptogenesis and decreases dendritic spine density in vivo in apoE transgenic and gene-targeted mice and in vitro in primary neuronal cultures (Brodbeck et al., 2011; Dumanis et al., 2009). In addition, neural stem cells in adult mice express apoE and apoE4 impairs adult hippocampal neurogenesis (Li et al., 2009), which might also contribute to apoE4-associated learning and memory deficits. Since there is no Aβ accumulation in any of these apoE4 mouse models, these data strongly suggest an Aβ-independent role of apoE4 in causing neuronal and behavioral deficits in vivo. In support of this notion, effects of apoE4 on cortical thickness, brain activity and mitochondrial function have been detected in children or college students (Filippini et al., 2009; Shaw et al., 2007), i.e., well before significant Aβ accumulation appears to occur in human brains (Braak et al., 2011).
It has been hypothesized that, in response to brain stresses or injuries, neuronal apoE expression is induced for purposes of repair or remodeling. Notably, this process also causes proteolytic cleavage of apoE4, resulting in fragments that lead to neuronal dysfunction and degeneration (Figure 2B and 2C) (Huang, 2006, 2010; Mahley et al., 2006). Indeed, neuronal apoE4 is more susceptible than neuronal apoE3 to proteolytic cleavage, resulting in the generation of C-terminally truncated neurotoxic fragments (Brecht et al., 2004). ApoE fragments are present at much higher levels in the brains of AD patients than in those of age- and sex-matched nondemented controls (Harris et al., 2003; Jones et al., 2011). Transgenic mice expressing the C-terminally truncated fragment of apoE have neurodegeneration in the hippocampus and show spatial learning and memory deficits (Andrews-Zwilling et al., 2010; Harris et al., 2003).
C-terminally truncated apoE4 increases tau phosphorylation and the formation of intracellular NFT-like inclusions in cultured neuronal cells and in transgenic mice (Figure 3B and 3C) (Huang, 2006, 2010; Mahley et al., 2006). Thus, neurotoxicity induced by the apoE4 fragments might be related to the formation of neurotoxic tau species. In line with this hypothesis, removing endogenous tau prevents neuronal and behavioral deficits in apoE4 fragment-transgenic mice (Andrews-Zwilling et al., 2010). ApoE4 fragments also target neuronal mitochondria, leading to mitochondrial dysfunction and neurotoxicity (Figure 3B and 3C) (Chang et al., 2005; Chen et al.). Notably, mitochondrial dysfunction in AD is greater in apoE4 than in apoE3 carriers (Gibson et al., 2000). ApoE4 is also associated with decreased cerebral glucose metabolism in both AD patients and nondemented subjects (Small et al., 2000), which likely reflects apoE4-dependent mitochondrial dysfunctions.
ApoE4-induced impairment of GABAergic interneurons
ApoE4 knock-in mice show an age-dependent decrease in hilar GABAergic interneurons, which correlates with the extent of apoE4-induced impairments of adult hippocampal neurogenesis and with learning and memory deficits (Andrews-Zwilling et al., 2010; Li et al., 2009). In transgenic mice expressing neurotoxic apoE4 fragments, the loss of hilar interneurons is more pronounced and also correlates with learning and memory deficits (Andrews-Zwilling et al., 2010). These adverse effects are prevented by tau removal (Andrews-Zwilling et al., 2010). Mice treated with the GABAA receptor potentiator pentobarbital exhibit normal neurogenesis and learning and memory (Andrews-Zwilling et al., 2010; Li et al., 2009). These findings strongly suggest that apoE4 causes age- and tau-dependent impairment of hilar GABAergic interneurons, leading to decreased neurogenesis in the hippocampus and to learning and memory deficits (Figure 3B).
Dysfunction of the GABAergic system may also contribute to cognitive impairment in humans. AD patients have decreased GABA and somatostatin levels in the brain and CSF, and these alterations are more severe in apoE4 carriers (Grouselle et al., 1998). ApoE4 is associated with increased brain activity at rest and in response to memory tasks (Dennis et al., 2010; Filippini et al., 2009), possibly reflecting impaired GABAergic inhibitory control. A single nucleotide polymorphism in the somatostatin gene increases the risk for AD in carriers of apoE4 but not of apoE3 (Vepsalainen et al., 2007). Furthermore, GABA levels in human CSF decrease with aging (Bareggi et al., 1982)—the strongest risk factor for AD. We hypothesize that apoE4 contributes to AD pathogenesis, at least partially, by causing age-dependent impairments of GABAergic interneurons (Figure 3B).
Tau and Other Co-Pathogens
AD is clearly a polyproteinopathy in which multiple proteins assume potentially pathogenic conformations and accumulate separately or together in the brain. Based on the pathological definition of the disease, AD is associated not only with the abnormal accumulation of amyloid plaques, but also with that of NFTs. NFTs form intracellularly and are made up primarily of aggregated tau bearing abnormal posttranslational modifications, including increased phosphorylation and acetylation (Cohen et al., 2011; Iqbal et al., 2010; Min et al., 2010). Several recent findings have challenged the traditional views that tau functions primarily to stabilize microtubules and that its aggregation in AD causes deficits through a loss-of-function mechanism (Morris et al., 2011). Studies in cell culture and genetically modified mouse models suggest that tau may normally facilitate or enhance excitatory neurotransmission by regulating the distribution of synaptic activity-related signaling molecules (Morris et al., 2011). However, when it is abnormally modified and assumes pathogenic conformations, tau becomes enriched in dendritic spines where it can interfere with neurotransmission (Hoover et al., 2010). Aβ oligomers promote this postsynaptic enrichment of tau through a process that involves members of the microtubule affinity-regulating kinase (MARK) family (Yu et al.; Zempel et al., 2010).
Interestingly, tau reduction prevents Aβ from causing neuronal deficits in cell culture and hAPP transgenic mice (Morris et al., 2011). Thus, while Aβ acts upstream of tau, its adverse effects depend in good part on tau (Figure 4). These conclusions are consistent with genetic studies: mutations in APP or presenilins that cause Aβ accumulation in the brain cause AD with amyloid plaques and NFTs (Bertram et al., 2010), whereas tau mutations cause NFTs but neither amyloid plaques nor AD (Figure 4). The latter mutations cause frontotemporal lobar degeneration instead (Ballatore et al., 2007). ApoE4 and its fragments increase tau phosphorylation and accumulation in the neuronal soma and dendrites (Andrews-Zwilling et al., 2010; Brecht et al., 2004; Harris et al., 2003). Moreover, tau reduction also prevents apoE4-dependent neuronal deficits in vitro and in vivo (Andrews-Zwilling et al., 2010), pinpointing tau as a key mediator or enabler of both Aβ- and apoE4-dependent pathogenesis (Figure 4). As reviewed above, apoE isoforms modulate both Aβ deposition as well as plaque-independent synaptic and cognitive deficits in hAPP mice, with apoE4 enhancing and apoE3 counteracting the abnormalities, further highlighting the co-pathogenic relationships among Aβ, tau and apoE4.
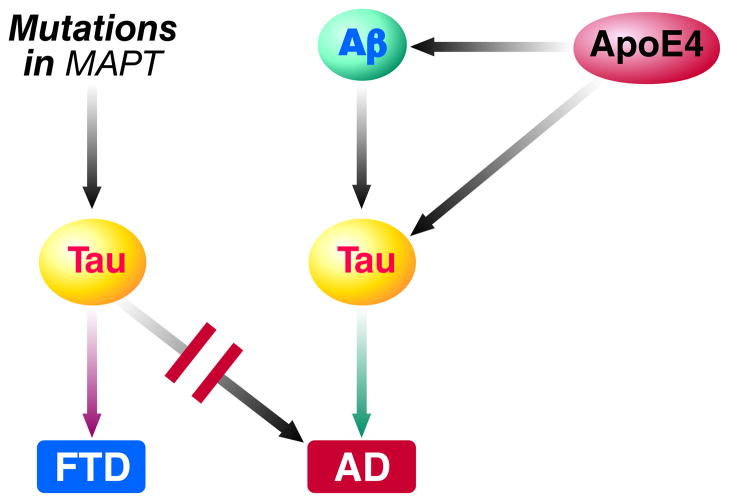
Figure 4. Roles of tau in different tauopathies.
Mutations in MAPT, the gene that encodes tau, result in the production of mutant tau and cause certain forms of frontotemporal dementia (FTD) but never AD. In AD, Aβ and apoE4 act upstream of wildtype tau and their adverse effects depend, at least partly, on tau whose structure or function may have been altered by abnormal posttranslational modification, mislocalization or other variables. Indeed, tau reduction prevents Aβ and apoE4 fragments from causing neuronal and behavioral deficits in mouse models. The exact mechanisms by which tau contributes to neuronal dysfunction and degeneration in AD, FTD and other tauopathies remain to be determined.
A large proportion of AD cases also have abnormal accumulations of the presynaptic protein α-synuclein and of the RNA-binding protein TDP-43 in brain (Higashi et al., 2007). As with tau, mutant forms of these proteins do not cause AD, but other neurodegenerative disorders. Aβ enhances the misfolding and accumulation of α-synuclein in vitro and in vivo, and studies in doubly transgenic mice suggest that these molecules can synergize to more severely impair neuronal functions (Masliah et al., 2001). Likewise, apoE4 fragments may enhance the accumulation of TDP-43 in neurons of FTD patients (Vossel et al.).
Potential Common Mechanisms
Like Aβ, tau and α-synuclein can exist in different assembly states, and several lines of evidence suggest that smaller aggregates such as soluble oligomers are more pathogenic than larger, insoluble, highly ordered, fibrillar aggregates (Morris et al., 2011; Winner et al., 2011). An interesting question that remains unresolved is whether the in vivo pathogenicity of these and other proteins associated with neurodegenerative disorders depends on specific conformations (e.g. β-pleated sheet structures) and whether the proteins require self-aggregation or binding to other proteins to assume these conformational states. At least for tau and α-synuclein, both of which are intrinsically disordered proteins, it is conceivable that even monomers could assume pathogenic conformations under circumstances that promote protein misfolding. Experimental evidence for such a scenario has recently been obtained for huntingtin bearing disease-associated polyglutamine repeat expansions (Miller et al., 2011).
Whether the various pathogenic protein conformations or assemblies impair neuronal functions through common, distinct or partly overlapping mechanisms also remains to be determined. The copathogenic interactions that have been identified among tau, apoE4, Aβ and α-synuclein suggest that there are probably downstream mechanisms on which the effects of these proteins converge (see above). Abnormalities in the activity or distribution of neurotransmitter receptors and downstream signaling cascades, excitotoxicity and dysregulation of intracellular calcium homeostasis, alterations in the intracellular transport of critical cargoes, mitochondrial impairments, epigenetic dysregulation, and engagement of pathogenic glial loops are among the possible convergence points. It is unfortunate that the identification of such diverse possibilities is often misinterpreted as controversy or lack of understanding. After all, even normal proteins often have multiple functions and different activities under different circumstances, cytokines such as TGF-β being a good example. There is no reason why the activity of pathogenic proteins should be less complex. Therefore, the various mechanisms listed above are clearly not mutually exclusive. In fact, some of them may be related, e.g., intracellular transport deficits and mitochondrial dysfunction.
In addition, abnormal folding and accumulation of multiple proteins could nonspecifically stress and ultimately overload the cellular protein handling machinery. Much evidence suggests that autophagy is the main mechanism by which cells clear abnormal protein aggregates (Harris and Rubinsztein). In the most common form of autosomal dominant AD, mutant PS1 may disrupt autophagy directly by impeding lysosomal proteolysis, whereas in other forms of AD autophagy impairments may involve different genetic or environmental factors (Nixon and Yang, 2011). Attempts to restore normal lysosomal proteolysis and autophagy in mouse models of AD have yielded promising therapeutic effects, lowering Aβ levels and improving neuronal function as well as cognitive performance (Nixon and Yang, 2011; Pickford et al., 2008; Yang et al., 2011a).
Another interesting aspect of Aβ, tau, α-synuclein and other proteins associated with neurodegenerative disorders is their ability to perpetuate pathology through cell-to-cell spread and seeding of abnormal protein aggregation in experimental models. These properties have been compared to those of prions, different forms of which cause Jacob-Creutzfeldt disease, scrapie and mad-cow disease (Braak and Del Tredici, 2011; Jucker and Walker, 2011). However, prions differ fundamentally from the other proteins in that they cause diseases that are communicable, which AD and most other neurodegenerative disorders are not.
It is well established that neurodegenerative disorders are strongly linked to aging. However, it remains uncertain whether this link is specifically caused by aging-related processes or simply reflects the time required for the relevant pathogenic processes to unfold. Genetic changes that accelerate the accumulation of pathogenic proteins in the brain can clearly override the aging “requirement” and cause AD or other neurodegenerative conditions in middle-aged or even young people. Nonetheless, diverse lines of experimental evidence support the notion that AD and other neurodegenerative conditions may be enabled by specific aging-related factors, such as gradual failure of neuroprotective or protein clearance mechanisms and the emergence of comorbidities (Herrup, 2010; Mawuenyega et al., 2010; Villeda et al., 2011). Inflammation may be a key component of unhealthy aging (Sastre et al., 2011).
Last but not least, both Aβ and apoE4 can contribute to network and cognitive dysfunction by impairing specific populations of inhibitory interneurons that normally regulate the activity of excitatory principal cells (Figure 2B, 2C and Figure 3B). Fast-spiking parvalbumin-positive GABAergic interneurons in the parietal cortex of hAPP mice have reduced levels of voltage-gated sodium channel (VGSC) subunits (Figure 2B, 2C), and similar alterations are present in patients with AD (Verret et al.). Increasing the level of these sodium channel subunits in the transgenic mice improves gamma oscillations, network activity and cognitive functions. Exploratory activity also increases gamma intensity and reduces network hypersynchrony in some of the mice. ApoE4 and its fragments cause age- and tau-dependent impairments of somatostatin-positive GABAergic interneurons in the hilus of the dentate gyrus, leading to learning and memory deficits (Figure 3B). Stimulation of GABA signaling reverses these deficits (Andrews-Zwilling et al., 2010). Thus, improving the function of interneurons may be an interesting new entry point for therapeutic interventions in AD.
PRECLINICAL AND CLINICAL TRIALS
Acetylcholine esterase inhibitors and memantine
Drugs that are currently approved by the FDA for the treatment of AD inhibit acetylcholine esterase to increase the levels of the neurotransmitter acetylcholine, which is depleted in AD brains, or antagonize NMDA-type glutamate receptors to prevent aberrant neuronal stimulation (Cummings, 2004). The impact of these drugs on disease manifestations is modest and transient, although observational studies suggest that combination treatment may increase the time before patients require nursing home care (Lopez et al., 2009). There is no convincing evidence, though, that these agents can prevent, halt or reverse the disease.
Targeting Aβ
Based on groundbreaking discoveries made during the last two decades, several therapeutics have been developed to decrease the production or enhance the clearance of Aβ. Drugs in the former category are designed to inhibit β- or γ-secretase, the enzymes that release Aβ from its precursor (Citron, 2010; De Strooper et al., 2010; Golde et al., 2011). Although the identity of these enzymes has been known for a long time, it has been difficult to develop drugs that penetrate the BBB and specifically inhibit the cleavage of APP without affecting the cleavage of alternative substrates such as Notch and voltage-gated sodium channel subunits (Citron, 2010; De Strooper et al., 2010; Golde et al., 2011). A phase III clinical trial of a γ-secretase inhibitor was recently stopped because of side effects that included worsening of cognitive impairments (Schor, 2011). Another category of drugs modulates γ-secretase cleavage of APP, decreasing the production of Aβ42/43 in favor of shorter Aβ species that may be less toxic than the longer species (Golde et al., 2011; Karran et al., 2011). However, it has not yet been rigorously excluded that the shorter Aβ species also contribute to neuronal dysfunction in vivo, a possibility that could limit the efficacy and safety of γ-secretase modulators. The γ-secretase modulator tarenflurbil was ineffective in a phase III trial (Green et al., 2009). Ongoing trials will assess whether more selective, Notch-sparing γ-secretase inhibitors or β-secretase (BACE1) inhibitors are more efficacious and safe.
Efforts to lower Aβ levels through enhanced clearance emerged from the demonstration that active immunization against Aβ cleared amyloid plaques in hAPP transgenic mice (Schenk et al., 1999). An active immunization trial in humans had to be stopped because of immunopathological side effects (Gilman et al., 2005). Passive immunization by repeated infusions of humanized monoclonal anti-Aβ antibodies in a phase II trial was better tolerated, although amyloid-related imaging abnormalities, including vasogenic edema and microhemorrhages, were noted on MRI scans in a proportion of patients (Salloway et al., 2009; Sperling et al., 2011b). This trial was subsequently extended to a phase III trial with decreased doses of the antibody in apoE4 carriers, who were particularly susceptible to the above complications, and results are expected to become available later this year. Additional investigational anti-Aβ therapeutics include drugs that aim to prevent the formation of pathogenic Aβ assemblies, such as scyllo-inositol, which showed no efficacy in a phase II trial (Salloway et al., 2011), and PBT2, which showed preliminary promise in a small phase IIa trial (Lannfelt et al., 2008).
An alternative or complementary approach to those described above may be to make the brain more resistant to whatever Aβ cannot be removed effectively and safely (Figure 2A, 2C, 2D). Several experimental strategies have been identified that prevent or reverse hAPP/Aβ-dependent neuronal and cognitive impairments in transgenic mice, including reductions of tau (Morris et al., 2011), group IVA phospholipase A2 (Sanchez-Mejia et al., 2008), cyclophilin D (Du et al., 2008), or Fyn (Chin et al., 2004); reversal of EphB2 or Nav1.1 depletions (Cisse et al., 2011; Verret et al.); and replacement of apoE4 with apoE3 (Buttini et al., 2002; Raber et al., 2000). More research is needed to further assess the therapeutic potential and safety of these strategies.
Targeting Tau
Although far fewer drug trials have focused on tau, interest in tau-related therapeutics has been growing steadily in recent years, in part, because of the difficulties encountered with anti-Aβ strategies (Brunden et al., 2009; Golde et al., 2011; Morris et al., 2011). In human AD patients, the phenothiazine methylene blue showed some promise for slowing disease progression in a phase II clinical trial conducted for one year (Gura, 2008). Methylene blue was originally thought to inhibit tau–tau interactions, but it may also reduce soluble tau through other mechanisms as it is known to have many targets (Schirmer et al.). Phase III trials with a newer formulation of methylene blue (LMTX) are planned.
Because it is uncertain which tau assembly or conformation is responsible for tau-dependent neuronal dysfunction and degeneration, it is equally uncertain whether the abundance of this structure is reduced by any of the available tau aggregation blockers. In fact, some tau aggregation inhibitors enhance the formation of potentially toxic tau oligomers (Taniguchi et al., 2005). This scenario is reminiscent of the current state of anti-Aβ treatments, where it is also unclear whether any of the anti-Aβ strategies that have undergone or are currently in clinical trials significantly reduce the abundance of the most pathogenic soluble Aβ assemblies in human brain tissues, whatever those may be (Benilova et al.).
Because several lines of evidence suggest that abnormal phosphorylation contributes to the pathogenicity of tau (Hoover et al., 2010; Morris et al., 2011; Zempel et al., 2010), tau kinases continue to be pursued as potential drug targets for AD, including GSK-3β, CDK5, MARK, and MAPK. However, all of these kinases have numerous other substrates besides tau, raising concerns about the safety of respective inhibitors, and so far no tau kinase inhibitor appears to have advanced to a late-phase clinical trial for AD.
A potential alternative to modulating tau phosphorylation is reducing overall tau levels. In mice, 50% reduction of endogenous tau is well tolerated, increases resistance to chemically induced seizures, and markedly reduces Aβ- and apoE4 fragment-induced neuronal and cognitive impairments in vivo (Morris et al., 2011). Assuming ongoing experiments confirm that reduction of overall tau levels is efficacious and safe also when initiated in adult and old animals with AD-related pathologies, it may be worthwhile to reduce tau levels by targeting tau itself or molecules that regulate its expression or clearance. Some relevant molecules have already been identified. For example, dyhydropyridines lower tau levels in cell cultures (Evans et al., 2011).
Tau is thought to be degraded via the ubiquitin-proteasome and lysosomal pathways. The ubiquitin ligase for tau has been identified as the C-terminus of HSP70-interacting protein (CHIP). Reduction of CHIP levels increased the accumulation of tau aggregates in human tau transgenic mice, and CHIP levels are reduced in AD brains (Sahara et al., 2005). Furthermore, as its name suggests, CHIP works in combination with heat shock proteins to regulate tau degradation (Dickey et al., 2007); levels of heat shock protein 90 (Hsp90) correlate inversely with the levels of soluble tau and tau oligomers (Sahara et al., 2007).
In AD brains, tau is hyperacetylated, which increases its half-life (Min et al., 2010), alters its microtubule binding and enhances aggregation (Cohen et al., 2011). Because both acetylation and ubiquitination target lysine residues, acetylation of tau by the acetyl transferase p300 inhibits ubiquitination and stabilizes tau (Min et al., 2010). In addition, acetylation of the 6 amino acid motif VQIINK (PHF6*) inhibits the binding of tau to microtubules and enhances tau aggregation (Cohen et al., 2011). This motif is critical for the formation of tau oligomers and filaments (Morris et al., 2011). Thus, the combination of inhibiting tau acetylation and enhancing proteasome function might synergize to lower the level of pathogenic tau species.
Tau degradation can also be enhanced by immune-mediated mechanisms. Active immunization targeting phosphorylated tau reduced filamentous tau inclusions and neuronal dysfunction in tau transgenic mice (Asuni et al., 2007). The mechanism by which intracellular proteins, including tau and α-synuclein, are cleared by immunization is not known but may involve lysosomal degradation (Masliah et al., 2005; Sigurdsson, 2009). Once antibodies enter the brain, they could be taken up by receptor-mediated endocytosis and activate autophagy (Sigurdsson, 2009) or interact with tau or α-synuclein in the extracellular milieu.
Microtubule disruption has been observed in several models of AD and FTLD, including tau transgenic mice and wildtype neuronal cultures exposed to Aβ oligomers (Brunden et al., 2010; Morris et al., 2011). Microtubule stabilizers have shown promise in preclinical and clinical trials for AD. For example, paclitaxel prevented Aβ-induced toxicity in cell culture (Zempel et al., 2010) as well as axonal transport deficits and motor impairments in tau transgenic mice (Zhang et al., 2005). Epothilone D, which has better BBB permeability, improves microtubule density and cognition in tau transgenic mice (Brunden et al., 2010). The peptide NAP stabilizes microtubules and reduces tau hyperphosphorylation (Vulih–Shultzman et al., 2007), suggesting that microtubule-stabilizing compounds can have more than one mechanism of action. NAP can be administered intranasally and showed some promise in a phase II clinical trial (Gozes et al., 2009).
Targeting ApoE4
Targeting Aβ-dependent effects of apoE4
Approaches targeting Aβ-dependent effects of apoE4 have focused on modifying apoE levels or its Aβ binding property to enhance Aβ clearance or decrease Aβ deposition (Kim et al., 2009). Studies in humans and transgenic mice show that brain Aβ levels and amyloid plaque formation are apoE isoform-dependent (apoE4 > apoE3 > apoE2) (Castellano et al., 2011; Kim et al., 2009). Brain apoE levels are also isoform-dependent, but in the opposite direction (apoE4 < apoE3 < apoE2) (Bales et al., 2009; Riddell et al., 2008). The apoE4 allele was deemed hypomorphic because apoE4 levels in plasma and brain tend to be lower than those of apoE3, which might reduce apoE4’s capacity for Aβ clearance. Based on this hypothesis, it has been proposed to increase apoE expression in brain in the hope that this would stimulate Aβ clearance and reduce Aβ levels (Bales et al., 2009; Riddell et al., 2008). However, recent studies directly tested this hypothesis in mice and reported that increasing expression levels of apoE3 or apoE4 in mutant hAPP or hAPP/PS1 transgenic mice actually increased amyloid deposition in their brains (Bien-Ly et al.; Kim et al., 2011). Thus, in contrast to the original hypothesis, this finding suggests that reducing, rather than increasing, apoE expression could be a promising approach to lowering brain Aβ levels and decreasing plaque loads. Studies are needed to further test this alternative hypothesis.
Other efforts to attenuate apoE4’s ability to promote Aβ deposition have focused on blocking the interaction between apoE and Aβ (Sadowski et al., 2006; Yang et al., 2011b). For example, the BBB-permeable synthetic peptide Aβ12-28P, which is homologous to the binding site of apoE on Aβ but bears a strategic single amino acid substitution, reduces Aβ toxicity in cell culture and amyloid deposition in hAPP transgenic mice (Sadowski et al., 2006; Yang et al., 2011b). However, these findings were obtained in mice that express murine rather than human apoE. The effect of murine apoE on Aβ metabolism clearly differs from that of human apoE in that mouse apoE promotes Aβ deposition, whereas human apoE promotes Aβ clearance (Bien-Ly et al., 2011; Holtzman et al., 1999). Therefore, it is conceivable that blocking the interaction between Aβ and human apoE at young ages might promote amyloid deposition.
Targeting Aβ-independent effects of apoE4
Several approaches have been proposed to target Aβ-independent effects of apoE4 (Figure 3C). One approach is to identify small molecule structure correctors capable of disrupting apoE4 domain interaction (Huang, 2006, 2010; Mahley et al., 2006). Since domain interaction is responsible for multiple detrimental effects of apoE4 (Brodbeck et al., 2011; Chen et al.; Huang, 2006, 2010; Mahley et al., 2006), blocking it should abolish or attenuate those detrimental effects. Towards this goal, small molecules with high potency have been identified by structure-assisted in-silico pharmacophore modelling, rational drug design and high-throughput screening. These compounds abolish apoE4 domain interaction in neuronal cultures, improve apoE4 intracellular trafficking, and prevent the detrimental effects of apoE4 on neurite outgrowth, mitchondrial motility and function, and Aβ production in cultured neuronal cells (Brodbeck et al., 2011; Chen et al.; Ye et al., 2005). Another approach is to identify the protease that cleaves apoE4 and generates neurotoxic fragments and to develop specific protease inhibitors (Huang, 2006, 2010; Mahley et al., 2006). Alternatively, humanized monoclonal antibodies specific for the neurotoxic apoE fragments could be used to clear the fragments or to block their detrimental actions. An additional approach is to protect mitochondria and the cytoskeleton from attack by the toxic apoE fragments (Huang, 2006; Mahley et al., 2006). Reducing apoE4 expression in neurons should also reduce the level of toxic apoE fragments and their downstream effects (Huang, 2006). Finally, synthetic apoE mimetic peptides have been shown to be neuroprotective in mouse models of acute brain injury and in a Drosophila model overexpressing hAPP (Laskowitz et al., 2007; Sarantseva et al., 2009). Although these approaches targeting Aβ-independent effects of apoE4 are promising, they are all at an early stage of development and need to be evaluated in animal models and, ultimately, in clinical trials to further assess their therapeutic potential.
Other Therapeutic Approaches
Because there is plenty of evidence for oxidative damage, inflammation and mitochondrial impairments in AD (Bezprozvanny, 2010; Galimberti and Scarpini, 2011; Perry et al., 2010), several attempts have been made to slow disease progression with antioxidants (Lee et al., 2010a), anti-inflammatory drugs (Cole and Frautschy, 2010) or putative mitochondrial protectors (Bezprozvanny, 2010). However, based on our interpretation of the literature, no effective treatment has emerged from these efforts so far. Hormone therapy has been similarly disappointing (Shumaker et al., 2003). A number of interesting experimental strategies are currently under investigation that target synaptic or network dysfunction (Koh et al., 2010; Palop and Mucke, 2010; Verret et al.), modulators of aging (Cohen et al., 2009; Gan and Mucke, 2008), or autophagy (Harris and Rubinsztein). Time will tell whether these or other new strategies can be developed into more effective therapeutics for AD.
LESSONS LEARNED AND NEW DEVELOPMENTS
The Basics
For many of the clinical trials that have been carried out on AD patients, there is no clear evidence that the treatment actually had the desired effect on the intended target within AD-vulnerable brain regions. Some of the drugs that were tested in advanced clinical trials may not even effectively penetrate the BBB. Furthermore, the extent of the required target modulation is often uncertain, simply because the pertinent pathobiology has not yet been worked out in adequate detail. For example, how much of a secretase inhibitor or an anti-Aβ antibody does it take to lower functionally relevant Aβ assemblies in the hippocampus and entorhinal cortex to a level where they can no longer impair synaptic functions? And what happens to alternate substrates or potentially cross-reactive epitopes at these doses? Do apoE isoforms differentially affect the actions or efficacies of the tested drugs? What exactly is it that anti-inflammatory drugs should accomplish in the brain to counteract AD and what would be a good outcome measure to ensure this goal was achieved? If enrichment of tau in dendritic spines is critical for its pathogenic effects, do tau-related drugs actually modulate tau in this subcellular compartment? There are myriad questions like these that have not yet been answered and that will likely have to be addressed before effective AD therapies can be developed. Indeed, there is a dire need for scientists to build a more solid knowledge base for AD and other neurodegenerative disorders to enable truly rational design and assessment of new therapeutics.
Too Little, Too Late
It may be possible to learn valuable lessons from other chronic diseases that become manifest primarily in older people, in particular cardiovascular disease. For example, effective management of hypertension often requires the combination of drugs that have different modes of action, such as diuretics, β-blockers and ACE inhibitors. Might cognitive impairments in AD require a similar combination of different drugs, e.g., aimed at Aβ, tau, and apoE4? If multiple risk factors are present at young age, the prevention of strokes, heart attacks and hypertensive cardiomyopathy often requires initiation of a multipronged approach many years, even decades, before these complications typically occur (stop smoking, lose weight, lower blood pressure and cholesterol, etc.). However, once these complications have occurred, they respond poorly or not at all to such interventions.
AD researchers increasingly view the cognitive failure that becomes evident at the clinical onset of AD as the late-stage complication of a process that has progressed silently for many years (Golde et al., 2011). They further suspect that risk factors for AD might be detectable decades before the first impairments are identified on cognitive testing. This line of thinking and new data supporting it have recently led to a substantial revision of the diagnostic research criteria for AD (Jack et al., 2011). The new criteria assume that clinical manifestations of AD are preceded by a long prodromal phase and place much greater emphasis on biomarkers such as MRI scans, PIB scans, and measurements of Aβ and tau in the cerebrospinal fluid. Promising efforts are underway to identify more sensitive and specific biomarkers that might allow for the earlier identification of people at risk for developing the disease and for evaluating the efficacy of new treatments in clinical trials (Hampel et al., 2010; Reddy et al., 2011). However, at this point we do not yet have the AD equivalents of blood pressure or cholesterol measurements, which have been so helpful in preventing cardiovascular disease.
To our knowledge, all previous and ongoing clinical trials for AD were initiated in symptomatic patients, whose disease may have already progressed to a point where treatments with preventive potential have become ineffective. The cohorts of patients that are being assembled in the dominantly inherited Alzheimer’s network (DIAN) (Bateman et al., 2011) and the Alzheimer’s Prevention Initiative (API) (Reiman et al., 2011) should change this situation, as they should allow for the design of carefully timed preventive trials in carriers of mutations that predictably cause early-onset AD within relatively narrow age ranges.
The increasing emphasis on prevention raises the intriguing question of whether it is at all possible to halt or reverse AD once cognitive impairments have emerged. While many AD researchers are increasingly skeptical about this possibility, the authors have a more optimistic perspective, primarily because of the extraordinary plasticity of the nervous system, which far exceeds that of other organs. We think that a cocktail of drugs that block not just one, but most root causes of the disease could have beneficial effects even in AD patients with cognitive deficits, especially if combined with drugs that optimize network activity and promote neural plasticity and repair. Combination treatment has certainly made a world of difference in the treatment of other challenging conditions such as cancer and AIDS. Notably, these biomedical success stories include major improvements in cognitive functions in people with well established HIV-associated dementia following combination antiretroviral therapy (Cysique and Brew, 2009).
Safety First?
However, most physicians and relatives taking care of elderly people have learned painfully that this fragile patient population is prone to treatment failures and side effects. Reasons include aging-related changes in metabolism and the accrual of comorbidities requiring multiple drugs that can interact unfavorably with new ones added to the mix. Evaluating the safety and efficacy of combination treatments on this complex background is a daunting task. The concept of early preventive interventions (see above) further raises the safety bar for AD drugs, as it implies treating large numbers of otherwise healthy people for prolonged periods of time. But how much risk is unacceptable if losing one’s mind is the alternative? Because the answer to this question varies widely among different people, regulatory agencies need to strike the right balance between protecting the public from harm and unduly restricting progress or freedom of choice. Advances in systems biology, the emergence of personalized medicine, and use of cells differentiated from patient-specific induced pluripotent stem (iPS) cells for preclinical drug tests (Bousquet et al., 2011; Brennand et al., 2011; Israel et al., 2012) will likely facilitate this challenging task in the future, as these promising developments could improve our ability to preselect those subjects for clinical trials that have the highest chance of benefiting from specific treatments and the least chance of suffering ill effects.
Heterogeneity of Patient Populations and Modifier Genes
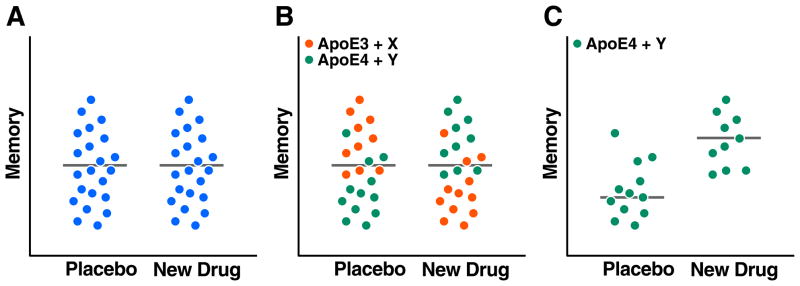
As described above, AD is a complex neurodegenerative disorder that is likely caused by interactions among multiple genetic, epigenetic, and environmental factors and pathways. This multifactoriality contributes to the heterogeneity of patient populations and makes it difficult to test drugs in clinical trials without pre-selecting appropriate patient groups and matching them up with the most suitable drugs (Figure 5). For example, are autosomal dominant early-onset AD and sporadic late-onset AD the same disease? Does AD presenting with amnestic versus executive deficits have the same or distinct etiologies? Firm answers to these questions are currently lacking but could have an important impact on the efficacy of drugs related to Aβ, apoE4 or other pathogens (Figure 5). The impact of apoE4 on AD risk is also affected by ethnicity (Farrer et al., 1997), introducing another layer of complexity. Therefore, beyond the identification of reliable early biomarkers for AD overall (see above), there is a need to define early biomarkers for potential AD subtypes.
Figure 5. Impact of Patient Heterogeneity and Modifier Genes on Drug Trials.
Hypothetical data clouds with dots representing memory measures obtained in individual human subjects. (A) Without better preselection of patients, potentially effective new drugs may show no efficacy in clinical trials because of the heterogeneity of the patient population tested. (B, C) Stratification of patient populations based on measurements of biomarkers and identification of specific genes could help match up subpopulations with the most suitable drug and significantly increase the chances of identifying better treatments for AD and related disorders.
Another important objective is to determine why some individuals are able to “escape” from the functional impact of the disease even when they have strong risk factors such as apoE4 or extensive AD pathology. Among the first factors to emerge in this quest is reelin, an extracellular matrix glycoprotein. It promotes synaptic plasticity, is depleted in vulnerable neuronal populations in AD patients and in hAPP mice, and can counteract Aβ-induced synaptic dysfunction (Chin et al., 2007; Durakoglugil et al., 2009). A recent GWAS identified variants of the reelin-encoding RELN gene that are associated with normal cognitive function in elderly people with substantial AD-like pathology (Kramer et al., 2011). Ongoing studies using whole genome sequencing and other systems biology approaches will likely reveal additional factors that prevent AD in some people against all odds and that, if effectively harnessed, may be able to prevent the conditions also in others.
Values and Limitations of Experimental Models
Evolutionary pressures do not allow for much slack and have preserved many processes and mechanisms across diverse species. However, there also are some striking differences among species that must be born in mind when trying to extrapolate from one to the other (Miller et al., 2010). Are there good experimental models for AD? The answer to this question depends entirely on what one expects from models. If one looks for models that simulate all aspects of the human condition and allow for reliable predictions of drug efficacy and safety in clinical trials, most would probably arrive at the conclusion that no good AD models exist. In contrast, if one looks for models that can help dissect the complexity of the human condition and provide useful information on the pathogenic impact and underlying mechanisms of specific factors, there are plenty.
Although many different types of models can be informative, including the most reductionist, it is critical to confirm findings across models and, ultimately, in the human condition. It is equally important to swiftly adjust working hypotheses based on new information gained in this process. For example, Aβ promotes the aggregation of α-synuclein not only in cell-free conditions, but also in neuronal cell cultures and transgenic mouse models (Masliah et al., 2001). In contrast, although tau effectively stabilizes microtubules in cell-free conditions, it does not appear to be required for this purpose in cell culture or adult mouse brains, calling into question the dogma that the main function of tau is to stabilize microtubules (Morris et al., 2011).
Advances in iPS cell technologies and related approaches now allow for the generation of human brain cells from patient-derived fibroblasts or blood cells and have already allowed for the identification of phenotypes with potential relevance to schizophrenia (Brennand et al., 2011) and AD (Israel et al., 2012). These exciting developments have renewed interest in cell culture models. Even conventional cell culture models have proved very useful in AD research. For example, they have taught us much about APP processing, Aβ production and differential effects of apoE isoforms, and many of the findings obtained in these models have held up well in animal models and humans (Bertram et al., 2010; Citron, 2010; De Strooper et al., 2010; Golde et al., 2011; Huang, 2006, 2010; Kim et al., 2009; Mahley et al., 2006; Morris et al., 2011; Palop and Mucke, 2010). Nevertheless, some caveats deserve to be mentioned. While dispersed cultures are well suited for studying cell-autonomous processes, they disrupt the intricate relationships that exist among diverse cell types in the brain and can drastically alter the activity of neurons and glia and their responses to pathogens. In addition, it remains to be determined how best to simulate brain aging in these models, which has such an important impact on the development of AD and other neurodegenerative disorders.
These aspects of the human condition are more easily simulated in animal models. Mice or rats genetically engineered to express human APP/Aβ, tau, apoE isoforms, α-synuclein or other AD-related factors in brain cells, either individually or in combination, have been particularly informative. Some of the insights provided by these models were highlighted in the text above and many others were reviewed in (Ashe and Zahs, 2010; Huang, 2006, 2010; Ittner and Gotz, 2011; Jucker, 2010; Kim et al., 2009; Mahley et al., 2006; Marchetti and Marie, 2011; Morris et al., 2011; Palop and Mucke, 2010; Querfurth and LaFerla, 2010). The following selection highlights but a few examples.
Neuronal expression of hAPP carrying mutations that cause autosomal dominant AD causes a range of AD-like abnormalities in transgenic mice, including pathologically elevated levels of Aβ in brain, impairments of learning and memory, behavioral alterations, synaptic deficits, aberrant network activity, alterations in neuronal activity-dependent proteins, amyloid plaques, neuritic dystrophy, vasculopathy, astrocytosis and microgliosis. Notably, several unexpected molecular alterations identified in hAPP transgenic mice have been validated in the human condition, including depletion of voltage-gated sodium channel subunits in cortical interneurons (Verret et al.), depletions of calbindin and EphB2 in dentate granule cells (Cisse et al., 2011; Palop et al., 2003), and hippocampal increases in metenkephalin, activated group IVA phospholipase A2, and collagen VI (Cheng et al., 2009; Meilandt et al., 2008; Sanchez-Mejia et al., 2008).
In spite of these striking similarities, several concerns have been raised about the relevance of these models to the human condition, in part because hAPP and Aβ are overexpressed in the mice but probably not in most cases of AD. However, overexpression of hAPP and Aβ also occurs in humans with APP gene duplications, and this overexpression causes syndromes that closely resemble sporadic AD (Rovelet-Lecrux et al., 2006). Another concern raised about hAPP mice is that most lines show little, if any, neuronal loss (Ashe and Zahs, 2010; Karran et al., 2011), a clear difference from the human condition. This discrepancy might represent a true species difference but might also suggest that Aβ simply does not kill neurons in vivo or that it requires longer to do so than the usual lifetime of a mouse.
It has also been pointed out that none of the treatments that have shown benefits in hAPP mice have so far shown benefits in the human condition (Karran et al., 2011). As outlined above, there are numerous reasons for drug failure in humans with AD that have nothing to do with species barriers. It is also true that the design of many preclinical trials is not rigorous enough and sensible recommendations to rectify this problem have been made (Jucker, 2010; Shineman et al., 2011). It should further be noted in this context that most transgenic models aim to evaluate specific pathogens in isolation, which is one of their strengths but could clearly be a weakness in the assessment of drugs for AD. Targeting a single factor in a multifactorial pathogenic cascade may well result in detectable benefits when this factor is isolated in an experimental model, but not when it is accompanied by other co-pathogens in the complex human condition.
Several reasons might account for why cognitive deficits can be detected in hAPP mice before plaque formation and in humans presumably only after plaque formation, including differences in the sensitivity of cognitive tests used, in the ability of mice and humans to compensate for hippocampal deficits, and in numerous variables that affect the deposition of Aβ into amyloid plaques or the formation of Aβ oligomers. Formation of plaques and Aβ oligomers can be reliably dissociated only in experimental models, where functional deficits have been shown to be plaque-independent (Palop et al., 2006; Palop and Mucke, 2010; Tomiyama et al., 2010).
Another concern is that the effect of murine apoE on Aβ metabolism clearly differs from human apoE, particularly with respect to Aβ deposition, which is more prominent in hAPP transgenic mice expressing murine apoE than in those expressing human apoE isoforms (Bien-Ly et al., 2011; Holtzman et al., 2000). Compared with mice lacking apoE, both human apoE3 and apoE4 stimulate Aβ clearance in mice, whereas murine apoE stimulates Aβ deposition (Bien-Ly et al., 2011; Holtzman et al., 2000). This difference has significant implications for understanding the effect of apoE on Aβ metabolism and for validating and interpreting clinical trials of anti-Aβ therapy. The great majority of preclinical trials related to Aβ were performed in hAPP mice expressing endogenous murine apoE (Zahs and Ashe, 2010). If murine apoE differs from human apoE in regulating Aβ metabolism, drugs that work well in hAPP mice with murine apoE might not work well in AD patients with human apoE. hAPP mice expressing different forms of human apoE may have greater predictive value.
CONCLUSIONS AND OUTLOOK
There often are no simple solutions to complex problems and AD is sadly a good case in point. Although progress in diverse disciplines of science, technology, and medicine has helped unravel many important aspects of this mysterious illness, the field has yet to translate these insights into more effective treatments for AD. We humbly submit the following suggestions as to how this process might be accelerated. Premature clinical trials should be avoided and scientific efforts intensified to broaden and deepen the understanding of AD pathobiology. More effective links should be established between basic scientists and clinical investigators and between academia and the pharmaceutical industry to expedite the discovery and validation of potential drug targets and the development of new therapeutics. Investigative and drug development efforts should be diversified to fully address the multifactoriality of the disease. It is likely that combination therapies with drugs targeting different causal or modifying factors (e.g. Aβ, tau, and apoE4) will be most effective. Efforts to discover better biomarkers should be continued and integrated with more innovative approaches to clinical trial design. The extraordinary potential of systems biology, personalized medicine, stem cell technology and cell reprogramming should be brought to bear on research and drug development for AD and related conditions. To benefit from these steps before the predicted rise in AD cases overwhelms health care systems around the world, they would have to be implemented expeditiously and on a broad scale. Concerted global efforts and substantial resources will be required to make this happen.
Acknowledgments
This work was supported by National Institutes of Health Grant AG022074 and a gift from the Stephen D. Bechtel, Jr. Foundation. We thank the authors’ lab members for many stimulating discussions of these topics, John Carroll for preparation of graphics, Gary Howard and Anna Lisa Lucido for editorial review, and Monica Dela Cruz for administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe KH, Zahs KR. Probing the biology of Alzheimer’s disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Hansen JC, Sullivan PM, Paul SM. Human APOE isoform-dependent effects on brain-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29:6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, et al. Apolipoprotein E is essential for amyloid deposition in the APPV717F transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C, Lee VMY, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Bareggi SR, Franceschi M, Bonini L, Zecca L, Smirne S. Decreased CSF concentrations of homovanillic acid and γ-aminobutyric acid in Alzheimer’s disease. Age- or disease-related modifications? Arch Neurol. 1982;39:709–712. doi: 10.1001/archneur.1982.00510230035010. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, Salloway S, Sperling RA, Windisch M, Xiong C. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic A[beta] oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat Neurosci. doi: 10.1038/nn.3028. Advance online publication, In press. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, Corrada MM, Head E, Kawas CH. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72:829–834. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: Back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. The rise and fall of Dimebon. Drug News Perspect. 2010;23:518–523. doi: 10.1358/dnp.2010.23.8.1500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Wang C, Huang Y. C-terminal-truncated apolipoprotein (apo) E4 inefficiently clears amyloid-β (Aβ) and acts in concert with Aβ to elicit neuronal and behavioral deficits in mice. Proc Natl Acad Sci USA. 2011;108:4236–4241. doi: 10.1073/pnas.1018381108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien-Ly N, Gillespie AK, Walker D, Yoon SY, Huang Y. Reducing human apolipoprotein E levels attenuates age-dependent Aβ accumulation in mutant human amyloid precursor protein transgenic mice. J Neurosci. doi: 10.1523/JNEUROSCI.0033-12.2012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Alzheimer’s pathogenesis: Is there neuron-to-neuron propagation? Acta Neuropathol. 2011;121:589–595. doi: 10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Wyss-Coray T, Buttini M, Mucke L, Mahley RW, et al. Neuron-specific apolipoprotein E4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck J, McGuire J, Liu Z, Meyer-Franke A, Balestra ME, Jeong DE, Pleiss M, McComas C, Hess F, Witter D, et al. Structure-dependent impairment of intracellular apolipoprotein E4 trafficking and its detrimental effects are rescued by small-molecule structure correctors. J Biol Chem. 2011;286:17217–17226. doi: 10.1074/jbc.M110.217380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers N, Sleegers K, Engelborghs S, Bogaerts V, Serneels S, Kamali K, Corsmit E, De Leenheir E, Martin JJ, De Deyn PP, et al. Genetic risk and transcriptional variability of amyloid precursor protein in Alzheimer’s disease. Brain. 2006;129:2984–2991. doi: 10.1093/brain/awl212. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for Alzheimer’s disease and related tauopathies. Nat Rev Drug Discov. 2009;8:783–793. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, Iba M, James MJ, Xie SX, Ballatore C, et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci. 2010;30:13861–13866. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: Pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Masliah E, Yu GQ, Palop JJ, Chang S, Bernardo A, Lin C, Wyss-Coray T, Huang Y, Mucke L. Cellular source of apolipoprotein E4 determines neuronal susceptibility to excitotoxic injury in transgenic mice. Am J Pathol. 2010;177:563–569. doi: 10.2353/ajpath.2010.090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, Mucke L, Mahley RW. Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: Isoform-specific effects on neurodegeneration. J Neurosci. 1999;19:4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid β peptides but not on plaque formation. J Neurosci. 2002;22:10539–10548. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camandola S, Mattson MP. Aberrant subcellular neuronal calcium regulation in aging and Alzheimer’s disease. Biochim Biophys Acta. 2011;1813:965–973. doi: 10.1016/j.bbamcr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, Thomas-Anterion C, Michon A, Martin C, Charbonnier F, et al. Early-onset autosomal dominant Alzheimer disease: Prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999;65:664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DE, Sabbagh MN, Ahern GL, Rapcsak SZ, Baxter LC, Yaari R, Woodruff BK, Hoffman-Snyder C, et al. Cerebrovascular risk factors and preclinical memory decline in healthy APOE epsilon4 homozygotes. Neurology. 2011;76:1078–1084. doi: 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, Demattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, et al. Human apoE isoforms differentially regulate brain amyloid-{beta} peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Ma TR, Miranda RD, Balestra ME, Mahley RW, Huang Y. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci USA. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HK, Liu Z, Meyer-Franke A, Brodbeck J, Miranda RD, McGuire JG, Pleiss MA, Ji ZS, Balestra ME, Walker DW, et al. Small-molecule structure correctors abolish detrimental effects of apolipoprotein E4 in cultured neurons. J Biol Chem. 2012;287:5253–5266. doi: 10.1074/jbc.M111.276162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci USA. 2010;107:12011–12016. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JS, Dubal DB, Kim DH, Legleiter J, Cheng IH, Yu GQ, Tesseur I, Wyss-Coray T, Bonaldo P, Mucke L. Collagen VI protects neurons against Aβ toxicity. Nat Neurosci. 2009;12:119–121. doi: 10.1038/nn.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Massaro C, Palop J, Thwin M, G-QY, Bien-Ly N, Bender A, Mucke L. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J Neurosci. 2007;27:2727–2733. doi: 10.1523/JNEUROSCI.3758-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Yu GQ, Kojima N, Masliah E, Mucke L. Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J Neurosci. 2004;24:4692–4697. doi: 10.1523/JNEUROSCI.0277-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouliaras L, Rutten BP, Kenis G, Peerbooms O, Visser PJ, Verhey F, van Os J, Steinbusch HW, van den Hove DL. Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Prog Neurobiol. 2010;90:498–510. doi: 10.1016/j.pneurobio.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Cisse M, Halabisky B, Harris JA, Devidze N, Dubal D, Lotz G, Kim DH, Hamto T, Ho K, Yu GQ, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M. Alzheimer’s disease: Strategies for disease modification. Nat Rev Drug Discov. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, Lee VM. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GM, Frautschy SA. Mechanisms of action of non-steroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2010;9:140–148. doi: 10.2174/187152710791011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Drug therapy - Alzheimer’s disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: A review. Neuropsychol Rev. 2009;19:169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Jr, Cox NJ, Dunbar-Jacob JM, Granieri EC, Hunt G, McGarry K, et al. National Institutes of Health State-of-the-Science Conference statement: Preventing alzheimer disease and cognitive decline. Ann Intern Med. 2010;153:176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Shulman JM, Chibnik LB, Keenan BT, Raj T, Wilson RS, Yu L, Leurgans SE, Tran D, Aubin C, et al. A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2011.09.033. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Vassar R, Golde T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. ApoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker H, Lo KY, Unger SM, Ferreira ST, Silverman MA. Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J Neurosci. 2010;30:9166–9171. doi: 10.1523/JNEUROSCI.1074-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement. 2010;6:303–311. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, et al. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS, Xu B, Rebeck GW, et al. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29:15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durakoglugil MS, Chen Y, White CL, Kavalali ET, Herz J. Reelin signaling antagonizes beta-amyloid at the synapse. Proc Natl Acad Sci USA. 2009;106:15938–15943. doi: 10.1073/pnas.0908176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Jinwal UK, Makley LN, Dickey CA, Gestwicki JE. Identification of dihydropyridines that reduce cellular tau levels. Chem Commun (Camb) 2011;47:529–531. doi: 10.1039/c0cc02253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. J Am Med Assoc. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D, Scarpini E. Inflammation and oxidative damage in Alzheimer’s disease: Friend or foe? Front Biosci (Schol Ed) 2011;3:252–266. doi: 10.2741/s149. [DOI] [PubMed] [Google Scholar]
- Gan L, Mucke L. Paths of Convergence: Sirtuins in aging and neurdegeneration. Neuron. 2008;58:10–14. doi: 10.1016/j.neuron.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genin E, Hannequin D, Wallon D, Sleegers K, Hiltunen M, Combarros O, Bullido MJ, Engelborghs S, De Deyn P, Berr C, et al. APOE and Alzheimer disease: A major gene with semi-dominant inheritance. Mol Psychiatry. 2011;16:903–907. doi: 10.1038/mp.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal K, Vogt DL, Liang M, Shen Y, Lamb BT, Pimplikar SW. Alzheimer’s disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc Natl Acad Sci USA. 2009;106:18367–18372. doi: 10.1073/pnas.0907652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Haroutunian V, Zhang H, Park LCH, Shi Q, Lesser M, Mohs RC, Sheu RKF, Blass JP. Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Ann Neurol. 2000;48:297–303. [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, et al. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer’s disease: The need for a paradigm shift. Neuron. 2011;69:203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Stewart A, Morimoto B, Fox A, Sutherland K, Schmeche D. Addressing Alzheimer’s disease tangles: From NAP to AL-108. Curr Alzheimer Res. 2009;6:455–460. doi: 10.2174/156720509789207895. [DOI] [PubMed] [Google Scholar]
- Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: A randomized controlled trial. JAMA. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouselle D, Winsky-Sommerer R, David JP, Delacourte A, Dournaud P, Epelbaum J. Loss of somatostatin-like immunoreactivity in the frontal cortex of Alzheimer patients carrying the apolipoprotein epsilon 4 allele. Neurosci Lett. 1998;255:21–24. doi: 10.1016/s0304-3940(98)00698-3. [DOI] [PubMed] [Google Scholar]
- Gura T. Hope in Alzheimer’s fight emerges from unexpected places. Nat Med. 2008;14:894. doi: 10.1038/nm0908-894. [DOI] [PubMed] [Google Scholar]
- Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, Herholz K, Bokde AL, Jessen F, Hoessler YC, et al. Biomarkers for Alzheimer’s disease: Academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- Harris F, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, Fish JD, Masliah E, Hopkins PC, Scearce-Levie K, et al. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci USA. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris FM, Tesseur I, Brecht WJ, Xu Q, Mullendorff K, Chang S, Wyss-Coray T, Mahley RW, Huang Y. Astroglial regulation of apolipoprotein E expression in neuronal cells. Implications for Alzheimer’s disease. J Biol Chem. 2004;279:3862–3868. doi: 10.1074/jbc.M309475200. [DOI] [PubMed] [Google Scholar]
- Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol. doi: 10.1038/nrneurol.2011.200. In press. [DOI] [PubMed] [Google Scholar]
- Harris JA, Devidze N, Halabisky B, Lo I, Thwin MT, Yu GQ, Bredesen DE, Masliah E, Mucke L. Many neuronal and behavioral impairments in transgenic mouse models of Alzheimer’s disease are independent of caspase cleavage of the amyloid precursor protein. J Neurosci. 2010;30:372–381. doi: 10.1523/JNEUROSCI.5341-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM. Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: ApoE4 mice show profound working memory impairments in the absence of Alzheimer’s-like neuropathology. Exp Neurol. 2001;170:326–344. doi: 10.1006/exnr.2001.7715. [DOI] [PubMed] [Google Scholar]
- Herrup K. Reimagining Alzheimer’s disease--an age-based hypothesis. J Neurosci. 2010;30:16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, Fagan AM, Chang LK, Sun Y, Paul SM. Expression of human apolipoprotein E reduces amyloid-β deposition in a mouse model of Alzheimer’s disease. J Clin Invest. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci USA. 1995;92:9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Molecular and cellular mechanisms of apolipoprotein E4 neurotoxicity and potential therapeutic strategies. Curr Opin Drug Discov Devel. 2006;9:627–641. [PubMed] [Google Scholar]
- Huang Y. Abeta-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2010;16:287–294. doi: 10.1016/j.molmed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji ZS, Miranda RD, Newhouse YM, Weisgraber KH, Huang Y, Mahley RW. Apolipoprotein E4 potentiates amyloid b peptide-induced lysosomal leakage and apoptosis in neuronal cells. J Biol Chem. 2002;277:21821–21828. doi: 10.1074/jbc.M112109200. [DOI] [PubMed] [Google Scholar]
- Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, et al. Abeta(1–42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nat Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med. 2010;16:1210–1214. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jiang H, Park S, Eltorai AE, Stewart FR, Yoon H, Basak JM, Finn MB, Holtzman DM. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-beta amyloidosis. J Neurosci. 2011;31:18007–18012. doi: 10.1523/JNEUROSCI.3773-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jensen JR, Cisek K, Funk KE, Naphade S, Schafer K, Kuret J. Imaging as a strategy for premortem diagnosis and staging of tauopathies. Curr Alzheimer Res. 2010;7:230–234. doi: 10.2174/156720510791050894. [DOI] [PubMed] [Google Scholar]
- Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- Kramer PL, Xu H, Woltjer RL, Westaway SK, Clark D, Erten-Lyons D, Kaye JA, Welsh-Bohmer KA, Troncoso JC, Markesbery WR, et al. Alzheimer disease pathology in cognitively healthy elderly: A genome-wide study. Neurobiol Aging. 2011;32:2113–2122. doi: 10.1016/j.neurobiolaging.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, McKenna SE, Song P, Wang H, Durham L, Yeung N, Christensen D, Vitek MP. COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J Neurotrauma. 2007;24:1093–1107. doi: 10.1089/neu.2006.0192. [DOI] [PubMed] [Google Scholar]
- Lee HP, Zhu X, Casadesus G, Castellani RJ, Nunomura A, Smith MA, Lee HG, Perry G. Antioxidant approaches for the treatment of Alzheimer’s disease. Expert Rev Neurother. 2010a;10:1201–1208. doi: 10.1586/ern.10.74. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Moussa CE, Lee Y, Sung Y, Howell BW, Turner RS, Pak DT, Hoe HS. Beta amyloid-independent role of amyloid precursor protein in generation and maintenance of dendritic spines. Neuroscience. 2010b;169:344–356. doi: 10.1016/j.neuroscience.2010.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5:634–645. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue MW, Schu M, Vardarajan BN, Buros J, Green RC, Go RC, Griffith P, Obisesan TO, Shatz R, Borenstein A, et al. A comprehensive genetic association study of Alzheimer disease in African Americans. Arch Neurol. 2011;68:1569–1579. doi: 10.1001/archneurol.2011.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Wahed AS, Saxton J, Sweet RA, Wolk DA, Klunk W, Dekosky ST. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80:600–607. doi: 10.1136/jnnp.2008.158964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Marie H. Hippocampal synaptic plasticity in Alzheimer’s disease: What have we learned so far from transgenic models? Rev Neurosci. 2011;22:373–402. doi: 10.1515/RNS.2011.035. [DOI] [PubMed] [Google Scholar]
- Martin I, Dawson VL, Dawson TM. Recent advances in the genetics of Parkinson’s disease. Annu Rev Genomics Hum Genet. 2011;12:301–325. doi: 10.1146/annurev-genom-082410-101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses AD. Neurodegeneration in the central nervous system of apoE-deficient mice. Exp Neurol. 1995;136:107–122. doi: 10.1006/exnr.1995.1088. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, et al. Effects of a-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. β-Amyloid peptides enhance α-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98:12245–12250. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilandt WJ, Yu GQ, Chin J, Roberson ED, Palop JJ, Wu T, Scearce-Levie K, Mucke L. Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2008;28:5007–5017. doi: 10.1523/JNEUROSCI.0590-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan Sanchez M, Heyn SN, Das D, Moghadam S, Martin KJ, Salehi A. Neurobiological elements of cognitive dysfunction in Down Syndrome: Exploring the role of APP. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.08.016. In press. [DOI] [PubMed] [Google Scholar]
- Miller J, Arrasate M, Brooks E, Libeu CP, Legleiter J, Hatters D, Curtis J, Cheung K, Krishnan P, Mitra S, et al. Identifying polyglutamine protein species in situ that best predict neurodegeneration. Nat Chem Biol. 2011;7:925–934. doi: 10.1038/nchembio.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Horvath S, Geschwind DH. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proc Natl Acad Sci USA. 2010;107:12698–12703. doi: 10.1073/pnas.0914257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Yang DS. Autophagy failure in Alzheimer’s disease-locating the primary defect. Neurobiol Dis. 2011;43:38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Jones B, Kekonius L, Chin J, Yu GQ, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher VP, Farrer MJ, Kessling AM, Fisher EMC, West RJ, Barber PC, Butler AC. Molecular mapping of alzheimer-type dementia in Down’s syndrome. Ann Neurol. 1998;43:380–383. doi: 10.1002/ana.410430316. [DOI] [PubMed] [Google Scholar]
- Putcha D, Brickhouse M, O’Keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J Neurosci. 2011;31:17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Fa M, Staniszewski A, Hashimoto G, Aziz F, Sakurai M, Ribe EM, Troy CM, Mercken M, et al. Endogenous amyloid-beta is necessary for hippocampal synaptic plasticity and memory. Ann Neurol. 2011;69:819–830. doi: 10.1002/ana.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Raber J, LeFevour A, Buttini M, Mucke L. Androgens protect against Apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: Increased susceptibility of females. Proc Natl Acad Sci USA. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Yu GQ, Buttini M, Mahley RW, Pitas RE, Mucke L. Alzheimer’s disease: Apolipoprotein E and cognitive performance. Nature. 2000;404:352–354. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Wilson R, Wilson J, Connell S, Gocke A, Hynan L, German D, Kodadek T. Identification of candidate IgG biomarkers for Alzheimer’s disease via combinatorial library screening. Cell. 2011;144:132–142. doi: 10.1016/j.cell.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, Quiroz YT, Kosik KS, Lopera F, Tariot PN. Alzheimer’s Prevention Initiative: A plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26(Suppl 3):321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remes AM, Laru L, Tuominen H, Aalto S, Kemppainen N, Mononen H, Nagren K, Parkkola R, Rinne JO. Carbon 11-labeled pittsburgh compound B positron emission tomographic amyloid imaging in patients with APP locus duplication. Arch Neurol. 2008;65:540–544. doi: 10.1001/archneur.65.4.540. [DOI] [PubMed] [Google Scholar]
- Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66:739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, et al. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronicke R, Mikhaylova M, Ronicke S, Meinhardt J, Schroder UH, Fandrich M, Reiser G, Kreutz MR, Reymann KG. Early neuronal dysfunction by amyloid beta oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol Aging. 2011;32:2219–2228. doi: 10.1016/j.neurobiolaging.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Rosendorff C, Beeri MS, Silverman JM. Cardiovascular risk factors for Alzheimer’s disease. Am J Geriatr Cardiol. 2007;16:143–149. doi: 10.1111/j.1076-7460.2007.06696.x. [DOI] [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- Sadowski MJ, Pankiewicz J, Scholtzova H, Mehta PD, Prelli F, Quartermain D, Wisniewski T. Blocking the apolipoprotein E/amyloid-beta interaction as a potential therapeutic approach for Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:18787–18792. doi: 10.1073/pnas.0604011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara N, Maeda S, Yoshiike Y, Mizoroki T, Yamashita S, Murayama M, Park JM, Saito Y, Murayama S, Takashima A. Molecular chaperone-mediated tau protein metabolism counteracts the formation of granular tau oligomers in human brain. J Neurosci Res. 2007;85:3098–3108. doi: 10.1002/jnr.21417. [DOI] [PubMed] [Google Scholar]
- Sahara N, Murayama M, Mizoroki T, Urushitani M, Imai Y, Takahashi R, Murata S, Tanaka K, Takashima A. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J Neurochem. 2005;94:1254–1263. doi: 10.1111/j.1471-4159.2005.03272.x. [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Keren R, Porsteinsson AP, van Dyck CH, Tariot PN, Gilman S, Arnold D, Abushakra S, Hernandez C, et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011;77:1253–1262. doi: 10.1212/WNL.0b013e3182309fa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mejia RO, Newman JW, Toh S, G-Q Y, Zhou Y, Halabisky B, Cisse M, Scearce-Levie K, Cheng IH, Gan L, et al. Phospholipase A2 reduction ameliorates cognitve deficits in mouse model of Alzheimer’s disease. Nat Neurosci. 2008;11:1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantseva S, Timoshenko S, Bolshakova O, Karaseva E, Rodin D, Schwarzman AL, Vitek MP. Apolipoprotein E-mimetics inhibit neurodegeneration and restore cognitive functions in a transgenic Drosophila model of Alzheimer’s disease. PLoS One. 2009;4:e8191. doi: 10.1371/journal.pone.0008191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Richardson JC, Gentleman SM, Brooks DJ. Inflammatory risk factors and pathologies associated with Alzheimer’s disease. Curr Alzheimer Res. 2011;8:132–141. doi: 10.2174/156720511795256062. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schirmer RH, Adler H, Pickhardt M, Mandelkow E. Lest we forget you - methylene blue. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.12.012. In press. [DOI] [PubMed] [Google Scholar]
- Schor NF. What the halted phase III gamma-secretase inhibitor trial may (or may not) be telling us. Ann Neurol. 2011;69:237–239. doi: 10.1002/ana.22365. [DOI] [PubMed] [Google Scholar]
- Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25:289–304. doi: 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: An observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Sheng ZH, Cai Q. Mitochondrial transport in neurons: Impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. doi: 10.1038/nrn3156. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shineman DW, Basi GS, Bizon JL, Colton CA, Greenberg BD, Hollister BA, Lincecum J, Leblanc GG, Lee LB, Luo F, et al. Accelerating drug discovery for Alzheimer’s disease: Best practices for preclinical animal studies. Alzheimers Res Ther. 2011;3:28. doi: 10.1186/alzrt90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. J Am Med Assoc. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM. Tau-focused immunotherapy for Alzheimer’s disease and related tauopathies. Curr Alzheimer Res. 2009;6:446–450. doi: 10.2174/156720509789207930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, Schiapparelli L, Salazar-Colocho P, Cuadrado-Tejedor M, Escribano L, Lopez de Maturana R, Del Rio J, Perez-Mediavilla A, Frechilla D. Overexpression of wild-type human APP in mice causes cognitive deficits and pathological features unrelated to Abeta levels. Neurobiol Dis. 2009;33:369–378. doi: 10.1016/j.nbd.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DHS, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, et al. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Jack CR, Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011b;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Munch G. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol Aging. 2011;32:763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, Hasegawa M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J Biol Chem. 2005;280:7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- Tomiyama T, Matsuyama S, Iso H, Umeda T, Takuma H, Ohnishi K, Ishibashi K, Teraoka R, Sakama N, Yamashita T, et al. A mouse model of amyloid {beta} oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, Takuma H, Kuwano R, Imagawa M, Ataka S, et al. A new amyloid beta variant favoring oligomerization in Alzheimer’s-type dementia. Ann Neurol. 2008;63:377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: A review. Prog Brain Res. 2007;161:303–316. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- Vepsalainen S, Helisalmi S, Koivisto AM, Tapaninen T, Hiltunen M, Soininen H. Somatostatin genetic variants modify the risk for Alzheimer’s disease among Finnish patients. J Neurol. 2007;254:1504–1508. doi: 10.1007/s00415-007-0539-2. [DOI] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, et al. Key role of interneuronal impairments in Alzheimer’s disease-related neural network and cognitive dysfunction. Cell. doi: 10.1016/j.cell.2012.02.046. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Ataka S, Mizuno T, Brooks WS, Wada Y, Kondo M, Jones G, Watanabe Y, Mulligan R, Nakagawa M, et al. High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol. 2009;66:1537–1544. doi: 10.1001/archneurol.2009.285. [DOI] [PubMed] [Google Scholar]
- Vossel K, Bien-Ly N, Bernardo A, Rascovsky K, Karydas A, Rabinovici G, Sidhu M, Huang E, Miller B, Huang Y, et al. ApoE and TDP-43 neuropathology in two siblings with familial FTLD-motor neuron disease. Neurocase. doi: 10.1080/13554794.2012.667124. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau reduction prevents Aβ-induced impairments in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulih–Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z, Gozes I. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther. 2007;323:438–449. doi: 10.1124/jpet.107.129551. [DOI] [PubMed] [Google Scholar]
- Weyer SW, Klevanski M, Delekate A, Voikar V, Aydin D, Hick M, Filippov M, Drost N, Schaller KL, Saar M, et al. APP and APLP2 are essential at PNS and CNS synapses for transmission, spatial learning and LTP. EMBO J. 2011;30:2266–2280. doi: 10.1038/emboj.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimo A, Prince M. World Alzheimer Report 2010: The Global Economic Impact of Dementia. Alzheimer’s Disease International. 2010:1–56. [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Petralia RS, Kurushima H, Patel H, Jung MY, Volk L, Chowdhury S, Shepherd JD, Dehoff M, Li Y, et al. Arc/Arg3.1 regulates an endosomal pathway essential for activity-dependent beta-amyloid generation. Cell. 2011;147:615–628. doi: 10.1016/j.cell.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Walker D, Bernardo A, Brodbeck J, Balestra ME, Huang Y. Intron-3 retention/splicing controls neuronal expression of apolipoprotein E in the CNS. J Neurosci. 2008;28:1452–1459. doi: 10.1523/JNEUROSCI.3253-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Jia L, Jia J. Association between somatostatin gene polymorphisms and sporadic Alzheimer’s disease in Chinese population. Neurosci Lett. 2009;465:181–183. doi: 10.1016/j.neulet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson DW, Bandyopadhyay U, Jiang Y, et al. Therapeutic effects of remediating autophagy failure in a mouse model of Alzheimer disease by enhancing lysosomal proteolysis. Autophagy. 2011a;7:788–789. doi: 10.4161/auto.7.7.15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ji Y, Mehta P, Bates KA, Sun Y, Wisniewski T. Blocking the apolipoprotein E/amyloid-beta interaction reduces fibrillar vascular amyloid deposition and cerebral microhemorrhages in TgSwDI mice. J Alzheimers Dis. 2011b;24:269–285. doi: 10.3233/JAD-2011-101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang Z, Wang B, Justice NJ, Zheng H. Amyloid precursor protein regulates Cav1.2 L-type calcium channel levels and function to influence GABAergic short-term plasticity. J Neurosci. 2009;29:15660–15668. doi: 10.1523/JNEUROSCI.4104-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Huang Y, Mullendorff K, Dong L, Giedt G, Meng EC, Cohen FE, Kuntz ID, Weisgraber KH, Mahley RW. Apolipoprotein (apo) E4 enhances amyloid β peptide production in cultured neuronal cells: ApoE structure as a potential therapeutic target. Proc Natl Acad Sci USA. 2005;102:18700–18705. doi: 10.1073/pnas.0508693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Polepalli J, Wagh D, Rajadas J, Malenka R, Lu B. A critical role for the PAR-1/MARK-tau axis in mediating the toxic effects of Abeta on synapses and dendritic spines. Hum Mol Genet. doi: 10.1093/hmg/ddr576. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs KR, Ashe KH. ‘Too much good news’ - are Alzheimer mouse models trying to tell us how to prevent, not cure, Alzheimer’s disease? Trends Neurosci. 2010;33:381–389. doi: 10.1016/j.tins.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Zempel H, Thies E, Mandelkow E, Mandelkow EM. A{beta} Oligomers Cause Localized Ca2+ Elevation, Missorting of Endogenous Tau into Dendrites, Tau Phosphorylation, and Destruction of Microtubules and Spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, Lee EB, Xie SX, Joyce S, Li C, et al. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc Natl Acad Sci USA. 2005;102:227–231. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Weisgraber KH. Understanding the association of apolipoprotein E4 with Alzheimer disease: Clues from its structure. J Biol Chem. 2009;284:6027–6031. doi: 10.1074/jbc.R800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]