Abstract
FT All Reaction Monitoring (FT-ARM) is a novel approach for the identification and quantification of peptides that relies upon the selectivity of high mass accuracy data and the specificity of peptide fragmentation patterns. An FT-ARM experiment involves continuous, data-independent, high mass accuracy MS/MS acquisition spanning a defined m/z range. Custom software was developed to search peptides against the multiplexed fragmentation spectra by comparing theoretical or empirical fragment ions against every fragmentation spectrum across the entire acquisition. A dot product score is calculated against each spectrum in order to generate a score chromatogram used for both identification and quantification. Chromatographic elution profile characteristics are not used to cluster precursor peptide signals to their respective fragment ions. FT-ARM identifications are demonstrated to be complementary to conventional data-dependent shotgun analysis, especially in cases where the data-dependent method fails due to fragmenting multiple overlapping precursors. The sensitivity, robustness and specificity of FT-ARM quantification are shown to be analogous to selected reaction monitoring-based peptide quantification with the added benefit of minimal assay development. Thus, FT-ARM is demonstrated to be a novel and complementary data acquisition, identification, and quantification method for the large scale analysis of peptides.
Keywords: data independent acquisition, multiplexed fragmentation, accurate mass measurements, all reaction monitoring, quantification, high resolution product ion scan
Introduction
To date, the most popular method for peptide and protein identification using mass spectrometry is bottom-up or shotgun proteomics.1 Shotgun proteomics technology is comprised of the following basic steps: enzymatic digestion of the sample, separation of the resultant peptides (liquid chromatography, capillary electrophoresis, etc.), and MS/MS peptide sequencing. MS/MS analysis of peptides is commonly achieved with an approach called data-dependent acquisition (DDA). DDA works by identifying the most abundant peptides present in a precursor scan. In a serial fashion, the top n precursors identified in the precursor scan are subject to a product ion scan to obtain fragmentation patterns (MS/MS) for the peptides. Narrow-band isolation of each peptide is performed to enable the highest quality MS/MS spectrum acquisition to aid in the identification process. The data generated from a shotgun proteomics experiment is then analyzed by database searching algorithms.2, 3,4–6 Database searching algorithms are designed to provide the most probable peptide match based upon the theoretical information derived from known or predicted protein polypeptide sequences. In practice, proteome measurements using DDA mass spectrometry have yielded up to 60% observation of the proteins predicted from the genome.7 However, 60% proteome coverage is not routinely achievable. Many reasons have been suggested for the low proteome coverage including poor quality MS/MS spectra8, 9 and incorrect genome annotation.10, 11 There is also strong evidence that the primary reason for the low coverage is the DDA paradigm.12, 13 DDA methods have been shown to limit the dynamic range of the analysis14 leading to inadequate sampling of the proteome, even for relatively simple organisms. For example, in yeast nearly the entire proteome (98%) was quantified with tandem affinity purification-tag (TAP-tag) Western-blotting15, yet such coverage has not been achieved by DDA methods with yeast whole cell lysate protein digests. Nonetheless, the TAP-tag work of Ghaemmaghami et al. serves as a “roadmap” for mass spectrometry based proteomics as suggested by the authors. Picotti et al.16 used this roadmap to demonstrate that detection of proteins across the entire dynamic range of the yeast proteome is possible with targeted mass spectrometry. Both of these publications15, 16 suggest that DDA methods can be complemented by parallel measurement strategies and new technology.
Multiplexed fragmentation, or simultaneous isolation and fragmentation of multiple peptides, has been developed to increase peptide identification rates in many laboratories. Recent multiplexed approaches include MSE 17–19 and others.20–24 During the course of our preparation of this manuscript, All Ion Fragmentation (AIF) was reported by Geiger et al.25 AIF was designed specifically for use with the Orbitrap Exactive. In this technique and MSE, MS scans are alternated with fragmentation scans where a large mass range is subject to fragmentation and the subsequent product ions are measured. Formation of the parent-product ion relationships must be established for subsequent database search identification. These relationships are reformed through chromatographic elution profile correlation. The pseudo MS/MS patterns generated from co-eluting fragments are extracted and submitted for database searching as though the data had been acquired with traditional DDA mass spectrometry. Both techniques, AIF and MSE, have shown that parallel peptide fragmentation has benefits over serial peptide fragmentation measurements. In the case of AIF, the instrument utilized is a single stage mass spectrometer so typical DDA mass spectrometry or product ion scanning is not possible with this instrument, which prevents a direct comparison. Geiger et al. indicate that DDA mass spectrometry on a two-stage mass spectrometer still outperforms AIF.
Here we present a novel approach toward peptide identification and quantification in complex mixtures using a combination of high mass accuracy data-independent acquisition (DIA) and spectral correlation analysis. We call this technique FT-All Reaction Monitoring (FT-ARM). In short, a relatively wide m/z range is accumulated and all ions within this mass range are subject to activation (collision-induced dissociation (CID), infrared multiphoton dissociation (IRMPD), etc.). Mass analysis of the fragment ions is performed with high mass accuracy (<5 ppm) such as is afforded by Fourier Transform Ion Cyclotron Resonance (FTICR), Orbitrap, or modern quadrupole time-of-flight (qTOF) instruments. A database of theoretical or empirical peptide fragmentation patterns are used to calculate the dot-product score for each peptide against each DIA scan event of a DIA-LC-MS experiment. The result of this analysis is a score chromatogram for each peptide contained in the database. A reverse sequence decoy database is searched to estimate false discovery rate (FDR). The FT-ARM process is fundamentally different than other DIA approaches, including MSE and all ion fragmentation (AIF), in that chromatographic elution profile characteristics are not used to cluster precursor peptide signals to their respective fragment ions. Instead FT-ARM relies upon the specificity in peptide fragmentation and high mass accuracy measurement and is therefore, independent of precursor mass. Simulations indicate that sufficient mass accuracy and number of matching fragment ions permits low false discovery rates (FDR), even when considering large databases. The area calculated from the score chromatogram for each peptide is shown to scale linearly as a function of concentration. Using this technology we are able to identify and quantify peptides present within a complex mixture in an unbiased way. Preliminary experiments using FT-ARM indicate that phosphorylated peptides can be detected when searching with unmodified peptide sequences. To illustrate quantification using FT-ARM within a complex background matrix, a Bovine serum albumin (BSA) digest was spiked into whole yeast cell lysate digest, and the BSA peptides were quantified using the FT-ARM process. This technique presents an attractive alternative to quantification using selected reaction monitoring (SRM) since FT-ARM simplifies proteotypic peptide26 selection and transition selection27 from the SRM assay development process. This strategy has already been adopted by others as an approach toward quantification using DIA data as demonstrated by incorporation of an FT-ARM like processing option in the popular MRM software Skyline27, 28.
Experimental
BSA Sample
Bovine serum albumin was used as received (Sigma Aldrich, St Louis, MO). A total of 8.0 mg BSA was suspended in 1.00 mL of 100 mM ammonium bicarbonate (pH 7.8) to generate a BSA stock solution that was diluted to obtain a 1.0 mg/mL BSA working solution. The BSA sample was reduced using 5mM dithiothreitol (DTT) for 30 minutes at room temperature. Cysteine residues were blocked using 15mM iodoacetamide (IAA) for 30 minutes in the dark at room temperature. The remaining IAA was quenched using 5 mM DTT for 15 minutes in the dark at room temperature. Protein digestion was achieved with 10 ug of sequencing grade trypsin (Pierce, Rockford, IL) with cleavage specificity to arginine and lysine amino acid residues. This reaction was performed at 37°C for 12 hours under constant agitation. The reaction was quenched by acidification (pH 2.0) and desalted using C18 Sepak (Waters Corporation, Milford, MA). The peptides were lyophilized and redissolved using 1.00 mL mobile phase A (99.9% ultrapure water, 0.1% formic acid).
Yeast Sample
Saccharomyces cerevisiae strain S288C (Baker’s yeast) was grown in glucose rich media to mid-log phase. Cells were collected by centrifugation and resuspended in 100 mM ammonium bicarbonate buffer at a concentration of 400 mg/ml. The cells were lysed using a bead-beater. The lysate was centrifuged at 1,000 g for five minutes to remove cell wall particles and unbroken cells. The lysate was centrifuged again for 30 minutes at 15,000g to separate the soluble and membrane protein fractions. The soluble fraction was assayed for protein concentration using Coomassie Plus Protein assay (Pierce, Location) and found to be ~5.0 mg/mL. The sample was reduced with 5 mM dithiothreitol (Sigma-Aldrich, St. Louis, MO) at room temperature for the duration of 30 minutes. The cysteine residues were blocked using iodoacetamide (Sigma-Aldrich, St. Louis, MO) 15 mM at room temperature for the duration of 30 minutes. The lysate was digested using 1:250 ratio of sequencing grade trypsin (Promega, Madison, WI). The reaction was allowed to proceed for 2 hours at 37°C while under constant agitation via orbital shaking. The digest was quenched and frozen at −20°C. This sample was desalted using C18 Sepak (Waters Corporation, Milford, MA). The peptides were lyophilized and redissolved in mobile phase A (99.9% ultrapure water, 0.1% formic acid).
E. coli Sample
Escherichia Coli strain K12 was grown in LB to mid-log phase. Cells were collected by centrifugation and resuspended in 100 mM ammonium bicarbonate buffer at a concentration of 400 mg/ml. The cells were lysed using a ultra-sonication. The lysate was centrifuged at 1,000 g for five minutes to remove cell wall particles and unbroken cells. The lysate was centrifuged again for 30 minutes at 15,000g to separate the soluble and membrane protein fractions. The soluble fraction was assayed for protein concentration using Coomassie Plus Protein assay (Pierce, Location) and found to be ~6.2 mg/mL. 1.0 mg from the stock solution was reduced with 5 mM dithiothreitol (Sigma-Aldrich, St. Louis, MO) at room temperature for the duration of 30 minutes. The cysteine residues were blocked using iodoacetamide (Sigma-Aldrich, St. Louis, MO) 15 mM at room temperature for the duration of 30 minutes. The lysate was digested using 1:250 ratio of sequencing grade trypsin (Promega, Madison, WI). The reaction was allowed to proceed for 2 hours at 37°C while under constant agitation via orbital shaking. The digest was quenched and frozen at −20°C. This sample was desalted using C18 Sepak (Waters Corporation, Milford, MA). The peptides were lyophilized and redissolved in mobile phase A (99.9% ultrapure water, 0.1% formic acid).
Quantification Samples
A set of dilutions were made to test the sensitivity of FT-ARM. The BSA digest stock solution was diluted 1:100, 1:1000, 1:10000, 1:100000, and 1:1000000. The amount of sample loaded onto the column using the starting amount of protein in the digest corresponds to 1.5, 0.15, 0.015, 0.0015, and 0.00015 pmol. This same set of dilutions was made into a yeast lysate background to test robustness of FT-ARM.
Large-scale quantification using FT-ARM was performed by diluting E. coli digest into a yeast digest background. These samples were prepared through the addition of 50 µg of yeast digest to 5, 10, 25, and 50 µg (10:1, 5:1, 2:1, and 1:1) of E. coli. Each sample composed of 50 µL of 1.0 mg/mL yeast lysate digest, the remainder of the composition is solvent A and E. coli digest 1.0 mg/mL depending on the dilution (ex. 50 µL yeast lysate, 25 µL ecoli lysate, and 25 µL solvent A = 2:1 dilution).
Liquid Chromatography
Liquid chromatography was performed using a Waters NanoAcquity UPLC (Waters Corporation, Milford, MA). Pulled tip columns were constructed in-house using a laser-pulling device (Sutter Instrument Company, Novato, CA) from 75 um ID × 360 um OD fused silica capillary. The packing material used for peptide separation was 100 Å C18 magic beads (Microm Bioresources Inc., Auburn, CA). A fused silica trap column was constructed from 100 um ID × 360 um OD fused silica capillary. A frit was made on one end of the trap with Kasil (PQ Corporation, Valley Forge, PA) to contain C18 packing material. The packing material used in the trap was 200 Å C18 magic beads (Microm Bioresources Inc., Auburn, CA). A binary solvent gradient was used for peptide separation. The mobile phase A consisted of 99.9% water with 0.1% formic acid. Mobile phase B consisted of 95.0% acetonitrile, 4.9% 18 MΩ water, and 0.1% formic acid. The gradient was setup as follows 5%–35% B in 30 minutes. Column washing was done by transitioning from 35% B to 80% B in 0.1 minutes and holding for 20 minutes. This was followed by re-equilibration for 30 minutes using 5% B. Large-scale identification experiments with yeast lysate digest and large-scale quantification experiments with E. coli lysate digest within a yeast background were conducted with a 2 hr. separation. The solvent compositions for each step was the same as with the 30 min. gradient, however, the analytical portion of the gradient was changed to 5–35% over 120 min.
Mass Spectrometry
All data were acquired on the LTQ-FT or the LTQ-Orbitrap mass spectrometers (Thermo Electron, San Jose, CA.). DDA experiments for identification comparison were conducted using a “top 5” approach in which a high resolution FTMS acquisition (30,000 resolution at 400 m/z) was followed by 5 ion trap MS/MS acquisitions. The AGC target value for the precursor scan was set to 1×106 counts (FTMS 30,000 RP) and for ion trap MS/MS scans to 3×104 counts. All MS/MS targets were chosen from the high resolution FTMS scan. Charge state screening was applied with consideration of only 2 and 3 plus isotope distributions. Dynamic exclusion was used with the following parameters: one repeat count, 15 second repeat duration, 500 exclusion list size, and 90 second exclusion duration.
All FT-ARM data were acquired using data-independent acquisition using 100 Da isolation windows unless otherwise specified. FT-ARM sensitivity characterization was performed by fragmenting all ions within a 100 Da window in the range 700–800 m/z. The resolution of the instrument was set to 25,000 (at 400 m/z) for the LTQ-FT or 30,000 (at 400 m/z) for the LTQ-Orbitrap. The automated gain control (AGC) settings utilized for this experiment were 1×66 ions with a maximum ion injection time of 1 second. For validation of FT-ARM targets from BSA, a DDA experiment was incorporated parallel to the DIA acquisition event. In this acquisition scheme, a precursor scan was performed over the m/z range 700–800 in the FT analyzer (1×106 ions, 1.0 s max ion injection time). Three of the most abundant ions were selected from this precursor scan for MS/MS sequencing in the linear RF ion trap (30,000 ions, 100 ms max ion injection time). Dynamic exclusion was used with 1 repeat count, a list of up to 500 entries, and exclusion duration set to 90 seconds. This was followed by a data-independent acquisition over the same m/z range 700–800 as described above. Experiments which utilized 12 Da DIA scan widths were setup the same as in the 100 Da window experiments described above, with the exception of dynamic exclusion. Dynamic exclusion was not enabled during these experiments since doubly or triply protonated peptide isotope distributions typically occupy spectral regions of 2.0–3.0 Da. Enabling dynamic exclusion on this mass range would impair the performance of DDA. Activation energy used was 60% relative energy with an activation time of 50 ms. The collision energy was optimized through obtaining the number of peptides identified at a fixed fragment ion mass tolerance, matchcount, and FDR.
To compare FT-ARM peptide identification capability versus DDA, spectra were acquired in two LC-DIA-MS/MS acquisitions using 5 × 100 Da windows spanning 500–1500 m/z (i.e. 2 DIA-LC-MS/MS and 2 DDA-LC-MS/MS). The first acquisition consisted of 100 Da windows from 500–1000 m/z, while the second set of acquisitions spanned 1000–1500 m/z. The resolution for these experiments was set to 30,000 at 400 m/z. An ACG target value of 1×106 ion counts was used. Peaklist generating software for this data was ReAdW (version 4.2.1), followed by MzXML2Search (version 4.4) both of which are packaged tools included in the Trans-Proteome Pipeline29. Mascot (version 2.3.01) was used to search the DDA data. Carbamidomethyl cysteine was the only static modification considered in all database searches. No variable modifications were searched. No missed cleavages were allowed. The precursor mass tolerance utilized in all Mascot searches was 5 ppm. Both forward and reverse sequence databases were searched and FDR estimated using these data. The quotient of reverse database hits over target hits was used to estimate FDR.
SRM data were acquired using a TSQ Vantage triple quadrupole mass spectrometer. A direct infusion experiment of BSA digest was used to confirm the most intense transitions for the BSA peptides (DAFLGSFLYEYSR:y6,y7,y8 and VPQVSTPTLVEVSR:y8,y9,y10). The ENO2_YEAST normalization peptide (AVDDFLISLDGTANK:y6,y7,y9) transitions were determined directly from previously acquired data-dependent acquisisions and used to normalize for comparison between FT-ARM and SRM. The mass spectrometer was set to monitor three transitions per peptide. The scan width for the experiment was set to 30 mDa. Dwell time for each transition was set to 100 ms per transition to provide high sensitivity. Optimized collision energies were provided from the linear calibration in the Skyline27 software package.
FT-ARM program
The FT-ARM program was written in the C++ programming language using Microsoft Visual Studio Developers Suite (Microsoft Corporation, Redmond, WA). This program was designed to accept two input data streams: theoretical or empirical reference spectra (.FASTA or .TXT peptide list format) and DIA multiplexed fragmentation target data acquired on the sample of interest. When operated using .FASTA or .TXT peptide lists, only 2 and 3 plus precursor m/z’s are calculated for peptide targets and determined to be within the defined DIA isolation window. The program first populates an array of masses and intensities for each target spectrum. Each spectrum in the target data is treated as a vector with n intensity values where n is the number of m/z values for which an ion signal exceeding an intensity threshold was observed. The reference spectra, derived from theoretical calculations or empirical data, are converted to vectors with the same properties. Theoretical reference spectra are generated directly from predicted monoisotopic b and y ions from the peptide sequences obtained from in silico database digestion. The program performs a dot-product calculation (Equation 1) between vectors from each reference spectrum and vectors from each measured target spectrum acquired during chromatographic separation.
| Equation (1) |
Where R is the reference spectrum vector, T is the target spectrum vector, Ri is the intensity of the ith m/z value of the reference spectrum, Ti is the ith m/z value of the target spectrum, and n is the number of peaks in the spectrum. Large positive dot-product scores are obtained at spectra/times when the peaks in the reference spectrum align with peaks in the target spectrum within the user specified mass tolerance. Peaks must be within a user specified mass tolerance (5 ppm for data presented here) in order to contribute to the dot product score. A match threshold for the number of b and y fragment ions matching a given reference spectrum is applied as a filter for each dot-product calculation. It was found in our analyses that match threshold values between 8 and 12 achieved (values used for data presented) the desired specificity without diminishing analysis sensitivity. If more than one peak exists within the user specified window the first peak in m/z space, most intense, or closest in ppm error to the target spectrum can be utilized. In this manuscript, we utilized the first peak in m/z space. However, it is possible for a fragment ion to contribute to more than one peptide score since peaks are not removed or subtracted from the analysis. Peptide targets and decoys are only considered if their hydrophobicity is within a user specified tolerance (± 10 hydrophobicity units) of a linear regression between retention time and hydrophobicity (SSRCalc)30. The current implementation forms the retention time and hydrophobicity regression based on the high confidence identifications from the CID acquisition; a future revision will calculate this regression solely from the FT-ARM analysis using a two-pass approach. The output from the program is an array containing the scan number, dot-product score, and match count (number of matching peaks within ppm tolerance of reference spectrum), retention time, and theoretical m/z for each query peptide. The dot product score is then weighted by the number of observed fragment ions generated for each time point in the data to increase the signal-to-noise ratio. It was determined that the algorithm is the most sensitive when the data is post-processed as little as possible (i.e. centroided, no peak smoothing, no deconvolution, no charge state determination). More importantly, the signal from score chromatograms can be integrated to obtain total area under the curve which is treated in much the same way as SRM acquired data. To achieve this, the areas obtained from quantified peptides were first normalized to reference peptide areas from the same analysis (or a stable heavy isotope standard). In the rare case that two different peptides presented to the mass spectrometer at the same time dissociate to yield fragment ions within 5 to 10 ppm of one another, the contribution to overall error in quantitation of either peptide is small due to the fact that the match count parameter typically requires 8 or more ions. The ratio of the area between these two peaks was normalized and used for comparison across samples. Identification using FT-ARM is performed by recording the maximum dot-product score for each peptide in both the target and decoy databases. FDR estimation is performed on the basis of maximum dot-product score for targets and decoys which reside within the DIA mass range acquired. A graphical user interface has been developed for use with this software to perform FT-ARM analysis, FDR estimation, and data comparison operations. The database used for all searches, both FT-ARM and DDA-Mascot, was NCBI Saccharomyces cerevisiae downloaded September 9, 2009. See Supporting Information for FT-ARM simulation and other data handling details.
Results and Discussion
FT-ARM analysis begins with selection of m/z range for the DIA scan event. Here we are using 12 or 100 Da isolation windows. Narrow isolation windows can increase the dynamic range of the peptides observed in the analysis since the fixed charge capacity of the linear ion trap in conjunction with AGC fundamentally limits the dynamic range of the measurement.31, 32 Ions present in the m/z range of interest are fragmented in the linear ion trap and transferred to the FT analyzer for high resolution measurement. This process is repeated during the entire LC separation. Precursor mass measurement is not necessary for FT-ARM. However, a precursor scan can be used to validate FT-ARM results using accurate precursor mass if desired. FT-ARM is analogous to SRM analysis on a triple quadrupole instrument. For example, in SRM analysis, low resolution precursor mass selection combined with two or more fragment ion masses also recorded with low mass accuracy provide specificity for identification and quantitation of peptides from complex samples. In FT-ARM, we have replaced the need for precursor mass selection with high resolution mass measurement on all fragment ions. Here ions are not physically filtered during the analysis as in the SRM assay. Highly accurate fragment mass measurements provide the selectivity required for identification and quantitation from complex samples, in the absence of peptide precursor mass determination. FT-ARM assays are conducted in a parallel as opposed to the serial transition measurements made in SRM assays. Prior identification of the most sensitive transitions is not required for FT-ARM, since “all transitions” are used in the FT-ARM scoring process. This can significantly reduce the time required to develop sensitive quantitative assays for targeted proteome quantification.
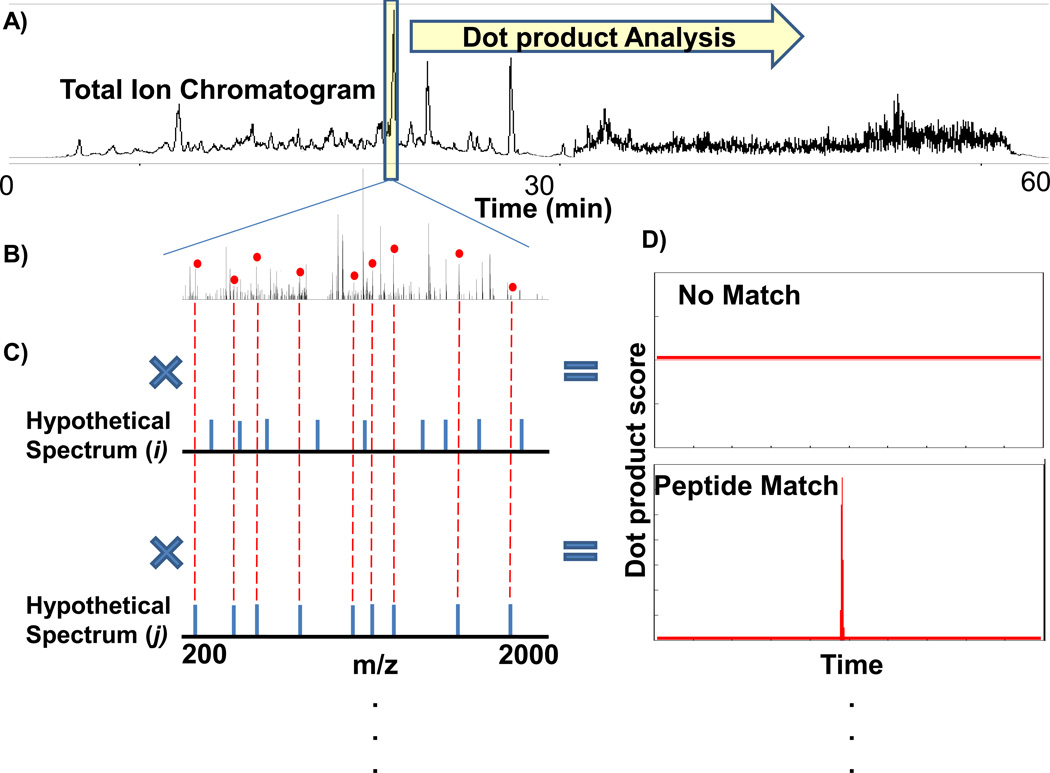
The FT-ARM process is shown in Fig. 1. Here a DIA LC-MS total ion chromatogram (TIC) is shown for yeast whole cell lysate digest spiked with BSA digest at a 1:100 ratio. The reference window in the center of the chromatogram in Fig. 1A is placed to simulate the position of a single FT-ARM calculation. A DIA spectrum acquired within this reference window is shown in Fig. 1B along with two theoretical reference spectra (Fig. 1C and Fig. 1D). The hypothetical spectrum i in this example contains no matching peaks in the target spectrum at this time point in the chromatogram, while j has many matching peaks as depicted with the red dots above the correctly matching peaks. The score chromatograms (Fig. 1D) show a matching peptide eluting (bottom inset) and no signal (top inset). Score chromatograms are integrated to obtain a score area which is proportional to the amount of analyte present in the mixture. Global FT-ARM datasets can be re-analyzed with a specific subset of hypothetical spectra computed for only the peptides of particular interest. Alternatively, for greater dynamic range targeted analyses, FT-ARM can also be operated such that only those m/z values of targeted peptides are accumulated and fragmented. Generally, FT-ARM data are acquired with an unbiased mass spectrometry approach and as such, these datasets can be re-mined with different hypothetical spectra or empirical peptide spectral databases. Global quantification of the peptides can be accomplished by generating hypothetical spectra for every peptide candidate in an entire organism.
Figure 1.
Diagram illustrating FT-ARM strategy based on accurate peptide fragment mass measurements. All ions are fragmented in every scan during LC separation to produce the total ion chromatogram in A). B) Complex fragmentation spectrum of all ions. C) Hypothetical peptide fragmentation spectra. D) Dot product analysis result called a Score Chromatogram.
The FT-ARM analysis scores all possible tryptic peptides in a given database against every acquired spectrum. This approach differs from DDA and conventional database searching algorithms, where the massive search space of entire databases containing all possible peptides is made efficient using precursor mass measurements to significantly reduce the pool of possible peptides which match an MS/MS spectrum. In contrast, FT-ARM analysis includes a single pass dot product calculation for each target spectrum against every peptide in the database. Despite the large search space, optimized design of the algorithm produces a modest runtime of ~5 minutes on a modern desktop computer for FT-ARM analysis when applied to whole yeast lysate digest over a 100 Da window (700–800 m/z). This search time was obtained when considering only the 2+ and 3+ peptides residing in this window. Thus, even though FT-ARM imposes greater computational demands, the overall analysis need not present a time bottle-neck in proteomics pipelines.
Peptide identification within a complex mixture, without narrow band isolation and without precursor mass confirmation, has not been demonstrated previously. FT-ARM peptide identification is demonstrated through its application to yeast whole cell lysate digest samples (Fig. 2). In this case, the experiment was designed to allow both DDA and DIA scan types so that identified peptides from each approach could be compared. FT-ARM does not require precursor masses or use the results from the DDA scans. The hybridized DDA-FT-ARM experiment was done by programming the mass spectrometer to first acquire a precursor spectrum over the range of 700–800 m/z, followed by three data-dependent scans (referencing the precursor scan), and finally, the data-independent scan window (700–800 m/z) for FT-ARM analysis (Fig. 2A). The total cycle time for this sequence of scans at maximum ion injection is 3s. Fig. 2B includes three traces: the total ion chromatogram (TIC) (Black), the extracted ion chromatogram (EIC) for the peptide of interest within 20 mDa (Red), and the FT-ARM score chromatogram (Blue) where all data have been normalized so they can be presented on the same plot. A peptide identified in this acquisition from the yeast protein enolase II was used in this example. The noise level found in the EIC for the peptide results from many confounding ions that appear with m/z similar to the identified peptide during the entire experiment. However, when the DIA scans are subject to FT-ARM analysis using the hypothetical b and y ions for this peptide, the score chromatogram generated is nearly noise free. This is important since quantification based upon FT-ARM relies on the score chromatogram area; it must be free from spurious or interfering peaks not directly resultant from the analyte of interest. Also, from this analysis we found that the retention time of the DDA MS/MS spectrum for which this same peptide was identified coincides with the signal acquired from the FT-ARM analysis, as one would expect. This example provides proof of concept for FT-ARM, i.e., identification of the tryptic peptide LGANAILGVSLAASR in the yeast digest was achieved by FT-ARM using only the accurate fragment masses, independent of precursor mass.
Figure 2.
A) Experimental scan sequence. B) Correlation between data-dependent acquisition (DDA) identification of a yeast tryptic peptide (LGANAILGVSLAASR) from a whole cell lysate digest. Extracted ion chromatogram (EIC) for the m/z of this peptide shown in red (20 mDa window). The FT-ARM score chromatogram of the same sequence is shown in blue. C) Mascot annotated spectrum for the identification of the peptide.
Low noise score chromatograms generated from FT-ARM result from high mass accuracy fragment ion measurement and match count (Supporting Information Figures 1). Highly accurate mass measurements have been used for increased specificity and simplification of complex mixture analysis.33 The effect of mass accuracy and match count on the FT-ARM analysis is shown using the same dataset as Fig. 2. As the mass tolerance is varied from 1000 ppm to 1 ppm, specificity in detection is gained.34 The other contributing factor in the specificity of FT-ARM is the match count parameter. In database searching algorithms, an analogous type of discrimination is utilized when attempting to make MS/MS identifications. An MS/MS pattern with few matching peaks receives a poor score and is not the most probable peptide match for the MS/MS pattern. Similarly, match count allows the user to specify a minimum number of m/z values to match the reference for a dot-product score to be recorded. The effect of varying the match count threshold when the ppm tolerance is fixed to 20 ppm is shown in Supporting Information Figure 2. As match count threshold is increased, the overall noise level decreases. This effect supplements accurate mass measurement by eliminating spurious peptide identification events in which only a small number of matching, high intensity peaks generate strong signal. Currently both parameters are implemented statically throughout the analysis, although they could be treated dynamically to best fit the data presented to the FT-ARM program. Additionally, relative ion intensities could be utilized, in an analogous way in MRM relative transition signal is used to limit false quantification35.
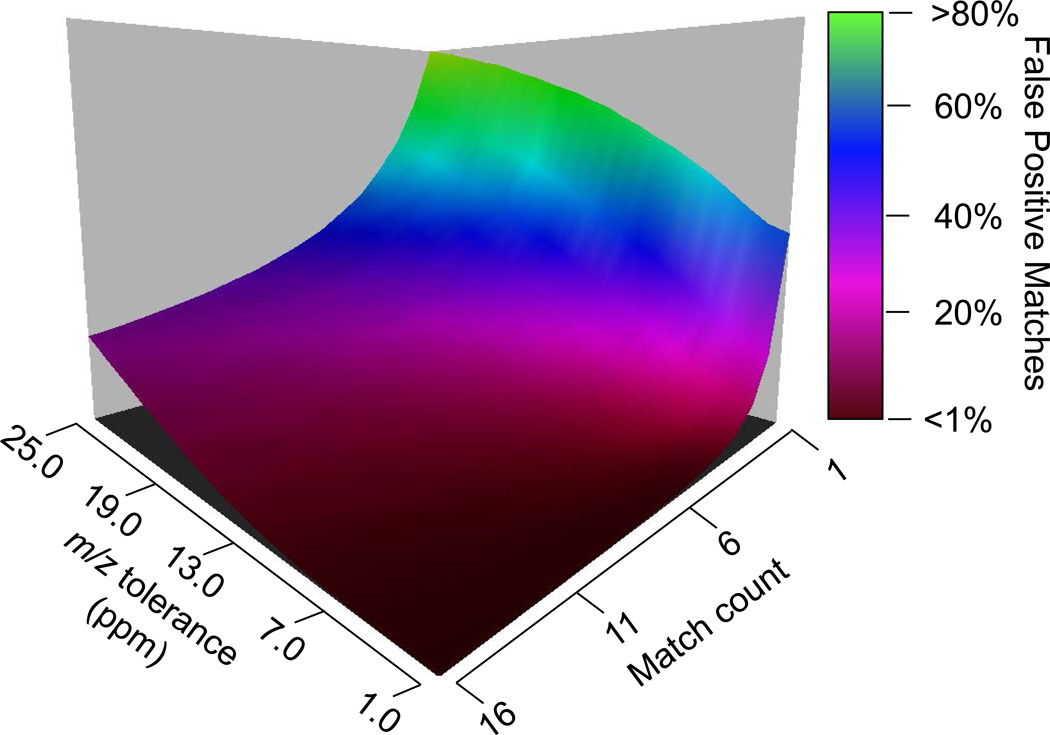
In an effort to understand the performance of this technique when applied to large-scale databases, a simulation was performed to ascertain the number of theoretical possible false matches for the FT-ARM process under defined circumstances. False matches occur when another peptide in the database or a higher order combination fragments from multiple peptides produce similar fragmentation patterns to the target peptide (i.e. b and y ions overlap at the specified mass tolerance). In the absence of precursor m/z information, peptides which fall within the same m/z window and have similar hydrophobicity could contribute to a peptide identity falsely and also invalid signal quantification. This simulation reveals how often this chimeric or multiplexed fragmentation patterns amongst peptides could align and generate a false positive match. In fact, predicted false positive matches are well below 1% when applying FT-ARM to the entire Saccharomyces cerevisiae database with at least 6 ppm fragment mass tolerance and match count of 5 or higher. It was assumed for this simulation that all peptide fragment ions in the m/z window of interest were detectable. This represents a worst case scenario, since all peptides in a FASTA database are not detectable with current mass spectrometry technologies for various reasons36. In Figure 3, the results from a simulation performed on the yeast database are presented as a function of m/z fragment ion tolerance (ppm) versus match count. This simulation was performed over the window of 700–800 m/z, in which, all 2–3+ tryptic peptides are considered. It reveals that given the appropriate m/z fragment ion tolerance and match count, FT-ARM is capable of providing highly specific identities. Modified peptides, missed cleavage sequences and other factors will further complicate databases and may yield higher false discovery rates. However, the simulations, like the results above demonstrate, suggest that the precursor-mass independent approach described in FT-ARM is feasible and will offer advantages for proteome analyses.
Figure 3.
A three dimensional plot presenting data from an FT-ARM simulation which allows for estimation of the number of potential false peptide matches in a fasta database. Empirical data presented in this manuscript has been collected in the regime in where this data suggests false matches are below 1%.
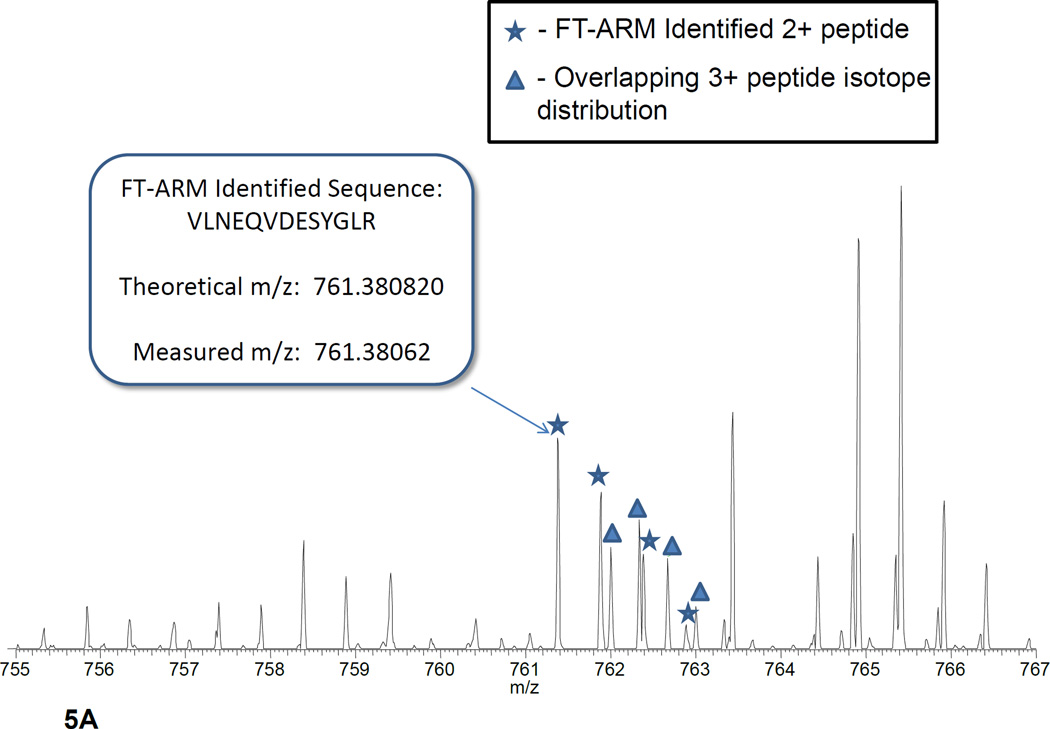
Identification of peptides from complex matrices is perhaps the best metric for the effectiveness of FT-ARM. In order to demonstrate the identification capability of FT-ARM, DIA LC-MS was acquired on yeast whole cell lysate digest. Separate experiments were used for both DDA Masco (1292 unique peptides) and DIA FT-AR (896 unique peptides), with data acquired using the scan cycle shown in Figure 4A. Both DDA and DIA data were obtained over the same mass range, overall acquisition time, and peptide charge state considered (2+ and 3+ only). The results from the analyses are summarized in Figure 4B. Identifications have been broken down into individual mass ranges. FDR was estimated using a decoy database strategy37 both in FT-ARM and Mascot analyses (see Supporting Information). Unlike the DDA analysis, the FT-ARM identifications were made without the need for narrow m/z isolation of each peptide or accurate precursor ion. Data acquired with 12 Da DIA scan widths over the range 700–800 m/z are shown in the inset. These data show that identifications increase as the DIA window size is decreased. The Venn diagram inset within Figure 4B shows the overlap between FT-ARM and Mascot peptide sequence identifications on the peptide level. About 40% of the peptides identifie (476 unique peptides) with DDA methods were also identified by FT-ARM. This level of overlap indicates that the FT-ARM technique provides a significant complementarity in number of additional peptide identities which were not identified by DDA and vice versa. In many cases, FT-ARM identifications were achieved where DDA failed due to overlapping isotope distributions that were isolated, as shown in Figure 5A. Manual inspection of random peptides uniquely identified by FT-ARM yielded the following reasons for missed peptide identifications in the DDA experiment: overlapping isotope distribution (34.9%), MS/MS acquisition but no identificatio (8.4%), and no MS/MS acquisitio (56.7%). Of the reasons, the first two are unlikely to be alleviated by mass lists or technical replicates using DDA. On the other hand, peptides uniquely identified by DDA were in some instances found to be the most abundant peptide in the precursor spectrum. Upon inspection of the flanking DIA scan events, fragment ions from the putative precursors are not present. Direct infusion experiments were performed to investigate this problem. Results from these efforts suggest that broadband isolation (100 Da) and CID activation in the ion trap is not occurring with the same efficiency as in narrow band isolation/activation and thus, product ions observed from narrow band isolation of the precursor are not present in the DIA scan (data not shown). These represent technical details that can be optimized to further improve the FT-ARM approach. Figure 5B illustrates the effect of reducing the DIA window size on the number of identifications/amu. In the case of FT-ARM, the IDs/amu grows exponentially with decreasing window size, whereas, DDA appears constant. In the case of DDA, the result is that identificaitons/amu effectively scales with the MS1 window size so the peptides identified per amu remains approximately constant.
Figure 4.
A) Experimental scan cycle for both DIA and DDA acquisitions B) Histogram of the identifications made using DDA mass spectrometry with Mascot versus DIA mass spectrometry with FT-ARM during equivalent acquisition periods. Inset shows identifications made when DIA acquisition window is reduced from 100 Da to 12 Da. Venn diagram inset showing the overlap between the identifications made with each approach at <5% estimated FDR. C) Histogram shows identifications made when DIA acquisition window is reduced from 100 Da to 12 Da. Venn diagram inset showing the overlap between the identifications made with each approach at <5% estimated FDR.
Figure 5.
A) Spectrum for which an FT-ARM unique identification was found. The overlapping isotope distributions shown did not yield identification with DDA methods. B) A plot showing the effect of the reduction of the DIA window size on the identifications per amu.
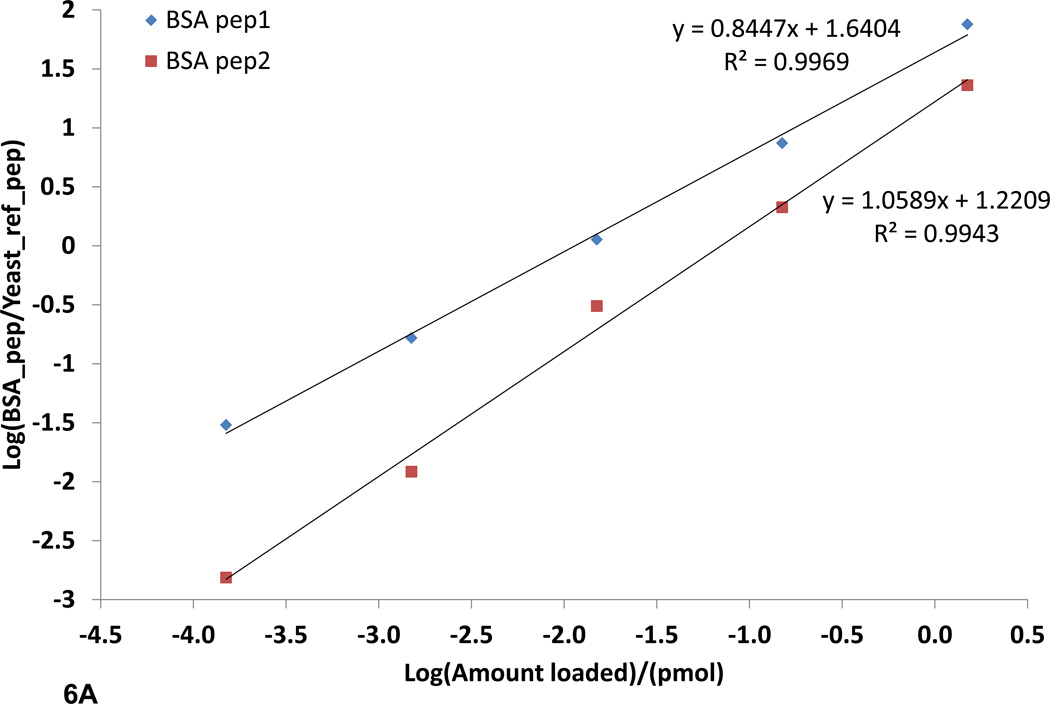
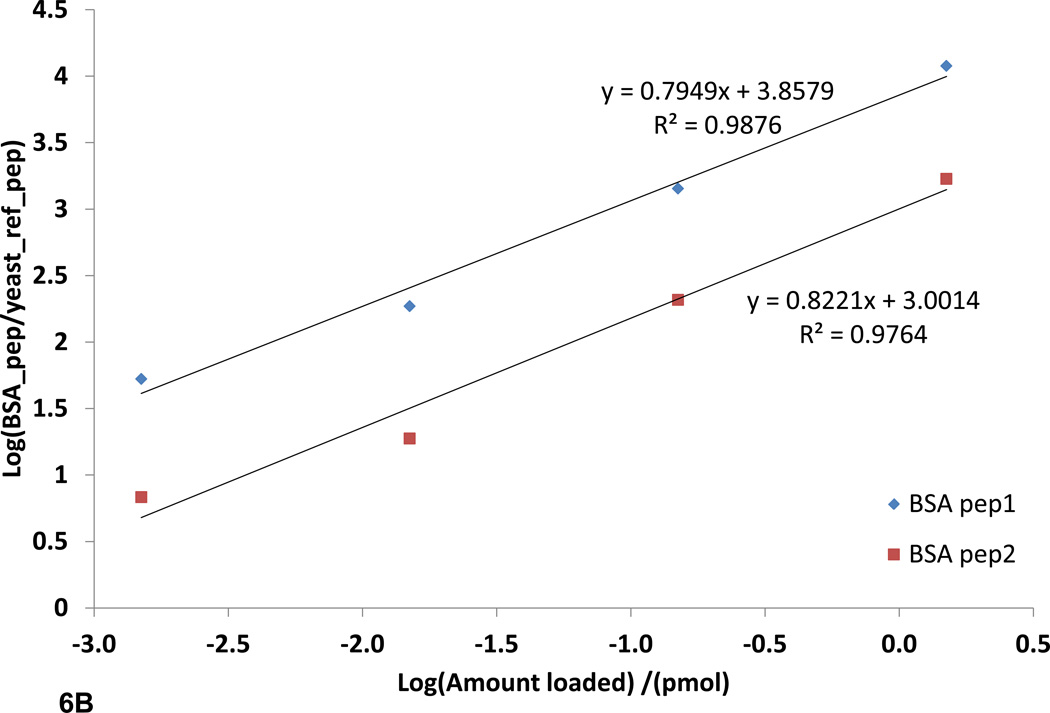
The ability to quantify peptides and proteins, in complex samples is of great importance in systems biology. SRM quantification is gaining traction in the field of proteomics due to its many desirable qualities such as high reproducibility, sensitivity, selectivity, and large linear dynamic range of five orders of magnitude or greater.16 This is very important in development of high throughput proteomics assays where minimal sample preparation is required. FT-ARM analysis shares these same properties; however, it also improves upon SRM analysis in two aspects. The first improvement is simplification of method development. SRM assays are time consuming to develop and often require empirical data for optimum sensitivity. It has been shown that tuning the instrument with synthetic peptides derived from a list of target peptides increases the sensitivity of this technique tremendously.16 This is primarily due to the inability for in silico prediction of the most sensitive transitions, or highest yielding CID products, to focus on for a given target peptide. FT-ARM analysis can make use of the entire hypothetical or empirical spectrum, and thus, all transitions are considered. The second improvement is multiplexed analysis. The mass analyzer used for SRM analysis is a triple quadrupole. The triple quadrupole has many benefits; however, it is by nature a spatially resolving mass analyzer, requiring physical separation of the ions. Physical ion separation limits SRM assays to a single analyte detected at any given instant. Efforts to increase the quadrupole scan speed and the ability to schedule SRM assays have increased the number of analytes quantifiable in a single acquisition. FT-ARM, in contrast, allows simultaneous detection of many fragments from all precursors within the selected m/z range and relies on accurate fragment mass to achieve specificity. Here a direct comparison of SRM vs. FT-ARM (100 Da DIA window) has been made to illustrate these methods can provide similar quantification information. For this comparison, a sample of BSA digest spiked into a yeast whole cell lysate digest (0.150–1500 fmol) was used. The yeast lysate digest provides a highly complex background matrix. In fact, careful measurements of the yeast proteome indicate that the dynamic range of SRM is likely greater than six orders of magnitude.15, 16 In Figure 6A and 6B, the two BSA peptides are quantified in the complex yeast background using FT-ARM and SRM with a TSQ Vantage. The peptides are quantified over four orders of magnitude. Linear regression data show that the relationship between FT-ARM signal and amount of analyte is linear in this complex background. The achieved FT-ARM dynamic range was observed to be nearly the same as that achieved using SRM by limiting the m/z range of the DIA to a smaller value such as 10 m/z. Detection of the lowest dilution level became possible using the narrower DIA window. This corresponds to 150 attomoles of BSA protein loaded on the column.
Figure 6.
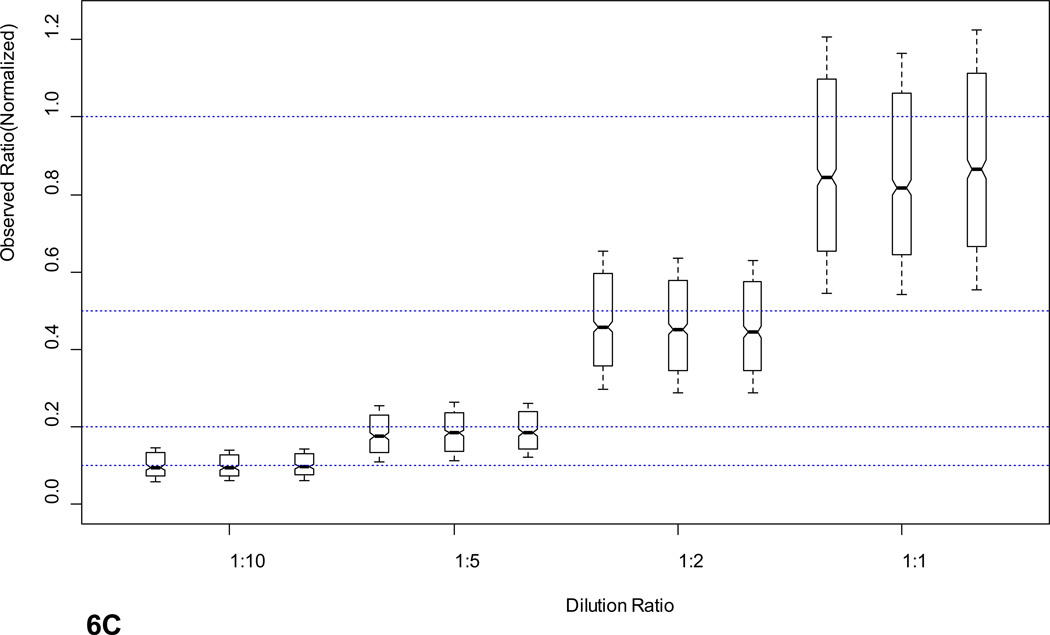
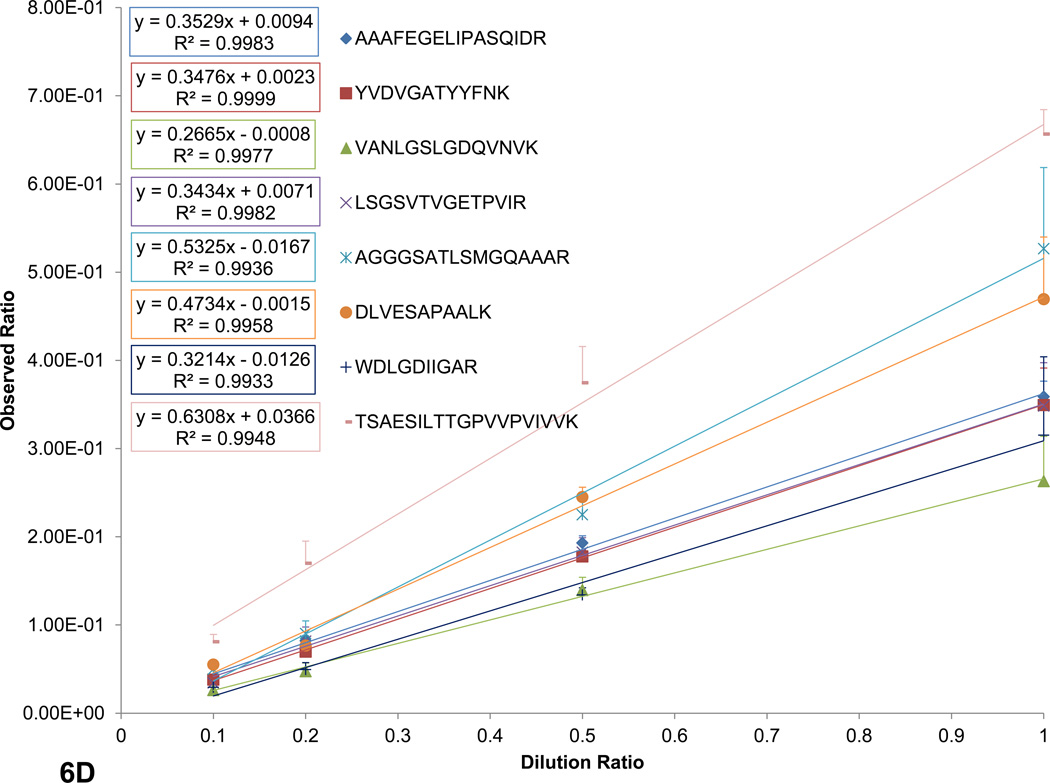
SRM and FT-ARM quantification of BSA digest spiked into yeast whole cell lysate digest (DAFLGSFLYEYSR and VPQVSTPTLVEVSR). A) Integrated area as function of concentration of BSA peptides using SRM. B) Integrated score area as function of concentration of BSA peptides using FT-ARM. C) Box and whisker plot for all peptides quantified from E. coli at <5% FDR normalized to 1. D) Random selection of peptides from the E. coli dataset in linear regression format of the average +/− σ at each dilution.
In order to illustrate the reproducibility, linearity, and robustness in quantification of large-scale measurements with FT-ARM, E. Coli lysate was diluted into a yeast lysate background. This sample set presents a significant challenge to the technique in terms of potential spurious quantitative signal. A summary of the ratios obtained for each E. coli peptide identified and quantified (<5% FDR; see Supporting Information) is presented in Figure 6C. Each dilution level was measured in triplicate to provide statistics. The median ratio for each dilution level is highly reproducible between replicates, as indicated by the notches in the box plot. These notches indicate at 95% confidence whether a median is statistically different. The box encompasses 50% of the measurements. Dotted blue lines indicate the expected ratio for each dilution level and “whiskers” represent +/− σ for the dataset. Although some variation in peptide response can be observed from this plot, most of the data (i.e. the box) corresponds very closely to the expected ratio. This response is comparable to other label free techniques including SRM, however, in this case no assay development was required to quantify hundreds of E. coli peptides within a very complex matrix. The measured ratios +/− σ for a random subset peptides has been shown in Figure 6D to illustrate linearity in peptide response in addition to reproducibility of measurements on the individual peptide level. As indicated by the peptides shown individual peptide quantification error is much lower. Average relative standard deviations for peptide level measurements are 11.3%. Precursor based label-free quantification studies report similar relative standard deviations (10–20%)38–40. Quantitative reproducibility and linearity are perturbed by overlapping fragment ions from contaminating ion signal. Since yeast peptides have an equal probability of yielding isobaric fragment ions (within the applied mass tolerance) as E coli peptides, linearity of quantification signals would not be possible due to the constant concentration of yeast peptides if fragment overlap contributed in any significant way to the FT-ARM score chromatograms. However, as predicted by simulation this phenomenon is not prevalent in our data.
Conclusions
FT-ARM is a novel mass spectrometry method and algorithm designed to combine peptide identification and quantification using multiplexed high mass accuracy fragment measurement. This approach presents an attractive complement to DDA mass spectrometry and SRM analysis. Dot-product score calculation allows signals specific to individual peptides to be observed even from highly complex mixtures. In addition, FT-ARM enables unique identification of a significant number of peptides that are missed by traditional DDA methods. This observation suggests that adding a DIA scan in conjunction with conventional DDA analysis will enhance the overall number of identifications in an analysis. Further refinement of the FT-ARM scoring algorithm will result in even greater numbers of identifications. Quantification is linear, highly sensitive, and measurable for every peptide using FT-ARM. Targeted quantification with FT-ARM can be performed without the need for FT-ARM identifications, if peptides were identified by another technique. Peptides from BSA were quantified within a yeast whole cell lysate background over the range of 1.5–1500 fmoles total BSA protein loaded. The dynamic range and sensitivity of FT-ARM appears to be limited by that of the linear ion trap and AGC. When the DIA window was limited to 10 Da, peptides from 150 attomoles of BSA total protein were detected. Robust, linear, and reproducible quantification was demonstrated on a large-scale with no assay development. FT-ARM methodology has been presented here as a proof of principal, and as such, underlying software will continue to be developed and optimized. FT-ARM is freely available and open source at http://brucelab.gs.washington.edu/.
Supplementary Material
Acknowledgments
This work is supported in part by National Institutes of Health grants 7S10RR025107, 5R01GM086688, 5R01RR023334, 1R01GM097112 and the University of Washington's Proteomics Resource (UWPR95794). The authors also would like to thank Dr. Priska D. von Haller for helpful discussion.
Footnotes
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org”
Bibliography
- 1.Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nature biotechnology. 2001;19(3):242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 2.Eng JK, Fischer B, Grossmann J, Maccoss MJ. A fast SEQUEST cross correlation algorithm. Journal of proteome research. 2008;7(10):4598–4602. doi: 10.1021/pr800420s. [DOI] [PubMed] [Google Scholar]
- 3.Eng JK, McCormack AL, Yates JR., III An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. Journal of the American Society for Mass Spectrometry. 1994;5(11):976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 4.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20(18):3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Craig R, Beavis RC. A method for reducing the time required to match protein sequences with tandem mass spectra. Rapid communications in mass spectrometry. 2003;17(20):2310–2316. doi: 10.1002/rcm.1198. [DOI] [PubMed] [Google Scholar]
- 6.Craig R, Beavis RC. TANDEM: matching proteins with tandem mass spectra. Bioinformatics. 2004;20(9):1466–1467. doi: 10.1093/bioinformatics/bth092. [DOI] [PubMed] [Google Scholar]
- 7.Cao XJ, Dai J, Xu H, Nie S, Chang X, Hu BY, Sheng QH, Wang LS, Ning ZB, Li YX, Guo XK, Zhao GP, Zeng R. High-coverage proteome analysis reveals the first insight of protein modification systems in the pathogenic spirochete Leptospira interrogans. Cell research. 2009;20(2):197–210. doi: 10.1038/cr.2009.127. [DOI] [PubMed] [Google Scholar]
- 8.Good DM, Wenger CD, Coon JJ. The effect of interfering ions on search algorithm performance for electron-transfer dissociation data. Proteomics. 2010;10(1):164–167. doi: 10.1002/pmic.200900570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renard BY, Kirchner M, Monigatti F, Ivanov AR, Rappsilber J, Winter D, Steen JA, Hamprecht FA, Steen H. When less can yield more - Computational preprocessing of MS/MS spectra for peptide identification. Proteomics. 2009;9(21):4978–4984. doi: 10.1002/pmic.200900326. [DOI] [PubMed] [Google Scholar]
- 10.Wright JC, Sugden D, Francis-McIntyre S, Riba-Garcia I, Gaskell SJ, Grigoriev IV, Baker SE, Beynon RJ, Hubbard SJ. Exploiting proteomic data for genome annotation and gene model validation in Aspergillus niger. BMC genomics. 2009;10:61. doi: 10.1186/1471-2164-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang KY, Georgianna DR, Heber S, Payne GA, Muddiman DC. Detection of alternative splice variants at the proteome level in Aspergillus flavus. Journal of proteome research. 2010;9(3):1209–1217. doi: 10.1021/pr900602d. [DOI] [PubMed] [Google Scholar]
- 12.Kiyonami R, Schoen A, Prakash A, Peterman S, Zabrouskov V, Picotti P, Aebersold R, Huhmer A, Domon B. Increased selectivity, analytical precision, and throughput in targeted proteomics. Molecular &cellular proteomics. 2010 doi: 10.1074/mcp.M110.002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panchaud A, Scherl A, Shaffer SA, von Haller PD, Kulasekara HD, Miller SI, Goodlett DR. Precursor acquisition independent from ion count: how to dive deeper into the proteomics ocean. Analytical chemistry. 2009;81(15):6481–6488. doi: 10.1021/ac900888s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li GZ, Vissers JP, Silva JC, Golick D, Gorenstein MV, Geromanos SJ. Database searching and accounting of multiplexed precursor and product ion spectra from the data independent analysis of simple and complex peptide mixtures. Proteomics. 2009;9(6):1696–1719. doi: 10.1002/pmic.200800564. [DOI] [PubMed] [Google Scholar]
- 15.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O'Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425(6959):737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 16.Picotti P, Bodenmiller B, Mueller LN, Domon B, Aebersold R. Full dynamic range proteome analysis of S. cerevisiae by targeted proteomics. Cell. 2009;138(4):795–806. doi: 10.1016/j.cell.2009.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva JC, Denny R, Dorschel C, Gorenstein MV, Li GZ, Richardson K, Wall D, Geromanos SJ. Simultaneous qualitative and quantitative analysis of the Escherichia coli proteome: a sweet tale. Molecular &cellular proteomics. 2006;5(4):589–607. doi: 10.1074/mcp.M500321-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Silva JC, Denny R, Dorschel CA, Gorenstein M, Kass IJ, Li GZ, McKenna T, Nold MJ, Richardson K, Young P, Geromanos S. Quantitative proteomic analysis by accurate mass retention time pairs. Analytical chemistry. 2005;77(7):2187–2200. doi: 10.1021/ac048455k. [DOI] [PubMed] [Google Scholar]
- 19.Silva JC, Gorenstein MV, Li GZ, Vissers JP, Geromanos SJ. Absolute quantification of proteins by LCMSE: a virtue of parallel MS acquisition. Molecular &cellular proteomics. 2006;5(1):144–156. doi: 10.1074/mcp.M500230-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Baek JH, Kim H, Shin B, Yu MH. Multiple products monitoring as a robust approach for peptide quantification. Journal of proteome research. 2009;8(7):3625–3632. doi: 10.1021/pr800853k. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn K, Mbeunkui F, Mitra SK, Mentzel T, Goshe MB. Improving protein and proteome coverage through data-independent multiplexed peptide fragmentation. Journal of proteome research. 2010;9(7):3621–3637. doi: 10.1021/pr100144z. [DOI] [PubMed] [Google Scholar]
- 22.Geromanos SJ, Vissers JP, Silva JC, Dorschel CA, Li GZ, Gorenstein MV, Bateman RH, Langridge JI. The detection, correlation, and comparison of peptide precursor and product ions from data independent LC-MS with data dependant LC-MS/MS. Proteomics. 2009;9(6):1683–1695. doi: 10.1002/pmic.200800562. [DOI] [PubMed] [Google Scholar]
- 23.Masselon C, Pasa-Tolic L, Lee SW, Li L, Anderson GA, Harkewicz R, Smith RD. Identification of tryptic peptides from large databases using multiplexed tandem mass spectrometry: simulations and experimental results. Proteomics. 2003;3(7):1279–1286. doi: 10.1002/pmic.200300448. [DOI] [PubMed] [Google Scholar]
- 24.Bern M, Finney G, Hoopmann MR, Merrihew G, Toth MJ, MacCoss MJ. Deconvolution of mixture spectra from ion-trap data-independent-acquisition tandem mass spectrometry. Analytical chemistry. 2010;82(3):833–841. doi: 10.1021/ac901801b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiger T, Cox J, Mann M. Proteomics on an Orbitrap benchtop mass spectrometer using all ion fragmentation. Molecular &cellular proteomics. 2010 doi: 10.1074/mcp.M110.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders WS, Bridges SM, McCarthy FM, Nanduri B, Burgess SC. Prediction of peptides observable by mass spectrometry applied at the experimental set level. BMC bioinformatics. 2007;8 Suppl 7:S23. doi: 10.1186/1471-2105-8-S7-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26(7):966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacCoss MJ. Personal Communication. 2011 [Google Scholar]
- 29.Keller A, Eng J, Zhang N, Li XJ, Aebersold R. A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Molecular systems biology. 2005;1 doi: 10.1038/msb4100024. 2005 0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krokhin OV, Spicer V. Peptide retention standards and hydrophobicity indexes in reversed-phase high-performance liquid chromatography of peptides. Anal Chem. 2009;81(22):9522–9530. doi: 10.1021/ac9016693. [DOI] [PubMed] [Google Scholar]
- 31.Belov ME, Anderson GA, Angell NH, Shen Y, Tolic N, Udseth HR, Smith RD. Dynamic range expansion applied to mass spectrometry based on data-dependent selective ion ejection in capillary liquid chromatography fourier transform ion cyclotron resonance for enhanced proteome characterization. Analytical chemistry. 2001;73(21):5052–5060. doi: 10.1021/ac010733h. [DOI] [PubMed] [Google Scholar]
- 32.Bruce JE, Anderson GA, Smith RD. "Colored" noise waveforms and quadrupole excitation for the dynamic range expansion of Fourier transform ion cyclotron resonance mass spectrometry. Analytical chemistry. 1996;68(3):534–541. doi: 10.1021/ac950823k. [DOI] [PubMed] [Google Scholar]
- 33.Pasa-Tolic L, Masselon C, Barry RC, Shen Y, Smith RD. Proteomic analyses using an accurate mass and time tag strategy. BioTechniques. 2004;37(4):621-4–626-33. doi: 10.2144/04374RV01. 636 passim. [DOI] [PubMed] [Google Scholar]
- 34.Spengler B. De novo sequencing, peptide composition analysis, and composition-based sequencing: a new strategy employing accurate mass determination by fourier transform ion cyclotron resonance mass spectrometry. Journal of the American Society for Mass Spectrometry. 2004;15(5):703–714. doi: 10.1016/j.jasms.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Abbatiello SE, Mani DR, Keshishian H, Carr SA. Automated detection of inaccurate and imprecise transitions in peptide quantification by multiple reaction monitoring mass spectrometry. Clin Chem. 2010;56(2):291–305. doi: 10.1373/clinchem.2009.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalski A, Cox J, Mann M. More than 100,000 detectable peptide species elute in single shotgun proteomics runs but the majority is inaccessible to data-dependent LC-MS/MS. J Proteome Res. 10(4):1785–1793. doi: 10.1021/pr101060v. [DOI] [PubMed] [Google Scholar]
- 37.Hather G, Higdon R, Bauman A, von Haller PD, Kolker E. Estimating false discovery rates for peptide and protein identification using randomized databases. Proteomics. 2010;10(12):2369–2376. doi: 10.1002/pmic.200900619. [DOI] [PubMed] [Google Scholar]
- 38.Ono M, Shitashige M, Honda K, Isobe T, Kuwabara H, Matsuzuki H, Hirohashi S, Yamada T. Label-free quantitative proteomics using large peptide data sets generated by nanoflow liquid chromatography and mass spectrometry. Mol Cell Proteomics. 2006;5(7):1338–1347. doi: 10.1074/mcp.T500039-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.Wang G, Wu WW, Zeng W, Chou CL, Shen RF. Label-free protein quantification using LC-coupled ion trap or FT mass spectrometry: Reproducibility, linearity, and application with complex proteomes. J Proteome Res. 2006;5(5):1214–1223. doi: 10.1021/pr050406g. [DOI] [PubMed] [Google Scholar]
- 40.Duan X, Young R, Straubinger RM, Page B, Cao J, Wang H, Yu H, Canty JM, Qu J. A straightforward and highly efficient precipitation/on-pellet digestion procedure coupled with a long gradient nano-LC separation and Orbitrap mass spectrometry for label-free expression profiling of the swine heart mitochondrial proteome. J Proteome Res. 2009;8(6):2838–2850. doi: 10.1021/pr900001t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.