Abstract
Neurodegenerative diseases, including Alzheimer’s disease (AD), target specific and functionally connected neuronal networks, raising the possibility that neurodegeneration may spread through abnormal patterns of neural network activity. AD is associated with high levels of amyloid-β (Aβ) peptides in the brain, synaptic depression, aberrant excitatory neuronal activity, and cognitive decline. However, the relationships among these alterations and their underlying mechanisms are poorly understood. In experimental models of AD, high concentrations of pathogenic Aβ assemblies reduce glutamatergic transmission and enhance long-term depression at the synaptic level. At the network level, they cause dysrhythmias, including neuronal synchronization, epileptiform activity, seizures, and postictal suppression. Both synaptic depression and aberrant network synchronization likely interfere with activity-dependent synaptic regulation, which is critical for learning and memory. Abnormal patterns of neuronal activity across functionally connected brain regions may also trigger and perpetuate trans-synaptic mechanisms of neurodegeneration. It remains to be determined if synaptic depression and network dysrhythmias are mechanistically related, which of them is primary or secondary, and whether normalization of one will prevent the other as well as cognitive dysfunction in AD.
INTRODUCTION
Alzheimer’s disease (AD) and other neurodegenerative diseases target specific, functionally connected neuronal networks (Braak and Braak 1991; Braak et al. 2003; Seeley et al. 2009), raising the possibility that neurodegeneration may spread through abnormal patterns of neural network activity (Fig. 1). Abnormal neuronal activity and firing patters could impair the formation of synapses and the regulation of synaptic strength, which is required for learning and memory and other forms of adaptive plasticity (for review, Turrigiano and Nelson 2004). Because both too much and too little stimulation can impair neuronal function and survival, neural network dysfunction could contribute directly to the neurodegenerative process, a notion that has important therapeutic and mechanistic implications.
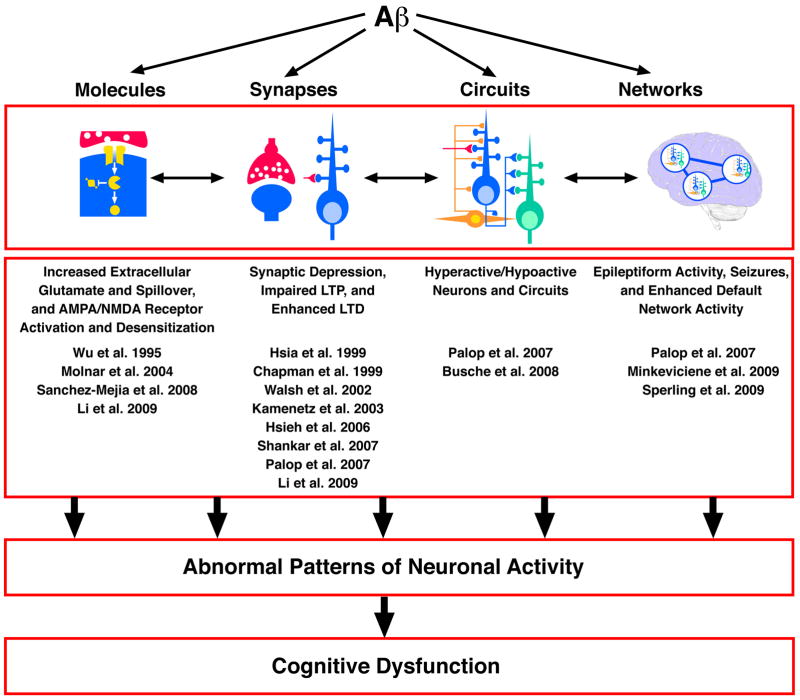
Figure 1. Aβ Can Affect Neuronal Processes at Multiple Levels of Complexity.
At the molecular level, Aβ may target membranes, receptors, transporters, or components of critical signaling cascades. At the synaptic level, Aβ reduces glutamatergic synaptic transmission and enhances long-term depression. At the circuit level, Aβ causes neurons to be hyperactive or hypoactive. At the network level, Aβ elicits dysrhythmias, including epileptiform activity. Although it is unlikely that Aβ exerts independent effects at each level of complexity, the extent to which these alterations are mechanistically related is currently unknown.
Activity-Driven Neuropathogenesis
The Hebbian notion that “neurons that fire together, wire together” is based on the observation that synchronization of pre- and postsynaptic firing activities strengthens synaptic connections, whereas mismatched firing weakens them. The end result is that neuronal activity patterns regulate synaptic strength and the functional output of neuronal circuits. This basic concept has potential implications for neurodegenerative disorders, which target specific, functionally connected neuronal networks (Braak and Braak 1991; Braak et al. 2003; Seeley et al. 2009). The effective regulation of synaptic connections and activities prevents overstimulation and ensures the production and uptake of vital growth factors (for review, Vicario-Abejon et al. 2002). Alterations that disrupt this regulation might result in the neuronal activity-dependent spread of neurodegeneration throughout vulnerable networks. This concept differs from the more conventional notion that pathogenic factors first and foremost cause the degeneration of neurons and synapses, and changes in network activity are secondary manifestations resulting from synaptic disconnections and circuit collapse. These possibilities are not mutually exclusive and could represent elements of the same vicious cycle.
In the framework of activity-driven neuropathogenesis, an abnormally active neuron might be every bit as disruptive to the function of the network as an abnormally silent one. Indeed, activity changes in just a single cortical pyramidal neuron can profoundly alter overall network activity and behavior (Li et al. 2009a). Three years ago, we hypothesized that destabilization of neuronal networks and compensatory responses contributes to cognitive impairments in AD and that such destabilization results, at least in part, from aberrant increases in neuronal activity induced by high Aβ levels (Palop et al. 2006). Since then, we demonstrated that high levels of Aβ elicit epileptiform activity and non-convulsive seizures in neocortical and hippocampal networks of human amyloid precursor protein (hAPP) transgenic mice (Fig. 2 and Palop et al. 2007). Epileptiform activity was also observed in hAPP/PS1 doubly transgenic mice (Minkeviciene et al. 2009). These Aβ effects may relate closely to the increased incidence of epileptic seizures seen in patients with AD (for review, Palop and Mucke 2009).
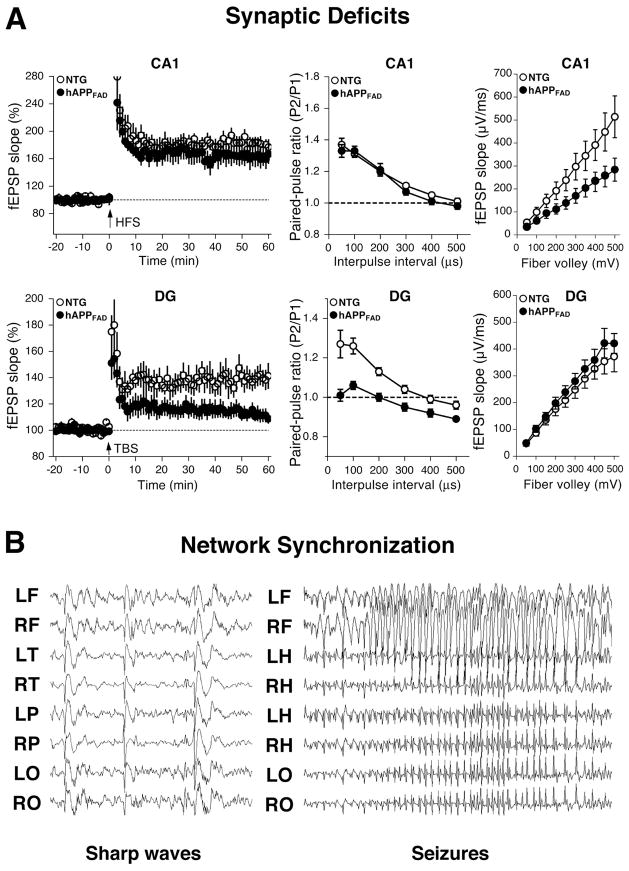
Figure 2. High Levels of Aβ Lead to Synapse-Specific Electrophysiological Deficits and Increased Network Synchronization in Animal Models.
A) hAPPJ20 mice with high levels of Aβ had different synaptic impairments in CA1 (top) and the dentate gyrus (DG) (bottom). At the Schaffer collateral to CA1 pyramidal cell synapse, long-term potentiation (LTP) (left) and paired-pulse facilitation (middle) were normal, whereas synaptic transmission strength (right) was impaired. At the perforant path to granule cell synapse in the DG, LTP (left) and paired-pulse modification (middle) were impaired, whereas synaptic transmission strength (right) was normal, as compared with NTG controls. B) Cortical and hippocampal EEG recordings demonstrating epileptiform spike discharges (left) and generalized non-convulsive seizures (right) in hAPPJ20 mice. fEPSP, field excitatory postsynaptic potentials; HFS, high frequency stimulation; TBS, theta-burst stimulation. L, left; R, right; F, frontal; T, temporal; P, parietal; O, posterior-parietal; and H, hippocampal. Adapted from (Palop et al., 2007).
Aberrant Excitatory Neuronal Activity, Synaptic Depression, and Homeostatic Responses
While pathogenic Aβ assemblies elicit aberrant excitatory activity at the network level, at specific excitatory synapses in the hippocampus, they cause synaptic depression, as reflected in impaired long-term potentiation, reduced synaptic transmission strength, and/or enhanced long-term depression (Chapman et al. 1999; Hsia et al. 1999; Kamenetz et al. 2003; Hsieh et al. 2006; Palop et al. 2007; Shankar et al. 2007; Li et al. 2009b). Whether these seemingly contradictory Aβ-dependent effects represent different elements of the same causal chain or independent branches of a more complex pathogenic web remains to be determined (Fig. 1). It is also unclear if the excitatory effects, suppressive effects, or both would have to be blocked to prevent Aβ-dependent cognitive dysfunction. In general, the relationship between cognitive/behavioral and electrophysiological abnormalities in AD and related mouse models remains to be elucidated (Palop et al. 2006; Palop and Mucke 2009).
Synaptic depression and aberrant excitatory network activity not only coexist in the context of high Aβ levels, but may also be causally linked in intricate ways (Table 1).
Table 1.
Are Synaptic Depression and Aberrant Excitatory Network Activity Mechanistically Related?
|
Particularly enticing to us is the possibility that depression of excitatory synaptic activity could lead to network disinhibition, if it affected inhibitory interneurons more than principal excitatory cells. Although this specific hypothesis has, to our knowledge, not yet been formally tested, several lines of evidence suggest that Aβ may indeed impair specific groups of inhibitory interneurons. For example, high levels of Aβ increase hippocampal and entorhinal levels of met-enkephalin, which could suppress the activity of inhibitory interneurons bearing μ-opioid receptors (Meilandt et al. 2008). Blocking these receptors with β-funaltrexamine (β-FNA) improved learning and memory of hAPP transgenic mice in the Morris water maze (Meilandt et al. 2008).
Another hypothesis we find attractive is that Aβ-induced increases in excitatory network activity lead to synaptic depression through homeostatic or compensatory mechanisms. In cultured neuronal networks, acute treatment with bicuculline, a GABAA antagonist, increases overall neuronal activity and firing rates. However, after a couple of days, neuronal activity returns to control levels. This homeostatic effect involves proportional decreases in miniature excitatory postsynaptic currents (mEPSC). Much evidence suggest that the firing rate is the key set point of these homeostatic responses (Turrigiano et al. 1998; Turrigiano and Nelson 2004). Surface receptors and synaptic strength may be homeostatically regulated to preserve a constant firing rate for each type of neuron.
Thus, Aβ-induced synaptic depression may be caused by a synaptic scaling mechanism that is responsive to the pro-excitatory effects of Aβ. Although little evidence suggests a pro-excitatory effect of Aβ at the synaptic level, Aβ acutely enhanced NMDA-dependent synaptic transmission in brain slices (Wu et al. 1995). Acute Aβ application also transiently increased the level of AMPA receptors on the neuronal surface, as well as the frequency of spontaneous excitatory postsynaptic currents (Fig. 3 and Sanchez-Mejia et al. 2008). In contrast, prolonged exposure of neurons to Aβ decreases surface levels of AMPA and NMDA receptors and suppresses synaptic transmission or plasticity (Walsh et al. 2002; Cleary et al. 2005; Snyder et al. 2005; Hsieh et al. 2006; Townsend et al. 2006; Shankar et al. 2007), raising the possibility of compensatory mechanisms. Consistent with this possibility, a recent study suggested that Aβ-induced synaptic depression results from an impairment of neuronal glutamate transporters, increased extracellular glutamate concentrations, and enhanced activation and desensitization of NMDA receptors (Li et al. 2009b). Aβ also induced AMPA receptor desensitization, as well as spiking activity in pyramidal neurons, when AMPA receptor desensitization was prevented with cyclothiazide (Li et al. 2009). Thus, Aβ-induced synaptic depression may result from homeostatic mechanisms, and failure of such mechanisms might contribute to neuronal hyperactivity.
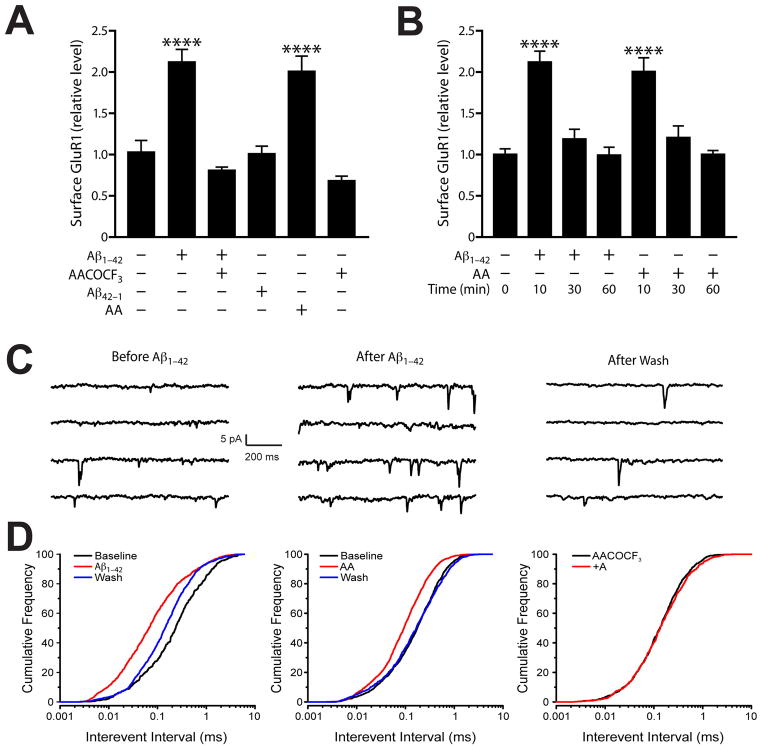
Figure 3. Acute Aβ Treatment Increases Surface AMPA Receptor Levels and Excitatory Synaptic Currents.
A–B) Surface levels of GluR1 were assessed by biotinylation assay at 0, 10, 30, or 60 min after addition of Aβ or arachidonic acid (AA) in primary neuronal cultures. Aβ and AA acutely increased surface levels of GluR1 at 10 min, but surface receptor levels returned to baseline after 30 and 60 min of Aβ or AA exposure (B). Inhibiting phospholipase A2, which releases AA from phospholipids, with AACOCF3 blocked the Aβ-induced increase in surface GluR1 levels (A). C–D) Brain slices were prepared from NTG mice. Neuronal activity was recorded from layer 5 pyramidal neurons in the presence of GABAzine (10 μM). C) Representative spontaneous excitatory postsynaptic currents (sEPSCs) traces recorded from a single voltage-clamped neuron before (left) and 5 min after (middle) application of Aβ, and 7 minutes after washout (right). For each condition, four segments of a continuous trace are shown. Note the increased frequency of sEPSCs after the Aβ treatment. D) Cumulative histograms demonstrating that sEPSC interevent intervals were reversibly decreased by Aβ (left) or AA (middle) and that the Aβ effect could be blocked by AACOCF3 (right). Adapted from (Sanchez-Mejia et al., 2008).
Homeostatic mechanisms exist at many different levels of complexity, from receptors to circuits and networks. hAPP mice with epileptiform activity in the neocortex and hippocampus show a profound remodeling of neuronal circuits in the hippocampus (Fig. 4), suggesting active efforts to suppress aberrant excitatory neuronal activity. These changes include ectopic expression of neuropeptide Y (NPY) by granule cells, sprouting of GABAergic terminals in the outer molecular layer, mossy fiber sprouting onto inhibitory basket cells, increases in the frequency and amplitude of granule cell miniature inhibitory postsynaptic currents (mIPSCs), and reductions of calbindin levels in granule cells similar to those seen in humans with AD (Palop et al. 2003; Palop et al. 2007). The struggle of compensatory inhibitory mechanisms against Aβ-induced aberrant excitation may delay network failure but result in profound fluctuations in neuronal activities. This hypothesis is supported by the following observations. While a small number of hAPP mice had massive increases in the expression of the neuronal activity marker Arc in granule cells, suggesting recent seizure activity, the majority of them had suppressed Arc expression, including a complete block to novelty-induced Arc induction (Palop et al. 2005, 2007). Furthermore, non-convulsive seizures in these mice were followed by episodes of marked postictal suppression (Palop et al. 2007). In the cortex of hAPP/PS1 transgenic mice, Busche et al. (2008) identified comparable proportions of abnormally hyperactive or hypoactive neurons.
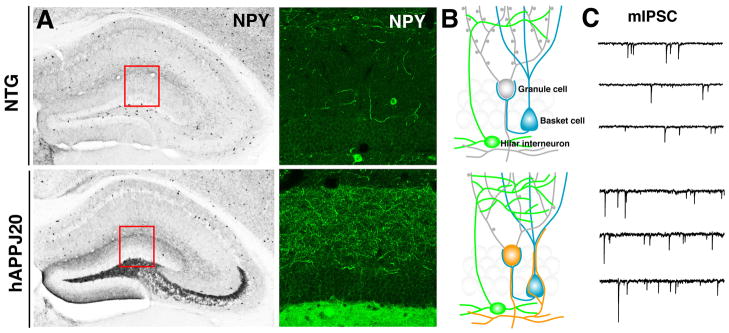
Figure 4. Inhibitory Hippocampal Remodeling and Increased Inhibition in hAPPJ20 Mice.
hAPPJ20 mice show robust remodeling of inhibitory circuits in the dentate gyrus, a brain region critically involved in learning and memory. A) Compared with NTG controls (top), hAPPJ20 mice (bottom) had increased NPY/GABAergic axons in the molecular layer and ectopic expression of NPY in mossy fibers. B) Diagram depicting axonal remodeling in hAPPJ20 mice, including GABAergic sprouting (green) onto granule cells (grey) and sprouting of NPY-positive mossy fibers (orange) onto basket cells (blue). C) Enhanced inhibitory input (mIPCS) onto granule cells in hAPPJ20 mice. Adapted from (Palop et al., 2007).
Taken together, these findings suggest that chronic or repeated Aβ-induced increases in excitatory activity result in profound morphological, biochemical, and neurophysiological alterations in neural networks. Many of these alterations likely represent compensatory or adaptive efforts to suppress neuronal overexcitation and, thus, may fulfill beneficial functions. However, at the same time, they may interfere with the agility of normal excitatory processes required for learning and memory.
Both Aβ and Apolipoprotein E4 May Promote Aberrant Increases in Neuronal Network Activity in Humans
As outlined above, Aβ can cause aberrant increases in neuronal activity in both acute and chronic models of AD. Interestingly, increased neuronal activity and epilepsy increase the production, release, and/or accumulation of Aβ (Mackenzie and Miller 1994; Kamenetz et al. 2003; Cirrito et al. 2005, 2008), raising the possibility of a vicious cycle in which Aβ promotes its own production through alterations in neuronal network activity.
Several studies indicate that Aβ deposition and aberrant excitatory neuronal activity are closely associated in cortical regions collectively known as the default network (Buckner et al. 2005; Mintun et al. 2006; Seeley et al. 2009; Sperling et al. 2009). The default network consists of functionally interconnected brain regions that are active during resting states and deactivated during many cognitive tasks (for review, Buckner et al. 2008). It includes the frontal, posterior-parietal, cingulate, and restrosplenial cortices. Interestingly, humans with AD or mild cognitive impairment (MCI) fail to fully deactivate the default network during diverse cognitive tasks (Lustig et al. 2003; Petrella et al. 2007; Pihlajamaki et al. 2008). Moreover, MCI patients showed aberrant activation in the lateral parietal and posterior cingulate cortex, and this aberrant activity correlated well with the amount of amyloid deposition in these regions (Sperling et al. 2009). It is worth noting in this context that hyperactive neurons in hAPP/PS1 mice tended to be located in close proximity to Aβ deposits (Busche et al. 2008).
Once AD has become manifest, it is difficult to determine if increases in network activity are a cause or consequence of Aβ accumulation and neurodegeneration. It is interesting in this regard that cognitively normal carriers of the APOE e4 allele, the main genetic risk factor for AD, showed abnormal increases in neural network activity when given working memory tasks (Wishart et al. 2006). It remains to be determined if the increased network activity seen in the context of high Aβ levels or apoE4 represents convergent and primary effects of these copathogens or compensatory efforts of the brain to overcome early impairments in neuronal functions. As discussed below, resolving this question has important therapeutic implications.
Could the Prevention of Aberrant Network Activity Prevent Cognitive Decline and Neurodegeneration in AD?
At a cell-autonomous level, aberrant excitatory activity could result in energy depletion, particularly if mitochondrial functions were already impaired at baseline. Notably, both Aβ and apoE4 can impair mitochondrial functions (Keller et al. 1997; Chang et al. 2005; Du et al. 2008; Mattson et al. 2008; Wang et al. 2008; Cho et al. 2009). At the circuit or network level, excessive release or accumulation of glutamate could result in excitotoxic damage and calcium dysregulation (Mattson et al. 1992; Mark et al. 1995; Rothman and Olney 1995; Bezprozvanny and Mattson 2008). Blocking Aβ-induced excesses in excitatory neuronal activity might prevent both cell-autonomous and trans-synaptic pathogenic consequences. But how can this feat be achieved? One approach to preventing excitotoxicity has been to block NMDA receptors with memantine (Lipton 2005; Kotermanski and Johnson 2009), a drug approved for use in AD. However, the therapeutic benefits of memantine in AD have been limited (for example, Porsteinsson et al. 2008), and this drug may not block the primary upstream mechanisms by which Aβ elicits aberrant excitatory neuronal activity. Indeed, alterations in NMDA receptor function may be only one of several processes triggered by these elusive mechanisms. Recent studies have made some headway toward their identification.
Reducing the levels of wildtype endogenous tau increased the resistance of non-transgenic mice to chemically induced seizures (Roberson et al. 2007). Consistent with the idea that Aβ impairs cognitive functions by eliciting aberrant excitatory activity, tau reduction also effectively prevented Aβ-induced cognitive deficits and aberrant excitatory neuronal activity in hAPP transgenic mice (Roberson et al. 2007 and unpublished results). Like Aβ, arachidonic acid acutely increases neuronal excitability (Fig. 3 and Sanchez-Mejia et al. 2008). Lowering arachidonic acid levels by reducing the activity of group IVA phospholipase A2 also prevented Aβ-dependent cognitive deficits in hAPP mice (Sanchez-Mejia et al. 2008). Aβ might also trigger aberrant neuronal activity by binding to the receptor for advanced glycation end products (Origlia et al. 2008), α7-nicotinic acetylcholine receptor (Dineley et al. 2001; Snyder et al. 2005), or prion protein (Lauren et al. 2009); impairments of astroglial or neuronal glutamate transporters (Harris et al. 1995; Keller et al. 1997; Masliah et al. 2000; Li et al. 2009b), glucose transporters (Keller et al. 1997), ATP transporters (Bezprozvanny and Mattson 2008), or ion-motive ATPase activities (Mark et al. 1995); and the formation of ion-fluxing pores in neuronal membranes (Yoshiike et al. 2007).
What effects these proximal actions of Aβ have on overall network activity will critically depend on whether Aβ blocks or activates the proteins it interacts with and which type of cell (e.g., inhibitory or excitatory) it affects the most. Additional studies are needed to address these questions and to determine the relative pathogenic importance of the processes described above, the extent to which they may be interconnected, and their potential as drug targets in the treatment and prevention of AD.
Acknowledgments
This work was supported by a Stephen D. Bechtel, Jr. Foundation Young Investigator Award to J.J.P. and by National Institutes of Health Grants AG022074 and NS041787 to L.M. We thank B. Halabisky and R. Sanchez-Mejia for helpful comments on the manuscript, G. Howard and S. Ordway for editorial review, and E. Juarez and M. Dela Cruz for administrative assistance.
References
- Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Chang S, Ma TR, Miranda RD, Balestra ME, Mahley RW, Huang Y. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci USA. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-b levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. b-Amyloid activates the mitogen-activated protein kinase cascade via hippocampal a7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer’s disease. J Neurosci. 2001;21:4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ME, Carney JM, Cole PS, Hensley K, Howard BJ, Martin L, Bummer P, Wang Y, Pedigo NWJ, Butterfield DA. b-Amyloid peptide-derived, oxygen-dependent free radicals inhibit glutamate uptake in cultured astrocytes: Implications for Alzheimer’s disease. Neuroreport. 1995;6:1875–1879. doi: 10.1097/00001756-199510020-00013. [DOI] [PubMed] [Google Scholar]
- Hsia A, Masliah E, McConlogue L, Yu G, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport, and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid b-peptide: Role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997 doi: 10.1046/j.1471-4159.1997.69010273.x. In press. [DOI] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer's drug memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Poo MM, Dan Y. Burst spiking of a single cortical neuron modifies global brain state. Science. 2009a;324:643–646. doi: 10.1126/science.1169957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009b;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. The molecular basis of memantine action in Alzheimer's disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res. 2005;2:155–165. doi: 10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol. 1994;87:504–510. doi: 10.1007/BF00294177. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Hensley K, Butterfield DA, Mattson MP. Amyloid beta-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci. 1995;15:6239–6249. doi: 10.1523/JNEUROSCI.15-09-06239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Alford M, Mallory M, Rockenstein E, Moechars D, Van Leuven F. Abnormal glutamate transport function in mutant amyloid precursor protein transgenic mice. Exp Neurol. 2000;163:381–387. doi: 10.1006/exnr.2000.7386. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. b-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilandt WJ, Yu G-Q, Chin J, Roberson ED, Palop JJ, Wu T, Scearce-Levie K, Mucke L. Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2008;28:5007–5017. doi: 10.1523/JNEUROSCI.0590-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Origlia N, Righi M, Capsoni S, Cattaneo A, Fang F, Stern DM, Chen JX, Schmidt AM, Arancio O, Yan SD, Domenici L. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J Neurosci. 2008;28:3521–3530. doi: 10.1523/JNEUROSCI.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J, Chin J, Roberson E, Wang J, Thwin M, Bien-Ly N, Yoo J, Ho K, Yu G-Q, Kreitzer A, Finkbeiner S, Noebels J, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer’s disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Bien-Ly N, Massaro C, Yeung BZ, Yu G-Q, Mucke L. Vulnerability of dentate granule cells to disruption of Arc expression in human amyloid precursor protein transgenic mice. J Neurosci. 2005;25:9686–9693. doi: 10.1523/JNEUROSCI.2829-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, BJ, Kekonius L, Chin J, Yu G-Q, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JR, Prince SE, Wang L, Hellegers C, Doraiswamy PM. Prognostic value of posteromedial cortex deactivation in mild cognitive impairment. PLoS One. 2007;2:e1104. doi: 10.1371/journal.pone.0001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, DePeau KM, Blacker D, Sperling RA. Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:283–292. doi: 10.1097/JGP.0b013e318162a0a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT. Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5:83–89. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu G-Q, Mucke L. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor - still lethal after eight years. Trends Neurosci. 1995;18:57–58. doi: 10.1016/0166-2236(95)93869-y. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mejia RO, Newman JW, Toh S, G-QY, Zhou Y, Halabisky B, Cisse M, Scearce-Levie K, Cheng IH, Gan L, Palop JJ, Bonventre JV, Mucke L. Phospholipase A2 reduction ameliorates cognitve deficits in mouse model of Alzheimer’s disease. Nat Neurosci. 2008;11:1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: A potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci. 2002;3:965–974. doi: 10.1038/nrn988. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA. 2008;105:19318–19823. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, Mamourian AC, Belloni DR, Rhodes CH, McAllister TW. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am J Psychiatry. 2006;163:1603–1610. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]
- Wu J, Anwyl R, Rowan MJ. beta-Amyloid selectively augments NMDA receptor-mediated synaptic transmission in rat hippocampus. Neuroreport. 1995;6:2409–2413. doi: 10.1097/00001756-199511270-00031. [DOI] [PubMed] [Google Scholar]
- Yoshiike Y, Kayed R, Milton SC, Takashima A, Glabe CG. Pore-forming proteins share structural and functional homology with amyloid oligomers. Neuromolecular Med. 2007;9:270–275. doi: 10.1007/s12017-007-0003-6. [DOI] [PubMed] [Google Scholar]