Abstract
BACKGROUND
Amelogenins are highly conserved proteins secreted by ameloblasts in the dental organ of developing teeth. These proteins regulate dental enamel thickness and structure in humans and mice. Mice that express an amelogenin transgene with a P70T mutation (TgP70T) develop abnormal epithelial proliferation in an amelogenin null (KO) background. Some of these cellular masses have the appearance of proliferating stratum intermedium, which is the layer adjacent to the ameloblasts in unerupted teeth. As Notch proteins are thought to constitute the developmental switch that separates ameloblasts from stratum intermedium, these signaling proteins were evaluated in normal and proliferating tissues.
METHODS
Mandibles were dissected for histology and immunohistochemistry using Notch I antibodies. Molar teeth were dissected for western blotting and RT-PCR for evaluation of Notch levels through imaging and statistical analyses.
RESULTS
Notch I was immunolocalized to ameloblasts of TgP70TKO mice, KO ameloblasts stained, but less strongly, and wild-type teeth had minimal staining. Cells within the proliferating epithelial cell masses were positive for Notch I and had an appearance reminiscent of calcifying epithelial odontogenic tumor with amyloid-like deposits. Notch I protein and mRNA were elevated in molar teeth from TgP70TKO mice.
CONCLUSION
Expression of TgP70T leads to abnormal structures in mandibles and maxillae of mice with the KO genetic background and these mice have elevated levels of Notch I in developing molars. As cells within the masses also express transgenic amelogenins, development of the abnormal proliferations suggests communication between amelogenin producing cells and the proliferating cells, dependent on the presence of the mutated amelogenin protein.
Keywords: abnormal proliferation, amelogenin, dental enamel, Notch I, null and transgenic mice
Introduction
The amelogenins are highly conserved proteins secreted by ameloblast cells within the enamel organ of developing teeth. These proteins are responsible for generating the full thickness of the enamel layer, as well as its intricate structure of decussating enamel rods (1–3). Amelogenins are secreted prior to eruption of the teeth into the oral cavity, and the enamel proteins are proteolytically processed in an organized manner by enamel proteases (4, 5). Simultaneous with enamel protein breakdown and removal, abundant amounts of mineral ions enter the enamel organic matrix and form crystals. These changes lead to the highly organized mineralized enamel layer in teeth that have erupted into the oral cavity. Enamel is the hardest tissue in the body and because ameloblast cells are lost as teeth erupt, this tissue must last throughout life without benefit of repair.
The X-chromosomal amelogenin (Amelx) null mouse (KO) has a deletion in its Amelx gene and therefore expresses none of the amelogenin proteins; these mice have defective dental enamel (6). Two strains of transgenic mice have also been generated that express either the abundant 180-amino acid amelogenin (TgM180), or M180 with a proline-to-threonine (TgP70T) mutation (7). This point mutation was originally identified in some human kindreds with the enamel defect amelogenesis imperfecta (8, 9). TgM180 mouse teeth have a relatively normal enamel phenotype; however, TgP70T mice have an enamel defect similar to that seen in humans with the same mutation (7).
When KO females were mated with TgM180 males, male offspring were either positive or negative for the transgene on a KO background, because of the location of the Amelx gene solely on the X-chromosome in mice. Male pups that were TgM180 positive on a KO background (TgM180KO) revealed partial rescue of the hypoplastic enamel KO phenotype in both enamel thickness and organization (10). However, TgP70T expression on a KO background did not lead to phenotypic rescue, but to abnormal proliferation of the stratum intermedium layer, and some regions within the resulting cellular masses were amelogenin protein positive by immunohistochemistry (7). The histologic findings were comparable to that seen in human calcifying epithelial odontogenic tumors (CEOT) that are rare, benign epithelial odontogenic tumors that are thought to be derived from stratum intermedium, which is the cell layer adjacent to the ameloblasts (11, 12).
The development of a tooth depends on sequential and reciprocal interactions between epithelial and mesenchymal layers (13). The Notch proteins are members of a conserved signaling pathway operating between cells that are in direct contact, and are thought to influence developmental decisions (14). In developing enamel organ cells, those cells destined to become stratum intermedium, the layer lying at the proximal end of the highly elongated ameloblast cells, become positive for Notch 1, whereas ameloblast cell precursors and ameloblasts themselves remain Notch negative (15, 16). Notch expression has also been detected in ameloblastomas and therefore it has been hypothesized that Notch signaling may regulate cell proliferation and differentiation of both normal and neoplastic odontogenic cells (17). Several of the amelogenins have been reported to have signaling functions as well (18).
The goal of our study was to evaluate Notch 1 in the ameloblast/stratum intermedium cell layers during enamel development in KO mice and in TgP70TKO proliferating epithelial masses and developing molars, to identify the potential role of Notch in the formation of these abnormal structures. Insight into the development of proliferating masses in these mice may lead to a greater understanding of stratum intermedium biology and the normal and aberrant roles of amelogenin proteins.
Materials and methods
Animal models
The generation of the Amelx KO, TgP70T and TgP70TKO mice was described previously (6, 7, 10) (Table 1). Mice were housed in an AAALAC accredited facility, and procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Table 1.
Mouse models
| Model | Description | Ref. |
|---|---|---|
| WT | Wild-type mouse | |
| TgM180 | Transgenic mouse expressing a 180 amino acid amelogenin | (7) |
| TgP70T | Transgenic mouse expressing a 180 amino acid amelogenin with a proline-to-threonine change at codon 70 | |
| KO | Amelogenin null mouse | (6) |
| Het | Female heterozygous for endogenous amelogenin gene | (10) |
| TgP70Thet | Similar to Het female, but with TgP70T | |
| TgP70TKO | Similar to KO male, but with TgP70T |
As the murine Amelx gene is found solely on the X-chromosome, we were able to mate KO females with transgenic males to generate male offspring that are either positive (TgP70TKO) or negative (KO) for the transgene. Females generated from this cross are heterozygous for the amelogenin gene and positive (TgP70Thet) or negative (het) for the transgene. Heterozygous females therefore have one normal amelogenin gene rather than two. Wild-type (WT) mice were used as controls.
DNA analysis
TgP70T transgenic males were mated with KO females. Sex of offspring was determined by dissection to identify ovary or testis. Genomic DNA was isolated from tail tissue and used for PCR with primers G929 and G911, and verified with primers G946 (19) and 733 A to identify transgenic animals.
733A: 5′-AATGGGGACCTGGATTTTGTTTGCCTGC
Histology and immunohistochemisry
Mandibles and maxillae from 3- to 4-day-old WT and the four types of KO x TgP70T offspring described above were fixed in 4% paraformaldehyde in PBS overnight at 4°C, decalcified, and embedded in paraffin. Consecutive 6-μm paraffin sections were prepared on Fisher ProbeOn Plus slides (Thermo Fisher Scientific Inc., Rockford, IL, USA). For each sample, one of the sections was stained with hematoxylin and eosin (H&E). The other sections were incubated with 3% H2O2 in PBS, followed by an overnight incubation at 4°C with primary antibody to Notch 1 (sc-6014; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1:50 dilution in 1.5% normal blocking serum in PBS, pH 7.4. Control sections were incubated with PBS. After several washes, the sections were incubated with biotinylated donkey anti-goat antibody (sc-2020; Santa Cruz Biotechnology) at a 1:200 dilution in PBS, washed again and incubated with avidin-biotin-peroxidase complex (Santa Cruz Biotechnology). Peroxidase activity was visualized with diaminobenzidine.
Western blot
For each strain, two mandibular first molars from 4-day-old pups were dissected and extracted in lysis buffer (10 mM Tris pH 7.6, 100 mM NaCl, 2 mM EDTA, 0.5% CHAPS) containing protease inhibitor cocktail (Roche Diagnostics GmbH, mannheim, Germany). Samples were homogenized, incubated for 30 min, and centrifuged 16 000 g for 20 min. Protein concentration was determined using the Bradford method, and 20 μg of each extract was loaded onto a 4–20% Tris-HEPES-SDS polyacrylamide gel (Thermo Fisher Scientific Inc.) followed by electrophoresis and transfer to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). Membranes were blocked with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h at room temperature and then incubated with Notch 1 antibody (sc-6014; Santa Cruz Biotechnology) at 1:200 dilution overnight at 4°C. Blots were incubated with horseradish peroxidase-conjugated donkey anti-goat secondary antibodies at a dilution of 1:4000 (sc-2020; Santa Cruz Biotechnology) for 1 h at room temperature and signals were detected using the ECL chemiluminescence detection system (SuperSignal® West Duration Substrate; Amersham Biosciences, Piscataway, NJ, USA). Blots were reprobed with anti-actin antibody (A2103; Sigma-Aldrich, st. Louis, MO, USA) as a control to normalize for protein loading without stripping. Band intensities were compared using ImageJ software (20).
RT-PCR
For each sample, two mandibular first molars were dissected from 3- to 4-day-old pups. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA was synthesized at 42°C for 50 min using Oligo (dT) 15 primer and SuperScript® III reverse transcriptase (Invitrogen). Polymerase chain reaction amplification with primers G1110 (forward, in Notch 1 exon 29) and G1111 (reverse, in Notch 1 exon 31) was performed for 32 cycles of 94°C for 1 min, 59°C for 1 min and 72°C for 2 min for semi-quantitative RT-PCR.
G1110: 5′-GAAGACCTGGAGACCAAGAAG
G1111: 5′-GAAGTCAGAGATGACAGCAGG
β-actin primers 738 and 739 were used in PCR reactions for the positive control (21).
The products were visualized on 2% agarose gels after ethidium bromide staining. For every reaction, a sample lacking reverse transcriptase was included as the negative control. Expected PCR products were 294 bp for Notch 1 and 353 bp for β-actin. Band intensities were quantified using ImageJ software and subsequently normalized by dividing the band density of the target gene by the intensity of its corresponding β-actin product, with analyses in triplicate.
Statistical analysis
The normalized band intensities were converted into a ratio and reported as means ± SE of the mean. The WT, KO male and het female samples all lacking the P70T transgene were combined to increase n for statistical analysis. ANOVA with Tukey’s Multiple Comparison test was used to indicate difference between groups with the level of significance at P < 0.05 (GraphPad 5.01; GraphPad Software Inc., La Jolla, CA, USA).
Results
Immunohistochemical analysis of Notch 1 in 3-day murine mandibles
Male TgP70T mice were mated with female KO mice, and the pups were analyzed by PCR for transgene status. Offspring were TgP70TKO males, null males without the transgene (KO), heterozygous females with the transgene (TgP70Thet) and heterozygous females without the transgene (het). As the endogenous amelogenin gene is located solely on the X-chromosome in mice, pups that were transgene-positive on the KO background could be easily identified.
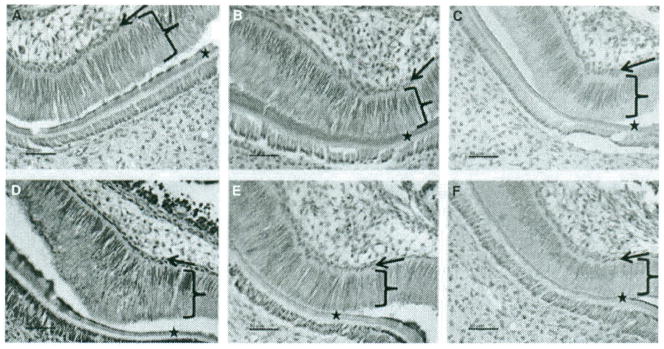
Mice with each genotype were sacrificed, and the jaws were fixed, embedded, sectioned, and stained with H&E or with Notch 1 antibody. Using H&E stain, the tall ameloblasts are shown with a thin layer of secreted enamel matrix adjacent to a thin layer of dentin and the odontoblast cell layer (Fig. 1A,B). Notch 1 had been immunolocalized to stratum intermedium cells, but not ameloblasts during fetal and early postnatal molar development in WT mice (15), in agreement with our results (not shown). In 3–4 day old TgP70TKO first molars, Notch 1 was localized to ameloblasts (bracket, Fig. 1D) and the adjacent stratum intermedium cells (arrow, Fig. 1D), with fainter staining in KO (Fig. 1E). Staining of WT and the negative control lacking primary antibody were negligible (Fig. 1C,F). TgP70TKO stained stronger than KO, and KO was more strongly stained than WT.
Figure 1.
Histology and immunohistochemistry for Notch 1 in first molars from 3-day-old WT and transgenic/KO mice. (A, D) TgP70TKO male; (B, E) KO male; (C, F) WT. (A, B) hematoxylin and eosin (H&E) stain; (D–F) immunohistochemistry using Notch 1 antibody; (C) negative control lacking primary antibody. Ameloblasts are indicated with brackets, stratum intermedium with arrows and the secreted enamel and dentin developing layers by asterisks. Magnification bars are indicated.
Histologic and immunohistochemical analysis of Notch 1 in abnormal epithelial cell masses in 4-week-old offspring from the KO/TgP70T transgene cross
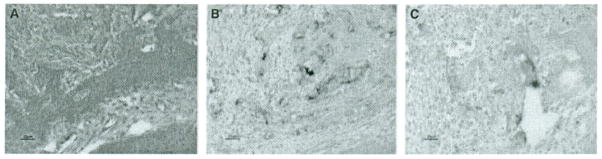
In these cellular masses, H&E staining revealed that the epithelial cells showed marked nuclear pleomorphism (Fig. 2A) and intercellular bridges (not shown). Amyloid-like material characteristic of CEOT was observed within the sheets or islands of epithelial cells (Fig. 2A,B), but the abnormal cells do not exactly parallel the phenotype seen in human CEOT as the secreted products do not stain with congo red (not shown). Notch 1 positive staining was seen in the abnormal tissues (Fig. 2B), whereas the negative control, which was incubated without primary antibody, did not stain, except for minor background (Fig. 2C). Abnormal epithelial cells were observed in upper or lower jaws of the TgP70TKO mice, and abnormal proliferation of stratum intermedium could be detected in mice with this genotype as young as 4 days old (7).
Figure 2.
Histology and immunohistochemistry for Notch 1 in consecutive sections through an abnormal cellular mass from a 4-week-old TgP70TKO mouse. (A) H&E stain; (B) immunohistochemistry using Notch 1 antibody; (C) negative control lacking primary antibody. Magnification bars are indicated.
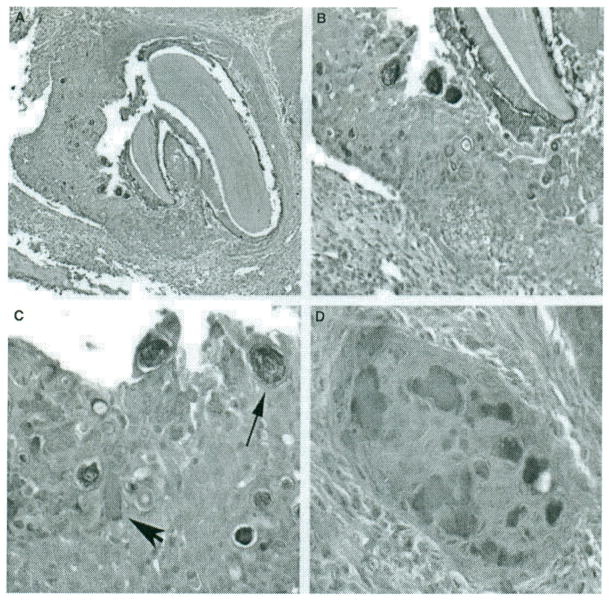
The appearance of abnormal cellular structures is illustrated in Fig. 3. Abnormal cells adjacent to a developing tooth in the left mandible of a 4-week-old TgP70Thet female mouse (A) are shown at higher magnification (B,C) where additional abnormal structures can be discerned (B,C). An island of epithelium, including some amorphous material in the right maxilla of the same mouse, is shown (D). As a Congo red stain did not highlight the amorphous substance in any of the samples tested (not shown), the exact nature of this material is currently unknown.
Figure 3.
The P70T transgene induces an aberrant odontogenic epithelial cell proliferation reminiscent of calcifying epithelial odontogenic tumor. (A) A dense sheet of epithelial cells is seen surrounding a developing tooth (H&E. 40x) within the left mandible of a TgP70Thet female mouse. (B and C) Proliferation of epithelial cells with interspersed Liesegang ring-like psammomatoid calcifications (arrow) and amorphous material resembling amyloid (arrowhead). (B) magnification. 100x; (C) magnification, 400x. (D) An island of epithelium containing aggregates of the amorphous material (400x), taken from a section of the right maxilla.
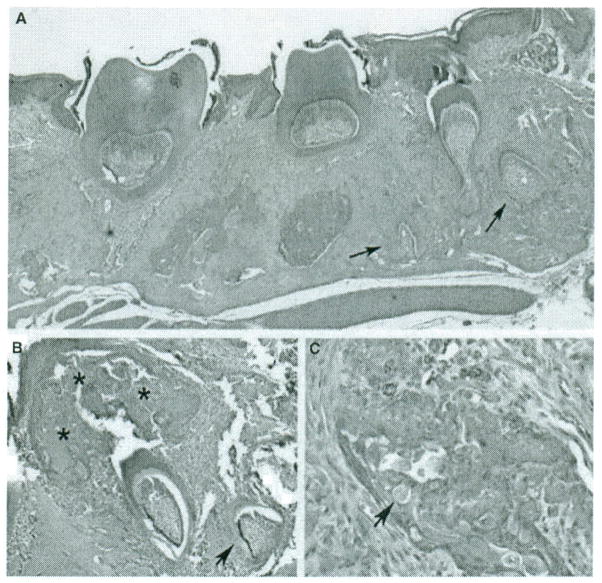
In male TgP70TKO mice, supernumerary teeth can develop by 4 weeks of age (arrows in Fig. 4A) in addition to the molars that have already erupted, a phenomenon we have observed in several individual mice with this genotype. In Fig. 4B, a benign epithelial proliferation is indicated by asterisks adjacent to a developing tooth. In addition, a mass with cells showing similarity to ghost cells frequently seen in human COC (calcifying odontogenic cyst) is shown in Fig. 4C.
Figure 4.
Introduction of mutant amelogenin disrupts normal odontogenic development. (A) Supernumerary teeth (arrows) were observed in the right maxilla of a male TgP70TKO mouse (H&E, 40x). (B) An aberrant benign epithelial proliferation (*) was noted overlying the crown of a developing tooth in a 4-week-old TgP70Thet female. Aggregates of calcified, enameloid-type material were scattered throughout the epithelium (40x). (C) Island of epithelium containing ghost cells (arrow) and the calcified substance (100x) from the mouse shown in B.
All mice with this genotype examined to date from 4–5 days to 6 weeks of age have abnormal proliferations, including males with a null Amelx background and females with a heterozygous Amelx background. These structures appear in both maxillae and mandibles and occasionally include supernumerary dental structures, but are not seen in TgP70T transgenic mice with a normal complement of amelogenin proteins.
Quantitative analysis of Notch 1 expression by western blot
To determine Notch 1 protein levels in transgenic mice, we performed western blot analysis of extracts from developing first molar teeth of 3- to 4-day-old pups which were offspring of KO females mated to TgP70T males, and age-matched WT pups. WT males and females, het females and KO males had similar low levels of Notch 1. P70TKO male mice had nearly twice the amount of Notch 1 as the group mentioned above (P < 0.05) and het female mice with the transgene had an intermediate level of Notch 1 (Fig. 5). This western approach was carried out five times using different litters of mice, with substantially the same results. Variation could be expected in the het female mice due to lyonization of X-chromosomal Amelx gene expression.
Figure 5.
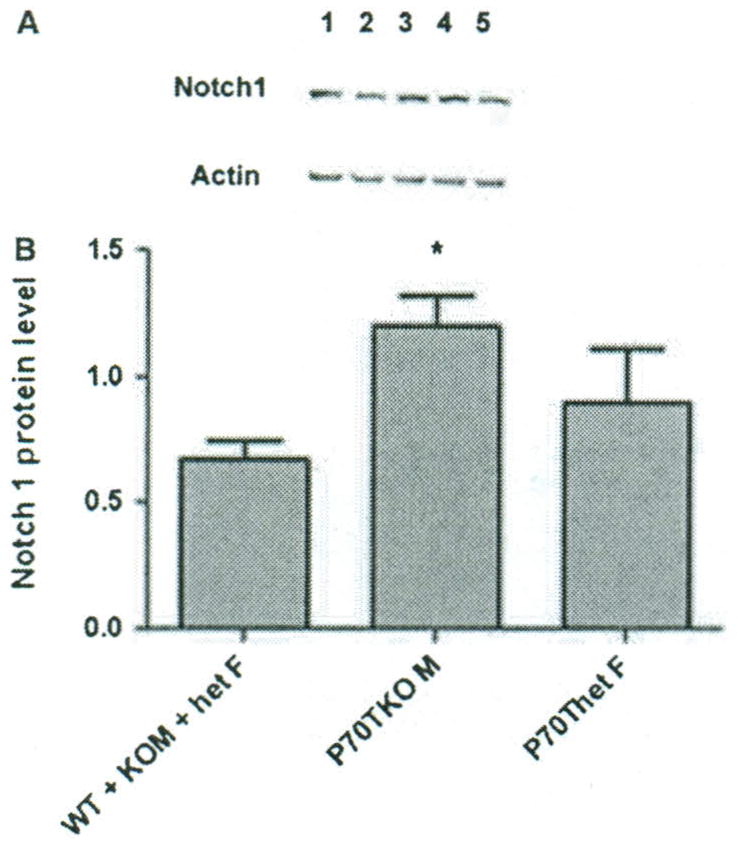
Western blot for comparison of Notch 1 protein in first molars. (A) Typical western blot in which identical amounts of extract from first mandibular molars were loaded onto the gel, and transfer to nitrocellulose was followed by probing with Notch 1 antibody. Subsequently the blot was probed with anti-actin antibody without stripping the membrane for normalization. Lane 1: TgP70TKO male; lane 2: KO male; lane 3: TgP70Thet female; lane 4: het female; lane 5, WT. (B) The histogram shows mean band intensities for Notch 1 in five experiments ± SEM. *A statistically significant difference between P70TKO male samples and the combined group of male and female wild-type. KO males and het females, lacking the transgene.
A similar approach was used to measure Notch 1 mRNA in first molar teeth by RT-PCR using the same genotypic strains as described above, with normalization by β-actin mRNA levels. Although the trend was identical, the differences did not meet the required level of significance (not shown).
Discussion
The amelogenins are enamel proteins that regulate the thickness and intricate structure of the dental enamel layer. We describe a mouse model for the human enamel defect amelogenesis imperfecta that unexpectedly develops abnormal epithelial structures in the presence of an amelogenin transgene with a single proline-to-threonine change, which is associated with elevated Notch 1 protein. Notch 1 is present at higher levels in molar teeth from TgP70TKO males compared with any of the other genotypes generated by these crosses, or by WT.
In various tissues, Notch signaling can affect proliferation, differentiation, and apoptosis, and can influence organogenesis and morphogenesis (14, 22). Notch 1, 2, 3 and 4 receptors have been identified in vertebrates, and at least seven membrane-bound ligands (JAGGED 1,2. DLL 1,3,4, DNER, contactin) have been recognized (23, 24). The Notch receptors and ligands are single-pass membrane proteins and the ligands on adjacent cells bind to epidermal growth factor-like repeats in the Notch receptor extracellular domain. This leads to several cleavages, and the Notch intracellular domain then migrates to the nucleus and associates with transcriptional co-activators to activate or inhibit developmental processes depending on the context (14).
In developing teeth, expression of Notch receptors and Dll1 ligand by two adjacent cell layers suggested a role for Delta–Notch signaling during odontoblast and ameloblast differentiation (25). Immunohistochemistry and in situ hybridization indicated that stratum intermedium cells express the Notch 1 protein and Notch target Hes 1 raRNA, whereas ameloblasts and their precursors also express another Notch receptor, Jagged 1 (16). Studies using DiI tracers in rat incisor organ culture suggested that stratum intermedium cells originate from the inner enamel epithelium (16). Using the dental epithelial cell line HAT-7, addition of recombinant Jagged 1 protein enhanced the appearance of stratum intermedium cells and neutralization with an anti-Jagged 1 antibody inhibited this effect, whereas over-expression of a Notch 1 internal peptide led to an increase in the number of stratum intermedium cells (16). It was hypothesized that the stratum intermedium lineage differentiates from the ameloblast lineage via Notch signaling (16).
The P70T transgene was designed so that expression would be similar to the endogenous Amelx gene, at a low level during fetal tooth development and then highly elevated at birth, primarily in molar ameloblasts. Although the endogenous primary Amelx transcript can be alternatively spliced into at least 15 mRNAs (26, 27), the mutated transgenic P70T is the only amelogenin present in TgP70TKO teeth because of the Amelx null genetic background. The elevation in TgP70T amelogenin expression occurs just before the appearance of abnormal cell masses adjacent to developing teeth. Elevated Notch 1 protein and mRNA were detected at this stage in developing molars of TgP70TKO mice where cells still had a relatively normal appearance.
The abnormal masses in adult mice included cells that were amelogenin positive using an anti-amelogenin antibody (7). Appearance of the abnormal murine epithelial cells was similar to the human CEOT, including intercellular linkages between tumor cells and expression of amelogenin which has been detected by immunohistochemistry of human CEOT neoplastic cells (7, 28, 29). It is possible that tumors have not been reported in individuals with the P70T mutation because they do not have a null genetic background, but express a full complement of amelogenin proteins, similar to TgP70T mice (7–9).
The Notch 1 antibody recognizes a 120 kDa transmembrane protein and was raised against a 20-amino acid peptide in the Notch 1 C-terminus. This antibody was used previously to detect over-expression of Notch 1 in various tumors, including human breast carcinomas and brain tumors (30, 31). Notch 1 is considered to be a regulatory member of one of several collaborating pathways according to tissue-specific context (32), and has been immunolocalized to epithelial cells within benign and malignant ameloblastic carcinomas of unknown etiology in two human patients (33).
In addition to their well-known role in dental enamel development, several of the amelogenin proteins also have been reported to have signaling properties during dental development (18) and when provided to cells in vitro or for repair of the periodontium in vivo (34–36). Ameloblasts are usually negative for Notch and positive for amelogenin, whereas the adjacent stratum intermedium cells have the opposite staining pattern. However, positivity for both Notch 1 and amelogenin is observed within the proliferating masses of cells adjacent to the ameloblast layer as well as in the ameloblast layer itself in the TgP70TKO mice. The lack of one or two normal amelogenin genes is associated with elevation of Notch 1 expression, but the expression of the mutated amelogenin transgene increases Notch 1 expression to the highest level.
A model to explain these findings would first consider the ameloblast precursor cells, which normally stop proliferating and become Notch 1 negative while expressing Notch receptor proteins (16). Differentiating ameloblasts begin to express the endogenous amelogenin gene at a low level during fetal development and after birth, amelogenin expression is highly elevated. The TgP70T is expressed in the same pattern, with low-level expression during late fetal development and elevation just after birth. Our hypothesis is that the P70T mutation, which inactivates a GlcNac binding site (37), weakens the ameloblast attachment to the developing enamel surface. This is similar to that observed in mice lacking another enamel protein, ameloblastin, in which ameloblasts detach and form tumors (38).
Normally, the stratum intermedium cells, which form a layer adjacent to ameloblasts distal to the enamel-secreting process, are Notch 1 positive; these cells are thought to represent a daughter cell layer that differentiates from ameloblast precursors. The two cell types, ameloblasts and stratum intermedium, seem to function cooperatively during enamel development with Notch signaling and other intercellular communications between cell layers (reviewed in 16). It has been suggested that stratum intermedium cells may also express a low level of amelogenin (39, 40) which may normally act as a signaling protein during development (18). Expression of a mutated amelogenin would lead to a disruption in the normal activities of at least one of these cell types, which influences the other, to proliferate abnormally with the appearance of stratum intermedium plus cells that are amelogenin positive. Further analysis may bring profound insights into the signaling capabilities of amelogenins, as well as the biology of stratum intermedium cells and their interactions with ameloblasts, both during normal development and in the abnormal proliferation/differentiation activities described here.
In conclusion, the results support a fundamental role for signaling between epithelial layers in the dental organ of developing teeth. Development of odontogenic aberrant structures in TgP70TKO mice depends on the presence of the mutated amelogenin TgP70T transgene, and is associated with changes in the Notch 1 signaling pathway. This mouse model provides a system in which the endogenous pattern of tight developmental control has been disrupted, and may lead to greater insights into signaling interactions that are required during normal dental epithelial morphogenesis.
Acknowledgments
We acknowledge the NIDCR of the NIH for support of this work through grant DE011089. We thank S. Decker, M. Aragon, and G. Hughes for technical assistance, M. Pugach for contributions toward the scientific discussion and A. Kuperstein for microCT analysis.
Footnotes
Conflict of interest
No conflict of interest is involved in this work.
References
- 1.Termine JD, Belcourt AB, Christner PJ, Conn KM, Nylen MU. Properties of dissociatively extracted fetal tooth matrix proteins. I. Principal molecular species in developing bovine enamel. J Biol Chem. 1980;255:9760–8. [PubMed] [Google Scholar]
- 2.Robinson C, Kirkham J, Brookes SJ, Bonass WA, Shore RC. The chemistry of enamel development. Int J Dev Biol. 1995;38:145–52. [PubMed] [Google Scholar]
- 3.Margolis HC, Beniash E, Fowler CE. Role of macromolecular assembly of enamel matrix proteins in enamel formation. J Dent Res. 2006;85:775–93. doi: 10.1177/154405910608500902. [DOI] [PubMed] [Google Scholar]
- 4.Smith C. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–61. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- 5.Simmer JP, Hu JCC. Expression, structure and function of enamel proteinases. Connect Tissue Res. 2002;43:441–9. doi: 10.1080/03008200290001159. [DOI] [PubMed] [Google Scholar]
- 6.Gibson CW, Yuan ZA, Hall B, et al. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem. 2001;276:31871–5. doi: 10.1074/jbc.M104624200. [DOI] [PubMed] [Google Scholar]
- 7.Gibson CW, Yuan ZA, Li Y, et al. Transgenic mice that express normal and mutated amelogenins. J Dent Res. 2007;86:331–5. doi: 10.1177/154405910708600406. [DOI] [PubMed] [Google Scholar]
- 8.Collier PM, Sauk JJ, Rosenbloom J, Yuan ZA, Gibson CW. An amelogenin gene defect associated with human X-linked amelogenesis imperfecta. Arch Oral Biol. 1997;42:235–42. doi: 10.1016/s0003-9969(96)00099-4. [DOI] [PubMed] [Google Scholar]
- 9.Ravassipour DB, Hart PS, Hart TC, et al. Unique enamel phenotype associated with amelogenin gene (AMELX) codon 41 point mutation. J Dent Res. 2000;79:1476–81. doi: 10.1177/00220345000790070801. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Suggs C, Wright JT, et al. Partial rescue of the amelogenin null dental enamel phenotype. J Biol Chem. 2008;283:15056–62. doi: 10.1074/jbc.M707992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pindborg JJ. A calcifying odontogenic tumor. Cancer. 1958;11:838–43. doi: 10.1002/1097-0142(195807/08)11:4<838::aid-cncr2820110423>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Franklin CD, Pindborg JJ. The calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol. 1976;42:753–65. doi: 10.1016/0030-4220(76)90098-0. [DOI] [PubMed] [Google Scholar]
- 13.Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 14.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 15.Mitsiadis TA, Lardelli M, Lendahl U, Thesleff I. Expression of Notch 1,2, and 3 is regulated by epithelial-mesenchymal interactions and retinoic acid in the developing mouse tooth and associated with determination of ameloblast cell fate. J Cell Biol. 1995;130:407–18. doi: 10.1083/jcb.130.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harada H, Ichimori Y, Yokohama-Tamaki T, et al. Stratum intermedium lineage diverges from ameloblast lineage via Notch signaling. Biochem Biophys Res Commun. 2006;340:611–6. doi: 10.1016/j.bbrc.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 17.Kumamoto H, Ohki K. Detection of Notch signaling molecules in ameloblastomas. J Oral Pathol Med. 2008;37:228–34. doi: 10.1111/j.1600-0714.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 18.Tompkins K, Alvares K, George A, Veis A. Two related low molecular mass polypeptide isoforms of amelogenin have distinct activities in mouse tooth germ differentiation in vitro. J Bone Miner Res. 2005;20:341–9. doi: 10.1359/JBMR.041107. [DOI] [PubMed] [Google Scholar]
- 19.Chen E, Yuan ZA, Wright JT, et al. The small bovine amelogenin LRAP fails to rescue the amelogenin null phenotype. Calcif Tissue Int. 2003;73:487–95. doi: 10.1007/s00223-002-0036-7. [DOI] [PubMed] [Google Scholar]
- 20.Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, MD, USA: 1997–2005. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 21.Yuan ZA, Collier PM, Rosenbloom J, Gibson CW. Analysis of amelogenin mRNA during bovine tooth development. Arch Oral Biol. 1996;41:205–13. doi: 10.1016/0003-9969(95)00119-0. [DOI] [PubMed] [Google Scholar]
- 22.Katsube K, Sakamoto K. Notch in vertebrates-molecular aspects of the signal. Int J Dev Biol. 2005;49:369–74. doi: 10.1387/ijdb.041950kk. [DOI] [PubMed] [Google Scholar]
- 23.Hu QD, Ang BT, Karsak M, et al. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115:163–75. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 24.Eiraku M, Tohgo A, Ono K, et al. DNER acts as a neuron-specific Notch ligand during Bergmann glial development. Nat Neurosci. 2005;8:873–80. doi: 10.1038/nn1492. [DOI] [PubMed] [Google Scholar]
- 25.Mitsiadis TA, Hirsinger E, Lendahl U, Goridis C. Delta-Notch signaling in odontogenesis: correlation with cyto-differentiation and evidence for feedback regulation. Dev Biol. 1998;204:420–31. doi: 10.1006/dbio.1998.9092. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Yuan ZA, Aragon MA, Kulkarni AB, Gibson CW. Comparison of body weight and gene expression in amelogenin null and wild-type mice. Eur J Oral Sci. 2006;114 (Suppl):190–3. doi: 10.1111/j.1600-0722.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 27.Bartlett JD, Ball RL, Kawai T, Tye CE, Tsuchiya M, Simmer JP. Origin, splicing and expression of rodent amelogenin exon 8. J Dent Res. 2006;85:894–9. doi: 10.1177/154405910608501004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saku T, Okabe H, Shimokawa H. Immunohistochemical demonstration of enamel proteins in odontogenic tumors. J Oral Pathol Med. 1992;21:113–9. doi: 10.1111/j.1600-0714.1992.tb00993.x. [DOI] [PubMed] [Google Scholar]
- 29.Kumamoto H, Yoshida M, Ooya K. Immunohistochemical detection of amelogenin and cytokeratin 19 in epithelial odontogenic tumors. Oral Dis. 2001;7:171–6. [PubMed] [Google Scholar]
- 30.Pece S, Serresi M, Santolini E, et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol. 2004;167:215–21. doi: 10.1083/jcb.200406140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu P, Shizhu Yu, Jiang R, et al. Differential expression of Notch family members in astrocytomas and medulloblastomas. Pathol Oncol Res. 2009;15:703–10. doi: 10.1007/s12253-009-9173-x. [DOI] [PubMed] [Google Scholar]
- 32.Talora C, Campese AF, Bellavia D, et al. Notch signaling and diseases: an evolutionary journey from a simple beginning to complex outcomes. Biochim Biophys Acta–Mol Basis Dis. 2008;1782:489–97. doi: 10.1016/j.bbadis.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Nakano K, Siar CH, Tsujigiwa H, Nagatsuka H, Nagai N, Kawakami T. Notch signaling in benign and malignant ameloblastic neoplasms. Eur J Med Res. 2008;13:476–80. [PubMed] [Google Scholar]
- 34.Swanson EC, Fong HK, Foster BL, et al. Amelogenins regulate expression of genes associated with cementoblasts in vitro. Eur J Oral Sci. 2006;114 (Suppl 1):239–43. doi: 10.1111/j.1600-0722.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- 35.Haze A, Taylor AL, Haegewald S, et al. Regeneration of bone and periodontal ligament induced by recombinant amelogenin after periodontitis. J Cell Mol Med. 2009;13:1110–24. doi: 10.1111/j.1582-4934.2009.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammarstrom L. Enamel matrix, cementum development and regeneration. J Clin Periodontal. 1997;9 (pt 2):658–68. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 37.Ravindranath RMH, Moradian-Oldak J, Fincham AG. Tyrosyl motif in amelogenins binds N-acetyl-D-glucosamine. J Biol Chem. 1999;274:2464–71. doi: 10.1074/jbc.274.4.2464. [DOI] [PubMed] [Google Scholar]
- 38.Fukumoto S, Kiba T, Hall B, et al. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol. 2004;167:973–83. doi: 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen E, Piddington R, Decker S, et al. Regulation of amelogenin gene expression during tooth development. Dev Dyn. 1994;199:189–98. doi: 10.1002/aja.1001990304. [DOI] [PubMed] [Google Scholar]
- 40.Iacob S, Veis A. Identification of temporal and spatial expression patterns of amelogenin isoforms during mouse molar development. Eur J Oral Sci. 2006;114 (Suppl 1):194–200. doi: 10.1111/j.1600-0722.2006.00287.x. [DOI] [PubMed] [Google Scholar]