Abstract
Microorganisms release effector molecules that modulate the host machinery enabling survival, replication, and dissemination of a pathogen. Here we characterized the extracellular proteome of Paracoccidioides brasiliensis at its pathogenic yeast phase. Cell-free culture supernatants from the Pb18 isolate, cultivated in defined medium, were separated into vesicle and vesicle-free fractions, digested with trypsin and analyzed by liquid chromatography-tandem mass spectrometry. In vesicle and vesicle-free preparations we identified, respectively, 205 and 260 proteins with two or more peptides, including 120 overlapping identifications. Almost 70% of the sequences were predicted as secretory, mostly using non-conventional secretory pathways, and many have previously been localized to fungal cell walls. A total of 72 proteins were considered as commonly transported by extracellular vesicles, considering that orthologues have been reported in at least two other fungal species. These sequences were mostly related to translation, carbohydrate and protein metabolism, oxidation/reduction, transport, response to stress, and signaling. This unique proteomic analysis of extracellular vesicles and vesicle-free released proteins in a pathogenic fungus provides full comparison with other fungal extracellular vesicle proteomes and broadens the current view on fungal secretomes.
Keywords: Extracellular vesicle proteome, Paracoccidioides brasiliensis, pathogenic fungus, proteomic analysis, vesicle-free released proteins
INTRODUCTION
Paracoccidioidomycosis (PCM) is a systemic granulomatous mycosis that occurs as an active disease in up to 2% of infected individuals living in endemic areas of Latin America.1 The disease is caused by isolates of the Paracoccidioides brasiliensis complex2 and of the newly classified species P. lutzii.3 The PCM infection occurs upon inhalation of fungal conidia produced by environmental mycelia, after subsequent transformation to the yeast pathogenic phase in the pulmonary alveoli. Lungs are generally the main organs affected; however, dissemination to other sites is also common. In juvenile forms of PCM, progression is rapid throughout organs of the lymphatic system. Modulation of the immune system is a key element to determine the infection onset and cellular immunity is the main source of host protection.4 Fungal components that elicit immune response and/or promote other types of interaction with the host are also responsible for shaping the virulence of different isolates.
Bacteria, fungi, and parasites release effector molecules that modulate the immune system and the host-parasite relationship, enabling survival, replication, and dissemination of a pathogen within the host. Classically, secreted proteins in eukaryotic cells bear an N-terminal signal sequence that targets to the endoplasmic reticulum-Golgi pathway of secretion.5 Leaderless or non-classical released proteins use different routes of transportation outside the plasma membrane that have partially been unraveled.6 One alternative route is through exosomes, which derive from intracellular multivesicular bodies.7 We have recently characterized extracellular vesicles isolated from culture supernatants of P. brasiliensis at the pathogenic yeast phase cultivated in defined medium.8 We observed that 100,000g-vesicle pellets contain antigenic components revealed by immunoblots with sera from PCM patients. We demonstrated that these vesicles carry highly immunogenic α-galactopyranosyl (α-Gal) epitopes, which are localized both at the vesicle surface and inside the lumen. At least part of these α-Gal residues is contained in O-linked oligosaccharides that ornament vesicle protein(s). Anti-α-Gal antibodies from patients with Chagas disease can agglutinate and evoke lysis of its etiological agent, the protozoan parasite Trypanosoma cruzi.9 Similarly to P. brasiliensis, T. cruzi also transports components carrying α-Gal epitopes in extracellularvesicles. 10
Fungal extracellular vesicles have recently been recognized as important structures for the trans-cell wall transport of virulence factors that modulate host’s immune response.11 Electron microscopy analysis identified heterogeneous populations of extracellular vesicles originally in Cryptococcus neoformans,12 which is an opportunistic Basidiomycete yeast that causes meningitis. Covering the polysaccharide cell wall that surrounds the plasma membrane in fungi, C. neoformans bears a thick capsule mainly composed by large chains of glucuronoxylomannan (GXM), which is the fungal most important virulence factor and immunomodulator. Not coincidentally, GXM is a major constituent of cryptococcal-released vesicles both in culture media and during in vitro macrophage infection.12,13 Melanin, which is a common virulence factor among fungi,14 and monohexosylceramide (CMH), a membrane glycolipid found in C. neoformans cell wall15 that is important in fungal infection,16 have also been detected in C. neoformans vesicles. 12,17
Besides polysaccharides, pigment, and glycolipids, fungal extracellular vesicles carry a multitude of proteins. Enzymatic activities of virulence factors, such as laccase (that synthesizes melanin), urease, and phosphatase, have been described in extracellular vesicles in C. neoformans.18 Proteomic analysis of extracellular vesicles by liquid chromatography-tandem mass spectrometry (LC-MS/MS) led to the identification of 101 proteins in C. neoformans18 and 279 proteins in Histoplasma capsulatum.19 H. capsulatum and P. brasiliensis are phylogenetically related thermo-dimorphic Ascomycetes that evoke granulomatous systemic mycoses with comparable traits. Using a similar strategy, a comparative extracellular vesicle proteomic analysis was carried out in the non-pathogenic Ascomycete Saccharomyces cerevisiae.20 The study addressed the biogenesis of fungal extracellular vesicles by comparing a set of mutants and their respective wild-type cells and resulted in the identification of 400 proteins. In all studies mentioned above, the authors identified proteins of diverse nature, e.g., related to metabolism, stress, translation, signaling, and defense mechanisms. Part of these proteins has been associated previously with C. neoformans virulence, supporting an important role for extracellular vesicles as transport vehicles of microorganism modulators to distant sites inside the host.11
Reports on extracellular proteomes comparing extracellular vesicles and vesicle-free proteins are rare,21 and so far have not been described in fungi. In the present work we characterized by LC-MS/MS the proteins found in vesicle and vesicle-free preparations of culture supernatant fluids from yeast-phase P. brasiliensis cultivated in defined medium. We then compared the identified proteins in P. brasiliensis with orthologues previously reported in other fungal extracellular vesicles.18-20 Proteomic analysis was facilitated by the genomic data currently available for isolates Pb18, Pb3 (P. brasiliensis), and Pb01 (P. lutzii) at the Dimorphic Fungal Database from the Broad Institute site (http://www.broadinstitute.org/annotation/genome/dimorph_collab/MultiHome.html). Pb18 is highly virulent in the mouse model4 and was used in our work. By comparing the current P. brasiliensis data with orthologues detected in other yeasts we not only provide an overall perspective on fungal vesicle proteins so far reported, but also help broaden the current view on in vitro fungal secretomes.22,23
2. MATERIALS AND METHODS
2.1. P. brasiliensis and Growth Conditions
P. brasiliensis, isolate Pb18, recently recovered from BALB/c lungs after experimental infection, was maintained in the yeast phase at 36 °C in slants of modified YPD medium (0.5% yeast extract, 0.5% casein peptone, 1.5% glucose, pH 6.5). For isolation of extracellular vesicles, yeast cells were transferred from 7-day-old slants into Erlenmeyer flasks containing 200 mL of defined Ham’s F12 medium (Invitrogen) supplemented with 1.5% glucose (F12/Glc) and cultivated for 4 days at 36 °C with shaking (log-phase pre-inoculum). Cells from four pre-inoculums were sedimented by gravity and the supernatants were discarded. The remaining cells were then inoculated into a single Erlenmeyer flask containing 500 mL of fresh medium and cultivated at high density for another 48 h for vesicle purification. At this point the cells were actively growing (98-99% viability as determined by Trypan blue). It is of note that in our previous publication8 a control of extracellular vesicle production using heat-killed cells as pre-inoculum did not show any detectable sterol bands in 100,000 g-pellets by high performance thin-layer chromatography (HPTLC), suggesting lack of background membrane debris.
2.2. Isolation of Extracellular Vesicles
Extracellular vesicles were isolated from culture supernatants following the protocol previously described.12 All the steps were carried out on ice or at 4 °C to avoid vesicle rupture. Cell-free supernatants were obtained by centrifugation at 4,000 g (15 min), followed by centrifugation at 15,000 g (30 min) to remove smaller debris. Pellets were discarded and the cell/debris-free supernatants were 20-fold concentrated using an Amicon ultrafiltration system (100-kDa cutoff, Millipore). Concentrated supernatants were centrifuged at 15,000 g (30 min) to remove aggregates and the resulting supernatant was then ultracentrifuged at 100,000g for 1 h to precipitate vesicles. Vesicle pellets were washed once in phosphate-buffered saline solution (PBS), and the final pellets were suspended in PBS and lyophilized for proteomic analysis.
2.3. Protein Digestion
We processed for proteomic analysis a pool of five preparations of: a) extracellular vesicles; b) ultrafiltration flow-through fluids; and c) ultracentrifugation supernatants. All samples were dissolved in water and precipitated with 10% trichloroacetic acid for 20 min on ice to remove salts from previous buffers. Proteins were recovered by centrifuging for 20 min at 16,000 g at 4 °C, and the pellet was washed once with cold acetone. Protein digestion was carried out in organic/aqueous solution as described elsewhere.24 The three samples were denatured at 95 °C for 5 min in buffered methanol/50 mM of NH4HCO3 (60:40, v/v); the disulfide bonds were reduced by adding a final concentration of 5 mM dithiothreitol and incubating for 30 min at 37 °C. Cysteine residues were alkylated by adding iodoacetamide to a final concentration of 10 mM and incubating for 90 min at room temperature in the dark. The digestion was performed with 1 μg of sequencing-grade trypsin (Promega), for 16 h at 37 °C. The reaction was terminated by addition of 0.05% trifluoroacetic acid, and the resulting peptides were desalted in a POROS R2 (Applied Biosystems) microcolumn, as described25 and dried in a vacuum concentrator prior to LC-MS/MS analysis.
2.4. LC-MS/MS Analysis and Protein Identification
Tryptic peptides were dissolved in 20 μL 0.1% formic acid (FA), and 5 μL were loaded into a reversed-phase trap column (1 cm × 75 μm, Luna C18, 5 μm, Phenomenex). LC was performed in a capillary reversed-phase column (20 cm × 75 μm, Luna C18, 5 μm, Phenomenex) coupled to a nanoHPLC (1D Plus, Eksigent). Bound peptides were eluted in a linear gradient (5 to 40%) of solvent B (solvent A: 5% acetonitrile, ACN/0.1% FA; solvent B: 80% ACN/0.1% FA) for 200 min and directly analyzed in an electrospray ionization-linear ion trap-mass spectrometer (LTQ XL/ETD, Thermo Fisher) equipped at the front end with a Triversa NanoMate nanospray source (Advion). The nanospray was set at 1.45 kV and 0.25 psi N2 pressure using a chip A (Advion). MS spectra were collected in centroid mode at the 400-1700 m/z range and the ten most intense ions were subjected twice to collision-induced dissociation (CID) with 35% normalized collision energy, before being dynamically excluded for 60 s.
All MS/MS spectra from peptides with 800 to 3,500 Da, more than 10 counts, and at least 15 fragments were converted into DTA files using Bioworks v.3.3.1 (Thermo Fisher). DTA files were submitted to database search using TurboSequest (Bioworks 3.3.1, Thermo Fisher Scientific). The database used in the analyses consisted of three Paracoccidioides genome sequences (http://www.broadinstitute.org/annotation/genome/dimorph_collab.1/MultiHome.html, Pb01 v1.0; Pb18 v1.0; Pb03 v1.0), besides human (International Protein Index, IPI human v 3.63) and porcine trypsin sequences from the GenBank, forming a database with 219,740 sequences. All the sequences were used in the correct and reverse orientations to enable the estimation of false-discovery rates (FDR). The database search parameters included: i) trypsin cleavage in both peptide termini with one missed cleavage site allowed; ii) carbamidomethylation of cysteine residues as a fixed modification; iii) oxidation of methionine residues as a variable modification; and iv) 2.0 Da and 1.0 Da for peptide and fragment mass tolerance, respectively. TurboSequest outputs were filtered with DCn ≥ 0.05, peptide probability ≤ 0.05, and Xcorr ≥ 1.5, 2.0, and 2.5 for singly-, doubly-, and triply-charged peptides, respectively. After filtering, the files were exported into XML formats and the peptide sequences were assembled into proteins using an in-house written script.26 Redundant protein hits were assembled into protein groups. The protein hits were re-filtered with the sum of peptide Xcorr ≥ 3.0. The FDR was estimated as described previously.18,19 Five hundred twenty-four proteins were P. brasiliensis proteins identified with 0.5% FDR (Table S1, Supporting information). Proteins defined by the highest number of peptides and the spectra detected are shown in Table S2, Supporting Information. To avoid overestimation of enriched functions based on redundant sequences or isoforms, proteins with ≥ 90% identity were removed from our dataset for further analyses. Proteins identified with more than one peptide in at least one fraction were used for further analysis and are shown in Table S3, Supporting Information.
2.5. Bioinformatic Analysis
Venn diagrams were prepared using either the Venn tool, available at http://omics.pnl.gov/software/VennDiagramPlotter.php, or the Power Point program for manual drawing. Protein sequences were submitted to glycosylphosphatidylinositol (GPI)-anchor prediction analysis using the FragAnchor algorithm27 available at http://navet.ics.hawaii.edu/~fraganchor/NNHMM/NNHMM.html, as described.28 Putative secretory proteins were predicted by the Fungal Secretome Database (FSD; http://fsd.snu.ac.kr/), which contains data of putative secreted proteins identified by a pipeline composed of nine prediction programs for secreted proteins in several fungal genomes29. After removing potential false positives, the proteins were predicted as secretory of the following types: SP or SP3, bearing a signal peptide; SL, proteins with predicted subcellular localization; and NS, non-classical secretory proteins according to the SecretomeP 1.0f. This program30 uses a combination of common features from experimentally proven secreted proteins that predict their probability of being extracellular, independent of the mechanism involved. The two best discriminatory features are the number of atoms and the number of positively charged residues, among others like the presence of predicted pro-peptides, regions of low complexity and presence of transmembrane helices.30
All protein sequences were submitted to Gene Ontology (GO) annotation,31 using the Blast2GO algorithm http://www.blast2go.de/.32 Enrichment analyses of functional clusters of Pb18 proteins in comparison with the genome were carried out using the orthologues from Aspergillus fumigatus and matched against the DAVID database v6.7 (Database for Annotation, Visualization and Integrated Discovery)33 available at http://david.abcc.ncifcrf.gov/. Identification of orthologous groups of vesicle proteins from P. brasiliensis, C. neoformans, H. capsulatum, and S. cerevisiae was performed using the OrthoMCL software v2.0.2,34 http://www.orthomcl.org/cgi-bin/OrthoMclWeb.cgi, with percent match cutoff = 50 and E-value exponent cutoff = -5.
2.6. Enzymatic Assays
Different concentrations of vesicle preparations, expressed as sterol contents in their membranes (estimated with the Amplex Red Cholesterol Assay kit, Invitrogen), were incubated in the presence of 0.2% (10 mM) L-DOPA in PBS for laccase activity determination; or 5 mg/ml of p-nitrophenyl phosphate in acetate buffer (pH 5.0) for phosphatase activity determination. The medium for urease activity contained 4% urea, 0.02% yeast extract, 0.002% phenol red, 0.273% KH2PO4, and 0.285% Na2HPO4. All reaction mixtures were incubated overnight at room temperature in the dark and the results recorded in a spectrophotometer at 405 nm (phosphatase), 450 nm (laccase), or 540 nm (urease). All experiments were performed in triplicates and differences between samples were statistically evaluated by the one-tailed Student’s t-test.
3. RESULTS
3.1. Overall Characteristics of P. brasiliensis Extracellular Proteins
In order to gain information about overall proteins exported to the extracellular milieu by P. brasiliensis we analyzed culture supernatants from the Pb18 isolate. Cell-free spent media from yeasts cultivated in defined medium were separated into vesicle (ves) and vesicle-free (ves-free) fractions, digested with trypsin and analyzed by LC-MS/MS. Vesicle proteome data correspond to the analysis of a pool containing five extracellular vesicle whole preparations (ves). Vesicle-free proteome is presented as combined data obtained from an aliquot of a pool of the corresponding a) ultrafiltration flow-through fluids and b) ultracentrifugation supernatants (details in Materials and Methods). Proteins identified with more than one peptide in either ves or ves-free fractions are denoted as “Yes” in Table S3, Supporting Information. We believe that by using a cutoff of one peptide for protein identification we are excluding eventual intracellular minor contaminants. Eighty-five proteins were identified exclusively in vesicle fractions, against 140 that were detected solely in ves-free fractions; we also found 120 sequences that have overlapped both fractions (Figure 1A and Table S3, Supporting Information).
Figure 1.

A, Venn diagrams showing the number of proteins detected in vesicle and vesicle-free (ves-free) fractions of the Pb18 extracellular proteome. B, Proportion of proteins in vesicle (ves) and ves-free preparations classified as secretory or not (no) when using the FSD: SP (containing signal peptide identified by SignaIP 3.0), SP3 (bearing signal peptide predicted by SigPred, SigCleave or RPSP), SL (sub-cellular localization predicted by PSort II and/or Target 1.1b), and NS (non-classical, predicted by SecretomeP 1.0f).
We then searched the FSD29 for predicted secretory proteins. Predicted secretion types are shown for each protein listed on Table S3, Supporting Information. Figure 1B shows that 69% of the ves-free proteins and 61% of the vesicle proteins were rated as secretory; however, only about 10% of the sequences in each fraction had a predicted signal peptide. These percentages take into consideration total proteins identified in each pool, i.e., 205 (ves) and 260 (ves-free). Most of the secretory proteins have been identified as using non-classical pathways of secretion. It is notable that a higher proportion of vesicle (39%) than ves-free (31%) proteins were predicted as non-secretory, suggesting that vesicular transport might be the sole route of extracellular delivery for part of these proteins. In Table S3, Supporting Information, we can observe that many proteins classified as non-secretory by FSD are prevalent in vesicle fractions, especially those related to protein and carbohydrate metabolism, response to stress, signaling process, cell division, transport, and other/unknown functions.
Glycosylphosphatidylinositols are glycolipid anchors that attach proteins to the cell surface. Potential GPI-anchored modified proteins contain a 15-30-mer peptide sequence of mainly hydrophobic amino acids at the C-terminus, preceded by three small amino acid residues that are recognized by the transamidase enzymatic complex, which transfers the glycolipid anchor to the mature polypeptide. In S. cerevisiae, GPI-anchored proteins were 4-fold enriched in vesicle preparations when compared to the genome background, suggesting that GPI-anchor modifications could target proteins to be transported within vesicles.20 We presently performed a GPI-anchoring prediction analysis using the FragAnchor algorithm.27 The results showed that 0.98% of P. brasiliensis released vesicle proteins (CRAL/TRIO domain-containing protein, PADG_07508, and zinc-regulated transporter 2, PADG_06417) had high probability of being GPI-anchored. This is almost 2-fold higher than the percentage predicted for Pb18 genome proteins. We performed a two-tailed Fisher test with GraphPad (http://www.graphpad.com/quickcalcs/contingency1.cfm) that showed that there was no significant enrichment (p = 0.4011).
Released proteins identified in P. brasiliensis were classified into functional categories using a Blast2GO tool.31 This analysis resulted in the annotation of 85.5% of the proteins into one or more GO (gene ontology) categories. Proteins listed in Table S3, Supporting Information, were grouped according to one of their GO categories. Following this classification, P. brasiliensis released proteins have diverse functions, such as protein, carbohydrate, lipid, and nucleotide metabolism, translation, response to stress, signaling, cell division, oxidation/reduction, transport, besides nuclear and cytoskeleton proteins (Table S3, Supporting Information). Signaling, cell division, and transport proteins were concentrated in vesicle-exclusive sequences, probably because they are either transmembrane or tend to be membrane-associated, like signaling proteins.35
3.2. Fungal extracellular vesicle orthologues
We next compared P. brasiliensis extracellular vesicle proteins with orthologues present in vesicles from C. neoformans,18 H. capsulatum,19 and S. cerevisiae.20 The analysis was performed with the OrthoMCL software that uses the Markov Clustering (MCL) algorithm,34 which was designed to group orthologous sequences in a genome-scale basis; it identifies both orthologues between species and functionally redundant, or “recent”, paralogs within species. The comparison has to be carefully considered due to variations in the LC-MS/MS conditions and amount of sample used in each study, thus although we only focused on the identified proteins, we cannot state that orthologues are not present in the other species. In addition, we compared P. brasiliensis sequences identified with two or more peptides with fungal proteins identified with one or more peptides.18-20 We found that 63% of the P. brasiliensis vesicle-associated sequences had orthologues in other fungal extracellular vesicles (Figure 2; Table S3, Supporting Information). Among them, 72 were common to P. brasiliensis and at least two other species, while 26 were identified in all four species analyzed. Nearly half of the P. brasiliensis released vesicle proteins listed in Table S3, Supporting information has vesicle orthologues in H. capsulatum. Most of the common vesicle proteins were also detected in ves-free fractions of P. brasiliensis supernatants (overlapping group), while 61 ves-free exclusive sequences had orthologues identified in vesicle preparations from the other fungal species. It is also notable that vesicular fungal orthologues were rarely found among hypothetical/predicted proteins of the P. brasiliensis “other/unknown” group. A complete list of vesicle orthologue sequences from other fungi, excluding P. brasiliensis, can be visualized in Table S4, Supporting Information. Note that only two proteins were described in three other fungal species, but not in P. brasiliensis (malate synthase and a protein component of the large (60S) ribosomal subunit).
Figure 2.
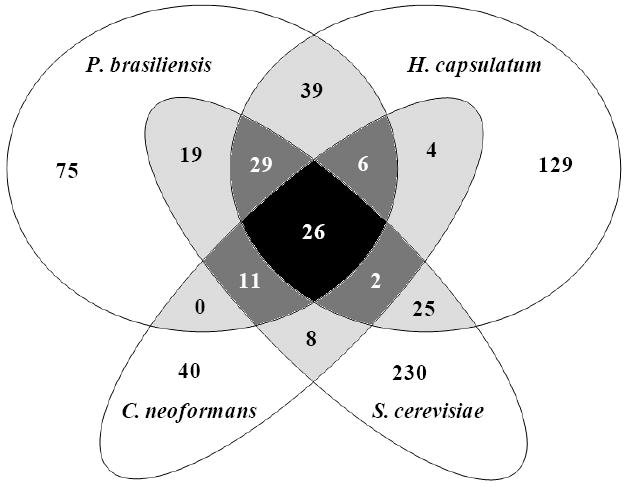
Analysis of orthologous proteins found in fungal extracellular vesicles. The Venn diagram shows the number of proteins detected in extracellular vesicle preparations from P. brasiliensis (this work) and orthologues described in vesicles from H. capsulatum19, C. neoformans18 and S. cerevisiae.20 Overlapping groups are shown in gray scale.
The 72 P. brasiliensis extracellular vesicle sequences mentioned previously that were commonly reported in at least two other fungal species are shown in Table 1. An enrichment analysis of functional clusters of these proteins in comparison with genome proteins using DAVID databases33 (Figure 3A) showed that groups involved in carbohydrate catabolic process, cell redox homeostasis, translation, citrate cycle, nucleotide/GTP binding and transport were enriched in vesicles over 2-fold when compared to their proportion in the genome. In terms of number of sequences within GO categories, translation and carbohydrate metabolism proteins were the most represented, followed by oxidation/reduction, transport, protein metabolism, response to stress, and signaling processes (Table 1).
Table 1.
List of P. brasiliensis extracellular vesicle (ves) non-redundant sequences identified with two or more peptides in Pb18 culture supernatants, and commonly found in at least two other fungal species. The groups were organized according to GO functions. Accession number of P. brasiliensis sequences and their orthologues in reported vesicle proteins from H. capsulatum19, C. neoformans18, and S. cerevisiae20 are indicated. The presence (Yes) or absence (NF) in vesicle-free fractions of P. brasiliensis culture supernatants is indicated. ND*, identified with one peptide. The FSD classification (see Figure 1 for details) is included. The gray scale indicates proteins exclusively detected in P. brasiliensis vesicles (dark gray), or in both fractions (medium gray). (accession number*) indicates that there are other orthologues in the group.
| FSD class | P. brasiliensis | Protein identification | ves | ves-free | H. capsulatum | C. neoformans | S. cerevisiae | |
|---|---|---|---|---|---|---|---|---|
| protein metabolism | ||||||||
|
| ||||||||
| 1 | - | PABG_04656 | serine hydroxymethyltransferase (534 aa) | Yes | NF* | HCAG_07418 | CNAG_04601 | YLR058C |
| 2 | SP3 | PAAG_09004 | puromycin-sensitive aminopeptidase (899 aa) | Yes | NF* | HCAG_06935 | YHR047C* | |
| 3 | NS | PABG_07587 | Cobalamin-independent synthase (757 aa) | Yes | NF* | HCAG_05565 | CNAG_01890 | YER091C |
| 4 | SP | PADG_07422 | subtilase-type proteinase psp3 (496 aa) | Yes | Yes | HCAG_00635 | YEL060C | |
| 5 | NS | PADG_06488 | peptidyl-prolyl cis-trans isomerase D (374 aa) | Yes | Yes | HCAG_04485* | YDR155C* | |
| 6 | NS | PADG_01605 | ubiquitin (306 aa) | Yes | Yes | HCAG_06019 | CNAG_00370 | |
| 7 | NS | PADG_01404 | aspartate aminotransferase (463 aa) | Yes | Yes | HCAG_08678 | YLR027C | |
|
| ||||||||
| carbohydrate metabolism | ||||||||
|
| ||||||||
| 8 | NS | PADG_04604 | transketolase (686 aa) | Yes | NF* | HCAG_05000 | CNAG_00965 | YBR117C* |
| 9 | NS | PADG_00668 | fructose-bisphosphate aldolase (361 aa) | Yes | NF* | HCAG_00010 | YKL060C | |
| 10 | - | PADG_06906 | triosephosphate isomerase (250 aa) | Yes | Yes | HCAG_02511 | YDR050C | |
| 11 | NS | PADG_02411 | glyceraldehyde-3-phosphate dehydrogenase (339 aa) | Yes | Yes | HCAG_04910 | CNAG_06699 | YGR192C* |
| 12 | - | PADG_04059 | enolase (439 aa) | Yes | Yes | CNAG_03072 | YGR254W* | |
| 13 | SP | PADG_07615 | glucan 1_3-beta-glucosidase (417 aa) | Yes | Yes | CNAG_00799 | YLR300W | |
| 14 | NS | PADG_07950 | glucokinase (510 aa) | Yes | Yes | HCAG_03191 | YCL040W | |
| 15 | - | PADG_01896 | phosphoglycerate kinase (418 aa) | Yes | Yes | HCAG_03385 | CNAG_03358 | YCR012W |
| 16 | - | PADG_07420 | transaldolase (323 aa) | Yes | Yes | HCAG_00638 | YLR354C | |
| 17 | NS | PADG_04898 | 3-isopropylmalate dehydratase large subunit (755 aa) | Yes | Yes | HCAG_05266 | YLR304C | |
| 18 | - | PADG_08387 | citrate synthase (475 aa) | Yes | Yes | HCAG_06981 | CNAG_00061 | YNR001C |
| 19 | NS | PADG_00451 | glucose-6-phosphate isomerase (569 aa) | Yes | Yes | HCAG_08202 | YBR196C | |
| 20 | - | PADG_04148 | alpha-mannosidase (1098 aa) | Yes | Yes | HCAG_04277 | YGL156W | |
|
| ||||||||
| lipid metabolism | ||||||||
|
| ||||||||
| 21 | NS | PADG_01363 | conserved hypothetical protein (107 aa) | Yes | Yes | HCAG_08732 | CNAG_06140 | |
|
| ||||||||
| translation | ||||||||
|
| ||||||||
| 22 | NS | PABG_03350 | 60S ribosomal protein L35 (127 aa) | Yes | NF* | HCAG_03415 | CNAG_00771 | |
| 23 | NS | PADG_02329 | 60S ribosomal protein L9-A (193 aa) | Yes | NF* | CNAG_00034 | YGL147C | |
| 24 | NS | PADG_04731 | ribosomal protein L14 (148 aa) | Yes | NF* | CNAG_04799 | YKL006W | |
| 25 | NS | PADG_07685 | 40S ribosomal protein S13-1 (152 aa) | Yes | NF* | CNAG_01153 | YDR064W | |
| 26 | NS | PADG_07803 | 60S ribosomal protein L12 (166 aa) | Yes | NF* | CNAG_01480 | YDR418W | |
| 27 | NS | PADG_00942 | 40S ribosomal protein S7 (202 aa) | Yes | NF* | HCAG_06613 | YOR096W | |
| 28 | - | PADG_01220 | conserved hypothetical protein (218 aa) | Yes | Yes | HCAG_07708 | CNAG_06095 | |
| 29 | NS | PADG_01427 | 40S ribosomal protein S12 (152 aa) | Yes | Yes | HCAG_06308 | YOR369C | |
| 30 | NS | PADG_02828 | 60S ribosomal protein L10a (218 aa) | Yes | Yes | HCAG_01850 | CNAG_02144 | |
| 31 | NS | PADG_06265 | elongation factor 1-gamma 1 (408 aa) | Yes | Yes | CNAG_00417 | YKL081W | |
| 32 | NS | PADG_02445 | 40S ribosomal protein S15 (154 aa) | Yes | Yes | CNAG_06633 | YOL040C | |
| 33 | NS | PADG_05721 | 60S ribosomal protein L4-A (373 aa) | Yes | Yes | HCAG_00468 | CNAG_04762 | YBR031W |
| 34 | - | PAAG_00594 | elongation factor 2 (832 aa) | Yes | Yes | HCAG_05988 | YOR133W | |
| 35 | - | PADG_00692 | elongation factor 1-alpha (461 aa) | Yes | Yes | HCAG_08798 | CNAG_06125 | YPR080W |
| 36 | NS | PADG_02142 | ribosomal protein L5 (300 aa) | Yes | Yes | HCAG_08444 | YPL131W | |
| 37 | SP | PADG_02446 | hypothetical protein (114 aa) | Yes | Yes | CNAG_05762 | YDR382W | |
| 38 | - | PADG_01407 | 40S ribosomal protein S3 (271 aa) | Yes | Yes | HCAG_00214 | YNL178W | |
|
| ||||||||
| response to stress | ||||||||
|
| ||||||||
| 39 | - | PADG_07715 | heat shock protein (672 aa) | Yes | Yes | HCAG_04686 | CNAG_06150 | YMR186W* |
| 40 | - | PADG_08369 | heat shock protein (596 aa) | Yes | Yes | HCAG_06961 | CNAG_03891 | YLR259C |
| 41 | - | PADG_08118 | hsp70-like protein (655 aa) | Yes | Yes | HCAG_05805 | CNAG_00334* | YAL005C* |
| 42 | SP | PADG_03562 | hsp70-like protein (678 aa) | Yes | Yes | HCAG_05805 | CNAG_00334* | YAL005C* |
| 43 | NS | PADG_00430 | heat shock protein SSC1 (681 aa) | Yes | Yes | HCAG_08176 | YJR045C | |
| 44 | - | PADG_02785 | heat shock protein Hsp88 (728 aa) | Yes | Yes | HCAG_00783 | YBR169C* | |
|
| ||||||||
| signaling process | ||||||||
|
| ||||||||
| 45 | SP3 | PADG_01243 | rab GDP-dissociation inhibitor (469 aa) | Yes | NF* | HCAG_07830 | YER136W | |
| 46 | NS | PADG_02833 | ADP-ribosylation factor (184 aa) | Yes | NF* | HCAG_04840 | YDL192W | |
| 47 | SP3 | PADG_08337 | GTP-binding protein rho1 (192 aa) | Yes | NF* | HCAG_05560 | CNAG_03315 | YGR152C |
| 48 | NS | PADG_07524 | nucleoside diphosphate kinase (153 aa) | Yes | Yes | HCAG_00544 | CNAG_04577 | YKL067W |
| 49 | - | PABG_01564 | inorganic pyrophosphatase (465 aa) | Yes | Yes | HCAG_04307 | YBR011C | |
| 50 | - | PADG_04440 | 14-3-3-like protein 2 (245 aa) | Yes | Yes | HCAG_04527 | CNAG_05235 | YDR099W* |
|
| ||||||||
| oxidation reduction | ||||||||
|
| ||||||||
| 51 | SP | PABG_03387 | superoxide dismutase (214 aa) | Yes | NF* | HCAG_01543* | YHR008C | |
| 52 | NS | PADG_03651 | 6-phosphogluconate dehydrogenase (492 aa) | Yes | NF* | HCAG_05884 | CNAG_02620 | YGR256W* |
| 53 | NS | PADG_03095 | mitochondrial peroxiredoxin PRX1 (223 aa) | Yes | Yes | HCAG_06210 | CNAG_03482* | YML028W |
| 54 | SP | PAAG_00986 | disulfide isomerase Pdi1 (539 aa) | Yes | Yes | HCAG_03630 | YCL043C | |
| 55 | NS | PADG_07210 | malate dehydrogenase (341 aa) | Yes | Yes | HCAG_03969 | CNAG_03225 | YKL085W |
| 56 | - | PABG_04154 | NADP-specific glutamate dehydrogenase (458 aa) | Yes | Yes | HCAG_05651 | CNAG_01577 | YAL062W* |
| 57 | NS | PADG_05504 | thioredoxin (118 aa) | Yes | Yes | CNAG_02801 | YGR209C | |
| 58 | - | PADG_06671 | 3-isopropylmalate dehydrogenase A (365 aa) | Yes | Yes | HCAG_02260 | CNAG_00450 | YCL018W |
|
| ||||||||
| transport | ||||||||
|
| ||||||||
| 59 | NS | PADG_08342 | GTP-binding protein YPTM2 (162 aa) | Yes | NF* | HCAG_06999 | YFL038C | |
| 60 | - | PADG_08391 | plasma membrane ATPase (930 aa) | Yes | NF* | HCAG_06977 | CNAG_06400 | YGL008C |
| 61 | NS | PABG_07782 | conserved hypothetical protein (311 aa) | Yes | NF* | HCAG_07936 | YMR183C | |
| 62 | - | PAAG_08620 | ADP_ATP carrier protein (310 aa) | Yes | Yes | HCAG_06283 | CNAG_06101 | YBL030C |
| 63 | NS | PAAG_06268 | cytochrome c (112 aa) | Yes | Yes | HCAG_05938 | YJR048W | |
| 64 | - | PADG_07042 | ATP synthase subunit 5 (231 aa) | Yes | Yes | HCAG_03815 | CNAG_01204 | |
| 65 | - | PADG_02561 | ATPase alpha subunit (557 aa) | Yes | Yes | HCAG_02813 | CNAG_05750 | YBL099W |
| 66 | NS | PADG_08349 | ATP synthase subunit beta (514 aa) | Yes | Yes | HCAG_06944 | CNAG_05918 | YJR121W |
|
| ||||||||
| cytoskeleton proteins | ||||||||
|
| ||||||||
| 67 | NS | PAAG_03031 | tubulin beta chain (448 aa) | Yes | Yes | CNAG_01840 | YFL037W | |
| 68 | NS | PADG_06295 | actin (347 aa) | Yes | Yes | HCAG_08210 | CNAG_00483 | YFL039C |
|
| ||||||||
| nuclear proteins | ||||||||
|
| ||||||||
| 69 | - | PADG_07134 | histone H4.2 (104 aa) | Yes | Yes | HCAG_03885 | CNAG_01648 | YBR009C |
| 70 | NS | PADG_05907 | histone H2B type 1-A (142 aa) | Yes | Yes | HCAG_03525 | YBL002W | |
|
| ||||||||
| other/ unknown function | ||||||||
|
| ||||||||
| 71 | - | PADG_01509 | DUF907 domain-containing protein (746 aa) | Yes | NF* | HCAG_03638 | YAL053W* | |
| 72 | NS | PADG_04288 | L-PSP endoribonuclease family protein (Hmf1) (168 aa) | Yes | Yes | HCAG_04397 | YER057C* | |
Figure 3.

Functional categorization of fungal extracellular vesicle proteins commonly found in P. brasiliensis and at least two other species (total of 72, Table 1); * p ≤ 0.05. The figure shows enriched functional clusters in comparison with the genome, represented by bars of fold-increase.
Some of these fungal proteins might have clear roles during infection. Proteins with antioxidant activities, such as superoxide dismutase (PABG_03387), mitochondrial peroxiredoxin (PADG_03095), and thioredoxin (PADG_05504), can defend microorganisms against reactive-oxygen species and are important for the survival of intracellular pathogens during infection.36 An orthologue of mitochondrial peroxiredoxin PRX1 (PADG_03095), for e.g., was differentially localized to the cell surface in the pathogenic hyphal phase of C. albicans37. The protein was also localized to the cell wall of P. brasiliensis yeasts (Longo et al., unpublished).
It was interesting to observe that cell division proteins (septins) were so far described basically in P. brasiliensis alone (Table S3, Supporting Information). Two of them have recently been characterized in P. brasiliensis: cdc4238 (cell division control protein, PABG_04744) interferes in the yeast cell polymorphism and virulence; cdc1239 (septin 4, PABG_05403) has been localized to the cell surface with a punctuated pattern suggestive of delivery in vesicles.
3.3. Detection of Enzymatic Activities in Extracellular Vesicles from P. brasiliensis
We tested whether P. brasiliensis releases vesicles containing active laccase, phosphatase, and urease. We found dose-dependent activities of phosphatase (Figure 4), whereas urease and laccase activities were not observed. Moreover, we could not find urease and laccase sequences in the extracellular vesicle proteome, but we identified a protein with predicted acid phosphatase activity (PADG_02403). In P. brasiliensis, a 75-kDa protein was localized at the cell surface bearing phosphatase activity;40 however, since its identity is unknown we could not correlate it with our proteomic data. The presence of laccase activity would be expected, since dopamine-derived melanin has been described in P. brasiliensis yeasts.14 On the other hand, extracellular urease activity has been described by our group as contributing to increased culture supernatant pH during late-log phase of growth.41
Figure 4.
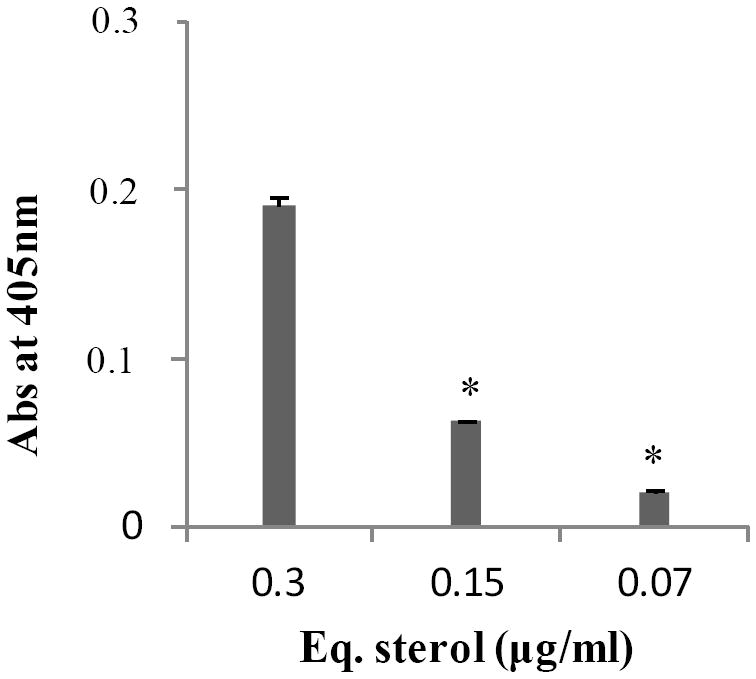
Phosphatase activity (A405) at different Pb18 extracellular vesicles concentrations expressed as equivalents of sterol contents. Background values were subtracted. * p ≤ 0.05.
4. DISCUSSION
This is an original description of a fungal extracellular proteome taking into consideration individual proteomic analyses of extracellular vesicle and vesicle-free preparations of culture supernatants. Our fungal model was P. brasiliensis, whose results were then compared with orthologous sequences described so far in extracellular vesicle proteomes from other fungi.18-20 Among the P. brasiliensis culture supernatant proteins here predicted to be secretory by the FSD,29 50% would use non-classical means of transportation (leaderless proteins). Based on the current knowledge, Nickel (2005)42 proposed four mechanisms for leaderless protein secretion: (i) export through the release of exosomes, (ii) direct translocation from the cytoplasm mediated by plasma membrane transporters, (iii) lysosomal secretion, and (iv) export mediated by plasma membrane blebbing. Fungal extracellular vesicle preparations are composed of heterogeneous populations differing in size and probably also in content,11,20 which we now show to partially vary among species as well. In addition, recent results pointed to the existence of multiple pathways of vesicle-mediated unconventional secretion in fungi, specifically, classical Golgi-derived exocytosis, exosome-related secretion, autophagy- and vesicle-dependent unconventional secretion.20,43 In S. cerevisiae, about 25% of vesicle proteins were predicted to bear a signal peptide, which represented an enrichment of about 3-fold when compared to whole cells. That added evidence that the Golgi apparatus could be involved in released vesicle biogenesis.20 In our overall analysis of fungal extracellular vesicle proteins (Table 1), 12.5% of the sequences had predicted signal peptide, including, for instance, an aminopeptidase (PAAG_09004), GTP-binding protein rho1 (PADG_08337), and superoxide dismutase (PABG_03387), thus corroborating this hypothesis.
Among the P. brasiliensis extracellular proteins here identified, 35% were detected in both vesicle and vesicle-free preparations. We believe that at least part of the overlapped sequences might be the result of vesicle-free proteins specifically bound, or possibly aggregated to, vesicles during the preparation process, especially during culture supernatant concentration. Very likely, though, is that proteins could have been released free from damaged vesicles during the purification process or even during growth. It is a fact that the yield of vesicle preparations is low and that the amount of protein in vesicle-free preparations is much higher. On the other hand, we now show that the extracellular vesicle content, which probably varies among different heterogeneous populations,11,20 partially varies among species as well. The existence of multiple pathways of vesicle-mediated unconventional secretion, as discussed above, would justify the vesicle protein heterogeneity observed intra- and inter-fungal species. That might also help to explain why we presently verified a number of orthologues of P. brasiliensis vesicle-free exclusive sequences in vesicle preparations from other yeasts. It is also possible that not all vesicle populations are pelleted after 1 h of ultracentrifugation. This hypothesis has to be tested, since it could justify the finding of membrane proteins in vesicle-free fractions. An enrichment in extracellular vesicles of proteins that are predominantly cytoplasmic may also occur during the inward budding process of multivesicular body formation that randomly engulfs small portions of the cytosol,7 as well as through a vesicle budding process that happens at the plasma membrane level in case of microvesicles.44 On the other hand, many of the extracellular proteins described here have mitochondrial function, and that could be the result of an autophagic delivery of cargoes.45 Note that the above speculations are far from trying to define vesicle biogenesis, considering we are probably detecting vesicle proteins deriving from diverse pathways.
Our proteomic analysis of culture supernatants from the P. brasiliensis pathogenic phase revealed a numerous and complex composition of proteins with diverse biological functions such as protein and carbohydrate metabolism, translation, response to stress, signaling, cell division, oxidation/reduction, and transport, corroborating previous reports.18-20 By comparing the current P. brasiliensis data with fungal orthologues detected in these reports we not only provided an overall perspective on fungal extracellular vesicle proteins so far reported, but also helped to broaden the current understanding of in vitro fungal secretomes from pathogenic fungi,22,23,46 considering that we analyzed vesicle and non-vesicle released proteins. Secretomes of whole cell-free supernatants have recently been reported in pathogenic H. capsulatum22 and Candida albicans.23,46 Holbrook et al. (2011)22 used very stringent criteria to define 33 proteins as extracellular in the H. capsulatum yeast phase with high confidence degree. Among them, 23 are orthologous to sequences here detected in both vesicle and vesicle-free preparations. Sorgo et al. (2010)23 compared C. albicans proteins from various growth conditions, differing in pH, temperature and presence of the hyphal inducer N-acetylglucosamine. The analysis revealed that growth conditions strongly affected the composition of the secretome, suggesting that released proteins could be involved in the adaptation and survival in different environments. Out of 51 extracellular proteins reported in C. albicans, only 13 are orthologous to those described here for P. brasiliensis, including glycosidases, proteinases, and superoxide dismutase.
We propose that the orthologues similarly detected in at least three distinct yeast extracellular vesicle proteomes (Table 1) commonly use this means of transportation towards the cell surface in different fungal species. It is worth mentioning that so far we have been unable to detect vesicles in the supernatants of the mycelium phase of P. brasiliensis (Silva and Puccia, unpublished results) and we have not yet characterized the mycelium-released proteins either. Included in these yeast vesicle proteins are proteins that could neutralize host defense mechanisms, as mentioned earlier for oxidation/reduction components, and others that could potentially help fungal dissemination, like proteases and lipases. A subtilase-type proteinase psp3 (PADG_07422) was common to three fungal released vesicle proteomes. We have previously characterized an exocellular serine-thiol (subtilase type) proteinase activity (PbST) in the yeast phase of P. brasiliensis that can selectively cleave components of the basal membrane of the extracellular matrix,41 therefore showing potential role in fungal dissemination. The identity of PbST could be proteinase psp3, since it shows a free cysteine residue in its sequence; however, this possibility still needs further experimental evidence. We can also cite four proteins within the carbohydrate metabolism GO group that have been microscopically localized to the P. brasiliensis cell wall and are implicated in adhesion to matrix-associated components, specifically, glyceraldehyde-3-phosphate dehydrogenase (PADG_02411),47 enolase (PADG_04059),48 gp43 (PADG_07615),49,50 and triosephosphate isomerase (PADG_06906).51
Gp43 is the best studied molecule in P. brasiliensis for its antigenic, immunomodulatory and protective properties. Although the molecule is in the group of beta-1,3-exoglucanases, it is not enzymatically active, and it has peculiar characteristics seen only in isolates of the P. brasiliensis complex.52 Gp43 has a signal peptide and can be abundantly found in the culture supernatants of some isolates, as well as partly on the cell wall.53 Transmission electron microscopy images show clusters of immunogold labeling with monoclonal antibodies outward from the cell wall, suggestive of being inside vesicles. A similar profile is depicted for histone 2B near the H. capsulatum cell wall.54 Orthologues of both molecules were identified in fungal supernatant vesicles (Table 1).
Extracellular vesicles seem to also export enzymes implicated in the carbohydrate architecture of the cell wall. We identified a cell wall alpha-1,3-glucan synthase MOK1-like (PADG_03169) in P. brasiliensis vesicles that has not been described in other fungal vesicle proteomes, in spite of the fact that both H. capsulatum and C. neoformans have high proportions of alpha-1,3-glucans in their cell wall. In addition, signaling GTP-binding protein Rho1 (PADG_08337) was found in all yeast vesicle proteomes; it is involved in cell wall integrity pathways as regulator of beta-1,3-glucan synthases in C. neoformans,55 S. cerevisiae,56 and P. brasiliensis.57
In this study we described an original proteomic analysis of extracellular vesicles and vesicle-free released proteins in a pathogenic fungus, which also provided full comparison with other fungal vesicle proteomes. We therefore believe that our work broadens the current view on fungal secretomes and helps understand the finding of some proteins with intracellular functions on the cell wall.58, 59, 60
Supplementary Material
Acknowledgments
We thank Marcio L. Rodrigues, Leonardo Nimrichter and Luiz R. Travassos for discussions and suggestions. We thank Marcio L. Rodrigues for critically reading the manuscript, and Christopher A. Desjardins for advice. This work has been funded by FAPESP, CNPq, and NIH (grants # 5G12RR008124-16A1 and 5G12RR008124-16A1S1). We are grateful to the Biomolecule Analysis Core Facility at the BBRC/Biology/UTEP (NIH grants 5G12RR008124-16A1 and 5G12RR008124-16A1S1) for the access to the LC-MS instrument.
Footnotes
Supplementary tables containing the complete set of results. This material is available free of charge via the Internet at http://pubs.acs.org. All LC–MS/MS raw datafiles were deposited on Tranche database (www.proteomecommons.org) under hash: 3CQXyXQ9XtCZNse0wa0i0CH4yHChUizwN8VxRUynHYWCAqHUtFIn9o9f3W0d/qAMmz w+INzkob2KonE8cRAL3E9Go08AAAAAAAACDA==
References
- 1.San Blas G, Niño-Vega G, Iturriaga T. Paracoccidioides brasiliensis and paracoccidioidomycosis, Molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med Mycol. 2002;40(3):225–242. doi: 10.1080/mmy.40.3.225.242. [DOI] [PubMed] [Google Scholar]
- 2.Matute DR, McEwen JG, Puccia R, Montes BA, San-Blas G, Bagagli E, Rauscher JT, Restrepo A, Morais F, Niño-Vega G, Taylor JW. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol Biol Evol. 2006;23(1):65–73. doi: 10.1093/molbev/msj008. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira MM, Theodoro RC, de Carvalho MJ, Fernandes L, Paes HC, Hahn RC, Mendoza L, Bagagli E, San-Blas G, Felipe MS. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol Phylogenet Evol. 2009;52(2):273–283. doi: 10.1016/j.ympev.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Calich VL, Vaz CA, Burger E. Immunity to Paracoccidioides brasiliensis infection. Res Immunol. 1998;149(4-5):407–417. doi: 10.1016/s0923-2494(98)80764-5. [DOI] [PubMed] [Google Scholar]
- 5.Glick BS, Malhotra V. The curious status of the Golgi apparatus. Cell. 1998;95(7):883–889. doi: 10.1016/s0092-8674(00)81713-4. [DOI] [PubMed] [Google Scholar]
- 6.Nickel W. Pathways of unconventional protein secretion. Curr Opin Biotechnol. 2010;21(5):621–626. doi: 10.1016/j.copbio.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes, from biogenesis and secretion to biological function. Immunol Lett. 2006;107(2):102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Vallejo MC, Matsuo AL, Ganiko L, Medeiros LC, Miranda K, Silva LS, Freymuller-Haapalainen E, Sinigaglia-Coimbra R, Almeida IC, Puccia R. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-Galactosyl epitopes. Eukaryot Cell. 2011;10(3):343–351. doi: 10.1128/EC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida IC, Milani SR, Gorin PA, Travassos LR. Complement-mediated lysis of Trypanosoma cruzi trypomastigotes by human anti-alpha-galactosyl antibodies. J Immunol. 1991;146(7):2394–2400. [PubMed] [Google Scholar]
- 10.Trocoli Torrecilhas AC, Tonelli RR, Pavanelli WR, da Silva JS, Schumacher RI, de Souza W, e Silva NC, de Almeida Abrahamsohn I, Colli W, Manso Alves MJ. Trypanosoma cruzi: parasite shed microvesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 2009;11(1):29–39. doi: 10.1016/j.micinf.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends Microbiol. 2009;17(4):158–162. doi: 10.1016/j.tim.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, Alvarez M, Nakouzi A, Feldmesser M, Casadevall A. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6(1):48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun. 2010;78(4):1601–1609. doi: 10.1128/IAI.01171-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taborda CP, da Silva MB, Nosanchuk JD, Travassos LR. Melanin as a virulence factor of Paracoccidioides brasiliensis and other dimorphic pathogenic fungi: a minireview. Mycopathologia. 2008;165(4-5):331–339. doi: 10.1007/s11046-007-9061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues ML, Travassos LR, Miranda KR, Franzen AJ, Rozental S, de Souza W, Alviano CS, Barreto-Bergter E. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun. 2000;68(12):7049–7060. doi: 10.1128/iai.68.12.7049-7060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rittershaus PC, Kechichian TB, Allegood JC, Merrill AH, Jr, Hennig M, Luberto C, Del PM. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest. 2006;116(6):1651–1659. doi: 10.1172/JCI27890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology. 2009;155(12):3860–3867. doi: 10.1099/mic.0.032854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7(1):58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, Almeida IC, Nosanchuk JD. Vesicular transport in Histoplasma capsulatum, an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008;10(8):1695–1710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira DL, Nakayasu ES, Joffe LS, Guimaraes AJ, Sobreira TJ, Nosanchuk JD, Cordero RJ, Frases S, Casadevall A, Almeida IC, Nimrichter L, Rodrigues ML. Characterization of yeast extracellular vesicles, evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One. 2010;5(6):e11113. doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galka F, Wai SN, Kusch H, Engelmann S, Hecker M, Schmeck B, Hippenstiel S, Uhlin BE, Steinert M. Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect Immun. 2008;76(5):1825–1836. doi: 10.1128/IAI.01396-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holbrook ED, Edwards JA, Youseff BH, Rappleye CA. Definition of the extracellular proteome of pathogenic-phase Histoplasma capsulatum. J Proteome Res. 2011;10(4):1929–1943. doi: 10.1021/pr1011697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorgo AG, Heilmann CJ, Dekker HL, Brul S, de Koster CG, Klis FM. Mass spectrometric analysis of the secretome of Candida albicans. Yeast. 2010;27(8):661–672. doi: 10.1002/yea.1775. [DOI] [PubMed] [Google Scholar]
- 24.Russell WK, Park ZY, Russell DH. Proteolysis in mixed organic-aqueous solvent systems: applications for peptide mass mapping using mass spectrometry. Anal Chem. 2001;73(11):2682–2685. doi: 10.1021/ac001332p. [DOI] [PubMed] [Google Scholar]
- 25.Jurado JD, Rael ED, Lieb CS, Nakayasu E, Hayes WK, Bush SP, Ross JA. Complement inactivating proteins and intraspecies venom variation in Crotalus oreganus helleri. Toxicon. 2007;49(3):339–350. doi: 10.1016/j.toxicon.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Nakayasu ES, Sobreira TJ, Torres R, Ganiko L, Oliveira PS, Marques AF, Almeida IC. Improved Proteomic Approach for the Discovery of Potential Vaccine Targets in Trypanosoma cruzi. J Proteome Res. 2011;11(1):237–246. doi: 10.1021/pr200806s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poisson G, Chauve C, Chen X, Bergeron A. FragAnchor, a large-scale predictor of glycosylphosphatidylinositol anchors in eukaryote protein sequences by qualitative scoring. Genomics Proteomics Bioinformatics. 2007;5(2):121–130. doi: 10.1016/S1672-0229(07)60022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayasu ES, Yashunsky DV, Nohara LL, Torrecilhas AC, Nikolaev AV, Almeida IC. GPIomics, global analysis of glycosylphosphatidylinositol-anchored molecules of Trypanosoma cruzi. Mol Syst Biol. 2009;5:261. doi: 10.1038/msb.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi J, Park J, Kim D, Jung K, Kang S, Lee YH. Fungal secretome database, integrated platform for annotation of fungal secretomes. BMC Genomics. 2010;11:105. doi: 10.1186/1471-2164-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bendtsen JD, Jensen LJ, Blom N, von HG, Brunak S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng Des Sel. 2004;17(4):349–356. doi: 10.1093/protein/gzh037. [DOI] [PubMed] [Google Scholar]
- 31.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology, tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conesa A, Götz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 33.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Stoeckert CJ, Jr, Roos DS. OrthoMCL, identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13(9):2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seabra MC. Membrane association and targeting of prenylated Ras-like GTPases. Cell Signal. 1998;10(3):167–172. doi: 10.1016/s0898-6568(97)00120-4. [DOI] [PubMed] [Google Scholar]
- 36.Campos EG, Jesuino RS, Dantas AS, Brigido MM, Felipe MS. Oxidative stress response in Paracoccidioides brasiliensis. Genet Mol Res. 2005;4(2):409–429. [PubMed] [Google Scholar]
- 37.Urban C, Sohn K, Lottspeich F, Brunner H, Rupp S. Identification of cell surface determinants in Candida albicans reveals Tsa1p, a protein differentially localized in the cell. FEBS Lett. 2003;544(1-3):228–235. doi: 10.1016/s0014-5793(03)00455-1. [DOI] [PubMed] [Google Scholar]
- 38.Almeida AJ, Cunha C, Carmona JA, Sampaio-Marques B, Carvalho A, Malavazi I, Steensma HY, Johnson DI, Leão C, Logarinho E, Goldman GH, Castro AG, Ludovico P, Rodrigues F. Cdc42p controls yeast-cell shape and virulence of Paracoccidioides brasiliensis. Fungal Genet Biol. 2009;46(12):919–926. doi: 10.1016/j.fgb.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Reis TF, Basso LR, Oliveira RR, Coelho PS. Septin localization in the dimorphic fungus Paracoccidioides brasiliensis. Yeast. 2011;28(12):843–854. doi: 10.1002/yea.1911. [DOI] [PubMed] [Google Scholar]
- 40.Xander P, Vigna AF, Feitosa LS, Pugliese L, Bailão AM, Soares CM, Mortara RA, Mariano M, Lopes JD. A surface 75-kDa protein with acid phosphatase activity recognized by monoclonal antibodies that inhibit Paracoccidioides brasiliensis growth. Microbes Infect. 2007;9(12-13):1484–1492. doi: 10.1016/j.micinf.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Puccia R, Carmona AK, Gesztesi JL, Juliano L, Travassos LR. Exocellular proteolytic activity of Paracoccidioides brasiliensis, cleavage of components associated with the basement membrane. Med Mycol. 1998;36(5):345–348. [PubMed] [Google Scholar]
- 42.Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6(8):607–614. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 43.Kmetzsch L, Joffe LS, Staats CC, de Oliveira DL, Fonseca FL, Cordero RJ, Casadevall A, Nimrichter L, Schrank A, Vainstein MH, Rodrigues ML. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol Microbiol. 2011;81(1):206–218. doi: 10.1111/j.1365-2958.2011.07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverman JM, Reiner NE. Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol. 2011;13(1):1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 45.Manjithaya R, Subramani S. Autophagy: a broad role in unconventional protein secretion? Trends Cell Biol. 2011;21(2):67–73. doi: 10.1016/j.tcb.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorgo AG, Heilmann CJ, Dekker HL, Bekker M, Brul S, de Koster CG, de Koning LJ, Klis FM. Effects of fluconazole on the secretome, the wall proteome, and wall integrity of the clinical fungus Candida albicans. Eukaryot Cell. 2011;10(8):1071–1081. doi: 10.1128/EC.05011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbosa MS, Báo SN, Andreotti PF, de Faria FP, Felipe MS, dos Santos FL, Mendes-Giannini MJ, Soares CM. Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect Immun. 2006;74(1):382–389. doi: 10.1128/IAI.74.1.382-389.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nogueira SV, Fonseca FL, Rodrigues ML, Mundodi V, Abi-Chacra EA, Winters MS, Alderete JF, de Almeida Soares CM. Paracoccidioides brasiliensis enolase is a surface protein that binds plasminogen and mediates interaction of yeast forms with host cells. Infect Immun. 2010;78(9):4040–4050. doi: 10.1128/IAI.00221-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanna SA, Monteiro Da Silva JL, Giannini MJ. Adherence and intracellular parasitism of Paracoccidioides brasiliensis in Vero cells. Microbes Infect. 2000;2(8):877–884. doi: 10.1016/s1286-4579(00)00390-7. [DOI] [PubMed] [Google Scholar]
- 50.Gesztesi JL, Puccia R, Travassos LR, Vicentini AP, de Moraes JZ, Franco MF, Lopes JD. Monoclonal antibodies against the 43,000 Da glycoprotein from Paracoccidioides brasiliensis modulate laminin-mediated fungal adhesion to epithelial cells and pathogenesis. Hybridoma. 1996;15(6):415–422. doi: 10.1089/hyb.1996.15.415. [DOI] [PubMed] [Google Scholar]
- 51.Peireira LA, Báo SN, Barbosa MS, da Silva JL, Felipe MS, de Santana JM, Mendes-Giannini MJ, Almeida Soares CM. Analysis of the Paracoccidioides brasiliensis triosephosphate isomerase suggests the potential for adhesin function. FEMS Yeast Res. 2007;7(8):1381–1388. doi: 10.1111/j.1567-1364.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 52.Puccia R, McEwen JG, Cisalpino PS. Diversity in Paracoccidioides brasiliensis. The PbGP43 gene as a genetic marker. Mycopathologia. 2008;165(4-5):275–287. doi: 10.1007/s11046-007-9055-2. [DOI] [PubMed] [Google Scholar]
- 53.Straus AH, Freymuller E, Travassos LR, Takahashi HK. Immunochemical and subcellular localization of the 43 kDa glycoprotein antigen of Paracoccidioides brasiliensis with monoclonal antibodies. J Med Vet Mycol. 1996;34(3):181–186. doi: 10.1080/02681219680000301. [DOI] [PubMed] [Google Scholar]
- 54.Nosanchuk JD, Steenbergen JN, Shi L, Deepe GS, Casadevall A. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J Clin Invest. 2003;112(8):1164–1175. doi: 10.1172/JCI19361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuchs BB, Tegos GP, Hamblin MR, Mylonakis E. Susceptibility of Cryptococcus neoformans to photodynamic inactivation is associated with cell wall integrity. Antimicrob Agents Chemother. 2007;51(8):2929–2936. doi: 10.1128/AAC.00121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirano H, Tanaka K, Ozaki K, Imamura H, Kohno H, Hihara T, Kameyama T, Hotta K, Arisawa M, Watanabe T, Qadota H, Ohya Y, Takai Y. ROM7/BEM4 encodes a novel protein that interacts with the Rho1p small GTP-binding protein in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16(8):4396–4403. doi: 10.1128/mcb.16.8.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sorais F, Barreto L, Leal JA, Bernabe M, San Blas G, Niño-Vega GA. Cell wall glucan synthases and GTPases in Paracoccidioides brasiliensis. Med Mycol. 2010;48(1):35–47. doi: 10.3109/13693780802713356. [DOI] [PubMed] [Google Scholar]
- 58.Nombela C, Gil C, Chaffin WL. Non-conventional protein secretion in yeast. Trends Microbiol. 2006;14(1):15–21. doi: 10.1016/j.tim.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Puccia R, Vallejo MC, Matsuo AL, Longo LVG. The Paracoccidioides cell wall: past and present layers towards understanding interaction with the host. Front Microbiol. 2011;2(257):1–7. doi: 10.3389/fmicb.2011.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guimarães AJ, Cerqueira MD, Nosanchuk JD. Surface architecture of Histoplasma capsulatum. Front Microbiol. 2011;2(225):1–14. doi: 10.3389/fmicb.2011.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


