Abstract
Background
Cannabinoid CB1 receptors (CB1R) mediate the effects of Δ9-tetrahydrocannabinol (THC), the psychoactive component in marijuana. Repeated THC administration produces tolerance and dependence, which limit therapeutic development. Moreover, THC produces motor and psychoactive side effects. β-arrestin2 mediates receptor desensitization, internalization and signaling, but its role in these CB1R effects and receptor regulation is unclear.
Methods
CB1R signaling and behaviors (antinociception, temperature, catalepsy) were assessed in β-arrestin2-knockout (βarr2-KO) and wild-type mice after THC administration. Cannabinoid-stimulated [35S]GTPγS and [3H]ligand autoradiography were assessed by Statistical Parametric Mapping (SPM) and region-of-interest analyses.
Results
β-arrestin2 deletion increased CB1R-mediated G-protein activity in subregions of the cortex, but did not affect CB1R binding, in vehicle-treated mice. βarr2-KO mice exhibited enhanced acute THC-mediated antinociception and hypothermia, with no difference in catalepsy. After repeated THC administration, βarr2-KO mice showed reduced CB1R desensitization and/or downregulation in cerebellum, caudal periaqueductal gray and spinal cord, and attenuated tolerance to THC-mediated antinociception. In contrast, greater desensitization was found in hypothalamus, cortex, globus pallidus and substantia nigra of βarr2-KO compared to wild-type mice. Enhanced tolerance to THC-induced catalepsy was observed in βarr2-KO mice.
Conclusions
β-arrestin2 regulation of CB1R signaling following acute and repeated THC administration was region-specific, and results suggest that multiple, overlapping mechanisms regulate CB1Rs. The observations that βarr2-KO mice display enhanced antinociceptive responses to acute THC and decreased tolerance to the antinociceptive effects of the drug, yet enhanced tolerance to catalepsy, suggest that development of cannabinoid drugs that minimize CB1R interactions with β−arrestin2 might produce improved cannabinoid analgesics with reduced motor suppression.
Keywords: Cannabinoid receptors, beta-arrestin, GPCR, tolerance, statistical parametric mapping
Introduction
CB1receptors (CB1R) are widely distributed in the CNS (1) and mediate the central effects of Δ9-tetrahydrocannabinol (THC) and cannabinoids (2). The endocannabinoid system is implicated in numerous physiological processes and is a potential therapeutic target for disorders including neurodegenerative and neuropsychiatric diseases and chronic pain (3). However, therapeutic use is limited by psychoactive and motor side effects. Moreover, repeated cannabinoid treatment produces tolerance to cannabinoid-mediated in vivo effects (4). Regionspecific CB1R desensitization and downregulation occur in conjunction with tolerance (5), but the molecular mechanisms that underlie CB1R adaptations and tolerance are not well defined.
CB1Rs primarily activate Gi/o-proteins, which regulate adenylyl cyclase, ion channels and kinases (6). Persistent cannabinoid exposure induces CB1R uncoupling from G-proteins (desensitization) (7), with subsequent receptor internalization (8) and degradation (downregulation) (9, 10). One mechanism for these adaptations occurs by G-protein-coupled receptor (GPCR) kinase (GRK)-mediated phosphorylation of activated receptors and subsequent β-arrestin binding (11). β-arrestin2 is one of two arrestin isoforms in the brain (12) and findings in cell models support a role for β-arrestin2 in CB1R adaptations. Co-expression of GRK3 and β-arrestin2 was required for rapid desensitization of CB1R-mediated potassium currents following exposure to WIN55,212-2 in Xenopus Oocytes (8). Similarly, expression of dominant negative β-arrestin2 attenuated desensitization of WIN55,212-2-mediated inhibition of glutamatergic neurotransmission in hippocampal neurons (13). Immunohistochemical studies show that CB1Rs are co-distributed with β-arrestin2 in certain CNS regions (12, 14), suggesting that β-arrestin2 might regulate CB1R signaling in the CNS.
As there are no pharmacological β-arrestin inhibitors, β-arrestin2 knockout (βarr2-KO) mice (15) provide a model to study its role in regulating GPCRs in vivo (16). Acute administration of THC to βarr2-KO mice revealed enhanced sensitivity to its antinociceptive and hypothermic effects (17). However, direct evidence for the role of β-arrestin2 in CB1R adaptations and tolerance following repeated THC is lacking.
We adapted Statistical Parametric Mapping (SPM) to analyze [35S]GTPγS autoradiography (18). SPM has the advantage of assessing changes in G-protein activation in an unbiased and anatomically inclusive manner. We applied SPM to examine the role of β-arrestin2 in CB1R regulation by comparing cannabinoid-stimulated [35S]GTPγS binding in brains from vehicle and THC-treated βarr2-KO and wild-type (WT) littermates. Combining this approach with behavioral assessment following THC administration allowed us to compare CB1R signaling with behavioral responses observed in the βarr2-KO mice. We demonstrate region-specific regulation of CB1Rs by β-arrestin2 that parallel changes in THC-mediated behavior and tolerance.
Methods and Materials
Detailed Methods are provided in the Supplement.
Mice
Male WT and βarr2-KO mice (littermates, 4 months) (15) were injected intraperitoneally with THC (10 mg/kg) or vehicle (1:1:18 ethanol:cremaphor:0.9% saline) twice daily for 6.5 days (subchronic treatment). Twenty-four hours after the final injection, mice were challenged with increasing doses of THC (3, 7, 20, 26 and 44 mg/kg, i.p.) every 40 minutes, with responses assessed 30 minutes after each injection. Studies followed the NIH Guidelines for the Care and Use of Laboratory Animals.
Behavior
Antinociception was assessed using the warm-water (52°C) tail-immersion assay (19). Duplicate measurements determined baseline responses, but mice were assessed only once following each injection to minimize tissue damage. A trained observer assessed immobility by determining the time mice spent motionless on a metal ring-stand over 5-minutes (20). Mice were gently restrained and body temperature was measured using a rectal probe thermometer (15). Mice were sacrificed by decapitation 24 hours after testing. The spinal cord and brain were extracted, frozen and stored at −80°C. For antinociception and catalepsy, data are presented as %MPE = 100%×[(experimental response latency – basal response latency)/(maximal possible response – basal response latency)]. Non-linear regression analysis was calculated using GraphPad Prism.
[35S]GTPγS and [3H]SR141716A binding
Whole spinal cord was collected (see Supplement), tissue was homogenized and agonist-stimulated [35S]GTPγS binding was conducted as published (10). Concentration-effect curves were generated using 0.01–3µM CP55,940 or 0.03–10µM WIN55,212-2. Percent stimulation = [(agonist-stimulated – basal)/basal]×100%. Curves were fit using non-linear regression in GraphPad Prism. [3H]SR141716A binding was performed as published (21) using [3H]SR141716A (0.1–2.5 nM) and non-specific binding was measured with 5µM SR141716A. Data were fit to a one-site model in GraphPad Prism. Statistical comparisons used Student-Newman Keuls post-hoc test.
[35S]GTPγS and [3H]CP55,940 autoradiography
Agonist-stimulated [35S]GTPγS autoradiography was conducted on duplicate serial sections as published (7, 18). Basal and CP55,940-stimulated [35S]GTPγS binding were conducted in adjacent sections. CP55,940 is a high-efficacy agonist for G-protein activation, but does not activate non-CB1sites in brain sections (18). Net stimulation (nCi/g) = (agonist–stimulated – basal). [3H]CP55,940 autoradiography was modified from (1, 22). Total binding was assessed with 3nM [3H]CP55,940 and non-specific binding was measured using 10µM CP55,940. Image reconstructions, SPM and region-of-interest (ROI) analysis were conducted as published (18, 23). ROI measurements were made on original unprocessed images, averaged across hemispheres, and analyzed by two-way ANOVA (significance p<0.05) and Student-Newman Keuls post-hoc comparisons.
Results
THC-induced responses in βarr2-KO mice
THC-mediated antinociception was assessed in vehicle- or THC-treated WT and βarr2-KO mice by cumulative dosing of THC. The basal latencies in WT and βarr2-KO mice subchronically treated with vehicle were 1.650±0.176 and 1.625±0.251, respectively. Basal latencies for WT and βarr2-KO mice treated with THC were 1.438±0.092 and 1.788±0.210, respectively. Cumulative dosing of THC produced a greater degree of antinociception in βarr2-KO mice subchronically treated with vehicle, compared to their WT littermates (Figure 1A: for genotype:F1,78=8.95, p=0.0037; for dose:F5,78=92.00, p<0.0001; for interaction:F5,78=5.11, p=0.0004). This difference was due to a difference in potency (ED50 value) between WT and βarr2-KO mice (Table 1). To determine the degree of antinociceptive tolerance, the ED50 of THC and 95% confidence intervals were calculated using nonlinear regression analysis of each curve. Comparison between genotypes revealed that subchronic THC treatment shifted the antinociceptive dose-response curve to the right to a much greater extent in WT (8.45-fold shift) compared to βarr2-KO mice (1.68-fold shift) (Table 1).
Figure 1.
Cumulative THC dose-response curves in WT and βarr2-KO mice subchronically treated with either vehicle or THC (10 mg/kg twice daily, i.p.). A. Following vehicle treatment, βarr2-KO mice display enhanced responses to THC compared to WT mice in the tail-flick antinociceptive test (for interaction of dose and genotype: F5,78 = 5.11, p = 0.0004, ***p<0.001 Bonferroni post-hoc analysis). Following THC pretreatment, βarr2-KO mice remain more responsive than WT mice (for genotype: F1,78 = 12.31, p = 0.0008, *p<0.05 Bonferroni post-hoc analysis). Both genotypes display tolerance following THC pretreatment (vehicle vs. THC: WT: for interaction of dose and pretreatment: F5,84 = 11.25, p <0.0001, ^p<0.05, ^^^p<0.001 Bonferroni post-hoc analysis. βarr2-KO: for pretreatment: F1-72 = 22.78, p <0.0001, #p<0.05, ##p<0.01 Bonferroni post-hoc analysis.) B. Following vehicle treatment, βarr2-KO mice display equivalent hypoactivity in response to THC compared to WT mice as assessed by the ring test for catalepsy (for genotype: F1,78=2.53, p=0.1159). Both genotypes displayed tolerance following the THC pretreatment (vehicle vs. THC: WT: for interaction of dose and pretreatment: F5-84=2.49, p=0.0376, ^^p<0.01 Bonferroni post-hoc analysis. βarr2-KO: for interaction of dose and pretreatment: F5-72= 4.91, p=0.0006, ###p<0.001 Bonferroni post-hoc analysis). Following THC pretreatment, βarr2-KO mice become more tolerant to the hypolocomotor effects of THC than WT mice (for genotype: F1,78 = 3.97, p=0.0497, #p<0.05 Bonferroni post-hoc analysis). C. Following vehicle treatment, βarr2-KO mice display greater hypothermia in response to THC compared to WT mice as determined by changes in rectal body temperature (for genotype: F1,78=8.15, p=0.0055). Following THC pretreatment, WT and βarr2-KO mice did not differ in their response profiles (for genotype: F1,78=0.09, p=0.9941). Both genotypes displayed tolerance following the THC pretreatment (vehicle vs. THC: WT: for interaction of dose and pretreatment: F5-84=13.26, p<0.0001, ^^^p<0.001 Bonferroni post-hoc analysis. βarr2-KO: for interaction of dose and pretreatment: F5–72=6.94, p<0.0001, ###p<0.001 Bonferroni post-hoc analysis). Data are presented as the mean ± S.E.M. (n = 8 WT, n = 7 βarr2-KO). The log values of the indicated doses are graphed on the abscissa and nonlinear regression curves are provided.
Table 1.
ED50 values (mg/kg) (±95% confidence intervals) for THC cumulative dosing curves obtained in WT and βarr2-KO mice for tail-flick, catalepsy and hypothermia assessments
| Behavioral Assessment |
Genotype | Vehicle ED50 (95% CI) |
THC ED50 (95% CI) |
Fold Shift in ED50 |
|---|---|---|---|---|
| Antinociception | WT | 13.85 (9.30–20.61) | 126.9 (81.50–197.4) | 8.45** |
| βarr2-KO | 6.37 (4.65–8.73) | 10.71 (2.60–44.10) | 1.68 | |
| Catalepsy | WT | 19.79 (11.56–33.88) | 41.94 (29.22–60.18) | 2.12** |
| βarr2-KO | 12.03 (6.04–23.96) | 57.32 (39.82-82.53) | 4.76* | |
| Hypothermia | WT | 50.24 (17.22–146.50) | NC | -- |
| βarr2-KO | 47.97 (14.06–163.60) | NC | -- |
(WT: vehicle vs. THC and KO: vehicle vs. THC, * p<0.05, ** p<0.01; sum of squares f test; n = 8/group) (NC, not converged)
Cannabinoids decrease rodent motility measured as time spent in a cataleptic state. Cumulative dosing of THC induced a similar degree of catalepsy in vehicle-treated WT and βarr2-KO mice (Figure 1B: genotype:F1,78=2.53, p=0.1159; dose:F5,78=65.19, p<0.0001; interaction:F5,78=0.71, p=0.6193). Subchronic THC treatment reduced the time that both WT and βarr2-KO mice spent immobile compared to vehicle-treated mice given the same acute dose of THC, indicating that both WT and βarr2-KO mice become tolerant to THC-mediated catalepsy (WT, treatment:F1,84=18.13, p<0.0001; dose:F5,84=84.00, p<0.0001; interaction:F5,84=2.49, p=0.0376; βarr2-KO, treatment:F1,72=53.27, p<0.0001; dose:F5,72=54.97, p<0.0001; interaction:F5,72=4.91, p=0.0006). Comparison of the THC-induced shift in the ED50 for each genotype revealed that, in contrast to antinociception, βarr2-KO mice displayed a greater degree of tolerance to THC-induced catalepsy (4.76-fold shift) than WT mice (2.12-fold shift) (F1,70=7.873; p<0.01 sum of least squares f test) (Table 1).
THC-induced hypothermia was also assessed in WT and βarr2-KO mice. Vehicle-treated βarr2-KO mice displayed greater decreases in body temperature across the THC dosing regimen, compared to vehicle-treated WT mice (Figure 1C: genotype:F1,78=8.15, p=0.0055; dose:F5,78=41.96, p<0.0001; interaction:F5,78=0.16, p=0.9775). Subchronic THC treatment induced significant tolerance to THC-mediated hypothermia in both genotypes (WT, treatment:F1,84=51.15, p<0.0001; dose:F5,84=32.38, p<0.0001; interaction:F5,84=13.26, p<0.0001; βarr2-KO, treatment:F1,72=65.02, p<0.0001; dose:F5,72=14.76, p<0.0001; interaction:F5,72=6.94, p<0.0001). The data for THC groups did not converge, therefore ED50 values could not be calculated and comparison of the degree of tolerance was not possible. However, statistical analysis of the two curves revealed no significant difference between THC-pretreated WT and βarr2-KO mice (genotype:F1–78=0.18, p=0.6765). Collectively these studies suggest region-specific CB1R regulation by β-arrestin2, because THC-induced antinociception, catalepsy and hypothermia are mediated by different neuronal populations in the CNS (24–26).
β-arrestin2 regulates CB1R desensitization in spinal cord
The finding that THC-mediated antinociception is enhanced, while development of tolerance is reduced in βarr2-KO mice suggests that β-arrestin2 might negatively regulate CB1Rs in the spinal cord because CB1Rs in this region contribute to tail-flick antinociception (27). Therefore, cannabinoid-stimulated [35S]GTPγS binding was assessed in spinal cords from WT and βarr2-KO mice. Basal [35S]GTPγS binding did not significantly differ between genotypes or treatments (WT/vehicle = 215±11, WT/THC = 208±18, βarr2-KO/vehicle = 221±20, and βarr2-KO/THC = 198±15 nCi/g). Residual THC would stimulate [35S]GTPγS binding above basal levels in vehicle-treated mice, thus THC washout was sufficient. CP55,940-mediated G-protein activity was first compared between vehicle-treated WT and βarr2-KO mice and showed no differences between genotypes (Figure 2A, Table 2). Subchronic treatment with THC significantly reduced CP55,940-stimulated [35S]GTPγS binding in WT mice, with an approximately 38% decrease in receptor-mediated activity (Figure 2A, Table 2, p<0.001). CB1R desensitization was attenuated in βarr2-KO mice, where the Emax value for CP55,940-stimulated [35S]GTPγS binding did not significantly differ from its vehicle control, but differed from THC-treated WT mice (Table 2, p<0.001). EC50 values for CP55,940-stimulated [35S]GTPγS binding did not significantly differ between treatment groups (Table 2), showing sufficient washout of THC. A significant interaction was found between drug and genotype [F1,24=5.68, p<0.05] in CP55,940-stimulated [35S]GTPγS binding, suggesting that β-arrestin2 contributes to CB1R desensitization in the spinal cord following THC treatment. Similar results were found using WIN55,212-2 (Table 2). These studies suggest that β-arrestin2-mediated CB1R desensitization might contribute to tolerance to THC-mediated antinociception, because both CB1R desensitization in the spinal cord and tolerance to the tail-flick test were significantly attenuated in βarr2-KO mice.
Figure 2.
Agonist-stimulated [35S]GTPγS concentration-effect curves using CP55,940 and CB1R binding using [3H]SR141716A in WT and βarr2-KO mice subchronically treated with either vehicle or THC (10 mg/kg twice daily, i.p.). A. Desensitization of CB1R-mediated G-protein activation was attenuated in spinal cords of βarr2-KO mice following subchronic treatment with THC. B. Subchronic administration of THC produced downregulation of CB1Rs in spinal cords of WT animals, but was attenuated in βarr2-KO mice. EC50 values for CP55,940- or WIN55,212-2-stimulated [35S]GTPγS binding did not significantly differ between treatment groups.
Table 2.
Agonist-stimulated [35S]GTPγS (top rows) and [3H]SR141716A (bottom rows) binding in spinal cords from WT and βarr2-KO mice following subchronic treatment with vehicle or THC. Emax values (expressed as %stimulation [(agonist-stimulated - basal)/basal × 100%]) and EC50 (nM) values were calculated with Prism using a non-linear fit. Bmax (pmol/mg) and KD (nM) values were calculated using a one-site hyperbola function.
| Group | EC50 (nM) | Emax (%Stim.) | % Vehicle |
|---|---|---|---|
| CP55,940 | |||
| WT Vehicle | 38 ± 17 | 45.04 ± 1.91 | 100 ± 4.25 |
| WT THC | 17 ± 11 | 27.88 ± 3.64***, ### | 61.89 ± 8.08 |
| βarr2-KO Vehicle | 24 ± 7 | 46.41 ± 2.13 | 100 ± 4.60 |
| βarr2-KO THC | 11 ± 3 | 41.10 ± 1.80 | 88.56 ± 3.88 |
| WIN55,212-2 | |||
| WT Vehicle | 85 ± 21 | 75.46 ± 6.82 | 100 ± 9.04 |
| WT THC | 198 ± 37 | 49.39 ± 4.41* | 65.45 ± 5.84 |
| βarr2-KO Vehicle | 338 ± 187 | 80.87 ± 11.55 | 100 ± 14.28 |
| βarr2-KO THC | 220 ± 69 | 72.53 ± 5.99 | 89.67 ± 7.41 |
| Group | KD (nM) | Bmax (pmol/mg) | % Vehicle |
| [3H]SR141716A | |||
| WT Vehicle | 1.03 ± 0.24 | 0.47 ± 0.04 | 100 ± 9.07 |
| WT THC | 0.91 ± 0.11 | 0.28 ± 0.02**, # | 59.40 ± 4.12 |
| βarr2-KO Vehicle | 1.43 ± 0.27 | 0.54 ± 0.04 | 100 ± 6.67 |
| βarr2-KO THC | 1.51 ± 0.34 | 0.44 ± 0.07 | 82.30 ± 12.21 |
Values represent the mean ± SEM (n = 6–7 per group).
p < 0.05,
p < 0.0l,
p < 0.001, versus respective vehicle control group;
p < 0.001 versus βarr2-KO THC (2-way ANOVA, Student-Newman Keuls Post-hoc).
β-arrestin2 regulates CB1R downregulation in spinal cord
β-arrestin2 facilitates endocytosis for many GPCRs (11). Internalized receptors can be degraded and the resulting downregulation could contribute to antinociceptive tolerance. To determine the role of β-arrestin2 in CB1R downregulation, [3H]SR141716A binding was performed in spinal cord. No differences were found in [3H]SR141716A binding between genotypes in vehicle-treated mice (Figure 2B, Table 2), showing that alterations in acute behavior were not due to a change in CB1R density in βarr2-KO mice. In contrast, subchronic THC treatment significantly reduced [3H]SR141716A binding in WT mouse spinal cord (Bmax = 59.4% of vehicle, p<0.01) an effect that was significantly attenuated in βarr2-KO mice (Bmax = 82.3% of vehicle). The Bmax value for THC-treated βarr2-KO mice did not differ from its vehicle control, but was significantly different from THC-treated WT mice (p<0.05). No differences in [3H]SR141716A KD values were found between groups (Figure 2B), indicating adequate washout of THC. These results indicate that β-arrestin2 is also involved in CB1R downregulation in the spinal cord, which could contribute to attenuation of THC-mediated antinociceptive tolerance.
β-arrestin2 regulates CB1R-mediated G-protein activity in a brain region-specific manner
THC-induced catalepsy (25), hypothermia (26) and antinociception (24) involve different populations of CB1Rs in the brain. Therefore, CB1R-mediated G-protein activity was assessed throughout the brains of vehicle- and THC-treated βarr2-KO and WT mice using CP55,940-stimulated [35S]GTPγS autoradiography with SPM and ROI analyses. CP55,940-stimulated [35S]GTPγS binding was widely distributed in brains from mice of both genotypes, with the highest levels in the basal ganglia, hippocampus, cerebellum and cortex, as previously reported (Figure 3) (18). SPM and ROI analyses of basal [35S]GTPγS binding showed no significant differences based on genotype or drug treatment in any region (Table S1 in the Supplement). CP55,940-stimulated [35S]GTPγS binding was first assessed in vehicle-treated mice of both genotypes. CP55,940-stimulated [35S]GTPγS binding appeared higher in cortical regions of βarr2-KO compared to WT mice (Figure 3) and SPM corroborated this observation. SPM revealed that CP55,940-stimulated [35S]GTPγS binding was significantly (p<0.05) greater in the cortex and caudal hippocampus of βarr2-KO compared to WT mice (Figure 4). This was confirmed by ROI analysis of individual sections, which showed significantly greater CP55,940-stimulated [35S]GTPγS binding in the piriform, auditory and visual cortices of βarr2-KO compared to WT mice (Figure 5, Table 3). Intra-regional effects were found within the hippocampus, where CB1R-mediated G-protein activity was significantly higher in βarr2-KO compared to WT mice only in the caudal hippocampus (Figure 4, Table 3). A non-significant trend toward greater CP55,940-stimulated [35S]GTPγS binding in βarr2-KO mice was observed in the cingulate cortex (p=0.051), somatosensory cortex (p=0.078) and amygdala (p=0.058) (Table 3). These results indicate region-specific regulation of acute CB1R signaling that was apparent in βarr2-KO mice.
Figure 3.
Net CP55,940-stimulated [35S]GTPγS binding reconstructions derived from the image average of all subjects for vehicle- and THC-treated mice of each genotype (n = 8). Original autoradiographic images are shown in grayscale and correspond to the scale (bottom right). AMYG, amygdala; A,V, auditory & visual cortex; CBLM, cerebellum; Cg, cingulate cortex; CPu, caudate-putamen; GP, globus pallidus; HIPP, hippocampus; HYPO, hypothalamus; PAG, periaqueductal gray; Pir, piriform cortex; SS, somatosensory cortex; SN, substantia nigra.
Figure 4.
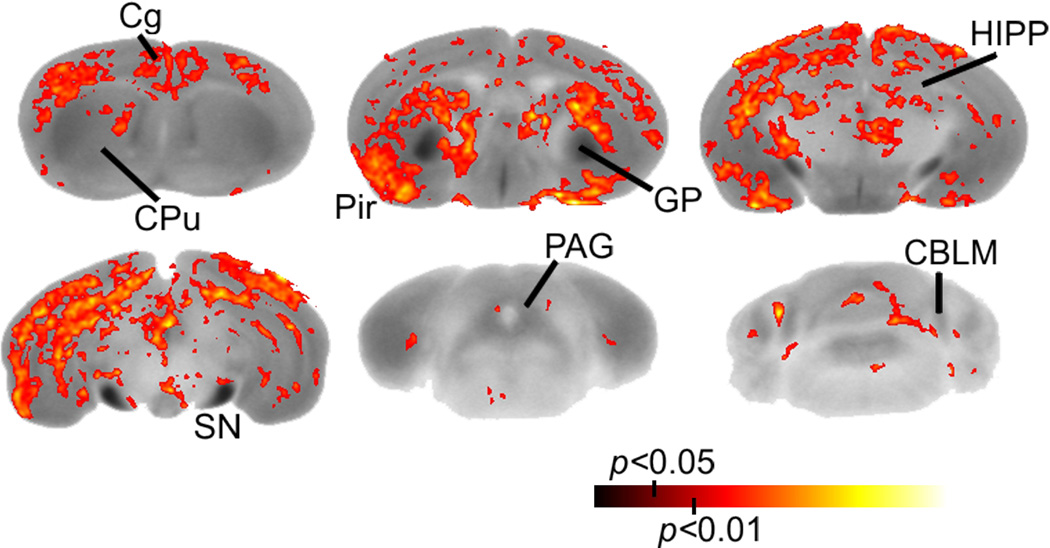
Genotype-specific differences in CP55,940-stimulated [35S]GTPγS binding were distributed primarily in the cortex and caudal hippocampus. Statistical parametric maps (significance is shown in red to yellow color and corresponds to the significance scale bar) show regions where significantly greater CP55,940-stimulated [35S]GTPγS binding was found in βarr2-KO compared to WT mice following vehicle treatment (n = 8 per group). Cg, cingulated cortex; CPu, caudate-putamen; GP, globus pallidus; Pir, piriform cortex; HIPP, hippocampus; SN, substantia nigra; PAG, periaqueductal gray; CBLM, cerebellum.
Figure 5.
Net CP55,940-stimulated [35S]GTPγS binding (mean ± SEM) in sampled brain regions (n = 8 per group) of WT and βarr2-KO mice following 6.5 day subchronic administration of either vehicle or 10 pmg/kg THC twice daily. *** p < 0.001, ** p < 0.01, * p < 0.05 versus respective vehicle; ### p < 0.001, # p < 0.05 versus WT vehicle; $ versus KO THC (Two way ANOVA, Student-Newman Keuls Post-hoc). PAG, periaqueductal gray; POA, preoptic area; SS, somatosensory cortex.
Table 3.
Net CP55,940-stimulated [35S]GTPγS binding in brain sections from WT and βarr2-KO mice following 6.5 day administration of either vehicle or THC (n = 8 per group). [35S]GTPγS binding is expressed as net agonist-stimulated [35S]GTPγS binding in nCi/g.
| Vehicle | THC | |||
|---|---|---|---|---|
| Region | WT | βarr2-KO | WT | βarr2-KO |
| Nucleus Accumbens | 150 ± 20 | 189 ± 24 | 136 ± 24 | 163 ± 33 |
| Caudate-Putamen | 256 ± 23 | 282 ± 24 | 230 ± 17 | 252 ± 25 |
| Globus Pallidus | 726 ± 36 | 751 ± 47 | 631 ± 41 | 593 ± 43* |
| Cingulate Cortex | 221 ± 39 | 318 ± 36 | 176 ± 29 | 235 ± 29 |
| Piriform Cortex | 225 ± 32 | 380 ± 18### | 195 ± 29 | 229 ± 25*** |
| Auditory & Visual Cortices | 188 ± 21 | 249 ± 26# | 165 ± 23 | 169 ± 9* |
| Somatosensory Cortex | 226 ± 25 | 281 ± 22 | 168 ± 15 | 169 ± 20** |
| Lateral Entorhinal Cortex | 350 ± 16 | 393 ± 19 | 252 ± 23** | 277 ± 23*** |
| Amygdala | 321 ± 17 | 385 ± 27 | 209 ± 26** | 228 ± 19*** |
| Hippocampus (rostral) | 248 ± 17 | 242 ± 32 | 147 ± 32* | 146 ± 23* |
| Hippocampus (caudal) | 279 ± 31 | 362 ± 27# | 203 ± 12* | 212 ± 14*** |
| Preoptic area | 141 ± 12 | 157 ± 29 | 172 ± 20 | 111 ± 30 |
| Hypothalamus | 216 ± 21 | 238 ± 32 | 169 ± 35 | 136 ± 20* |
| Thalamus | 90 ± 15 | 90 ± 15 | 79 ± 12 | 110 ± 21 |
| Substantia Nigra | 814 ± 26 | 856 ± 30 | 776 ± 58 | 733 ± 24* |
| PAG (rostral) | 146 ± 19 | 171 ± 28 | 81 ± 16* | 88 ± 9** |
| PAG (caudal) | 207 ± 26 | 189 ± 22 | 100 ± 12**,$ | 158 ± 12 |
| Cerebellum | 440 ± 19 | 437 ± 23 | 314 ± 32* | 434 ± 38 |
Values represent mean ± SEM.
p < 0.05,
p < 0.01,
p < 0.001 versus respective vehicle;
p < 0.05,
p < 0.001 versus WT vehicle;
p < 0.05 versus KO THC (n = 8 per group, two way ANOVA, Student-Newman Keuls Post-hoc).
To investigate the role of β-arrestin2 in CB1R regulation after repeated THC, CP55,940-stimulated [35S]GTPγS binding was compared between vehicle- and THC-treated WT or βarr2-KO mice. SPM showed regions in which THC-induced decreases in CP55,940-stimulated [35S]GTPγS binding were significant (p<0.05) (Figure 6). SPM and ROI analysis also revealed differences between βarr2-KO and WT mice in both the regional extent and magnitude of CB1R desensitization. Genotype-specific attenuation in desensitization was seen in some regions in βarr2-KO mice, similar to spinal cord. In the cerebellum, SPM showed that CP55,940-stimulated [35S]GTPγS binding was significantly reduced in brains from THC-treated WT mice (Figure 6), and ROI analysis confirmed that CB1R-mediated G-protein activity in THC-treated WT mice was reduced to 71±7% of levels in vehicle-controls (Figure 5, Table 3). In contrast, no significant difference in CP55,940-stimulated [35S]GTPγS binding in the cerebellum was observed between THC- and vehicle-treated βarr2-KO mice using SPM or ROI analysis (Figure 5, Table 3). A significant interaction of drug treatment×genotype was found [F1,28=4.51, p<0.05], suggesting that β-arrestin2 regulates CB1R desensitization in the cerebellum following this THC treatment paradigm. SPM revealed intra-regional differences in the PAG (Figure 6). Similar significant decreases in CP55,940-stimulated [35S]GTPγS binding were detected in the rostral PAG (~Bregma −3.40) from THC-treated βarr2-KO and WT mice. In contrast, CP55,940-stimulated [35S]GTPγS binding in the caudal PAG was not significantly affected by THC treatment in βarr2-KO mice (~Bregma −4.60), suggesting that CB1R desensitization was attenuated in βarr2-KO mice. This was confirmed by ROI analysis, which showed that THC treatment significantly reduced CP55,940-stimulated [35S]GTPγS binding in the caudal PAG of WT mice (48±5% of vehicle), whereas activity was not significantly different between vehicle-and THC-treated βarr2-KO mice (84±6% of vehicle) (Table 3, Figure 5). In contrast, ROI analysis revealed a similar magnitude of desensitization in the rostral PAG of WT (55%±6 of vehicle) and βarr2-KO (51±5% of vehicle) mice.
Figure 6.
SPM analysis revealed both region- and genotype-specific differences in desensitization of CB1R-mediated G-protein activation in the reconstructed mouse brain of WT and βarr2-KO mice following 6.5 day subchronic administration of either vehicle or 10 mg/kg THC (n = 8 per group) twice daily. Brain regions within each genotype demonstrating significant desensitization (p < 0.05, 2-way ANOVA, n = 8) are colored in blue/green and correspond to the significance scale (bottom). AMYG, amygdala; A,V, auditory & visual cortex; CBLM, cerebellum; Cg, cingulate cortex; CPu, caudate-putamen; GP, globus pallidus; HIPP, hippocampus; Hypo, hypothalamus; LEnt, lateral entorhinal cortex; PAG, periaqueductal gray; Pir, piriform cortex; cPAG, caudal periaqueductal gray; rPAG, rostral periaqueductal gray; SN, substantia nigra; SS, somatosensory cortex.
SPM identified several regions in which CB1R desensitization appeared similar in βarr2-KO and WT mice (Figure 6). In the amygdala, ROI analysis showed that THC treatment reduced cannabinoid-stimulated [35S]GTPγS binding to 59±5% and 65±8% of vehicle control in βarr2-KO and WT mice, respectively (Figure 5, Table 3). Significant reductions in CP55,940-stimulated [35S]GTPγS binding were also found in the lateral entorhinal cortex and rostral hippocampus, and did not significantly differ between genotypes (Figure 5, Table 3).
CP55,940-stimulated [35S]GTPγS binding was also similar between genotypes in the caudate-putamen and nucleus accumbens, where CB1R-mediated G-protein activity did not differ between THC- and vehicle-treated groups of either genotype (Figures 5–6, Table 3). Different results were observed in the globus pallidus and substantia nigra when comparing genotypes. CP55,940-stimulated [35S]GTPγS binding did not differ between THC- and vehicle-treated WT mice in these regions, showing a lack of CB1R desensitization. However, CP55,940-stimulated [35S]GTPγS binding was significantly reduced in the substantia nigra (~85% of vehicle) and globus pallidus (~79% of vehicle) of THC-treated compared to vehicle-treated βarr2-KO mice (Figures 5–6, Table 3). Therefore, deletion of β-arrestin2 appeared to enhance CB1R desensitization in the globus pallidus and substantia nigra, without altering acute CB1R-mediated G-protein activity in vehicle-treated mice.
SPM analysis revealed enhanced desensitization in several other regions of βarr2-KO compared to WT mice (Figure 6). In the hypothalamus, CP55,940-stimulated [35S]GTPγS binding in THC-treated mice was 57±8% and 79±16% of vehicle in βarr2-KO and WT mice, respectively (Figure 5, Table 3) and occurred in the absence of genotype-dependent differences in vehicle-treated mice (Table 3). Subchronic THC also induced CB1R desensitization in the piriform, auditory and visual cortices of βarr2-KO, but not WT, mice (Figure 5, Table 3). SPM revealed intra-regional differences in desensitization within the hippocampus, wherein desensitization was significantly greater in magnitude in the caudal hippocampus of βarr2-KO mice (Figures 6). ROI analysis showed a significantly greater reduction in CP55,940-stimulated [35S]GTPγS binding in the caudal hippocampus in βarr2-KO mice (58.6±4% of vehicle), compared to WT mice (72.6±4% of vehicle) (Figure 5, Table 3).
Increases in the apparent efficacy of CP55,940-stimulated [35S]GTPγS binding in some regions of βarr2-KO brains appeared to be associated with the enhancement or unmasking of CB1R desensitization in these same regions following THC administration. The relative magnitude of desensitization in βarr2-KO mice when normalized to WT animals was positively correlated with the relative efficacy of CP55,940-stimulated [35S]GTPγS binding in βarr2-KO mice when normalized to WT animals (p=0.0011, R2=0.60; Figure 7, Table 5). For example, a 1.5-fold greater relative efficacy of CP55,940-stimulated [35S]GTPγS binding in βarr2-KO mice compared to WT mice corresponded to an approximately 2-fold greater magnitude of desensitization in βarr2-KO relative to WT mice.
Figure 7.
Relative efficacy of CP55,940-stimulated [35S]GTPγS binding is positively correlated with the relative magnitude of CB1R desensitization in βarr2-KO mice following subchronic THC administration. Each point in the graph represents a brain area (see Table 5) corresponding to its mean Desensitization Ratio and mean Relative efficacy. The Desensitization Ratio was calculated by normalizing the magnitudes of desensitization in βarr2-KO mice to the mean magnitude of desensitization in WT mice. Relative efficacy was calculated by normalizing the magnitudes of CP55,940-stimulated [35S]GTPγS binding in vehicle treated βarr2-KO mice by the mean magnitude of CP55,940-stimulated [35S]GTPγS binding in vehicle treated WT mice. Calculated ratios in the graph represent the mean ± SEM (n = 6–8). Linear regression analysis was performed in Graphpad Prism 5.
Table 5.
Relative Efficacy and Desensitization Ratio values for each brain region from WT and βarr2-KO mice treated for 6.5 days with either vehicle or THC (n = 6–8 per group). Calculation of values is explained in the legend of Figure 7.
Relative Efficacy and Desensitization Ratio values represent the mean ± SEM (n = 6–8).
| Brain Region | Relative Efficacy |
Desensitization Ratio |
|---|---|---|
| Nucleus Accumbens | 1.26 ± 0.16 | 2.21 ± 0.75 |
| Caudate-Putamen | 1.10 ± 0.09 | 1.00 ± 0.85 |
| Cingulate Cortex | 1.44 ± 0.16 | 1.26 ± 0.44 |
| Piriform Cortex | 1.69 ± 0.08 | 3.01 ± 0.49 |
| Auditory & Visual Cortices | 1.33 ± 0.14 | 2.65 ± 0.30 |
| Somatosensory Cortex | 1.24 ± 0.10 | 1.53 ± 0.27 |
| Lateral Entorhinal Cortex | 1.12 ± 0.05 | 1.06 ± 0.21 |
| Amygdala | 1.20 ± 0.08 | 1.16 ± 0.14 |
| Hippocampus (rostral) | 0.98 ± 0.13 | 0.97 ± 0.24 |
| Hippocampus (caudal) | 1.30 ± 0.10 | 1.51 ± 0.14 |
| Hypothalamus | 1.10 ± 0.15 | 2.01 ± 0.38 |
| PAG (rostral) | 1.17 ± 0.19 | 1.10 ± 0.12 |
| PAG (caudal) | 0.91 ± 0.11 | 0.31 ± 0.12 |
| Cerebellum | 0.99 ± 0.05 | 0.03 ± 0.30 |
PAG, periaqueductal gray
β-arrestin2 is involved in CB1R downregulation in the cerebellum
The role of β-arrestin2 in CB1R downregulation was assessed using [3H]CP55,940 autoradiography. [3H]CP55,940 binding did not differ between genotypes in vehicle-treated mice (Table 4), suggesting that genotype-dependent differences in CP55,940-stimulated [35S]GTPγS binding were not due to differences in CB1R levels. [3H]CP55,940 binding was significantly decreased in the cerebellum of WT mice following THC treatment (55±10% of vehicle), whereas [3H]CP55,940 binding was similar in both treatment groups in βarr2-KO mice. [3H]CP55,940 binding did not significantly differ between THC- and vehicle-treated mice of either genotype in any other region examined (Table 4). A significant interaction of drug treatment×genotype was found in the cerebellum [F1,28=4.277, p<0.05], suggesting that β-arrestin2 is involved in CB1R downregulation in the cerebellum.
Table 4.
[3H]CP55,940 binding in brain sections from WT and βarr2-KO mice treated for 6.5 days with either vehicle or THC (n = 8 per group). [3H]CP55,940 binding is expressed as nCi/mg.
| Vehicle | THC | |||
|---|---|---|---|---|
| Region | WT | βarr2-KO | WT | βarr2-KO |
| Caudate-Putamen | 2.39 ± 0.36 | 2.10 ± 0.43 | 1.76 ± 0.46 | 2.05 ± 0.38 |
| Globus Pallidus | 5.51 ± 0.86 | 5.29 ± 0.89 | 4.90 ± 1.11 | 4.88 ± 0.72 |
| Cingulate Cortex | 1.63 ± 0.29 | 1.41 ± 0.22 | 1.22 ± 0.24 | 1.14 ± 0.13 |
| Somatosensory Cortex | 1.57 ± 0.27 | 1.57 ± 0.25 | 1.23 ± 0.22 | 1.23 ± 0.16 |
| Amygdala | 1.77 ± 0.40 | 1.72 ± 0.24 | 1.20 ± 0.30 | 1.64 ± 0.22 |
| Hippocampus | 1.66 ± 0.29 | 1.48 ± 0.21 | 1.24 ± 0.28 | 1.43 ± 0.19 |
| Hypothalamus | 1.75 ± 0.42 | 1.67 ± 0.23 | 1.25 ± 0.35 | 1.27 ± 0.29 |
| Thalamus | 1.12 ± 0.16 | 1.17 ± 0.18 | 1.17 ± 0.26 | 1.24 ± 0.12 |
| Substantia Nigra | 5.66 ± 0.80 5 | 5.52 ± 0.82 | 5.28 ± 1.09 | 5.15 ± 1.07 |
| PAG | 1.76 ± 0.37 | 1.47 ± 0.21 | 1.21 ± 0.27 | 1.51 ± 0.30 |
| Cerebellum | 3.18 ± 0.46 2 | 2.63 ± 0.40 | 1.74 ± 0.32* | 2.80 ± 0.36 |
[3H]CP55,940 binding values represent the mean ± SEM. p < 0.05 versus respective vehicle (Two way ANOVA, Student-Newman Keuls Post-hoc).
Discussion
This study shows that β-arrestin2 regulates CB1R-mediated G-protein activity in a CNS region-specific manner and that these differences are reflected in THC-mediated responses observed in βarr2-KO mice. Genotype-specific differences were observed after acute THC, as βarr2-KO mice displayed enhanced THC-mediated hypothermia, antinociception and receptor-mediated G-protein activity in the forebrain compared to their WT littermates. Following repeated THC administration, β-arrestin2 deletion attenuated tolerance to THC-mediated antinociception and CB1R desensitization and/or downregulation in the cerebellum, caudal PAG, and spinal cord. These findings are consistent with the prediction that β-arrestin2 regulates CB1R adaptations that occur following subchronic THC administration. CB1R desensitization was similar between genotypes in other regions, indicating that other mechanisms might mediate desensitization or compensate for β-arrestin2 deletion. Interestingly, CB1R desensitization was enhanced in a subset of regions, some of which also showed genotype-specific differences in acute signaling.
THC-mediated hypothermia and antinociception were enhanced in βarr2-KO compared to WT mice, whereas catalepsy was similar between genotypes. These results agree with Breivogel et al (17), who showed that THC-mediated antinociception and hypothermia were enhanced by β-arrestin2 deletion. Antinociception and hypothermia produced by the high efficacy agonist CP55,940 did not differ between WT and βarr2-KO mice (17), indicating that effects of β-arrestin2 on CB1Rs are ligand-dependent. Microinjection studies showed that the preoptic anterior hypothalamus is involved in cannabinoid-mediated hypothermia (26), but cannabinoid-stimulated G-protein activity did not significantly differ between genotypes in the preoptic area or hypothalamus. Similarly, CB1Rs in the spinal cord and PAG mediate cannabinoid-induced antinociception (24, 27, 28), but acute cannabinoid-stimulated G-protein activity did not differ between genotypes in these areas despite an enhancement in THC-induced antinociception. Physiological responses result from the interplay of various brain regions, so it is possible that enhanced CB1R signaling in the forebrain contributes indirectly to the observed enhancements in antinociception and hypothermia. Alternatively, THC might indirectly produce these responses via modulation of other receptor systems that are regulated directly by β-arrestin2. In either case, our results suggest that β-arrestin2 dampens THC-induced antinociception and hypothermia. Interestingly, morphine-mediated antinociception and hypothermia were also enhanced in βarr2-KO mice (15, 19, 29), supporting the idea that β-arrestin2 negatively regulates receptor signaling in these functional circuits. However, enhanced antinociceptive responses in βarr2-KO mice are not due to non-specific dysregulation in these systems, as other opioid agonists, such as fentanyl or methadone, produced similar responses in βarr2-KO and WT mice (30, 31).
A possible explanation for the finding that CB1R-mediated G-protein activity was enhanced in some regions of βarr2-KO mice is that CB1R constitutive activity or high endocannabinoid tone might induce β-arrestin2-mediated desensitization/internalization. In fact, previous studies showed that CB1Rs undergo constitutive endocytosis with substantial localization in intracellular vesicles (32), although there is disagreement on the role of CB1R activation in this process (33, 34). If deletion of β-arrestin2 increases the population of functional CB1Rs localized to the plasma membrane, as suggested by enhanced CP55,940-stimulated [35S]GTPγS binding in regions such as cortex and caudal hippocampus, then more receptors might be available for agonist-stimulated activation of G-proteins (35), thereby increasing signaling efficacy and in vivo potency of the agonist. Alternatively, CB1Rs in these regions might exhibit desensitization to the acute cumulative-dosing THC exposure in WT mice, although we previously found no significant effect of acute THC treatment on CB1R-mediated G-protein activation (7).
Deletion of β-arrestin2 attenuated THC-induced desensitization of CB1R-mediated G-protein activity in the cerebellum, caudal PAG, and spinal cord, a similar finding to reports in cell models (8, 13). Tolerance to THC-mediated antinociception was also attenuated in βarr2-KO mice, which likely can be attributed to the reduced CB1R desensitization/downregulation observed in the spinal cord and caudal PAG following THC treatment, as these regions mediate cannabinoid-induced antinociception (24, 28). Findings in cerebellum are consistent with the expression of β-arrestin2 and CB1R mRNA in this region as demonstrated by the Allen brain atlas (http://mouse.brain-map.org/brain/Arrb2.html) (36). These results suggest that the development of cannabinoids that minimize CB1R interactions with β-arrestin2 might enhance antinociception while reducing the development of tolerance, thereby producing more effective analgesics.
Previous studies found that CB1Rs in the basal ganglia appear resistant to desensitization compared with other regions (5, 21, 37). Similarly, CB1Rs in the caudate-putamen, globus pallidus and substantia nigra from WT mice were not desensitized following subchronic THC treatment in this study. Surprisingly, however, CB1R desensitization was observed in the globus pallidus and substantia nigra of βarr2-KO mice. While expression of β−arrestin2 mRNA in the striatum is low (36), our results suggest a functional interaction with CB1Rs. β−arrestin1 mRNA expression is high in this region (36), thus β−arrestin1 could contribute to enhanced desensitization. The globus pallidus has been implicated in THC-induced catalepsy (25), which might explain the observation that tolerance to THC-induced catalepsy was enhanced in βarr2-KO compared to WT mice. This finding suggests that β-arrestin2 inhibits desensitization in these striatal output nuclei and thereby contributes to the reduced CB1R adaptations usually reported in these regions. Thus, β-arrestin2 would promote cataleptic side effects of THC by inhibiting the development of tolerance.
The finding that desensitization was unaffected or enhanced in certain regions of βarr2-KO mice indicates that non-β-arrestin2-mediated CB1R regulation contributes to these adaptations. β-arrestin1 exhibits significant homology to β-arrestin2 (12) and has a widespread CNS localization that overlaps with CB1Rs (12, 38). Deletion of β-arrestin1 enhanced isoproterenol-mediated cardiac responses (39) and decreased apomorphine-induced climbing in the absence of baseline differences in locomotion (11). These findings suggest that β-arrestin1 both negatively and positively regulates receptor function. Several kinases regulate CB1R desensitization and tolerance and might contribute to desensitization observed in βarr2-KO mice. cAMP-dependent protein kinase (PKA) appears to contribute to desensitization (40) and PKA inhibitors reverse tolerance to THC-induced antinociception, catalepsy, and hypoactivity (41, 42). Protein kinase C (PKC) phosphorylates CB1R in cell models (43). CB1R adaptation and tolerance also involve ERK in a region- and effect-specific manner (44, 45). Deletion of G-protein-associated sorting protein1 (GASP1), which targets receptors to lysosomes for degradation, attenuates CB1R downregulation and tolerance to cannabinoid-mediated antinociception (46). Together, these results suggest that additional mechanisms can regulate CB1R activity, either in a parallel or overlapping manner with β-arrestin2.
In summary, the present studies demonstrate multiple effects of β-arrestin2 deletion on CB1R function, and confirm its role in attenuating acute THC-mediated antinociception and hypothermia. Moreover, results demonstrate both positive and negative modulatory effects of β-arrestin2 on THC tolerance, such that tolerance to antinociception was reduced whereas tolerance to catalepsy was enhanced in βarr2-KO mice. These findings corresponded with reduced CB1R desensitization in the caudal PAG and spinal cord, and enhanced CB1R desensitization in striatal output nuclei, respectively. These findings suggest that developing cannabinoid agonists that minimize interactions between CB1R and β-arrestin2 might be therapeutically beneficial, as data suggest that these agonists might enhance antinociception but attenuate antinociceptive tolerance. The side effect profile might also improve because deletion of β-arrestin2 enhanced tolerance to THC-induced cataleptic effects, suggesting that motor side effects might be minimized. These findings demonstrate that interactions between CB1Rs and β-arrestin2 should be considered in future drug development.
Supplementary Material
Acknowledgements
The authors thank James Gillespie and Patraic Lichtman for assistance with experiments and image processing. This study was supported by USPHS Grants DA014277 (LJS), DA025321 (DES), DA14460 (LMB), F31-DA021952 (KMR), F30-DA023758 (PTN), and an A.D. Williams Award from Virginia Commonwealth University (LJS).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. The AAPS journal. 2005;7:E625–E654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handb Exp Pharmacol. 2005:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- 5.Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Critical reviews in neurobiology. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 6.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 7.Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin W, Brown S, Roche JP, Hsieh C, Celver JP, Kovoor A, et al. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J Neurosci. 1999;19:3773–3780. doi: 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oviedo A, Glowa J, Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res. 1993;616:293–302. doi: 10.1016/0006-8993(93)90220-h. [DOI] [PubMed] [Google Scholar]
- 10.Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, et al. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70:986–996. doi: 10.1124/mol.105.019612. [DOI] [PubMed] [Google Scholar]
- 11.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 12.Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 13.Kouznetsova M, Kelley B, Shen M, Thayer SA. Desensitization of cannabinoid-mediated presynaptic inhibition of neurotransmission between rat hippocampal neurons in culture. Mol Pharmacol. 2002;61:477–485. doi: 10.1124/mol.61.3.477. [DOI] [PubMed] [Google Scholar]
- 14.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 15.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 16.Schmid CL, Bohn LM. Physiological and pharmacological implications of beta-arrestin regulation. Pharmacology & therapeutics. 2009;121:285–293. doi: 10.1016/j.pharmthera.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breivogel CS, Lambert JM, Gerfin S, Huffman JW, Razdan RK. Sensitivity to delta9-tetrahydrocannabinol is selectively enhanced in beta-arrestin2 −/− mice. Behavioural pharmacology. 2008;19:298–307. doi: 10.1097/FBP.0b013e328308f1e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen PT, Selley DE, Sim-Selley LJ. Statistical Parametric Mapping reveals ligand and region-specific activation of G-proteins by CB1 receptors and non-CB1 sites in the 3D reconstructed mouse brain. Neuroimage. 2010;52:1243–1251. doi: 10.1016/j.neuroimage.2010.04.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pertwee RG. The ring test: a quantitative method for assessing the 'cataleptic' effect of cannabis in mice. Br J Pharmacol. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, et al. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moise AM, Eisenstein SA, Astarita G, Piomelli D, Hohmann AG. An endocannabinoid signaling system modulates anxiety-like behavior in male Syrian hamsters. Psychopharmacology (Berl) 2008;200:333–346. doi: 10.1007/s00213-008-1209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen PT, Holschneider DP, Maarek JM, Yang J, Mandelkern MA. Statistical parametric mapping applied to an autoradiographic study of cerebral activation during treadmill walking in rats. Neuroimage. 2004;23:252–259. doi: 10.1016/j.neuroimage.2004.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- 25.Pertwee RG, Wickens AP. Enhancement by chlordiazepoxide of catalepsy induced in rats by intravenous or intrapallidal injections of enantiomeric cannabinoids. Neuropharmacology. 1991;30:237–244. doi: 10.1016/0028-3908(91)90150-a. [DOI] [PubMed] [Google Scholar]
- 26.Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2[(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)- 6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J Pharmacol Exp Ther. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- 27.Smith PB, Martin BR. Spinal mechanisms of delta 9-tetrahydrocannabinol-induced analgesia. Brain Res. 1992;578:8–12. doi: 10.1016/0006-8993(92)90222-u. [DOI] [PubMed] [Google Scholar]
- 28.Lichtman AH, Martin BR. Spinal and supraspinal components of cannabinoid-induced antinociception. J Pharmacol Exp Ther. 1991;258:517–523. [PubMed] [Google Scholar]
- 29.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 30.Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004;66:106–112. doi: 10.1124/mol.66.1.106. [DOI] [PubMed] [Google Scholar]
- 31.Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leterrier C, Bonnard D, Carrel D, Rossier J, Lenkei Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J Biol Chem. 2004;279:36013–36021. doi: 10.1074/jbc.M403990200. [DOI] [PubMed] [Google Scholar]
- 33.Leterrier C, Laine J, Darmon M, Boudin H, Rossier J, Lenkei Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J Neurosci. 2006;26:3141–3153. doi: 10.1523/JNEUROSCI.5437-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald NA, Henstridge CM, Connolly CN, Irving AJ. An essential role for constitutive endocytosis, but not activity, in the axonal targeting of the CB1 cannabinoid receptor. Mol Pharmacol. 2007;71:976–984. doi: 10.1124/mol.106.029348. [DOI] [PubMed] [Google Scholar]
- 35.Grimsey NL, Graham ES, Dragunow M, Glass M. Cannabinoid Receptor 1 trafficking and the role of the intracellular pool: implications for therapeutics. Biochem Pharmacol. 80:1050–1062. doi: 10.1016/j.bcp.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 37.Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- 38.Gurevich EV, Benovic JL, Gurevich VV. Arrestin2 and arrestin3 are differentially expressed in the rat brain during postnatal development. Neuroscience. 2002;109:421–436. doi: 10.1016/s0306-4522(01)00511-5. [DOI] [PubMed] [Google Scholar]
- 39.Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, et al. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circulation research. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- 40.Huang CC, Chen YL, Lo SW, Hsu KS. Activation of cAMP-dependent protein kinase suppresses the presynaptic cannabinoid inhibition of glutamatergic transmission at corticostriatal synapses. Mol Pharmacol. 2002;61:578–585. doi: 10.1124/mol.61.3.578. [DOI] [PubMed] [Google Scholar]
- 41.Bass CE, Welch SP, Martin BR. Reversal of delta 9-tetrahydrocannabinol-induced tolerance by specific kinase inhibitors. Eur J Pharmacol. 2004;496:99–108. doi: 10.1016/j.ejphar.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Lee MC, Smith FL, Stevens DL, Welch SP. The role of several kinases in mice tolerant to delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2003;305:593–599. doi: 10.1124/jpet.102.044446. [DOI] [PubMed] [Google Scholar]
- 43.Garcia DE, Brown S, Hille B, Mackie K. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J Neurosci. 1998;18:2834–2841. doi: 10.1523/JNEUROSCI.18-08-02834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubino T, Forlani G, Vigano D, Zippel R, Parolaro D. Modulation of extracellular signal-regulated kinases cascade by chronic delta 9-tetrahydrocannabinol treatment. Molecular and cellular neurosciences. 2004;25:355–362. doi: 10.1016/j.mcn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Rubino T, Forlani G, Vigano D, Zippel R, Parolaro D. Ras/ERK signalling in cannabinoid tolerance: from behaviour to cellular aspects. J Neurochem. 2005;93:984–991. doi: 10.1111/j.1471-4159.2005.03101.x. [DOI] [PubMed] [Google Scholar]
- 46.Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, et al. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.