Abstract
A versatile reaction cascade leading to highly substituted 1,2,3,6-tetrahydropyridines has been developed. It comprises rhodium(I)-catalyzed C–H activation–alkyne coupling followed by electrocyclization and subsequent acid/borohydride-promoted reduction. This one pot procedure affords the target compounds in up to 95% yields and with >95% diastereomeric purity.
C–H bond functionalization has proven to be a powerful strategy for the assembly of pharmaceutically relevant classes of nitrogen heterocycles from simple and readily available precursors.1,2 We and others have in particular capitalized upon this approach to prepare highly substituted pyridines from alkynes and α,β-unsaturated imines, which in turn are derived from amines and diverse enones and enals (eq 1).3 Resonance stabilization of the heteroaromatic product provides a key driving force to enable this overall transformation to be accomplished by multiple mechanistically distinct pathways.
Herein, we utilize the same readily available starting materials to provide efficient access to highly substituted piperidine derivatives, a heterocycle class that is prevalent in a large number of bioactive natural products and drugs.4,5 Specifically, we report on a one pot cascade process to prepare tetrahydropyridines substituted at multiple sites in good yields and with very high diastereoselectivities (eq 2). This sequence enables the preparation of fully differentiated hexasubstituted piperidine derivatives, a level of differential substitution that to our knowledge has not previously been reported.6
 |
(1) |
 |
(2) |
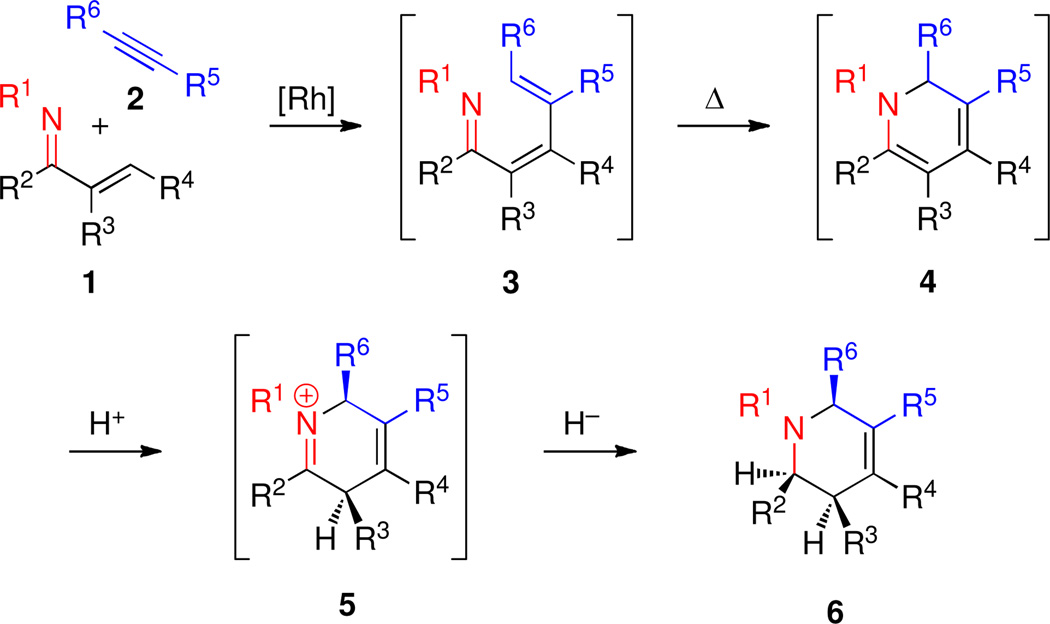
Rh-catalyzed β-C–H bond activation of α,β-unsaturated imines 1 followed by addition across alkynes 2 gives azatriene intermediates 3 that undergo electrocyclization in situ to give 1,2-dihydropyridines 4.3b–d We envisioned that these 1,2-dihydropyridines 4 could serve as very useful intermediates in a sequence leading to highly substituted piperidine derivatives as long as selective functionalization of the double bonds could be accomplished with high stereoselectivity. One avenue for achieving this goal would be stereoselective protonation of the enamine double bond followed by stereoselective reduction of the resulting iminium intermediate 5 to provide 1,2,3,6-tetrahydropyridines 6 (Scheme 1). The reduction of N-alkyl 1,2-dihydropyridines to 1,2,3,6-tetrahydropyridines via iminium intermediates is documented to proceed in good yields.7 However, for the vast majority of examples no stereocenters are introduced, and we could not identify any examples where this reduction sequence results in the introduction of two new stereocenters.
Scheme 1.
Reaction Cascade for the One Pot, Stereoselective Synthesis of Piperidine Derivatives.
We therefore first chose to investigate reduction conditions using dihydropyridine 4e as a test substrate. Alkenylation of imine 1e in toluene at 80 °C using 2.5 mol % of [Rh(coe)2Cl]2 and 5 mol % of the 4-Me2N-C6H4-PEt2 ligand followed by in situ electrocyclization proceeded cleanly within 2 h to give 4e in >90% NMR yield. Dihydropyridine 4e was then subjected to a variety of reduction conditions (Table 1). At the outset, the toluene solution of 4e was added to a suspension of NaBH4 in ethanol at 0 °C, a procedure based upon previously reported conditions for reducing 1,2-dihydropyridines unsubstituted at the 5- and 6-positions. Unfortunately, only partial reduction to a mixture of tetrahydropyridines along with unidentified byproducts was observed (Table 1, entry 1).7 However, when the toluene solution of 4e and an excess of acetic acid were added to the NaBH4 suspension, GC-MS analysis indicated a much cleaner conversion to a mixture of four products, the major component of which was identified as 6e (vide infra). Apparently, regio- and stereoselective protonation–reduction was considerably facilitated by a Brønsted acid.
Table 1.
Influence of Reduction Conditions on Yield and dr of Tetrahydropyridine 6e.a
 | ||||
|---|---|---|---|---|
| Entry | Reducing agent | Solvent/acidb | yield (%)c |
d.r.c |
| 1 | NaBH4 | PhMe–EtOH/ – | (77)d | (54:46)d |
| 2 | NaBH4 | PhMe–EtOH/AcOH | 86 | 94:6 |
| 3 | NaBH4 | PhMe–EtOH/pivOH | 84 | 65:35 |
| 4 | Bu4NBH4 | PhMe–EtOH/AcOH | 85 | 92:8 |
| 5 | Na(CN)BH3 | PhMe–EtOH/AcOH | 87 | 68:32 |
| 6 | Na(AcO)3BH | PhMe–EtOH/AcOH | 85 | 95:5 |
| 7 | Me4N(AcO)3BH | PhMe–EtOH/AcOH | 78 | 96:4 |
| 8 | Me4N(AcO)3BH | PhMe–DCM/AcOH | 81 | 89:11 |
| 9 | Na(AcO)3BH | PhMe–EtOH/TsOH | (35)d | (54:46)d |
| 10 | Na(AcO)3BH | PhMe–EtOH/TFA | 17 | (27:73) |
Reduction conditions: 20 µmol of dihydropyridine, 5 equiv of acid, 3 equiv of reducing agent, 0 °C for 2 h, then 0 °C to RT overnight.
PhMe from Rh-mediated reaction, PhMe:EtOH or CH2Cl2 = 1:1; pivOH = pivalic acid, TsOH = p-toluensulfonic acid; TFA = trifluroacetic acid.
Determined by GC-MS using 2,6-dimethoxytoluene as an internal standard; yield = total yield of tetrahydropyridine isomers with regard to imine starting material, dr = ratio of depicted all-cis product to sum of other diastereomers. The estimated error for GC integrals is ±3%.
Approximate numbers; unidentified byproducts with overlapping retention times also formed.
Optimization of the reduction conditions indicated that both the nature of the acid and the reducing agent had an influence on the product distribution, but no significant counterion effect was observed (entries 3, 4, and 5). In addition, we suspected that the actual reducing species in entry 2 was (AcO)3BH−.8 Indeed, the use of (AcO)3BH−/AcOH afforded 6e in high yield and diastereoselectivity; stronger acids lead to markedly worse results (entries 6–10). Based on these findings, the conditions listed in entry 6 were chosen for reductions involving other dihydropyridines.
A diverse set of imines 1 and alkynes 2 were next evaluated to test the scope of the cascade reaction (Table 2). The imines were obtained by condensation of the corresponding α,β-unsaturated ketone and a primary amine, the enones being commercially available or readily accessible by an aldol condensation.9 Upon completion of the alkenylation and cyclization steps, crude solutions of the dihydropyridines and acetic acid were added to a suspension of Na(AcO)3BH in ethanol at 0 °C, and the resulting reaction mixtures were stirred at 0 °C to ambient temperature overnight.
Table 2.
Substrate Scope of the Cascade Transformation.a
 | |||||
|---|---|---|---|---|---|
| Imine R1–R4 | Alkyne R5 R6 | Pdt | Imine | Alkyne | Pdt |
| 1a Bn H H Me | 2a Et Et | 6a | 2a | 6l | |
| 1b Bn H H Ph | 2a | 6b | |||
| 1c Bn Me H Ph | 2a | 6c | 2a | 6m | |
| 1d Bn Me Me Ph | 2a | 6d | |||
| 1e Bn Me Me Me | 2a | 6e | 2a | 6n | |
| 1f Cy Me Me Me | 2a | 6f | |||
| 1g Ph Me Me Me | 2a | 6g | 2a | 6o | |
| 1e | 2b Ph Ph | 6h | |||
| 1e | 2c i-Pr Me | 6i |  |
2a | 6p |
| 1e | 2d t-Bu Me | 6j | |||
| 1e | 2e i-Pr CO2Me | 6k | 1j | 2e | 6q |
 | |||||
Yields correspond to the overall yield of analytically pure product after silica gel chromatography and are based upon the α,β-unsaturated imine starting material. The diastereoselectivity was determined by 1H NMR analysis of clearly resolved piperidine hydrogens. For full experimental details, see the Supporting Information.
Alkyne regioselectivity 2:1, combined yield for separated regioisomers.
Combined yield for regioisomerically pure, diastereomeric mixture.
Under the optimized reaction conditions, less substituted imines 1a–c afforded tetrahydropyridines 6a–c in excellent overall yields. For 6c, where a single additional stereocenter was introduced, good diastereoselectivity was also observed. Most importantly, all hexasubstituted products showed outstanding diastereoselectivities with only a single diastereomer detectable by 1H and 13C NMR spectroscopy except for the hindered tert-butyl substituted product 6j and bicyclic product 6l, for which a 10:5:3 and a 10:1:1 ratio of stereoisomers were observed, respectively.10a
A variety of N-substituents were well tolerated, including N-benzyl (6a–e,h–q), branched N-alkyl (6f), and N-phenyl (6g) derivatives. Although 3-hexyne was employed as the alkyne input for the majority of examples, diphenylacetylene also provided the tetrahydropyridine 6h in high yield and with excellent stereoselectivity. The unsymmetrical alkyne, isopropyl methyl acetylene, 2c, afforded a 2:1 regioisomeric mixture of products that could be separated by silica gel chromatography.10b In contrast, t-butyl methyl acetylene, 2d, gave a single regioisomer upon C–H activation–cyclization; however, a mixture of diastereomers was obtained after reduction (vide infra). Notably, unsymmetrical alkyne 2e bearing an ester functionality afforded 6k as a single regio- and diastereoisomer.10c A number of 4-phenyl and 4-heteroaryl tetrahydropyridines have been recognized as pharmacologically potent compounds.2,11 For this reason, we prepared imines 1i–l containing furyl, pyrrolyl, and indolyl moieties, respectively, in addition to the phenyl-substituted derivatives 1b–d. The corresponding tetrahydropyridine products were isolated in 52–95% yield (6b–d and 6m–q). For 6p no over-reduction of the indole ring system was observed.12 Combination of imine 1e with alkyne 2e served to highlight the potential of the cascade process for introducing a maximum number of different piperidine substituents in a concise sequence. To the best of our knowledge, 6q is the first example of a hexasubstituted, fully differentiated piperidine derivative.
The relative configuration of the saturated ring carbon atoms was established by X-ray crystallography: the structure of 6h was solved as the free amine and 6e and 6g as the corresponding ammonium salts, unambiguously revealing all-cis stereochemistry.13 Based on these results and the similarities of the NMR spectra of all tetrahydropyridines, in addition to assuming similar reduction pathways, we assigned the all-cis configuration to the other products by analogy.
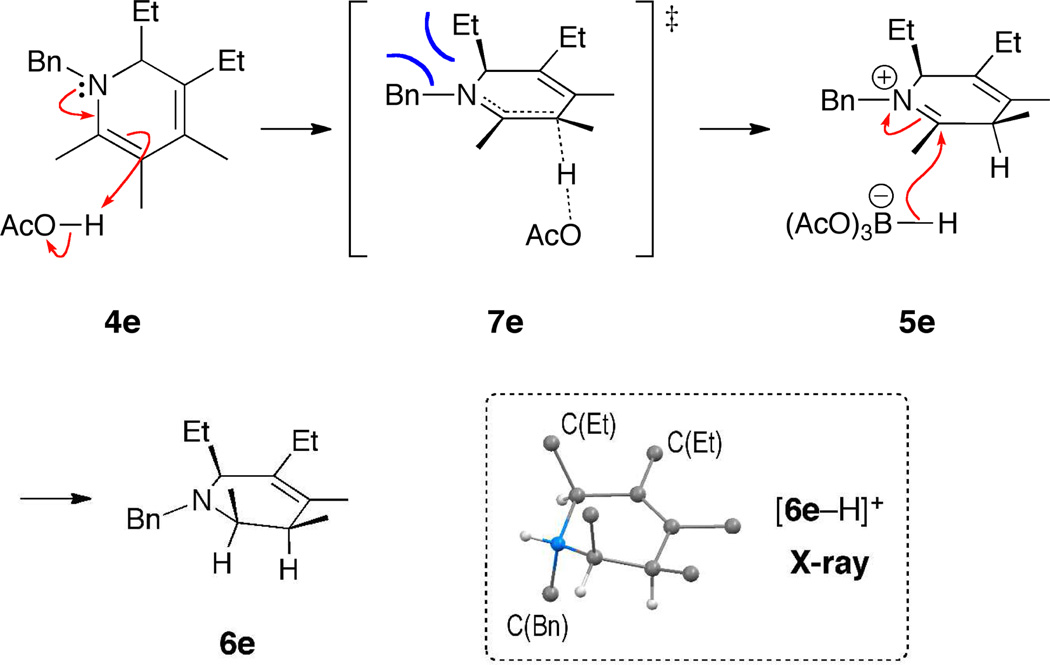
We rationalize the observed stereochemical outcome by a kinetically controlled protonation followed by face-selective borohydride reduction (shown for product 6e in Scheme 2). The transition state 7e leading from dihydropyridine 4e to iminium ion 5e exhibits N–C(2) double bond character.14 Due to allylic strain between the N-benzyl and C(5)-ethyl substituents, the conformation with Et–C(5) in a pseudoaxial position is preferred. Approach of the acid and proton transfer then occur in an anti fashion, affording cis-iminium ion 5e. This species is eventually reduced by (AcO)3BH−, which delivers its hydride from the less hindered side to give all-cis product 6e.
Scheme 2.
Proposed Mechanism for the Stereoselective Reduction and Ball-and-Stick Representation of [6e–H]+.13
Additionally, we sought to extend the synthetic utility of the cascade sequence by providing an initial demonstration that nucleophiles other than hydride can be added to protonated dihydropyridines with high selectivity. Specifically, when isolated 4e was treated stepwise with 2-naphthylsulfonic acid and allylcerium chloride, heptasubstituted piperidine derivative 8 was obtained in good yield as a single diastereomer (eq 3).15,16 The relative configuration of 8 was established by X-ray crystallography and points to a mechanism similar to that operative in the reactions leading to tetrahydropyridines 6.
In conclusion, we have developed a cascade transformation that enables the one pot preparation of highly substituted piperidine derivatives 6 starting from imines and alkynes in good overall yields and with uniformly excellent diastereoselectivities. The broad scope and versatility of the cascade process was demonstrated by the introduction of a variety of alkyl, aryl and heteroaryl substituents at multiple sites in the tetrahydropyridine products.
 |
(3) |
The synthetic potential of dihydropyridine intermediates 4 was further accentuated by the demonstration that not only hydride but also carbon nucleophiles can be added with high diastereoselectivity to give heptasubstituted piperidine derivative 8. Further expansion of this sequence to a broader set of carbon nucleophiles is actively being pursued as is the application of this cascade transformation to the rapid preparation of bioactive compounds.
Supplementary Material
Acknowledgement
This work was supported by NIH Grant GM069559 (to J.A.E.). The Director, Office of Energy Research, Office of Basic Energy Sciences, Chemical Sciences Division, U.S. Department of Energy, under Contract DE-AC02-05CH11231 is acknowledged by R.G.B. S.D. is grateful to the Swiss National Science Foundation for a postdoctoral fellowship (PBZHP2-130-966).
Footnotes
Supporting Information. Full experimental details and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Robert G. Bergman, Email: rbergman@berkeley.edu.
Jonathan A. Ellman, Email: jonathan.ellman@yale.edu.
References
- 1.A large and increasing number of reports on the synthesis of heterocycles involving C–H activation has been published in the past few years. For recent examples, see the following publications and references therein: Jayakumar J, Parthasarathy K, Cheng C-H. Angew. Chem. Int. Ed. 2012;51:197–200. doi: 10.1002/anie.201105755. Yang G, Zhang W. Org. Lett. 2012;14:268–271. doi: 10.1021/ol203043h. Mahoney SJ, Fillion E. Chem. Eur. J. 2012;18:68–71. doi: 10.1002/chem.201103155. Guimond N, Gorelsky SI, Fagnou K. J. Am. Chem. Soc. 2011;133:6449–6457. doi: 10.1021/ja201143v. Huestis MP, Chan L, Stuart DR, Fagnou K. Angew. Chem. Int. Ed. 2011;50:1338–1341. doi: 10.1002/anie.201006381. Oberg KM, Rovis T. J. Am. Chem. Soc. 2011;133:4785–4787. doi: 10.1021/ja200766k. Hyster TK, Rovis T. Chem. Sci. 2011;2:1606–1610. doi: 10.1039/C1SC00235J. Du Y, Hyster TK, Rovis T. Chem. Commun. 2011;47:12074–12076. doi: 10.1039/c1cc15843k. Morimoto K, Hirano K, Satoh T, Miura M. J. Org. Chem. 2011;76:9548–9551. doi: 10.1021/jo201923d. Rakshit S, Grohmann C, Besset T, Glorius F. J. Am. Chem. Soc. 2011;133:2350–2353. doi: 10.1021/ja109676d. Li X, Zhao M. J. Org. Chem. 2011;76:8530–8536. doi: 10.1021/jo201530r. Shiota H, Ano Y, Aihara Y, Fukumoto Y, Chatani N. J. Am. Chem. Soc. 2011;133:14952–14955. doi: 10.1021/ja206850s. Zhu C, Xie W, Falck JR. Chem. Eur. J. 2011;17:12591–12595. doi: 10.1002/chem.201102475.
- 2.For recent C–H activation reviews covering heterocycle syntheses, see: Engle KM, Mei T-S, Wasa M, Yu J-Q. Acc. Chem. Res. 2011 doi: 10.1021/ar200185g. published online Dec. 14, 2011. Colby DA, Tsai AS, Bergman RG, Ellman JA. Acc. Chem. Res. 2011 doi: 10.1021/ar200190g. published online Dec. 8, 2011. Yeung CS, Dong VM. Chem. Rev. 2011;111:1215–1292. doi: 10.1021/cr100280d. Wencel-Delord J, Dröge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740–4761. doi: 10.1039/c1cs15083a. McMurray L, O’Hara F, Gaunt MJ. Chem. Soc. Rev. 2011;40:1885–1898. doi: 10.1039/c1cs15013h. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624–655. doi: 10.1021/cr900005n. Xu L-M, Li B-J, Yang Z, Shi Z-J. Chem. Soc. Rev. 2010;39:712–733. doi: 10.1039/b809912j. Satoh T, Miura M. Chem. Eur. J. 2010;16:11212. doi: 10.1002/chem.201001363. Li C-J. Acc. Chem. Res. 2009;42:335–344. doi: 10.1021/ar800164n. Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242–3272. doi: 10.1039/b816707a. Rubin M, Sromek AW, Gevorgyan V. Synlett. 2003;15:2265–2291.
- 3.For the synthesis of pyridines via C–H activation, see: Hyster TK, Rovis T. Chem. Commun. 2011;47:11846–11848. doi: 10.1039/c1cc15248c. Colby DA, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2008;130:3645–3651. doi: 10.1021/ja7104784. Parthasarathy K, Jeganmohan M, Cheng C-H. Org. Lett. 2008;10:325–328. doi: 10.1021/ol7028367. For leading references on alternative multicomponent strategies for the synthesis of pyridines, see Chen Ming. Z, Micalizio GC. J. Am. Chem. Soc. 2012;134:1352–1356. doi: 10.1021/ja2105703. Trost BM, Gutierrez AC. Org. Lett. 2007;9:1473–1476. doi: 10.1021/ol070163t. Yamamoto Y, Kinpara K, Ogawa R, Nishiyama H, Itoh K. Chem. Eur. J. 2006;12:5618–5631. doi: 10.1002/chem.200600176. Movassaghi M, Hill MD. J. Am. Chem. Soc. 2006;128:4592–4593. doi: 10.1021/ja060626a. McCormick MM, Duong HA, Zuo G, Louie J. J. Am. Chem. Soc. 2005;127:5030–5031. doi: 10.1021/ja0508931. Tanaka R, Yuza A, Watai Y, Suzuki D, Takayama Y, Sato F, Urabe H. J. Am. Chem. Soc. 2005;127:7774–7780. doi: 10.1021/ja050261e. Takahashi T, Tsai F-Y, Li Y, Wang H, Kondo Y, Yamanaka M, Nakajima K, Kotora M. J. Am. Chem. Soc. 2002;124:5059–5067. doi: 10.1021/ja017507+.
- 4.For leading references on piperidines, see: Fattorusson E, Taglialatela-Scafati O, editors. Modern Alkaloids: Structure, Isolation, Synthesis and Biology. Wiley-CH: Weinheim; 2007. Michael JP. Nat. Prod. Rep. 2008;25:165. doi: 10.1039/b612166g. and earlier articles of this series. Mitchinson A, Nadin A. J. Chem. Soc. Perkin Trans. 2000;1:2862–2892. Laschat S, Dickner T. Synthesis. 2000;13:1781–1813.
- 5.For reviews on the chemistry and biological activity of tetrahydropyridines, see: Mateeva NN, Winfield LL, Redda KK. Curr. Med. Chem. 2005;12:551–571. doi: 10.2174/0929867310504050551. Felpin F-X, Lebreton J. Curr. Org. Synth. 2004;1:83–109.
- 6.For recent tandem and multicomponent approaches to tetrahydropyridines, see : Harrison DP, Sabat M, Myers WH, Harman WD. J. Am. Chem. Soc. 2010;132:17282–17295. doi: 10.1021/ja107536w. Tsukamoto H, Kondo Y. Angew. Chem. Int. Ed. 2008;47:4851–4854. doi: 10.1002/anie.200800823. Kalbarczyk KP, Diver ST. J. Org. Chem. 2009;74:2193–2196. doi: 10.1021/jo802582k. Khan AT, Khan MM, Bannuru KKR. Tetrahedron. 2010;66:7762–7772. Clarke PA, Zaytsev AV, Whitwood AC. Synthesis. 2008;21:3530–3532. For recent stereoselective syntheses of mutiply substituted 1,2,3,6-tetrahydropyridines, see: Wong H, Garnier-Amblard EC, Liebeskind LS. J. Am. Chem. Soc. 2011;133:7517–7527. doi: 10.1021/ja201012p. Ghorai MK, Halder S, Das RK. J. Org. Chem. 2010;75:7061–7072. doi: 10.1021/jo101680f. Lemonnier G, Charette AB. J. Org. Chem. 2010;75:7465–7467. doi: 10.1021/jo1015344. Toumieux S, Compain P, Martin OR. J. Org. Chem. 2008;73:2155–2162. doi: 10.1021/jo702350u. Kobayashi T, Nakashima M, Hakogi T, Tanaka K, Katsumura S. Org. Lett. 2006;8:3809–3812. doi: 10.1021/ol061405c.
- 7. Legault C, Charette AB. J. Am. Chem. Soc. 2003;125:6360–6361. doi: 10.1021/ja0348647. Comins DL, Weglarz M. J. Org. Chem. 1991;56:2506–2512. Comins DL, Weglarz MA, O’Connor S. Tetrahedron Lett. 1988;29:1751–1754. Thiessen LM, Lepoivre JA, Alderweireldt FC. Bull. Soc. Chim. Belg. 1975;84:689–695. For an early review of the chemistry of dihydropyridines, including ionic reductions, see: Eisner U, Kuthan J. Chem. Rev. 1972;72:1–42. For the stereoselective exhaustive hydrogenation of heteroarenes, see: Glorius F. Org. Biomol. Chem. 2005;3:4171–4175. doi: 10.1039/b512139f.
- 8.BH4− is readily converted to (RCO2)3BH− when treated with carboxylic acids: Evans DA, Chapman KT, Carreira EM. J. Am. Chem. Soc. 1988;110:3560–3578. Gribble GW, Nutaitis CF. Org. Prep. Proced. Int. 1985;17:317–384.
- 9.See the Supporting Information for details regarding the preparation of imines 1 and alkyne 4c. For some imines, E/Z isomers are observed by NMR. However, this does not affect the rate of C–H activation because isomerization is much more rapid than the time scale at which the reaction occurs. See, e.g., Jennings WB, Boyd DR. J. Am. Chem. Soc. 1972;94:7187–7188.
- 10.(a) Diastereomeric ratios of crude products on a preparative scale were checked by 1H NMR spectroscopy for 6e, 6h and 6n in order to exclude high drs as a result of selective isolation of one diastereoisomer; they were found to be >95%. (b) When alkyne 2c was coupled with an α,β-unsaturated imine lacking γ-substitution, high regioselectivity was observed. See, entry 2 in Table 2, ref 3b. (c) The reduced yield is not due to selective isolation of 6k as evidenced by crude NMR spectra.
- 11.For two recent reports on biologically active 4-(indol-3-yl)-substituted 1,2,3,6-tetrahydropyridines, see: Annedi SC, Maddaford SP, Mladenova G, Ramnauth J, Rakhit S, Andrews JS, Lee DKH, Zhang D, Porreca F, Bunto D, Christie L. J. Med. Chem. 2011;54:7408–7416. doi: 10.1021/jm201063u. Nolan TL, Lapinsky DJ, Talbot JN, Indarte M, Liu Y, Manepelli S, Geffert LM, Amos ME, Taylor PN, Madura JD, Surratt CK. ACS Chem. Neurosci. 2011;2:544–552. doi: 10.1021/cn200044x.
- 12.Gribble GW. Chem. Soc. Rev. 1998;27:395–404. [Google Scholar]
- 13.See the Supporting information for ORTEP representations, details of the crystallographic analyses, and CIF files. For the structure depicted in Scheme 2, only ring atoms and atoms directly attached to them are shown.
- 14.Energetically, 7e is closer to 5e than to 4e, and therefore it is reasonable to assume structural similarity to 5e based on the Hammond postulate.
- 15.For a recent example of additions of organometallic reagents to iminium ions, see: Hata S, Koyama H, Shimizu M. J. Org. Chem. 2011;76:9670–9677. doi: 10.1021/jo201692x.
- 16.For reviews on organocerium reagents in organic synthesis, see: Bartoli G, Marcantoni E, Marcolini M, Sambri L. Chem. Rev. 2010;110:6104–6143. doi: 10.1021/cr100084g. Liu H-J, Shia K-S, Shang X, Zhu B-Y. Tetrahedron. 1999;55:3803–3830.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.