Abstract
Purpose of review
Asthma is an inflammatory respiratory condition with significant associated morbidity and mortality that is increasing in prevalence. Air pollution is an important factor in both the development of asthma and in asthma exacerbations. Oxidative stress as a result of exposure to air pollution and underlying genetic polymorphisms that may play a role in susceptibility to this oxidative stress are the subject of current investigation.
This article reviews the data regarding the effects of air pollution on the innate immune response and potential clinical and treatment implications of how genetic polymorphisms affect this response.
Recent findings
Recent investigation reveals how pollutant-induced oxidative stress impacts airway inflammatory responses. Work by our study group demonstrates that asthmatic patients have an exaggerated inflammatory response to air pollution induced oxidative stress. New trials investigating antioxidants as potential therapeutic interventions may target this specific issue.
Summary
Air pollution plays a critical role in asthma and may affect certain patients more than others. Further investigation into the genetic polymorphisms that affect inflammatory responses may help target patient populations at greatest risk for air pollution induced asthma and may provide new therapeutic options for these patient populations.
Keywords: asthma, oxidative stress, ozone, LPS
Introduction
The increase in prevalence of asthma and other respiratory diseases contributes to patient morbidity and mortality as well as to increased cost in the form of medications, hospitalizations, health care utilization, and school/work absenteeism. While mechanisms behind this increase in prevalence are not fully elucidated, air pollutants generated by industrial progress have been implicated as an important etiologic consideration. Ozone, nitrogen dioxide, and particulate matter are air pollutants that are major constituents of smog and byproducts of fossil fuel combustion.
Ozone is one of the most commonly encountered pollutants, and epidemiologic studies have demonstrated that ozone is a trigger for asthma exacerbations in children, even at levels below the US Environmental Protection Agency (EPA) standards of 120ppb (1 hour average) and 75ppb (8 hour average)[1–4]. Silverman et. al found that among asthmatic children aged six to eighteen, there was a 20% increase in general hospitalizations and a 19% increased risk for ICU admissions for each 22ppb increase in ozone[5]. Particulate matter, such as that from diesel exhaust, is another important environmental exposure that leads to an increased risk for asthma development and exacerbation [6–9]. Endotoxin, also known as LPS, is a component of the outer membrane of gram negative bacteria and is derived from animals and agricultural activities. It has been identified on both large and small particulate matter particles, as well as in tobacco smoke. Exposure to LPS in early childhood has been associated with asthma exacerbations and increased prevalence of asthma [10,11]. Ryan et al. demonstrated that while exposure to traffic related particles increased the risk of wheeze alone, exposure to house dust LPS had a synergistic effect and increased this risk further [12]. A meta-analysis by Mendy et. al demonstrated that LPS exposure was positively correlated with wheeze in infants and toddlers [13].
The purpose of this review is to highlight recent research that has identified a). how oxidative stress responses evoked by pollutant exposure promotes airway inflammation, b). patient populations that are more susceptible to environmental oxidative stress, and c). effects of environmental air pollution.”
Effects of Air Pollution on Innate Immune Responses: Recent Findings with Ozone
The respiratory system is particularly susceptible to air pollution by nature of consistent exposure to the environment, with increased exposure with physical activity. Normally, protection of the host occurs via several pathways involving both the innate and adaptive immune system. Components of the innate immune system may be modified by ozone and LPS in populations with underlying respiratory disease and may contribute to adverse health effects[14] .
The cellular inflammatory response to air pollution in humans is the subject of current investigation and multiple studies have demonstrated that both ozone and LPS exposure augment the influx of neutrophils in the airway [15–17]. The role of neutrophils in airway symptoms, however, is less well defined, though the generation of reactive oxygen species is one possible mechanism by which neutrophils may play a role in airway induced hyper-reactivity[14].
Reactive oxygen species (ROS) have long been known to induce epithelial cell inflammation by directly causing tissue injury. Ozone challenge of epithelial cells results in NFκB activation and production of a variety of pro-inflammatory mediators such as IL-8, IL-6, PGE2, and LTC4 that recruit inflammatory cells [18–22]. Toll like receptors, first line effector molecules in innate immunity, are also believed to play a critical role in airway hyper-reactivity and inflammatory responses induced by air pollution [23,24] through epithelial cell release of substances into the airway lining fluid that activate resident airway macrophages [16,25–31].
A study by Williams et. al in 2007 demonstrated that TLR2 and TLR4 knock-out mice had a decrease in ozone induced airway hyper-reactivity [24]. We and others found similarities in the airway inflammatory response induced by ozone and LPS [15]. Both ozone and LPS challenge augmented sputum neutrophilia and subjects' responses for each of these pollutants were significantly correlated with each other, suggesting one potential common mechanism to account for similarities in airway neutrophil responses by ozone and LPS.
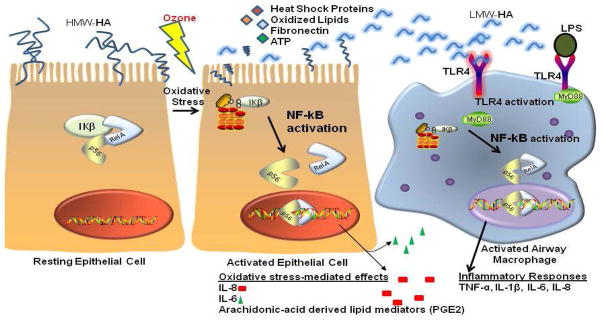
Because LPS signals through toll-like receptor 4 (TLR4) on the cell surface on macrophages, this signaling pathway is a putative candidate to account for similarities between ozone and LPS airway inflammatory responses. Indeed, mechanistic studies in mice suggest that at least some ozone responses are mediated through TLR4 [32–34]. Toll-like receptors recognize pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs) released by injured cells such as heat shock proteins, oxidized lipids, fibrinogen, and low molecular weight hyaluronan (HA) [35] (Figure 1). They are critical in initiating inflammatory responses to a variety of stimuli, characterized by production of pro-inflammatory mediators such as IL-1β, TNF-α, and IL-6.
Figure 1. Innate Immune Activation after Pollutant Exposure.
Left panels: Ozone activates resting airway epithelial cells via the transcription factor NFkB to produce a variety of pro-inflammatory mediators. It also damages epithelial cells, with release of damage associated molecular patterns (DAMPs) such as heat shock proteins, ATP, fibronectin, and oxidized lipids.
Right panel: Resident airway macrophages are activated through TLR4. TLR4 can be activated by airborne LPS found on particulate matter and tobacco smoke.
High molecular weight hyaluronic acid (HA) can be cleaved into low molecular weight forms by oxidative stress.
It is currently thought that epithelial cells can release DAMPs after experiencing oxidative stress, such as found with ozone exposure. These DAMPS can then activate TLR4 signaling pathways on airway macrophages.
Hyaluronic acid (HA), a glycosaminoglycan that is a component of the airway epithelial extracellular matrix, has recently emerged as a mediator of ozone-induced airway hyperreactivity & inflammatory responses [26,36]. High molecular weight HA is present on the apical surface of airway epithelial cells; its low molecular weight form is an endogenous ligand for TLR4 [27,31]. In airway epithelium, these low molecular weight fragments can be generated by ROS-induced depolymerization of hyaluronan [25,29], and by hyaluronidase activity associated with upregulation of TNF-α in concert with IL-1 β [30]. Garantziotis et. al demonstrated that TLR4 deficient mice are partially protected from airway hyperreactivity after ozone exposure and after instillation of hyaluronic acid, supporting the idea that TLR4 contributes to the development of ozone-induced airway hyperreactivity [26,36]. Various groups have shown that low molecular weight fragments of HA have pro-inflammatory actions [28]. In rodent models, Hollingsworth and colleagues showed that low molecular weight HA is increased in bronchoalveolar lavage fluid after ozone challenge, and that HA mediates inflammatory responses in the respiratory tract via CD44 [36] and TLR4 [26] signaling mechanisms. We recently reported that normal volunteers, allergic nonasthmatics, and mild allergic asthmatics had increased HA in their respiratory tract lining fluid after ozone exposure [16].
The increased susceptibility of asthmatics to ozone-induced airway disease was previously thought to be secondary to exacerbation of allergic airway inflammation. Ozone has been found to enhance airway eosinophilia [37,38] and to increase the sensitivity to subsequent allergen exposure [39]. Recent evidence has emerged that exacerbations may also be secondary to augmented innate immune inflammatory responses in allergic asthmatics. In controlled chamber studies, both normal volunteers and allergic asthmatics suffer from increased neutrophilic airway inflammation [16,40]. However, in a study of allergic asthmatics and normal volunteers, our group found that only the allergic asthmatics had increased cell surface expression of TLR4 on mature macrophages from induced sputum after 400 ppb ozone exposure, and increased levels of the pro-inflammatory cytokines IL-1β, IL-6, and IL-8 in the respiratory tract lining fluid after ozone exposure [16]. We hypothesized that in addition to exacerbation of underlying allergic inflammation, asthmatics also have enhanced activation of innate immune inflammatory pathways. We recently found that despite similarities in neutrophil and macrophage proportions in induced sputum samples after ozone exposure, gene array profiles were distinctly different. Compared to normal volunteers, allergic asthmatics showed increased immune signaling involving the NFkB network [41], supporting the notion that asthmatics suffer from increased innate immune activation after ozone exposure. Future work will need to focus on mechanisms explaining this enhanced innate immune response in asthmatics.
Oxidative Stress Genes and Susceptibility to Air Pollution
As inhalation of air pollutants promotes the production of radical oxygen species, genetic polymorphisms that result in antioxidant deficiencies may result in increased airway inflammation and hyperreactivity in response to environmental agents. A number of intracellular antioxidant enzymes including NQO1, GSTM1, GSTP1, and HO-1 regulate cellular and mucosal oxidant stress [42–46]. These enzymes are regulated by the master transcription factor NRF2 which translocates to the nucleus after oxidative stress. Cells that encounter oxidative stress activate NRF2 binding to the Antioxidant Response Element (ARE), leading to the transcription of a broad range of antioxidant genes. This cellular response is designed to defend against the harmful effects of oxidative agents.
Polymorphisms of genes of glutathione S-transferases (GSTs) have been investigated because of the important role of these enzymes in antioxidant defense. The GSTM1, or glutathione-S-transferase Mu1, null genotype has been associated with increased response to environmental agents. Studies have demonstrated an increased risk of wheezing in children exposed to tobacco smoke during the perinatal period as well as an increased risk of acute exacerbation of asthma in response to ozone exposure in subjects with the null genotype [47]. Recent investigation by Dillon et. al revealed that subjects with the GSTM1 null genotype have an increased inflammatory response with elevated levels of IL-1β and TNFα in the sputum to inhaled LPS (at 20,000 endotoxin units) [48]. This level of LPS exposure models the amount that a worker would be exposed to during an 8 hour work shift at an animal farming operation and genotypic differences may thus be an important factor in explaining why some people tolerate this level of endotoxin exposure with fewer adverse effects than others.
GSTM1 and GSTP1 have also been found to modify the adjuvant effect of diesel exhaust particles on allergic inflammation. A study by Gilliland et. al in 2004 demonstrated that subjects with the null genotype for GSTM1 and GSTP1 codon 105 variants had enhanced nasal allergic responses in the presence of diesel exhaust particles [49]. Subjects with these genetic variants also demonstrate larger responses to allergens with secondhand tobacco smoke with increases in nasal-allergen specific IgE and histamine [50].
The GSTM1 genotype plays a role in inflammation following ozone exposure as well. Subjects with the GSTMI null genotype have increased neutrophil influx to the airway 24 hours following exposure to ozone at a level of 400 ppb [51]. A study by Wu et. al also demonstrated that GSTM1 is a risk factor for ozone-induced inflammatory responses, as knockdown of GSTM1 lead to enhanced IL-8 production from human bronchial epithelial cells exposed to ozone [52]. IL-8, which is a proinflammatory mediator known to be a potent neutrophil activator, is an important biomarker for ozone-induced airway inflammation.
Potential Therapeutic Interventions against Pollutant-Induced Inflammation
While specific agents targeting pollutant-induced asthma exacerbation are not currently clinically available, understanding the potential mechanisms for pollutant-exacerbated asthma allows investigation into potential therapeutic interventions. As discussed above, air pollutants exert inflammatory effects as well as inducing oxidative stress. For this reason, anti-inflammatory agents and antioxidants have the potential to decrease the effects of pollutants on airway epithelial cells.
While anti-inflammatory agents may help to mitigate the effect of ozone on decrements in lung function, this effect appears to be mediated by analgesic function as opposed to anti-inflammatory function [53–55]. Studies have demonstrated that ozone induced pain-related symptoms may result in inhibition of maximal inspiration due to the stimulation of airway C fibers [56]. Prior work has demonstrated that non-steroidal anti-inflammatory agents such as ibuprofen and indomethacin inhibit the nociceptive effect of ozone on spirometry concomitantly with inhibition of cyclo-oxygenase products of arachidonic acid. This study by Hazucha et. al did not, however, demonstrate changes in neutrophilic inflammation with ibuprofen administration[54].
Other commonly utilized anti-inflammatory agents have been demonstrated to decrease airway hyperreactivity in response to pollutants. Sodium cromoglycate has been demonstrated to inhibit LPS-induced bronchial obstruction in asthmatic subjects who were pre-treated with this agent, suggesting a possible function in treating asthmatics exposed to LPS [57]. The utilization of corticosteroids for pollution-exacerbated asthma is less well elucidated. Inhaled corticosteroids blunted sputum neutrophilia in response to ozone exposure in asthmatics [58] and in normal volunteers [59], but did not prevent the functional airway response. Novel anti-inflammatory agents tailored specifically to the inflammatory pathways that mediate pollutant-induced effects are currently under investigation. Lazaar et. al recently described a selective CXCR2 antagonist that was able to inhibit CXCL1-induced CD11b expression on peripheral blood neutrophils with subsequent decrease in neutrophil activation and recruitment in ozone-induced airway neutrophilia, suggesting a potential role for this antagonist in neutrophil-predominant diseases[60].
In light of the effect of reactive oxygen species on airway inflammation and the effect of genetic polymorphisms in antioxidant enzymes, antioxidant interventions have become a popular therapeutic target. Different research teams have reported that α-tocopherol and vitamin C combination therapy was effective in mitigating the effect of ozone-induced lung function decrements in asthmatics [61–64], or in normal volunteers after they had undertaken an antioxidant depleted diet for 3 weeks to mimic a state of poor antioxidant nutritional status [63]. However, extracellular antioxidant supplementation had no effect on airway inflammatory cell recruitment after ozone [63]. Oral supplementation with sulforaphane, an antioxidant compound derived from specially bred broccoli, upregulates expression of NRF2-regulated Phase II enzymes (GSTM1, GSTP1, HO1, and NQO1). Application of sulforaphane induced phase II enzymes in B cells and effectively blocked diesel exhaust particle enhancement of IgE-production [65]. In primary bronchial epithelial cells exposed to diesel extract, sulforaphane pre-treatment inhibited pro-inflammatory cytokine production by diesel [66]. It has been shown in vivo that Phase II enzymes in nasal epithelial cells can be induced by 3 days of oral SFN supplementation [67]. Further work is required to determine which antioxidant interventions may be best suited for particular pollutant exposures, the best route of administration, and which populations may benefit the most from particular interventions.
Conclusion
Recent work has highlighted the innate immune inflammatory responses experienced in the airways using both murine and human models of pollutant exposures. Airway inflammation can be caused through oxidative damage to the airway epithelium, as well as through activation of resident airway cells that can then respond to substances released by damaged epithelial cells. Because antioxidant enzymes such as the well studied GSTM1 have been found to influence airway pollutant responses, the role of oxidative stress genes in conferring susceptibility to pollutant-induced inflammation has come to the forefront of research efforts in hopes of targeting susceptible populations.
In addition to studying the effect of these antioxidant genes in normal volunteers, studies are now focused on understanding the effect of antioxidant tone in populations that are especially susceptible to pollutant-induced inflammation, such as asthmatics and those with COPD. Asthmatic children have been found to have altered glutathione homeostasis (i.e. reduced levels), thought to be secondary to excessive free radical formation that then leads to a state of increased oxidative stress with deranged redox signaling and control [68,69]. Children with severe asthma were found to have decreased NRF2 transcriptional activity in airway cells compared to mild-moderate asthmatic children [69]. It has also been shown that maternal antioxidant gene polymorphisms in NRF2, GSTM1, and GSTT1 can influence their children’s risk of asthma and wheezing with a prenatal pharmacologic oxidative stress through acetaminophen consumption [70]. In addition, GSTPi has been found to be decreased in children with asthma, with GSTPi having a role in reducing oxidative stress through cysteine oxidation [71]. What is unknown at present is the interaction between the baseline oxidative stress experienced by asthmatics and their antioxidant response after an environmental pollutant exposure. Such work will delineate the most effective strategies to prevent and treat environmentally-induced asthma exacerbations.
Key points.
In addition to direct damage of airway epithelium, ozone-induced inflammation is mediated through TLR4 signaling pathways
Asthmatics have increased innate immune inflammatory responses after a controlled ozone exposure compared to healthy individuals
Oxidative Stress Genes have been implicated in conferring susceptibility to pollutant-induced airway inflammation
Antioxidant interventions may show promise against pollutant-induced inflammation.
Acknowledgments
Funding Sources: MLH is supported by NIH KL2RR025746. Although the research described in this article has been funded wholly or in part by the United States Environmental Protection Agency through cooperative agreement CR833463-01 with the Center for Environmental Medicine, Asthma, and Lung Biology at the University of North Carolina at Chapel Hill, it has not been subjected to the Agency's required peer and policy review, and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred. Mention of trade names or commercial products does not constitute endorsement or recommendation for use
Abbreviations
- LPS
endotoxin
- ROS
reactive oxygen species
- HA
hyaluronic acid
- TLR4
toll-like receptor 4
- ppb
part per billion
- IL-8
interleukin-8
- IL-6
interleukin-6
- PGE2
prostaglandin E2
- LTC4
leukotriene C4
- PAMPs
pathogen associated molecular patterns
- DAMPs
damage associated molecular patterns
- NQO1
NAD(P)H dehydrogenase, quinone 1
- GSTM1
glutathione S-transferase mu 1
- GSTP1
Glutathione S-transferase P1
- HO-1
heme oxygenase 1
- NRF2
NF-E2-related factor 2
Footnotes
Conflicts of interest
The authors have declared that they have no conflicts of interest.
References & Recommended Reading
- 1.Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA. 2004;292:2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, Leaderer BP. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- 3.Gold DR, Damokosh AI, Pope CA, 3rd, Dockery DW, McDonnell WF, Serrano P, Retama A, Castillejos M. Particulate and ozone pollutant effects on the respiratory function of children in southwest Mexico City. Epidemiology. 1999;10:8–16. [PubMed] [Google Scholar]
- 4.White MC, Etzel RA, Wilcox WD, Lloyd C. Exacerbations of childhood asthma and ozone pollution in Atlanta. Environ Res. 1994;65:56–68. doi: 10.1006/enrs.1994.1021. [DOI] [PubMed] [Google Scholar]
- 5**.Silverman RA, Ito K. Age-related association of fine particles and ozone with severe acute asthma in New York City. J Allergy Clin Immunol. 2010;125:367–373. e365. doi: 10.1016/j.jaci.2009.10.061. This study demonstrates that ozone and fine particulate matter increase asthma exacerbations in children. [DOI] [PubMed] [Google Scholar]
- 6.Cakmak S, Dales RE, Coates F. Does air pollution increase the effect of aeroallergens on hospitalization for asthma? J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Pope CA., 3rd Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who's at risk? Environ Health Perspect. 2000;108 (Suppl 4):713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Portnov BA, Reiser B, Karkabi K, Cohen-Kastel O, Dubnov J. High prevalence of childhood asthma in Northern Israel is linked to air pollution by particulate matter: evidence from GIS analysis and Bayesian Model Averaging. Int J Environ Health Res. 2011 doi: 10.1080/09603123.2011.634387. This study shows that exposure to particulate matter at a lower concentration than current international air pollution standards is a risk factor for childhood asthma in urban areas. [DOI] [PubMed] [Google Scholar]
- 9.Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol. 2005;115:221–228. doi: 10.1016/j.jaci.2004.11.047. quiz 229. [DOI] [PubMed] [Google Scholar]
- 10.Rylander R. Endotoxin in the environment--exposure and effects. J Endotoxin Res. 2002;8:241–252. doi: 10.1179/096805102125000452. [DOI] [PubMed] [Google Scholar]
- 11.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med. 2005;172:1371–1377. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan PH, Bernstein DI, Lockey J, Reponen T, Levin L, Grinshpun S, Villareal M, Hershey GK, Burkle J, LeMasters G. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. Am J Respir Crit Care Med. 2009;180:1068–1075. doi: 10.1164/rccm.200808-1307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Mendy A, Gasana J, Vieira ER, Forno E, Patel J, Kadam P, Ramirez G. Endotoxin exposure and childhood wheeze and asthma: a meta-analysis of observational studies. J Asthma. 2011;48:685–693. doi: 10.3109/02770903.2011.594140. This metanalysis concludes that exposure to endotoxin is a risk factor for wheeze in early childhood. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hegelan M, Tighe RM, Castillo C, Hollingsworth JW. Ambient ozone and pulmonary innate immunity. Immunol Res. 2011;49:173–191. doi: 10.1007/s12026-010-8180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Hernandez ML, Harris B, Lay JC, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Alexis NE, Peden DB. Comparative airway inflammatory response of normal volunteers to ozone and lipopolysaccharide challenge. Inhal Toxicol. 2010;22:648–656. doi: 10.3109/08958371003610966. This study demonstrates that ozone and LPS induce neutrophilic inflammation and modify markers of innate immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Hernandez ML, Lay JC, Harris B, Esther CR, Jr, Brickey WJ, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Alexis NE, et al. Atopic asthmatic subjects but not atopic subjects without asthma have enhanced inflammatory response to ozone. J Allergy Clin Immunol. 2010;126:537–544. e531. doi: 10.1016/j.jaci.2010.06.043. This study shows that atopic asthmatics have increased airway inflammatory responses in response to ozone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishna MT, Madden J, Teran LM, Biscione GL, Lau LC, Withers NJ, Sandstrom T, Mudway I, Kelly FJ, Walls A, et al. Effects of 0. 2 ppm ozone on biomarkers of inflammation in bronchoalveolar lavage fluid and bronchial mucosa of healthy subjects. Eur Respir J. 1998;11:1294–1300. doi: 10.1183/09031936.98.11061294. [DOI] [PubMed] [Google Scholar]
- 18.Devlin RB, McKinnon KP, Noah T, Becker S, Koren HS. Ozone-induced release of cytokines and fibronectin by alveolar macrophages and airway epithelial cells. Am J Physiol. 1994;266:L612–619. doi: 10.1152/ajplung.1994.266.6.L612. [DOI] [PubMed] [Google Scholar]
- 19.Jaspers I, Chen LC, Flescher E. Induction of interleukin-8 by ozone is mediated by tyrosine kinase and protein kinase A, but not by protein kinase C. J Cell Physiol. 1998;177:313–323. doi: 10.1002/(SICI)1097-4652(199811)177:2<313::AID-JCP13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 20.Jaspers I, Flescher E, Chen LC. Ozone-induced IL-8 expression and transcription factor binding in respiratory epithelial cells. Am J Physiol. 1997;272:L504–511. doi: 10.1152/ajplung.1997.272.3.L504. [DOI] [PubMed] [Google Scholar]
- 21.McKinnon KP, Madden MC, Noah TL, Devlin RB. In vitro ozone exposure increases release of arachidonic acid products from a human bronchial epithelial cell line. Toxicol Appl Pharmacol. 1993;118:215–223. doi: 10.1006/taap.1993.1027. [DOI] [PubMed] [Google Scholar]
- 22.Nichols BG, Woods JS, Luchtel DL, Corral J, Koenig JQ. Effects of ozone exposure on nuclear factor-kappaB activation and tumor necrosis factor-alpha expression in human nasal epithelial cells. Toxicol Sci. 2001;60:356–362. doi: 10.1093/toxsci/60.2.356. [DOI] [PubMed] [Google Scholar]
- 23.Becker S, Fenton MJ, Soukup JM. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Respir Cell Mol Biol. 2002;27:611–618. doi: 10.1165/rcmb.4868. [DOI] [PubMed] [Google Scholar]
- 24.Williams AS, Leung SY, Nath P, Khorasani NM, Bhavsar P, Issa R, Mitchell JA, Adcock IM, Chung KF. Role of TLR2, TLR4, and MyD88 in murine ozone-induced airway hyperresponsiveness and neutrophilia. J Appl Physiol. 2007;103:1189–1195. doi: 10.1152/japplphysiol.00172.2007. [DOI] [PubMed] [Google Scholar]
- 25.Casalino-Matsuda SM, Monzon ME, Conner GE, Salathe M, Forteza RM. Role of hyaluronan and reactive oxygen species in tissue kallikrein-mediated epidermal growth factor receptor activation in human airways. J Biol Chem. 2004;279:21606–21616. doi: 10.1074/jbc.M309950200. [DOI] [PubMed] [Google Scholar]
- 26.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is Necessary for Hyaluronan-mediated Airway Hyperresponsiveness After Ozone Inhalation. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200903-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 27.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 28.Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16:693–701. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- 29.Manzanares D, Monzon ME, Savani RC, Salathe M. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am J Respir Cell Mol Biol. 2007;37:160–168. doi: 10.1165/rcmb.2006-0413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monzon ME, Manzanares D, Schmid N, Casalino-Matsuda SM, Forteza RM. Hyaluronidase expression and activity is regulated by pro-inflammatory cytokines in human airway epithelial cells. Am J Respir Cell Mol Biol. 2008;39:289–295. doi: 10.1165/rcmb.2007-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 32.Cho HY, Kleeberger SR. Genetic mechanisms of susceptibility to oxidative lung injury in mice. Free Radic Biol Med. 2007;42:433–445. doi: 10.1016/j.freeradbiomed.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Hollingsworth JW, 2nd, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med. 2004;170:126–132. doi: 10.1164/rccm.200311-1499OC. [DOI] [PubMed] [Google Scholar]
- 34.Kleeberger SR, Reddy S, Zhang LY, Jedlicka AE. Genetic susceptibility to ozone-induced lung hyperpermeability: role of toll-like receptor 4. Am J Respir Cell Mol Biol. 2000;22:620–627. doi: 10.1165/ajrcmb.22.5.3912. [DOI] [PubMed] [Google Scholar]
- 35*.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. This review article discusses the role of TLRs in enhancing sensitivity to microbial challenge. [DOI] [PubMed] [Google Scholar]
- 36.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, et al. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem. 2009;284:11309–11317. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 37.Peden DB, Boehlecke B, Horstman D, Devlin R. Prolonged acute exposure to 0. 16 ppm ozone induces eosinophilic airway inflammation in asthmatic subjects with allergies. J Allergy Clin Immunol. 1997;100:802–808. doi: 10.1016/s0091-6749(97)70277-x. [DOI] [PubMed] [Google Scholar]
- 38.Vagaggini B, Taccola M, Cianchetti S, Carnevali S, Bartoli ML, Bacci E, Dente FL, Di Franco A, Giannini D, Paggiaro PL. Ozone exposure increases eosinophilic airway response induced by previous allergen challenge. Am J Respir Crit Care Med. 2002;166:1073–1077. doi: 10.1164/rccm.2201013. [DOI] [PubMed] [Google Scholar]
- 39.Kehrl HR, Peden DB, Ball B, Folinsbee LJ, Horstman D. Increased specific airway reactivity of persons with mild allergic asthma after 7.6 hours of exposure to 0. 16 ppm ozone. J Allergy Clin Immunol. 1999;104:1198–1204. doi: 10.1016/s0091-6749(99)70013-8. [DOI] [PubMed] [Google Scholar]
- 40*.Vagaggini B, Bartoli ML, Cianchetti S, Costa F, Bacci E, Dente FL, Di Franco A, Malagrino L, Paggiaro P. Increase in markers of airway inflammation after ozone exposure can be observed also in stable treated asthmatics with minimal functional response to ozone. Respir Res. 2010;11:5. doi: 10.1186/1465-9921-11-5. This study shows that asthmatics with a greater functional response to ozone also have greater neutrophil recruitment into airways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernandez MLBW, Alexis NE, Fry RC, Rager JE, Zou B, Ting JPY, Zhou Ha, Peden DB. Airway cells from atopic asthmatics exposed to ozone display an enhanced innate immune gene profile. J Allergy Clin Immunol. 2012 Jan;129(1):259–261.e2. doi: 10.1016/j.jaci.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.David GL, Romieu I, Sienra-Monge JJ, Collins WJ, Ramirez-Aguilar M, del Rio-Navarro BE, Reyes-Ruiz NI, Morris RW, Marzec JM, London SJ. Nicotinamide adenine dinucleotide (phosphate) reduced:quinone oxidoreductase and glutathione S-transferase M1 polymorphisms and childhood asthma. Am J Respir Crit Care Med. 2003;168:1199–1204. doi: 10.1164/rccm.200305-684OC. [DOI] [PubMed] [Google Scholar]
- 43.Gilliland FD, Gauderman WJ, Vora H, Rappaport E, Dubeau L. Effects of glutathione-S-transferase M1, T1, and P1 on childhood lung function growth. Am J Respir Crit Care Med. 2002;166:710–716. doi: 10.1164/rccm.2112065. [DOI] [PubMed] [Google Scholar]
- 44.Gilliland FD, Li YF, Dubeau L, Berhane K, Avol E, McConnell R, Gauderman WJ, Peters JM. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2002;166:457–463. doi: 10.1164/rccm.2112064. [DOI] [PubMed] [Google Scholar]
- 45.Higgins LG, Hayes JD. The cap'n'collar transcription factor Nrf2 mediates both intrinsic resistance to environmental stressors and an adaptive response elicited by chemopreventive agents that determines susceptibility to electrophilic xenobiotics. Chem Biol Interact. 2010 doi: 10.1016/j.cbi.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 46.McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- 47.London SJ, Romieu I. Gene by environment interaction in asthma. Annu Rev Public Health. 2009;30:55–80. doi: 10.1146/annurev.publhealth.031308.100151. [DOI] [PubMed] [Google Scholar]
- 48*.Dillon MA, Harris B, Hernandez ML, Zou B, Reed W, Bromberg PA, Devlin RB, Diaz-Sanchez D, Kleeberger S, Zhou H, et al. Enhancement of systemic and sputum granulocyte response to inhaled endotoxin in people with the GSTM1 null genotype. Occup Environ Med. 2011;68:783–785. doi: 10.1136/oem.2010.061747. This study demonstrates that genotypic differences play a role in response to endotoxin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilliland FD, Li YF, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet. 2004;363:119–125. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- 50.Gilliland FD, Li YF, Gong H, Jr, Diaz-Sanchez D. Glutathione s-transferases M1 and P1 prevent aggravation of allergic responses by secondhand smoke. Am J Respir Crit Care Med. 2006;174:1335–1341. doi: 10.1164/rccm.200509-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexis NE, Zhou H, Lay JC, Harris B, Hernandez ML, Lu TS, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, et al. The glutathione-S-transferase Mu 1 null genotype modulates ozone-induced airway inflammation in human subjects. J Allergy Clin Immunol. 2009;124:1222–1228. e1225. doi: 10.1016/j.jaci.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Wu W, Doreswamy V, Diaz-Sanchez D, Samet JM, Kesic M, Dailey L, Zhang W, Jaspers I, Peden DB. GSTM1 modulation of IL-8 expression in human bronchial epithelial cells exposed to ozone. Free Radic Biol Med. 2011;51:522–529. doi: 10.1016/j.freeradbiomed.2011.05.006. This study shows that GSTM1 deficiency results in increased ozone-induced IL-8 expression, providing in vitro evidence that this genotypic variation contributes to ozone induced inflammatory response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexis N, Urch B, Tarlo S, Corey P, Pengelly D, O'Byrne P, Silverman F. Cyclooxygenase metabolites play a different role in ozone-induced pulmonary function decline in asthmatics compared to normals. Inhal Toxicol. 2000;12:1205–1224. doi: 10.1080/08958370050198548. [DOI] [PubMed] [Google Scholar]
- 54.Hazucha MJ, Madden M, Pape G, Becker S, Devlin R, Koren HS, Kehrl H, Bromberg PA. Effects of cyclo-oxygenase inhibition on ozone-induced respiratory inflammation and lung function changes. Eur J Appl Physiol Occup Physiol. 1996;73:17–27. doi: 10.1007/BF00262805. [DOI] [PubMed] [Google Scholar]
- 55.Passannante AN, Hazucha MJ, Bromberg PA, Seal E, Folinsbee L, Koch G. Nociceptive mechanisms modulate ozone-induced human lung function decrements. J Appl Physiol. 1998;85:1863–1870. doi: 10.1152/jappl.1998.85.5.1863. [DOI] [PubMed] [Google Scholar]
- 56.Hazucha MJ, Bates DV, Bromberg PA. Mechanism of action of ozone on the human lung. J Appl Physiol. 1989;67:1535–1541. doi: 10.1152/jappl.1989.67.4.1535. [DOI] [PubMed] [Google Scholar]
- 57.Michel O, Ginanni R, Sergysels R. Protective effect of sodium cromoglycate on lipopolysaccharide-induced bronchial obstruction in asthmatics. Int Arch Allergy Immunol. 1995;108:298–302. doi: 10.1159/000237168. [DOI] [PubMed] [Google Scholar]
- 58.Vagaggini B, Taccola M, Conti I, Carnevali S, Cianchetti S, Bartoli ML, Bacci E, Dente FL, Di Franco A, Giannini D, et al. Budesonide reduces neutrophilic but not functional airway response to ozone in mild asthmatics. Am J Respir Crit Care Med. 2001;164:2172–2176. doi: 10.1164/ajrccm.164.12.2009090. [DOI] [PubMed] [Google Scholar]
- 59.Alexis NE, Lay JC, Haczku A, Gong H, Linn W, Hazucha MJ, Harris B, Tal-Singer R, Peden DB. Fluticasone propionate protects against ozone-induced airway inflammation and modified immune cell activation markers in healthy volunteers. Environ Health Perspect. 2008;116:799–805. doi: 10.1289/ehp.10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60**.Lazaar AL, Sweeney LE, MacDonald AJ, Alexis NE, Chen C, Tal-Singer R. SB-656933, a novel CXCR2 selective antagonist, inhibits ex vivo neutrophil activation and ozone-induced airway inflammation in humans. Br J Clin Pharmacol. 2011;72:282–293. doi: 10.1111/j.1365-2125.2011.03968.x. This study provides evidence that an antagonist blocking CXCR2 may reduce ozone-induced airway inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, Reyes-Ruiz NI, Estela del Rio-Navarro B, Hernandez-Avila M, London SJ. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax. 2004;59:8–10. [PMC free article] [PubMed] [Google Scholar]
- 62.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Tellez-Rojo MM, Moreno-Macias H, Reyes-Ruiz NI, del Rio-Navarro BE, Ruiz-Navarro MX, Hatch G, Slade R, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002;166:703–709. doi: 10.1164/rccm.2112074. [DOI] [PubMed] [Google Scholar]
- 63.Samet JM, Hatch GE, Horstman D, Steck-Scott S, Arab L, Bromberg PA, Levine M, McDonnell WF, Devlin RB. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am J Respir Crit Care Med. 2001;164:819–825. doi: 10.1164/ajrccm.164.5.2008003. [DOI] [PubMed] [Google Scholar]
- 64.Trenga CA, Koenig JQ, Williams PV. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Arch Environ Health. 2001;56:242–249. doi: 10.1080/00039890109604448. [DOI] [PubMed] [Google Scholar]
- 65.Wan J, Diaz-Sanchez D. Phase II enzymes induction blocks the enhanced IgE production in B cells by diesel exhaust particles. J Immunol. 2006;177:3477–3483. doi: 10.4049/jimmunol.177.5.3477. [DOI] [PubMed] [Google Scholar]
- 66.Ritz SA, Wan J, Diaz-Sanchez D. Sulforaphane-stimulated phase II enzyme induction inhibits cytokine production by airway epithelial cells stimulated with diesel extract. Am J Physiol Lung Cell Mol Physiol. 2007;292:L33–39. doi: 10.1152/ajplung.00170.2006. [DOI] [PubMed] [Google Scholar]
- 67.Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130:244–251. doi: 10.1016/j.clim.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LA. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123:146–152. e148. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69**.Fitzpatrick AM, Stephenson ST, Hadley GR, Burwell L, Penugonda M, Simon DM, Hansen J, Jones DP, Brown LA. Thiol redox disturbances in children with severe asthma are associated with posttranslational modification of the transcription factor nuclear factor (erythroid-derived 2)-like 2. J Allergy Clin Immunol. 2011;127:1604–1611. doi: 10.1016/j.jaci.2011.03.031. This study shows that antioxidant defense is impaired in children with severe asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70**.Shaheen SO, Newson RB, Ring SM, Rose-Zerilli MJ, Holloway JW, Henderson AJ. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. J Allergy Clin Immunol. 2010;126:1141–1148. e1147. doi: 10.1016/j.jaci.2010.08.047. This study demonstrates a relationship between maternal antioxidant gene polymorphisms and acetaminophen use in late pregnancy and development of asthma in the child. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71*.Schroer KT, Gibson AM, Sivaprasad U, Bass SA, Ericksen MB, Wills-Karp M, Lecras T, Fitzpatrick AM, Brown LA, Stringer KF, et al. Downregulation of glutathione S-transferase pi in asthma contributes to enhanced oxidative stress. J Allergy Clin Immunol. 2011;128:539–548. doi: 10.1016/j.jaci.2011.04.018. This study suggests that downregulation of GSTPi after allergen exposure contributes to increased oxidative stress and the asthma phenotype. This may provide a therapeutic target for affected individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]