Abstract
Contrasting information suggests either almost complete depletion of sarcoplasmic reticulum (SR) Ca2+ or significant residual Ca2+ concentration after prolonged depolarization of the skeletal muscle fiber. The primary obstacle to resolving this controversy is the lack of genetically encoded Ca2+ indicators (GECI) targeted to the SR that exhibit low-Ca2+ affinity, a fast biosensor:Ca2+ off-rate reaction, and can be expressed in myofibers from adult and older adult mammalian species. This work used the recently designed low-affinity Ca2+ sensor (Kd=1.66 mM in the myofiber) CatchER (calcium sensor for detecting high concentrations in the ER) targeted to the SR, to investigate whether prolonged skeletal muscle fiber depolarization significantly alters residual SR Ca2+ with aging. We found CatchER a proper tool to investigate SR Ca2+ depletion in young-adult and older adult mice, consistently tracking SR luminal Ca2+ release in response to brief and repetitive stimulation. We evoked SR Ca2+ release in whole-cell voltage-clamped flexor digitorum brevis (FDB) muscle fibers from young and old FVB mice and tested the maximal SR Ca2+ release by directly activating the ryanodine receptor (RyR1) with 4-Chloro-m-cresol (4-CmC) in the same myofibers. Here, we report for the first time, that the Ca2+ remaining in the SR after prolonged depolarization (2s) in myofibers from aging (~220 µM) was larger than young (~132µM) mice. These experiments indicate that SR Ca2+ is far from fully depleted under physiological conditions throughout life, and support the concept of excitation-contraction uncoupling in functional senescent myofibers.
Keywords: Skeletal muscle, calcium sensor, CatchER, sarcoplasmic reticulum, aging
INTRODUCTION
Measuring SR residual Ca2+ concentration in depolarized skeletal muscle fiber is essential to understanding the influence of luminal SR Ca2+ on SR Ca2+ release and force deterioration with aging. Developing reliable molecular tools to detect Ca2+ kinetics in intracellular organelles is imperative to determining its role in cell signaling, physiological responses to training and exercise, and the pathogenesis of muscular dystrophy, central core disease, malignant hyperthermia, and fatigue syndromes [28, 44]. The SR is the main source of Ca2+ for skeletal muscle contraction, but the role of its intricate terminal cisternae/longitudinal architecture and Ca2+ buffering by endogenous Ca2+-binding proteins, such as calsequestrin and calreticulin, in intracellular Ca2+ homeostasis is poorly understood. Particularly, the elevated SR Ca2+ concentration requires a low-affinity biosensor to reliably monitor Ca2+ concentration and kinetics.
Modifications in Ca2+ concentration are the signal that regulates a number of functions in excitable and nonexcitable cells [15, 16, 36]. However, loading the skeletal muscle SR with Ca2+sensitive indicators presents technical difficulties that seriously hamper our ability to determine Ca2+ concentration and release kinetics and to answer questions relevant to muscle physiology and pathology. Assessing residual SR Ca2+ in excitable cells is relevant for the study of luminal Ca2+ buffer capacity, Ca2+-dependent regulation of IP3R and RyR function, store-operated Ca2+ entry, termination of SR Ca2+ release, and age-dependent decline in SR Ca2+ release [12]. Therefore, measuring Ca2+ movements using engineered protein targeted to the ER/SR is increasingly important [4, 12, 19, 27, 34].
Previous studies have shown that decreased expression of the Cav1.1 subunit diminishes SR Ca2+ release in muscle fibers from aging mice [11, 12], a process known as excitation-contraction uncoupling (ECU) [3]. ECU is partially due to increased Cav 1a expression with aging [35]. Recently, we estimated SR Ca2+ depletion in response to 4-CmC or myofiber depolarization in myofibers from young-adult mice electroporated with the Ca2+ biosensor D1ER [12]. We concluded that significant depletion of SR Ca2+ under physiological conditions is unlikely in young mice [12]. However, quantifying SR Ca2+ concentration after prolonged myofiber depolarization was not possible due to limitations in calibrating D1ER in the myofiber as described previously [27]. Quantifying residual SR Ca2+ requires measuring its resting concentration, release, and content in the same fiber using CatchER as the Ca2+ biosensor.
We recently reported that the novel EGFP-derived biosensor CatchER can monitor Ca2+ concentration and dynamics in high Ca2+ concentration compartments, such as the SR. It exhibits relatively low affinity in the skeletal muscle, as its Kd, calibrated in the myofiber, is 1.66 mM, and an unprecedented off-rate at ~700 s−1which makes it one of the more advanced and useful tools for examining changes in SR Ca2+ concentration in response to pulses both as prolonged as those applied to elicit muscle tetanus and as short as an action potential [34].
Here, we show that CatchER exhibits a consistent response to muscle fiber stimulation, which allows us to quantify SR Ca2+ content, the rate of Ca2+ flux in the SR lumen, and the residual SR Ca2+ concentration in response to prolonged electrical stimulation beyond physiological demands in voltage-clamped adult mouse flexor digitorum brevis (FDB) muscle fibers. Maximal SR Ca2+ depletion was also examined by bypassing the physiological Cav1.1/ryanodine receptor-1 (RyR1) coupling to stimulate the RyR1 directly. For this application, we used the specific RyR1 agonist 4-Chloro-m-cresol (4-CmC) based on the reproducibility of its action and the lack of any reported effect on SR Ca2+ refilling.
We report a significant larger residual SR Ca2+ concentration in fibers from old compared to young adult mice, finding that supports the concept of excitation-contraction uncoupling in senescent mice.
MATERIALS AND METHODS
Flexor digitorum brevis muscle fibers
Single skeletal muscle fibers from the FDB were obtained from 3–5-month-old C57BL6 mice raised in the Wake Forest University School of Medicine (WFUSM) Animal Research Program. Mice were killed by cervical dislocation following isoflurane anesthesia. For aging studies, we used young (3–5-month) and old (22–25-month) FVB (Freund virus B) mice from the WFUSM colony. Animal handling followed a protocol approved by the WFUSM Animal Care and Use Committee.
FDB fiber electroporation and SR Ca2+ fluorescence recording
The pcDNA3.1 plasmid carrying the CatchER biosensor was electroporated into the FDB muscle as described [5]. FDB fibers were enzymatically dissociated and recorded 2–3 weeks after electroporation [34]. Dissociated FDB fibers were voltage-clamped in the whole-cell configuration of the patch-clamp technique [9], according to procedures specific for muscle fibers [40]. Potential voltage-errors associated with whole-cell recording in large cells were minimized by selecting small FDB fibers and adequately compensating for whole-cell capacitance transients. The pipette electrode was filled with the following solution (mM): 130 Cs-aspartate, 2 MgCl20.2 or 20 Cs2EGTA as specified below, 10 HEPES, 5 Na-ATP, and 0.5 GTP; pH was adjusted to 7.3 with CsOH. High EGTA was used to reproduce experimental conditions in a series of studies on FDB muscle fibers in which SR Ca2+ release was recorded in the cytosol [12, 34, 41]. The electrode resistance ranged from 450 to 650 kΩ. The external solution contained (in mM): 100 TEA (tetraethylammonium hydroxide)-OH, 50 Na2SO4, 2 MgSO4, 2 CaSO4, 2 3–4DAP, and 5 Na-HEPES. Solution pH was adjusted to 7.3 with CH4SO3 [12, 40]. These solutions ensured good control of resting leakage currents, and the preparation was preserved throughout the experiment. Methanesulphonic acid is the counterion to TEA. Drift and noise imposed by silver-chloride wires were corrected by standard methods. All experiments were recorded at room temperature (21–22°C). To permeate the myofibers, we used a solution containing 90 mM K-glutamate, 1.02 mM MgCl2, 5 mM NaCl, 10 mM Hepes, 1 mM 1,2-bis(oaminophenoxy)ethane-n,n,n′,n′-tetraacetic acid (BAPTA), 0.323 mM CaCl2, 0.025 N-benzyl-p-toluene sulphonamide (BTS), 2% (vol/vol) poly (N-vinyl-2-pyrrolidone) (PVP) (1 mM free Mg2+, 0.0001 mM free Ca2+), pH 7.2, adjusted with KOH [43]. Free [Ca2+] and [Mg2+] in solution were calculated by the Max-Chelator program.
Fluorescence was recorded using an Axiovert 200 microscope with a 20x/0.75 (Zeiss, Oberkochen, Germany) and a Radiance 2100 (Bio-Rad, Zeiss) confocal system. Fibers were imaged through a C-Apochromat 40x water-immersion objective (NA 1.2, Zeiss) or a 20x Fluar (NA 0.75) using a krypton-argon laser at 488-nm excitation wavelength. CatchER’s fluorescence emission was measured at 528 ± 25 nm wavelength. For most experiments, the laser was attenuated to 6–12% with a neutral density filter. Fibers were imaged in line-scan (x-t) mode. The fiber was always oriented parallel to the x scan direction. Linescan images were acquired with 256 pixels (0.236 mm/pixel) in the x- and 512 pixels (0.833 ms/pixel) in the t-direction. For image acquisition, we used LaserSharp 2000 software (Bio-Rad, Zeiss). Cytosolic Ca2+ transients were recorded using 5µM Rhod-2AM (Invitrogen, Carlsbad, CA) loaded for 30 min on CatchER-expressing FDB fibers. Fibers were excited at 568 nm using a krypton laser and recorded at 600 nm.
SR Ca2+ release and cytosolic Ca2+ transients were recorded sequentially (20 s interval). No differences in the amplitude and kinetics of SR Ca2+ release monitored with CatchER were apparent in Rhod-2-loaded and unloaded fibers (data not shown).
CatchER calibration in mouse FDB muscle fibers was reported in detail previously (Supporting Information in [34]).
Statistics
Values are given as mean ± SEM with the number of observations (n). Statistical analysis was performed using the Student’s unpaired t-test and the Mann-Whitney rank-sum test when values were not normally distributed. P < 0.05 was considered significant.
RESULTS
RyR1 mediates SR Ca2+ release recorded with CatchER
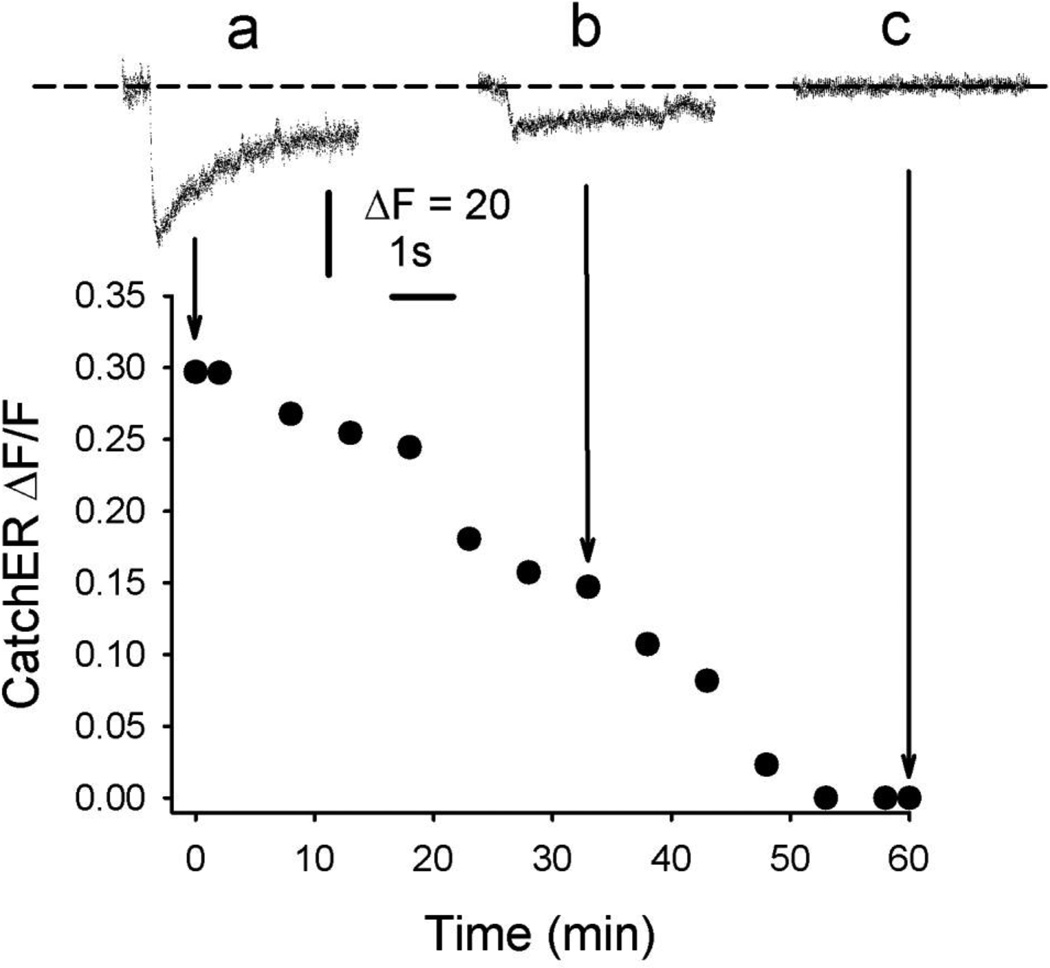
To verify that RyR1 mediates the Ca2+ release detected with CatchER, we exposed fibers to the channel blocker ryanodine. Nadir SR Ca2+ fluorescence decreased with time after applying 5µM ryanodine extracellularly. Figure 1 illustrates CatchER fluorescence in response to a series of 20mV/100ms pulses delivered before (a) and after (b, c) applying ryanodine to fibers from young adult mice. The amplitude of CatchER fluorescence 28 and 55 min after applying the drug compared to the amplitude of the signal at time zero was 45 ± 4% and 0.3 ± 0.02 % (n = 3). During ryanodine application, basal ΔF/F did not change significantly; it was 1.61 ± 0.27 and 1.49 ± 0.28 at times 0 and 55 min, respectively. Since RyR1 is the only RyR isoform expressed in adult skeletal muscle, we conclude that it mediates decreases in SR Ca2+ in response to sarcolemmal depolarization [2, 22, 26]. Declines in CatchER fluorescence, in response to the agonist 4-CmC, support this conclusion (see below). The slow time scale of the effect indicates slow diffusion of the agent. The kinetics of ryanodine binding to its receptor may be a contributing factor, as reported [12]. Control experiments in which ryanodine was omitted exhibited a maximal decline of 20 ± 2.3 % (n =5) in CatchER’s ΔF/F at the end of 60 min.
Figure 1. RyR1 mediates SR Ca2+ release.
Time course of CatchER ΔF/F in control (first 5 min) and after adding 5µM ryanodine to the bath solution. Traces a, b, and c represent SR Ca2+ transients monitored with CatchER in control, 33, and 60 min, respectively, in 3 experiments with a representative fiber.
High cytosolic EGTA concentration does not affect SR Ca2+ recovery
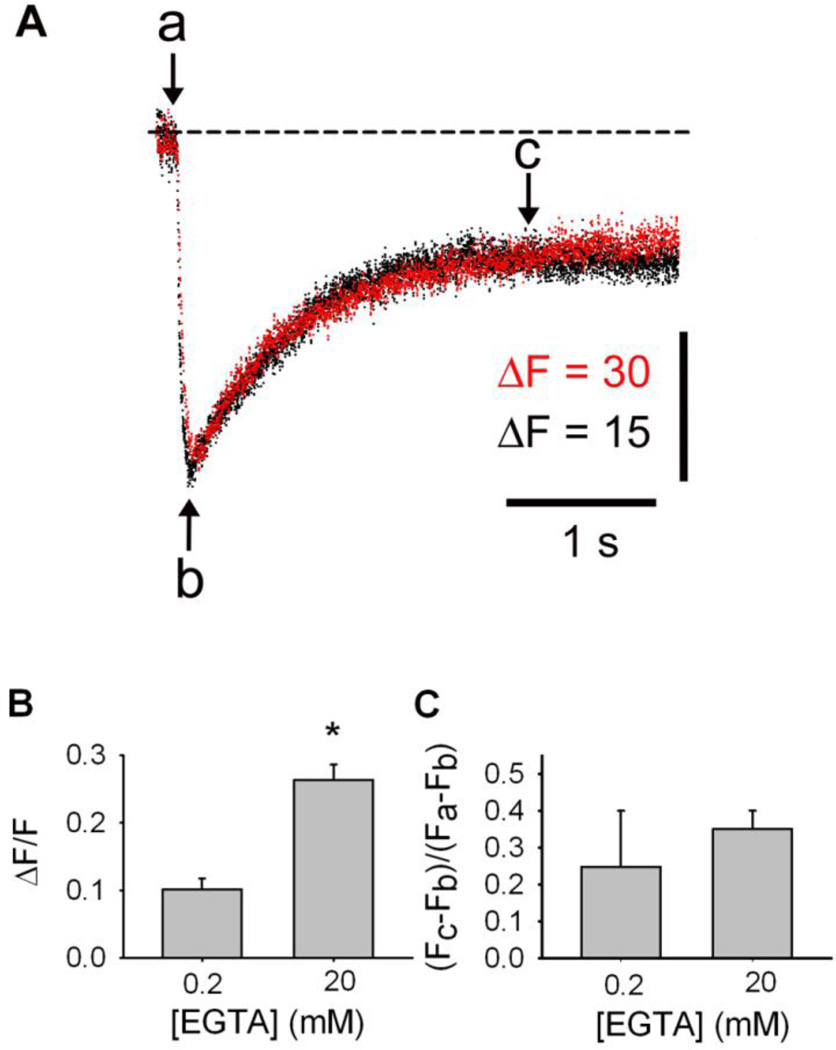
Like D1ER [12], CatchER displays a fluorescence signal larger in the presence of 20 mM than in 0.2 mM cytosolic EGTA; despite EGTA’s Ca2+ buffer capacity, SR Ca2+ recovery is not altered. Figure 2A shows the time course of CatchER fluorescence in response to 20mV/100ms command pulses recorded in 0.2 (black trace) and 20 mM (red trace) EGTA in fibers from young-adult mice. CatchER signal in 0.2 mM EGTA was scaled up to overlap with its recording in 20 mM EGTA to compare their kinetics. High cytosolic EGTA concentration (20 mM) does not seem to prevent SR Ca2+ recovery and elicits average fluorescence signals 2.6 times larger than those recorded in 0.2 mM EGTA, which is statistically significant (Fig. 2B). Why SR Ca2+ depletion is more pronounced with high EGTA concentration is not obvious. Perhaps in the low EGTA solution, Ca2+ inactivates RyR1. To examine whether strong cytosolic Ca2+ chelation modifies SR Ca2+ uptake, we analyzed SR Ca2+ recovery 2.5 s after the end of the depolarizing pulse. Figure 2C shows that SR Ca2+ recovery in 0.2 and 20mM EGTA did not differ significantly. In addition, 38 fibers recorded with 20mM EGTA responded to electrical stimulation, while 2 out of 19 in 0.2 mM EGTA did not. Differences in Ca2+ recovery beyond c in the two experimental conditions were not statistically significant. Based on these results, we used a pipette solution containing 20mM EGTA for the remaining experiments.
Figure 2. High cytosolic EGTA concentration increases CatchER transient amplitude but does not affect SR Ca2+ recovery.
A. SR Ca2+ transients recorded in electroporated FDB fibers and recorded in whole cell patch-clamp using 0.2 (black trace) or 20mM (red trace) EGTA in the pipette solution. B. CatchER ΔF/F – [EGTA] relationship. CatchER fluorescence at nadir of the response (b) is significantly larger in 20 than 0.2mM EGTA (p < 0.001, n = 11 in 0.2 mM EGTA; n = 13 in 20mM EGTA). C. SR Ca2+ recovery is expressed as (Fc-Fb)/(Fa-Fb), where a is the average of the basal fluorescence points, and b and c are CatchER fluorescence at nadir and 2s after repolarization starts, respectively. SR Ca2+ recovery is independent of cytosolic [EGTA] (p > 0.05).
Brief and prolonged SR Ca2+ release tracked with CatchER
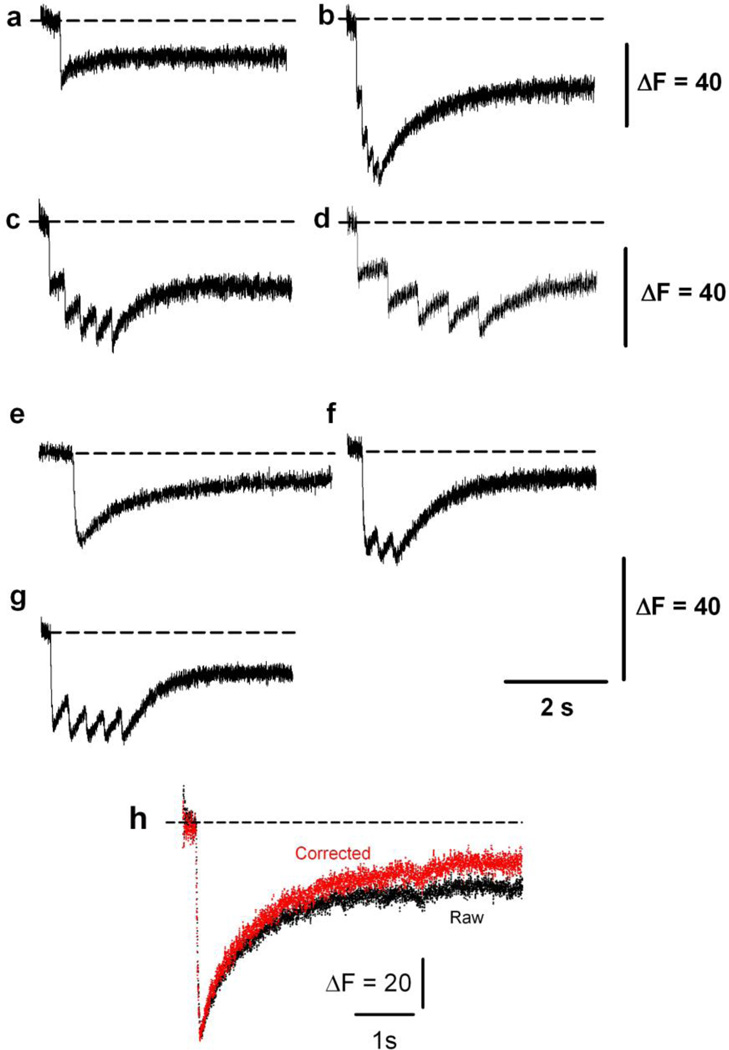
Previously reported Ca2+ biosensors targeted to the SR have been used to track slow changes in luminal SR Ca2+ [1, 12, 27, 32, 33, 42, 44]. We demonstrated that CatchER detected SR Ca2+ in response to a single action potential [44]. Here, we examined whether it could detect a variety of pulses from brief repetitive to a single prolonged stimulation under voltage-clamp. Figure 3 shows changes in CatchER’s fluorescence in response to various command pulses to 20mV. These data indicate that CatchER responds adequately to pulses of different duration (a: 10ms; e: 100ms), frequency (b: 10; c: 3,3; d: 1.6; and f: 5 Hz), or duration and frequency (g: 50 ms pulse at 3.3 Hz) and can thus monitor SR Ca2+ dynamics under a variety of physiological conditions. Figure 3 shows that SR Ca2+ is incompletely recovered within the recording time, regardless of the pulse applied to the cell. We rule out cell damage as a potential mechanism because all myofibers included in this study maintained membrane electrical properties within 10% of the original values throughout the experiment. Photobleaching partially explains the slow fluorescence recovery. Figure 3H shows a typical change in CatchER fluorescence in response to a 30mV/100ms pulse (raw trace). Correcting for photobleaching (corrected trace) partially speeds recovery (21 ± 3.2 %; n = 17 fibers), or perhaps calsequestrin binds Ca2+ with higher affinity than CatchER [10], delaying recovery of the biosensor’s fluorescence. We also cannot rule out the influence of the CatchER: Ca2+ on-rate reaction.
Figure 3. CatchER tracks SR Ca2+ release in response to single or repetitive prolonged stimulation.
Transient changes in CatchER’s fluorescence response to various 20mV command pulses: (a) a 10ms pulse, (b) five 10ms pulses at 10Hz, (c) a 10 ms pulse at 3.3 Hz, (d) a 10 ms pulse at 1.6 Hz, (e), a 100 ms pulse, (f) 100 ms pulse at 5 Hz, and (g) 50 ms pulse at 3.3 Hz. H. CatchER’s fluorescence response to a 30mV/100ms pulse (raw trace, black) and after correcting for photobleaching (corrected trace, red).
Voltage-dependent CatchER luminal signal and cytoplasmic Ca2+ transients
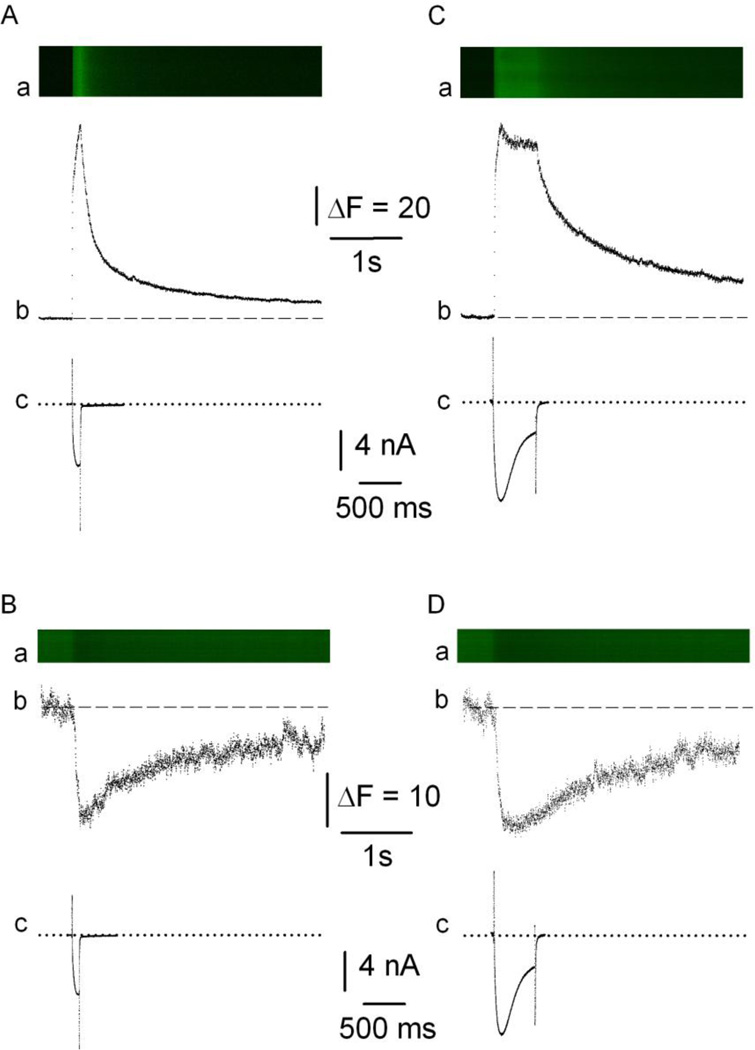
Figure 4 shows young adult mouse FDB fibers expressing CatchER loaded with 5µM Rhod2AM and depolarized with 100 (A, B) or 500 (C, D) ms command pulses from Vh = −80mV to 20 mV applied in the whole-cell configuration of patch-clamp. Panels A and C correspond to Rhod2 while B and D to CatchER. Top panels (a) show images in line-scan mode with vertical and horizontal axes corresponding to space and time, respectively. Fluorescence increases (A, C) or decreases (B, D) depend on whether cytosolic Rhod-2 or SR CatchER fluorescence was recorded, respectively. The use of 20mM EGTA in the internal (pipette) solution provided a dual benefit: it prevented Rhod-2 saturation and the movement of artifacts during fiber depolarization. Middle panels (b) display time-dependent changes in the Ca2+ indicator’s fluorescence. The decreased fluorescence signal indicates that Ca2+ dissociates from CatchER upon fiber depolarization. The rate of fluorescence signal rise for CatchER and Rhod-2 is similar, while the cytosolic fluorescence decay is faster than the luminal Ca2+ recovery. The rising phase of the Rhod-2 signal was fitted to a double-exponential function with time constants of 2 ± 0.11 and 139 ± 15ms and 5 ± 0.31 and 246 ± 39ms in response to 100 and 500 ms pulses, respectively. The decay phase also shows two time constants of 103 ± 17 and 1132 ± 168 ms and 163 ± 19 and 1193 ± 145 (n = 7), respectively. The CatchER rising and recovery phases were fitted with a single time constant of 26 ± 3.5 and 19 ± 2.3 ms and 1315 ± 147 and 2512 ± 323 (n = 12) in response to 100 and 500 ms pulses, respectively. Differences in Ca2+ affinity and Ca2+ buffer capacity of SR and cytoplasm can explain differences in the time course of CatchER and Rhod-2 fluorescence. Figure 4c illustrates the Ca2+ currents in real- time to compare the time course of the electrical and optical signals. The time-to-peak (in ms) of the Rhod-2 signal were 98 ± 4 and 113 ± 8, while for CatchER, the values were 91 ± 5 and 139 ± 23 in response to 100 and 500ms pulses, respectively. Values are mean ± SEM for 14 fibers expressing CatchER and loaded with Rhod-2. Apparent differences between time-to-peak of Rhod-2 and CatchER signals were not statistically significant.
Figure 4. SR and cytosolic Ca2+ transients recorded in the same FDB fibers.
Ca2+ transients were recorded in response to a command pulse (20mV and 100-ms [A, B] or 500-ms [C, D] duration [Vh = −80mV]). Panel a shows images in confocal line-scan mode with vertical and horizontal axes corresponding to space and time, respectively; b, Ca2+ transients’ fluorescence profile measured with Rhod-2 (A, C) or CatchER (B,D); c, Ca2+ currents. Dashed and dotted lines indicate basal fluorescence before pulse application and isoelectric current, respectively. ΔF/F for Ab, Bb, Cb, and Db were 6.65, 0.26, 6.7, and 0.28, respectively. The rising phase of the Rhod-2 signal was fitted to an exponential function with two time constants of 3 and 146ms and 4 and 246ms in response to 100 and 500 ms pulses, respectively. The decay phase also shows two time constants of 114 and 1025ms and 159 and 1246, respectively. Time to peak is 98 and 111ms, respectively. The CatchER rising and recovery phases were fitted with a single time constant of 31 and 23ms and 1214 and 2733 in response to 100 and 500 ms pulses, respectively. Time to peak is 100 and 127ms, respectively.
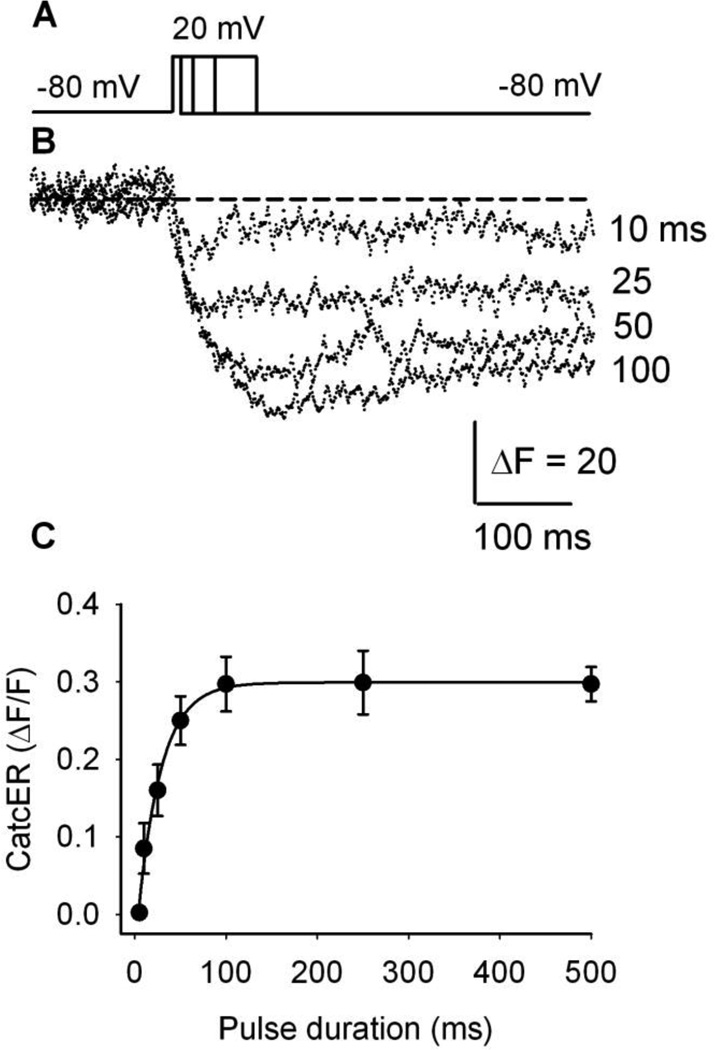
CatchER’s fluorescence amplitude increases with the duration of sarcolemmal depolarization
We recently showed that CatchER’s fluorescence decreases with sarcolemmal depolarization, and the fluorescence-membrane voltage relationship follows a Boltzmann equation in fibers from young and old mice [34]. To further determine whether CatchER responds to SR Ca2+ release as a function of pulse duration, we voltage-clamped 12 fibers from young adult mice at −80mV and stimulated them with 20mV command pulses of increasing duration (10, 25, 50, and 100ms) (Fig. 5A, B). As shown in Figure 5C, CatchER’s fluorescence elicited by progressively longer pulses showed a graded increase in signal amplitude, reaching a plateau for pulses longer than 100 ms (250 and 500ms).
Figure 5. CatchER’s peak Ca2+ transient depends on pulse duration.
A. Various pulse durations (10, 25, 50, and 100 ms) tested in FDB fibers, voltage-clamped at −80 mV (holding potential), and pulsed to 20mV. The pulse waveform is depicted in the top panel. B. Maximal CatchER transient relationship to pulse duration. Data points represent the mean ± SEM of 12 fibers.
Ca2+ remaining in the SR after prolonged depolarization differs in myofibers from young and old mice
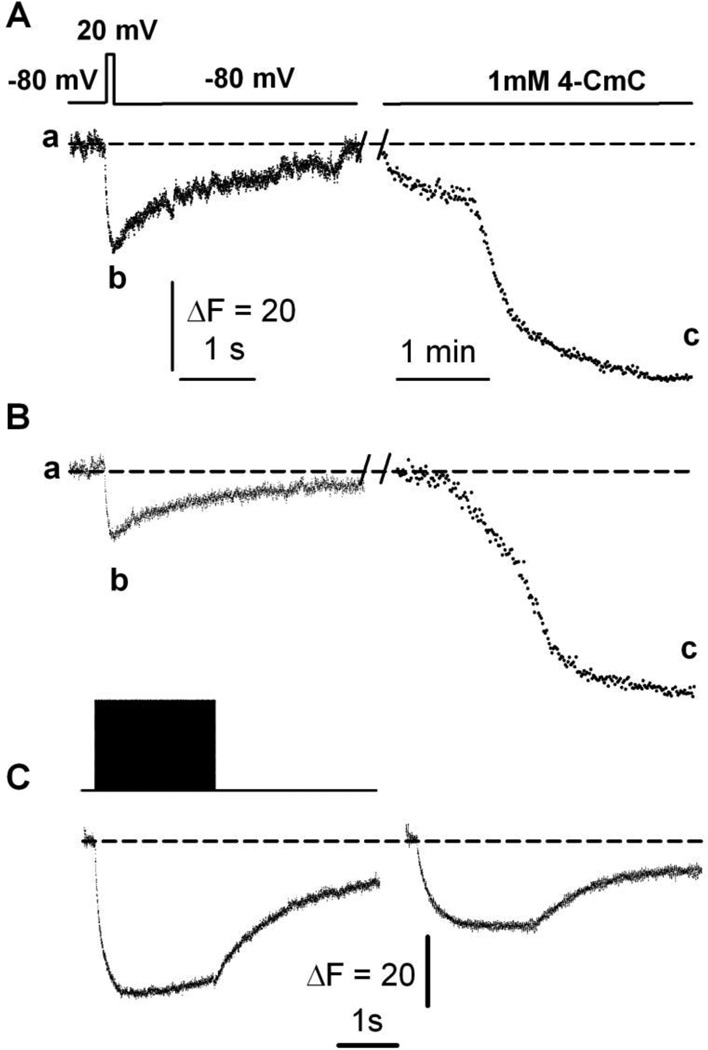
Figure 6A shows SR Ca2+ fluorescence in response to a 20mV/100 ms pulse as depicted on the top trace in a fiber from a young adult mouse. After ~2min, the same fiber was exposed to 1mM 4-CmC for the whole recording period, as indicated on the top bar, to bypass the excitation-SR Ca2+ release process and elicit SR Ca2+ release by acting directly on RyR1 (protocol 1). CatchER fluorescence seems to reach a deeper nadir than the response to the electrical pulse. The ratio between the peak response to electrical stimulation (b) and 4-CmC (c) was 0.44 ± 0.09 (n = 6).
Figure 6. Maximal SR Ca2+ depletion evoked by 4-CmC.
A. CatchER fluorescence transient (bottom trace) in response to 20mV/100 ms command pulse (top trace) in a voltage-clamped myofiber from a young-adult mouse. The same fiber was exposed to 1mM 4-CmC with ~2min interval. The dashed line indicates the basal fluorescence (a), while the nadir of the response to electrical stimulation or 4-CmC application is shown in (b) and (c), respectively. B. CatchER fluorescence transient in response to a 20mV/100 ms command pulse in a voltage-clamped myofiber from an old mouse. The same fiber was exposed to 1mM 4-CmC at ~2min intervals. C. CatchER fluorescence (bottom) in response to repetitive stimulation with 2-ms pulses at 150 Hz for 2 s at 20mV (top) in myofibers from young (left) and old (right) mice.
To investigate whether a remaining Ca2+ pool persists in the SR after 4-CmC application, electrical stimulation was prolonged to 500ms, and 15µM cyclopiazonic acid (CPA) were added with 1mM 4-CmC to block SERCA-mediated SR Ca2+ uptake in addition to agonist-dependent SR Ca2+ release (protocol 2). Increased pulse duration and the combination of the two pharmacological agents resulted in a similar SR Ca2+ depletion (data not shown).
Similarly, Figure 6B shows SR Ca2+ fluorescence in response to a 20mV/100 ms pulse in a fiber from an old mouse. While CatchER’s response to the electrical pulse is smaller, its response to 4-CmC does not differ significantly from that obtained in young fibers [34]. The ratio between the nadir in response to electrical stimulation (b) and 4-CmC (c) was 0.21 ± 0.07 (n = 7).
Figure 6C shows the response of myofibers from young (left) and old (right) mice to a 2s/20mV train of 3 ms pulses at 150 Hz. The b/c ratio was 0.41 ± 0.08 (n = 15 fibers) and 0.24 ± 0.06 (n = 17 fibers), respectively. These experiments indicate that a Ca2+ pool remains in the SR lumen after myofiber depolarization through prolonged activation of the excitation-SR Ca2+ release mechanism, consistent with declining voltage-gated SR Ca2+ release with aging [11].
To further examine the residual luminal Ca2+ in the SR after prolonged Ca2+ release, we applied an alternative technique. After SR Ca2+ release was measured FDB fibers from young and old mice expressing CatchER were exposed to 0.01% saponin for 2 min in permeabilization solution (see Methods). The permeabilized myofiber was exposed to 10−6 M ionomycin diluted in the previous solution to equilibrate among the extracellular space, cytosol, and lumen of the SR. Free [Mg2+] was set at 1 mM, and free [Ca2+] was set at 10−7 mM with BAPTA. Although apparently more stringent, this technique did not promote a significant difference in residual Ca2+ compared to the 4-CmC protocols. The Ca2+ concentration remaining in the SR after Ca2+ release in response to prolonged repetitive electrical stimulation (2s) was (in µM): 132 ± 29 and 220 ± 37 in myofibers from young (n = 9 fibers) and old (n = 7 fibers) mice, respectively. As resting SR Ca2+ concentration did not differ in myofibers from young and old mice [34], our data are consistent with a larger SR luminal Ca2+ depletion in fibers from young mice or an impaired voltage-gated SR Ca2+ release in aging mice [11].
DISCUSSION
The main findings of this work are: 1) CatchER can track changes in SR Ca2+ lumen in response to pulses varying in amplitude, duration, and frequency; 2) photobleaching partially accounts for CatchER’s slow fluorescence recovery; 3) SR Ca2+ release monitored with CatchER is solely mediated by RyR1; 4) SR luminal Ca2+ release measured with CatchER mirrors the cytosolic Ca2+ transient, measured with Rhod-2; and 5) the fraction of residual SR Ca2+ is larger in myofibers from old than young mice, supporting excitation-contraction uncoupling in senescent myofibers.
CatchER is a low-affinity Ca2+ biosensor suitable for measuring luminal SR Ca2+
CatchER allowed us to monitor changes in Ca2+ concentration dynamics in a practically unexplored environment—skeletal muscle SR. We recently described the structure and optical properties of this GECI and the mechanism of its response to Ca2+ [34]. Previous attempts to measure SR Ca2+ in amphibian and mammalian species have been discussed elsewhere [12]. Although CatchER’s single-excitation/single-emission optical spectrum is limited compared to the single excitation/dual emission D1ER [12, 18, 27], it has a number of advantages, including responsiveness, reproducibility of the signal, and low Ca2+ affinity. These properties allowed us to measure high SR luminal Ca2+ concentrations and their dynamics in a considerable number of myofibers after FDB muscle in vivo electroporation. Difficulties in reliably calibrating D1ER, the most commonly used biosensor for Ca2+ analysis in the muscle, have been reported [12, 27]; however, its ratiometric signal recorded in muscle fiber provided useful information about relative SR Ca2+ changes [12]. Relative changes in citrine/CFP ratio, rather than SR Ca2+ concentrations, have been reported using D1ER in arterial smooth muscle [42], HEK-293 cells expressing RyR2 [13], and vascular endothelial cells [21]. Here, we were able to quantify residual SR Ca2+ concentration after SR Ca2+ released in response to fiber electrical and pharmacological stimulation in young and old mice, which was impractical with previous GECI, representing a net improvement over previous approaches. Also, CatchER expression by electroporation and recording without previous prolonged loading procedures make it a promising tool to investigate the role of SR Ca2+ movements under a variety of physiological and disease conditions.
The concentration of residual SR Ca2+ after Ca2+ release in skeletal myofibers from senescent mice is unknown
A series of reports estimated SR Ca2+ content and depletion using cytosolic synthetic Ca2+ indicators in young rodents. In voltage-controlled SR Ca2+ release in mouse FDB fibers, a 100ms voltage pulse that maximally activates Ca2+ release, according to the authors, reduces the initial SR content about 80% [37]. Note that they estimated an SR Ca2+ content of 3 mM, consistent with previous reports [8] [7, 17, 20, 24, 30]. However, more recent works using engineered Ca2+ biosensors or Fluo-5N targeted to the SR showed values in the micromolar range [27, 34, 43]. We have shown that SR Ca2+ depletion is unlikely, even in response to prolonged depolarization using the biosensor D1ER, which was later confirmed using D4cpv [19, 33]. Using CatchER, we recorded a resting SR Ca2+ concentration of 512 and 573 µM in myofibers from young and old mice, respectively [34]. Some studies have reported an SR Ca2+ concentration of ~1mM in frog muscle fibers [31, 38], while a 3–4-fold lower value (~308µM) was noted using the Ca2+ biosensor D1ER [27]. We found higher basal SR Ca2+ concentration than that reported in mouse tibialis anterior muscle [27]. This difference may be explained by difficulties in measuring D1ER’s Rmax [27] or saturation due to multiple binding sites, as its reported dissociation constant is ~60µM [18].
Indirect observations suggest that SR Ca2+ depletion does not limit its release in muscle fibers from aging mice [11]; however, no direct measure of residual Ca2+ content after prolonged depolarization was reported. That SR Ca2+ release decreases with aging would account for increased residual SR Ca2+. Measurements of the residual [Ca2+]/resting [Ca2+] relationship required the development of a low-affinity biosensor targeted to the SR. Calibrations in FDB fiber showed that CatchER’s Kd is 1.66mM, which make it an excellent instrument to address this question.
Recently, SR Ca2+ concentration, fractional Ca2+ release, and resting SR Ca2+ concentration were recorded in FDB fibers from young-adult mice using the synthetic Ca2+ indicator Fluo-5N [43]. In contrast with the results reported here, the authors reported 88% SR Ca2+ depletion in response to 10s/50Hz tetanic stimulation. Although they used long pulses, the reported level of depletion is larger than that recorded with D1ER or CatchER; luminal Ca2+ depletion seems to plateau when measured with these biosensors, possibly due to an equilibrium in SR Ca2+ release and uptake. The Fluo-5N SR-loading method allowed the first quantitative estimates of static content and dynamic changes in SR Ca2+ concentration, but the prolonged dye-loading process limits its use in physiological recordings.
We demonstrated previously that resting SR Ca2+ concentration does not differ in fibers from young and old mice; however, SR residual Ca2+ was not quantified in old mice. Here, for the first time, we report that residual Ca2+ increases with aging, which fully supports our theory that SR Ca2+ availability does not limit SR Ca2+ release.
Residual SR Ca2+ after prolonged myofiber depolarization
We demonstrated previously using confocal microscopy that CatchER expresses in the myofiber SR [34]. Here, we show that blocking RyR1 results in complete ablation of CatchER’s fluorescence signal. We rule out the possibility that CatchER is located in the cytosol because, in addition to the morphological evidence, basal fluorescence is intense, which indicates that the biosensor is exposed to high Ca2+ concentration. Thus, exposed to high Ca2+ concentrations, CatchER signal decreases in response to sarcolemmal depolarization. In contrast, the cytosolic compartment of FDB fibers is strongly buffered, and basal free Ca2+ is below 100nM [12], preventing significant CatchER fluorescence under resting conditions.
Whether SR Ca2+ recovery in response to fiber excitation fully represents SR Ca2+ uptake from the cytosol, or the signal is influenced by Ca2+ redistribution between bound and unbound conformations of the biosensor, calsequestrin’s buffer capacity, or Ca2+ diffusion from the longitudinal to the terminal cisternae SR is not known. CatchER measurements in a calsequestrin knockout mouse model together with a more refined optical detector, such as a two-photon or a spot scanning confocal microscope [6], in stretched muscle fibers might answer these questions. Previous studies assessed SR Ca2+ in amphibian and mammalian skeletal and cardiac muscle SR [12, 14, 23, 27, 29]. The use of cameleon Ca2+ sensors represented an advance over previous methods. First, they can be selectively targeted to intracellular organelles in intact cells or tissues in vivo or in vitro. Second, their ratiometric property minimizes confounding factors, such as mechanical artifacts and probe concentration.
In this work, we found a significant residual SR Ca2+ after prolonged myofiber depolarization, and we recorded a larger SR Ca2+ depletion in myofibers from young compared to old mice. Instead of single pulse, we used a more physiological repetitive stimulation. A train of pulses was sustained until CatchER’s signal reached a plateau. Under these experimental conditions, a more significant residual Ca2+ was recorded in the SR from old mice. Residual SR Ca2+ was quantified based on converting CatchER’s signal into Ca2+ concentration according to published methods [34, 43]. These experiments support the conclusion that CatchER is suitable for measuring Ca2+ in a high concentration cellular environment; that residual SR Ca2+ concentration is significant beyond physiological demands; and that it is higher in myofibers from old compared to young adult mice. CatchER’s relatively low affinity measured in mouse FDB myofibers [34] makes it appropriate for detecting SR Ca2+ concentration and dynamics. The myofiber sustains SR release in response to repetitive stimulation probably due to efficient SR Ca2+ pump-mediated Ca2+ uptake. Differences in residual SR Ca2+ in response to sarcolemmal depolarization in myofibers from young and old mice reflect excitation-contraction uncoupling, the molecular substrate of which is the age-dependent decline in Cav1.1 expression and a larger number of uncoupled RyR1 [25, 39]. We propose that releasing a larger residual Ca2+ pool in myofibers by increasing Cav1.1 expression will improve myofiber specific force and power with aging.
ACKNOWLEDGMENTS
The present study was supported by grants from the National Institutes of Health/National Institute on Aging AG07157, AG33385, and AG15820 to Osvaldo Delbono; and EB007268 and GM081749 to Jenny Yang, and the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332).
Abbreviations
- ER/SR
endoplasmic/sarcoplasmic reticulum
- EGFP
enhanced green fluorescence protein
- FDB
flexor digitorum brevis
- RyR1
ryanodine receptor-isoform-1
- 4-CmC
4-Chloro-m-cresol
- TEA
tetraethylammonium
- di-8-ANEPPS
di-8-amino naphthyl ethenyl pyridinium
- CPA
cyclopiazonic acid
- CatchER
calcium sensor for detecting high concentrations in the ER
- GECI
genetically encoded Ca2+ biosensor
REFERENCES
- 1.Canato M, Scorzeto M, Giacomello M, Protasi F, Reggiani C, Stienen GJ. Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc Natl Acad Sci U S A. 2010;107:22326–22331. doi: 10.1073/pnas.1009168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conti A, Gorza L, Sorrentino V. Differential distribution of ryanodine receptor type 3 (RyR3) gene product in mammalian skeletal muscles. Biochem J. 1996;316(Pt 1):19–23. doi: 10.1042/bj3160019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delbono O. Excitation-Contraction Coupling Regulation in Aging Skeletal Muscle. In: Lynch GS, editor. Sarcopenia-Age-Related Muscle Wasting and Weakness. Mechanisms and Treatment. Victoria: Springer; 2011. pp. 113–134. [Google Scholar]
- 4.Demaurex N, Frieden M. Measurements of the free luminal ER Ca(2+) concentration with targeted "cameleon" fluorescent proteins. Cell Calcium. 2003;34:109–119. doi: 10.1016/s0143-4160(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 5.DiFranco M, Neco P, Capote J, Meera P, Vergara JL. Quantitative evaluation of mammalian skeletal muscle as a heterologous protein expression system. Protein Expr Purif. 2006;47:281–288. doi: 10.1016/j.pep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 6.DiFranco M, Quinonez M, DiGregorio DA, Kim AM, Pacheco R, Vergara JL. Inverted double-gap isolation chamber for high-resolution calcium fluorimetry in skeletal muscle fibers. Pflugers Arch. 1999;438:412–418. doi: 10.1007/s004240050929. [DOI] [PubMed] [Google Scholar]
- 7.Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493(Pt 2):357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez A, Rios E. Perchlorate enhances transmission in skeletal muscle excitation-contraction coupling. J Gen Physiol. 1993;102:373–421. doi: 10.1085/jgp.102.3.373. DOI Electronic Resource Number. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 10.Hidalgo C, Donoso P, Rodriguez PH. Protons induce calsequestrin conformational changes. Biophys J. 1996;71:2130–2137. doi: 10.1016/S0006-3495(96)79413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez-Moreno R, Wang ZM, Gerring R, Delbono O. Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys J. 2008;94:3178–3188. doi: 10.1529/biophysj.107.118786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jimenez-Moreno R, Wang ZM, Messi ML, Delbono O. Sarcoplasmic reticulum Ca2+ depletion in adult skeletal muscle fibres measured with the biosensor D1ER. Pflugers Arch. 2010;459:725–735. doi: 10.1007/s00424-009-0778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones LM, Yang W, Maniccia AW, Harrison A, van der Merwe PA, Yang JJ. Rational design of a novel calcium-binding site adjacent to the ligand-binding site on CD2 increases its CD48 affinity. Protein Sci. 2008;17:439–449. doi: 10.1110/ps.073328208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabbara AA, Allen DG. Measurement of sarcoplasmic reticulum Ca2+ content in intact amphibian skeletal muscle fibres with 4-chloro-m-cresol. Cell Calcium. 1999;25:227–235. doi: 10.1054/ceca.1999.0023. [DOI] [PubMed] [Google Scholar]
- 15.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 16.Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochimica et Biophysica Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- 17.Owen VJ, Lamb GD, Stephenson DG, Fryer MW. Relationship between depolarization-induced force responses and Ca2+ content in skeletal muscle fibres of rat and toad. J Physiol. 1997;498(Pt 3):571–586. doi: 10.1113/jphysiol.1997.sp021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci U S A. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13:521–530. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Pape PC, Jong DS, Chandler WK. Calcium release and its voltage dependence in frog cut muscle fibers equilibrated with 20 mM EGTA. J Gen Physiol. 1995;106:259–336. doi: 10.1085/jgp.106.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KS, Poburko D, Wollheim CB, Demaurex N. Amiloride derivatives induce apoptosis by depleting ER Ca(2+) stores in vascular endothelial cells. Br J Pharmacol. 2009;156:1296–1304. doi: 10.1111/j.1476-5381.2009.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne AM, Zheng Z, Gonzalez E, Wang ZM, Messi ML, Delbono O. External Ca2+-dependent excitation-contraction coupling in a population of ageing mouse skeletal muscle fibres. Journal of Physiology. 2004;560.1:137–157. doi: 10.1113/jphysiol.2004.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 24.Posterino GS, Lamb GD. Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres. J Physiol. 2003;551:219–237. doi: 10.1113/jphysiol.2003.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renganathan M, Messi ML, Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. Journal of Membrane Biology. 1997;157:247–253. doi: 10.1007/s002329900233. [DOI] [PubMed] [Google Scholar]
- 26.Rossi D, Sorrentino V. Molecular genetics of ryanodine receptors Ca2+-release channels. Cell Calcium. 2002;32:307–319. doi: 10.1016/s0143416002001987. [DOI] [PubMed] [Google Scholar]
- 27.Rudolf R, Magalhaes PJ, Pozzan T. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J Cell Biol. 2006;173:187–193. doi: 10.1083/jcb.200601160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudolf R, Mongillo M, Rizzuto R, Pozzan T. Looking forward to seeing calcium. Nat Rev Mol Cell Biol. 2003;4:579–586. doi: 10.1038/nrm1153. [DOI] [PubMed] [Google Scholar]
- 29.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 30.Shirokova N, Garcia J, Pizarro G, Rios E. Ca2+ release from the sarcoplasmic reticulum compared in amphibian and mammalian skeletal muscle. J Gen Physiol. 1996;107:1–18. doi: 10.1085/jgp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somlyo AV, Gonzalez-Serratos HG, Shuman H, McClellan G, Somlyo AP. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981;90:577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sztretye M, Yi J, Figueroa L, Zhou J, Royer L, Rios E. D4cpv-calsequestrin: a sensitive ratiometric biosensor accurately targeted to the calcium store of skeletal muscle. J Gen Physiol. 2011;138:211–229. doi: 10.1085/jgp.201010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sztretye M, Yi J, Figueroa L, Zhou J, Royer L, Allen P, Brum G, Rios E. Measurement of RyR permeability reveals a role of calsequestrin in termination of SR Ca(2+) release in skeletal muscle. J Gen Physiol. 2011;138:231–247. doi: 10.1085/jgp.201010592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang S, Wong H-C, Wang ZM, Huang Y, Zou J, Zhuo Y, Pennati A, Gadda G, Delbono O, Yang JJ. Design and Application of a Class of Sensors to Monitor Ca2+ Dynamics in High Ca2+ Concentration Cellular Compartments. Proceedings of the National Academy of Sciences 2011 Sep 27. 2011 doi: 10.1073/pnas.1103015108. Epub 2011 Sep 13.: 16265-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor JR, Zheng Z, Wang ZM, Payne AM, Messi ML, Delbono O. Increased CaVbeta1A expression with aging contributes to skeletal muscle weakness. Aging Cell. 2009;8:584–594. doi: 10.1111/j.1474-9726.2009.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treves S, Vukcevic M, Maj M, Thurnheer R, Mosca B, Zorzato F. Minor sarcoplasmic reticulum membrane components that modulate excitation-contraction coupling in striated muscles. J Physiol. 2009;587:3071–3079. doi: 10.1113/jphysiol.2009.171876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ursu D, Schuhmeier RP, Melzer W. Voltage-controlled Ca2+ release and entry flux in isolated adult muscle fibres of the mouse. J Physiol. 2005;562:347–365. doi: 10.1113/jphysiol.2004.073882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volpe P, Simon BJ. The bulk of Ca2+ released to the myoplasm is free in the sarcoplasmic reticulum and does not unbind from calsequestrin. FEBS Lett. 1991;278:274–278. doi: 10.1016/0014-5793(91)80134-o. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z-M, Messi ML, Delbono O. L-type Ca2+ channel charge movement and intracellular Ca2+ in skeletal muscle fibers from aging mice. Biophysical Journal. 2000;78:1947–1954. doi: 10.1016/S0006-3495(00)76742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang ZM, Messi ML, Delbono O. Patch-clamp recording of charge movement, Ca2+ current and Ca2+ transients in adult skeletal muscle fibers. Biophysical Journal. 1999;77:2709–2716. doi: 10.1016/s0006-3495(99)77104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods CE, Novo D, DiFranco M, Capote J, Vergara JL. Propagation in the transverse tubular system and voltage dependence of calcium release in normal and mdx mouse muscle fibres. J Physiol. 2005;568:867–880. doi: 10.1113/jphysiol.2005.089318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, Jaggar JH. IP3 Constricts Cerebral Arteries via IP3 Receptor-Mediated TRPC3 Channel Activation and Independently of Sarcoplasmic Reticulum Ca2+ Release. Circ Res. 2008;102:1118–1126. doi: 10.1161/CIRCRESAHA.108.173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziman AP, Ward CW, Rodney GG, Lederer WJ, Bloch RJ. Quantitative measurement of Ca(2)(+) in the sarcoplasmic reticulum lumen of mammalian skeletal muscle. Biophys J. 2010;99:2705–2714. doi: 10.1016/j.bpj.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou J, Hofer AM, Lurtz MM, Gadda G, Ellis AL, Chen N, Huang Y, Holder A, Ye Y, Louis CF, Welshhans K, Rehder V, Yang JJ. Developing sensors for real-time measurement of high Ca2+ concentrations. Biochemistry. 2007;46:12275–12288. doi: 10.1021/bi7007307. [DOI] [PubMed] [Google Scholar]