Abstract
Background
Recent studies have shown that human copper transporter 1 (hCtr1), the major copper influx transporter, is involved in the transport of platinum-based antitumor agents. We investigated the predictive and prognostic values of hCtr1, and cooper efflux transporters ATP7A and ATP7B, in patients with locally advanced non-small cell lung cancer (NSCLC) receiving first-line platinum-based chemotherapy.
Methods
From 2004 to 2009, we identified 54 consecutive stage III NSCLC patients who underwent first-line platinum-based doublet chemotherapy. Immunohistochemical studies of hCtr1, ATP7A and ATP7B on the paraffin-embedded pre-treatment tumor samples were performed and correlated with chemotherapy response and survival.
Results
Overexpression of hCtr1, ATP7A and ATP7B were observed in 68%, 48% and 74% of the participants, respectively. hCtr1 overexpression was associated with better chemotherapy responses (P < 0.01); whereas ATP7A and ATP7B were not. Patients with hCtr1 overexpressing tumors had better progression-free survival (PFS) and overall survival (OS) (P = 0.01 and 0.047, respectively). In multivariate analyses for chemotherapy response and PFS, only hCtr1 overexpression emerged as a favorable independent predictive and prognostic factor (all P < 0.01).
Conclusion
This is the first report to state that hCtr1 is not only an independent predictor of platinum-based chemotherapy response but also a prognostic factor in stage III NSCLC.
Keywords: Human copper transporter 1, Cisplatin, ATP7A, ATP7B, Non-small cell lung cancer, Prognosis
1. Introduction
Lung cancer is the leading cause of cancer-related death in both men and women worldwide. Approximately 80–85% of all lung cancers are non-small-cell lung cancer (NSCLC) [1]. The improvement of treatment strategies for NSCLC is of great interest for both patients and treating physicians. Although surgical resection is the optimal treatment, the majority of patients have disease that are not resectable as a result of their extent or superimposed medical illnesses [1]. Patients will generally require systemic chemotherapy in both the adjuvant and palliative settings [2].
Platinum-based antitumor agents, especially cisplatin, have been recognized as an important antitumor agent in lung cancer [3]. The combination of two cytotoxic drugs, a platinum and a non-platinum agent, is the standard first-line treatment of patients with advanced NSCLC [4]. This treatment yields response rates of approximately 25–30% and a median overall survival of 9–11 months [5,6]. However, many patients eventually relapse and develop resistance to the treatment. It is well known that cisplatin acts an multiple cellular targets representing diverse signal transduction pathways [7]. It is therefore conceivable that multiple mechanisms have been proposed for cisplatin resistance [7,8].
One mechanism associated with resistance to cisplatin is reduced intracellular accumulation owing to impaired drug intake, enhanced outward transport, or both. Mechanisms for transporting cisplatin were not known until recent demonstrations that import and export transporters involved in maintenance of copper homeostasis are also involved in the transport of these drugs [9]. Human copper transporter 1 (hCtr1), the major copper influx transporter, has been convincingly demonstrated to transport cisplatin and its analogues [10]. Although expression of hCtr1 is ubiquitous because all the tissues require copper, studies showed that expression levels of hCtr1 were highly variable among normal tissues and also human malignancies [11]. It has been reported that the regulation of hCtr1 expression by Cu is controlled at both transcriptional [12] and post-translational levels [13] such as enhanced hCtr1 degradation by endocytosis-mediated protein degradation; whereas only posttranslational control of hCtr1 expression by cisplatin has been reported [14]. Studies also suggested that the two copper efflux transporters ATP7A and ATP7B regulate the efflux of cisplatin [9]. The expressions of ATP7B [15,16] and hCtr1 [17] in the gynecologic cancers have been investigated and the results suggested that their expressions are associated with chemo-sensitivities in platinum-based therapy. However, the clinical significance of these transporters in lung cancer is still unknown. In this study, we investigated the expressions of hCtr1, ATP7A and ATP7B, in patients with advanced non-small cell lung cancer (NSCLC), and assessed the predictive and prognostic values of these transporters to first-line platinum-based doublet chemotherapy.
2. Patients and methods
2.1. Patients
Between 2004 and June 2009, we identified 54 consecutive patients with histologically proven stage IIIA to IIIB NSCLC according to the American Joint Cancer Committee (AJCC) 2002 criteria at the National Cheng Kung University Hospital in Tainan, Taiwan. Twenty-eight patients had tumor samples from bronchoscopic or thoracoscopic biopsies. The remaining 26 patients received computerized tomography (CT)-guided core biopsies of their primary tumors. Treatment decisions were made by an institutional tumor board consisting of thoracic surgeons, medical oncologists, and radiation oncologists. Patients were eligible for the study according to the following criteria: they were not indicated for definitive chemoradiotherapy with either borderline surgically respectable tumors or malignant pleural effusion, they had previously untreated stage IIIA/IIIB diseases, they received at least 2 courses of platinum-based doublet but not single or triplet chemotherapy on an intent-to-treat basis, they had measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) [18], and their paraffin-embedded pre-treatment tumor blocks were available for this study. Exclusion criteria were patients who had previously known history of cancer, or patients who had no subseauent clinical follow-up data.
All patient data, including staging information, treatment, and outcome variables, were collected retrospectively. The protocol for chart and radiography review and archived tumor specimen use was approved by the institutional review board at the National Cheng Kung University Hospital.
2.2. Chemotherapy
All patients were treated with first-line platinum-based doublet chemotherapy. The regimens of chemotherapy were cisplatin 60 mg/m2 or carboplatin at a dose calculated to produce an area under the serum concentration–time curve of 6.0 min mg/mL, and a non-platinum agent, repeated every 3 weeks. Each treatment was repeated for two or more cycles, unless the patient met the criteria for progressive disease (PD) or experienced unacceptable toxicity. Chemotherapy dosage was modified by toxicities in subsequent courses.
2.3. Therapeutic efficacy assessment
Computerized tomography (CT) scanning of the chest including liver was performed before and every 3 months during treatment until completion of chemotherapy, and every 3–6 months thereafter until disease progression or the initiation of subsequent anticancer therapy. Chest X-rays were repeated monthly between each chest CT scan evaluation for the assessment of disease progression. At the clinicians' discretion, radiological tumor assessment could be repeated early on the basis of clinical need or suspicion of disease progression. The assessment strategies were the same for those who completed therapy and those who did not. The sum of maximal diameters of all measurable tumor lesions was recorded at baseline and after treatment. These measurements were used to calculate the largest percentage of shrinkage or smallest percentage of growth using the baseline assessment as reference. The best response to therapy was categorized using RECIST 1.0 criteria [18].
2.4. Immunohistochemistry
Immunohistochemistry was performed on 4-μm-thick tissue sections prepared from a representative paraffin block of each patient's tumor. After blocking endogenous peroxidase activity, the sections were subjected to heat-induced antigen retrieval using an autoclave, and then incubated overnight at 4 °C with primary antibody for hCtr1 (Novus Biologicals, Littleton, CO) at a dilution of 1:500, ATP7A (Abcam, Cambridge, UK) and ATP7B (Lifespan Biosciences, Seattle, WA) at a dilution of 1:100. Detection was done using a biotin-labeled secondary antibody and ABC complex (LSAB kit; DAKO, Carpinteria, CA) with diaminobenzidine substrate (DAB) as the chromogen. Finally, the sections were lightly counterstained with hematoxylin. Positive and negative controls were included in all runs. Two independent (JJY and WCC) performed the analysis of immunohistochemistry and they were blinded to the clinical characteristics and outcomes.
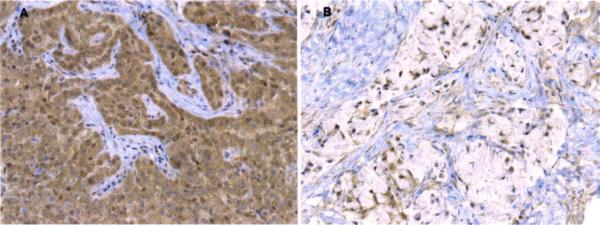
Each slide was evaluated using light microscopy and the staining was scored semiquantitatively by assessing the intensity (on a 0–3 sca1e)and by estimating the percentage of positive cytoplasmic or membranous staining cells (on a 1–4 scale: 1.1–25% staining; 2, 26–50% staining; 3, 51–75% staining; or 4, >75% staining). Weakly positive cytoplasmic staining (intensity score 1) was observed in some of the pneumocytes and bronchiolar epithelial cells adjacent to tumors. With respect to both intensity and frequency, overexpression was defined as tumors with diffuse cytoplasmic staining of moderate/strong intensity (≥25% cells and intensity score ≥2) in this study [11,19]. Fig. 1 shows representative cases of over- and low-expressions of hCtr1 in tumors.
Fig. 1.
Immunohistochemical stains for hCtr1 in representive non-small-cell lung carcinomas. (A) Carcinoma cells showing htCr1 overexpression manifested by diffuse cytoplasmic staining of strong intensity (200×). (B) Carcinoma cells showing low hCtr1 immunostaining activity (200×).
2.5. Statistical analysis
The primary goal of this study was to determine if these copper transporters protein levels were predictive of the best tumor response. Associations between clinical characteristics and immunostaining results were evaluated by chi-square or Fisher's exact tests. Response rates were compared using Fisher's exact test. Progression-free survival (PFS) was calculated as the time interval between the dates of pathological diagnosis and disease progression or death, whichever occurred first. Overall survival (OS) was calculated as the time interval between the dates of pathological diagnosis and death or last follow-up. Survival curves for PFS and OS were constructed using the Kaplan–Meier method, and log-rank tests were carried out to evaluate differences between groups. Univariate logistic regression analyses followed by multiple logistic regression analyses were applied to evaluate the role of immunostainings and clinicopathological parameters as predictors of treatment response. Associations between potential predictors and survival were evaluated by Cox proportional hazards models, using univariate analyses followed by multivariate analyses. All statistical tests were performed using StatView software (Version 5.0, SAS Institute Inc., Cary, NC, USA) at the two-tailed significance level of 0.05.
3. Results
3.1. Patients and treatment
The median follow-up time was 31.8 months for the living patients (range, 17.6–82.3 months). The patients were 36–76 years old (median: 59 years) at diagnosis. There were 33 male and 21 female patients. Nine patients had stage IIIA disease, and 45 patients had stage IIIB. There were 42 adenocarcinoma, 10 squamous cell carcinoma, one large cell tumor, and one unspecified non-small-cell tumor. All patients received cisplatin/carboplatin-containing doublets chemotherapy. Thirty patients were treated with gemcitabine and cisplatin (55.6%). Twenty patients received docetaxel and cisplatin (37%). One patient was treated with vinorelbine and cisplatin, one with paclitaxel and cisplatin, and two with paclitaxel and carboplatin. The courses of chemotherapy ranged from 2 to 6, with a median of 3. The best measurable response rates at the end of chemotherapy by CT scanning were 50% in 27 patients. Ten patients had stable disease and 17 patients had progressive disease.
3.2. Predictive impact of hCtr1, ATP7A and ATP7B immunostainings on measurable tumor response
Overexpression of hCtr1, ATP7A and ATP7B immunostainings was observed in 68%, 48% and 74% of the participants, respectively. There were no significant associations between expressions of hCtr1, ATP7A and ATP7E (all P>0.05). None of these copper transporters expressions had significant associations with gender, age, histological type, tumor size, nodal status or stage of disease (Table 1). hCtr1 overexpression was significantly associated with better chemotherapeutic responses, whereas the expressions of ATP7A and ATP7B were not. Among the 27 patients with partial responses of chemotherapy, 23 (85%) had overexpressed hCtr1 immunostainings. While among the 17 patients with progressive disease after chemotherapy, only 6 (35%) patients had overexpressing hCtr1 immunostainings (P<0.01). The statuses of hCtr1, ATP7A and ATP7B immunostainings in relation to histopathological and clinical data are summarized in Table 1.
Table 1.
Characteristics of patients with stage III non-small-cell lung cancer (NSCLC) receiving first-line CDDP-based chemotherapy and expressions of tumor hCtr1, ATP7A and ATP7B.
| Characteristics | All patients | hCtr1 |
ATP7A |
ATP7B |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Over-expression | Low-expression | P | Over-expression | Low-expression | P | Over-expression | Low-expression | P | ||
| All patients | 54 | 37 | 17 | 26 | 28 | 40 | 14 | |||
| Age in years | ||||||||||
| ≤50 | 11 | 8 | 3 | 0.74 | 4 | 7 | 0.38 | 8 | 3 | 0.91 |
| >50 | 43 | 29 | 14 | 22 | 21 | 32 | 11 | |||
| Gender | ||||||||||
| Male | 33 | 20 | 13 | 0.12 | 13 | 20 | 0.11 | 23 | 10 | 0.36 |
| Female | 21 | 17 | 4 | 13 | 8 | 17 | 4 | |||
| Histology | ||||||||||
| Adeno Ca | 42 | 31 | 11 | 0.29 | 20 | 22 | 0.99 | 33 | 9 | 0.13 |
| Squamous Ca | 10 | 5 | 5 | 5 | 5 | 5 | 5 | |||
| Others | 2 | 1 | 1 | 1 | 1 | 2 | 0 | |||
| T stage | ||||||||||
| T1 | 2 | 2 | 0 | 0.71 | 0 | 2 | 0.36 | 2 | 0 | 0.33 |
| T2 | 9 | 6 | 3 | 6 | 3 | 6 | 3 | |||
| T3 | 5 | 4 | 1 | 2 | 3 | 5 | 0 | |||
| T4 | 38 | 25 | 13 | 17 | 20 | 27 | 11 | |||
| N stage | ||||||||||
| N0 | 14 | 10 | 4 | 0.33 | 6 | 8 | 0.73 | 10 | 4 | 0.53 |
| N2 | 22 | 17 | 5 | 12 | 10 | 18 | 4 | |||
| N3 | 18 | 10 | 8 | 8 | 10 | 12 | 6 | |||
| Clinical stage | ||||||||||
| IIIA | 9 | 7 | 2 | 0.51 | 4 | 5 | 0.81 | 7 | 2 | 0.78 |
| IIIB | 45 | 30 | 15 | 22 | 23 | 33 | 12 | |||
| Best measurable response | ||||||||||
| CR | 0 | 0 | 0 | <0.01 | 0 | 0 | 0.11 | 0 | 0 | 0.44 |
| PR | 27 | 23 | 4 | 14 | 13 | 22 | 5 | |||
| SD | 10 | 8 | 2 | 7 | 3 | 7 | 3 | |||
| PD | 17 | 6 | 11 | 5 | 12 | 11 | 6 | |||
CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease.
In univariate logistic regression analysis, hCtr1 overexpression was an independent predictor of platinum-based chemotherapy treatment response (odds ratio [OR], 5.34; 95% CI, 1.45–19.65; P=0.01); but neither ATP7A nor ATWB was. Multivariate logistic regression analyses also showed that hCtr1 overexpression was an independent predictor of platinum-based chemotherapy treatment response (OR, 11.26; 95% CI, 1.92–66.02; P<0.01) (Table 2). Expressions of ATP7A and ATP7B were not significant predictors of chemotherapy response in multivariate analyses either.
Table 2.
Logistic regression of hCtr1 immunostaining, and clinicopathological parameters as predictive factors of cisplatin-based chemotherapy response.
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| hCtr1 (over-vs. low-expression) | 5.34 (1.45–19.65) | 0.01 | 11.26 (1.92–66.02) | <0.01 |
| ATP7A (over- vs. low-expression) | 1.35 (0.46–3.93) | 0.59 | 1.09 (0.30–3.99) | 0.89 |
| ATP7B (over- vs. low-expression) | 2.20 (0.63–7.74) | 0.22 | 2.24 (0.47–10.58) | 0.31 |
| Sex (mate vs. female) | 1.17 (0.39–3.50) | 0.78 | 1.51 (0.36–6.30) | 0.57 |
| Age (years) (≥50 vs. <50) | 2.01 (0.51–7.90) | 0.32 | 1.83 (0.38–9.50) | 0.47 |
| Histology (adenocarcinoma vs. others) | 0.41 (0.11–1.59) | 0.20 | 0.16 (0.02–1.10) | 0.07 |
| Stage (IIIB vs. IIIA) | 1.31 (031–5.51) | 0.72 | 2.59 (0.45–14.93) | 0.29 |
OR: odds ratio; CI: confidence interval.
3.3. Impact of hCtr1, ATP7A and ATP7B immunostainings on survival
The PFS and OS at 1 year for all patients were 22.2% and 74.1%, respectively. Overall median PFS and OS were 7.7 and 25.3 months for hCtr1 overexpression and 5.3 and 14.9 months for hCtr1 low expression patients, respectively. The 1-year PFS was 27% for hCtr1 overexpression patients, compared to 11.8% for hCtr1 low expression patients (P=0.01) (Fig. 2A). The 1-year and 3-year OS were 81.1% and 38.2% for hCtr1 overexpression patients, compared to 58.8% and 17.6% for hCtr1 low expression patients (P=0.047) (Fig. 3A). No association between ATP7A or ATP7B expression and PFS or OS (all P>0.05) was found (Figs. 2B, C and 3B, C).
Fig. 2.
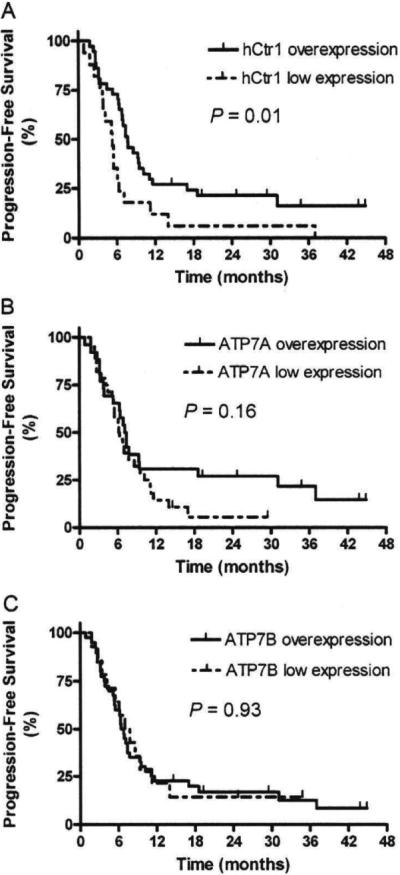
Progression-free survival (PFS) curves in non-small-cell Lung carcinoma patients with hCtr1 overexpression tumors and hCtr1 low expression tumors (A). ATP7A overexpression tumors and ATP7A low expression tumors (B). ATP7B overexpression tumors and ATP7B low expression tumors (C).
Fig. 3.
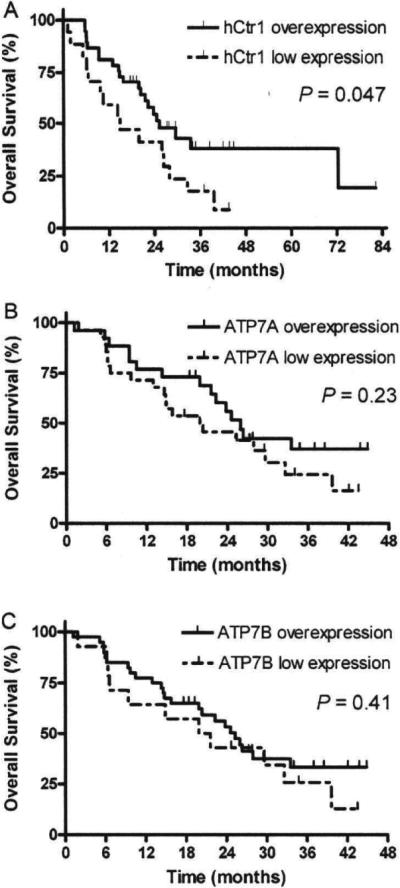
Overall survival (OS) curves in non-small-cell lung carcinoma patients with hCtr1 overexpression tumors and hCtr1 low expression tumors (A), ATP7A overexpression tumors and ATP7A low expression tumors (B). ATP7B overexpression tumors and ATP7B low expression tumors (C).
In the univariate Cox proportional hazard model, hCtr1 overexpression was a significant prognostic factor influencing PFS (hazard ratio [HR], 0.48; 95% CI, 0.26–0.87; P=0.02) (Table 3). However, neither ATP7A nor ATP7B expression was a significant factor. To further evaluate the independent parameters such as patient characteristics, tumor factors, and expression profiles of hCtr1, ATP7A and ATP7B on PFS, a multivariate Cox proportional hazards regression analysis was performed. This analysis included those factors from univariate analysis. As a consequence, expression statuses of hCtr1, ATP7A and ATP7B, gender, age (≥50 vs. <50), histology (adenocarcinoma vs. others) and stage IIIB vs. IIIA were all included as covariates in the Cox praportional hazard model. We found that in the multivariate Cox model, hCtr1 overexpression (HR, 0.37; 95% CI, 0.18–0.77; P<0.01) was the only significant prognostic factor of PFS after controlling for gender, age, histology, stage of disease, and expression statuses of ATP7A and ATP7B (Table 3). Furthermore, we conducted a stepwise analysis and found that hCtr1 overexpression was the only significant prognostic factor influencing PFS in all steps and the only variable left in the final model. The HRs associated with hCtr1 overexpression remained quite stable throughout the process.
Table 3.
Cox proportional hazard model analysis of potential prognostic factors influencing progression-free survival (PFS).
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| hCtrl (over- vs. low-expression) | 0.48 (0.26–0.87) | 0.02 | 0.37 (0.18–0.77) | <0.01 |
| ATP7A (over- vs. low-expression) | 0.65 (0.36–1.19) | 0.17 | 0.73 (038–1.41) | 0.35 |
| ATP7B (over- vs. low-expression) | 0.97 (0.50–1.88) | 0.93 | 1.43 (0.67–3.07) | 0.36 |
| Sex (male vs. female) | 1.17 (0.65–2.13) | 0.60 | 0.99 (0.51–1.95) | 0.99 |
| Age (years) (≥50 vs. <50) | 0.57 (0.28–1.17) | 0.12 | 0.61 (0.28–1.35) | 0.22 |
| Histology (adenocarcinoma vs. others) | 1.52 (0.75–3.08) | 0.25 | 1.68 (0.75–3.74) | 0.21 |
| Stage (IIIB vs. IIIA) | 1.06 (0.47–2.38) | 0.89 | 0.91 (0.37–2.21) | 0.83 |
HR: hazard ratio: CI: confidence interval.
4. Discussion
The present study provides the protein expression analyses of the copper influx transporter, hCtr1, and the efflux transporters, ATP7A and ATP7B, in NSCLC patients receiving first-line platinum-based doublet chemotherapy by immunohistochemical stainings. These transporters not only control the acquisition and elimination of copper ion, but also for the platinum-based antitumor agents. These transporters together regulate the overall intracellular steady-state cisplatin levels. It has been documented that intracellular cisplatin level is an important determinant for cell killing activities of cisplatin [20]. Thus, determining the expression levels of these transporters in tumor cells may provide important insights into drug resistance mechanism in cisplatin-based cancer chemotherapy. We investigated the expression of these transporters in lung cancer and found significant hCtr1 and ATP7B overexpression in 37 (68%) and 40 (74%), respectively, out of 54 consecutive stage III NSCLC patients who underwent first-line platinum-based doublet chemotherapy. In contrast, we found much less elevated expression of ATP7A (48%) in this cohort. Our results show that overexpression of hCtr1 was associated with better chemotherapy responses, but ATP7A and ATP7B were not. Patients with hCtr1-overexpressing tumors also had better PFS and OS. This is the first report that hCtr1 is not only an independent predictor for good response of platinum-based chemotherapy but also a significant prognostic factor for PFS and OS in stage III NSCLC patients.
Ishida et al. recently reported that low levels of Ctr1 mRNA are associated with poor clinical response to platinum-based therapy in ovarian cancer [17]. In another study, we found that elevated expression of hCtr1 is associated with favorable PFS in local recurrent cervical carcinoma patients who received salvage radiation therapy and cisplatin chemotherapy (HHWC et al., unpublished data). These results, together with those presented in this communication, suggest that hCtr1 expression may have prognostic value for predicting the treatment efficacy in cancer chemotherapy using cisplatin.
Correlations of overexpression of ATP7B with unfavorable clinical outcome in various cancers refractory to cisplatin therapy have been reported [15,16,21]. Human ovarian cancer patients with ATP7B-positive tumors have significantly poorer responses to cisplatin-based chemotherapy than those bearing ATP7B-negative tumors [16]. Moreover, using real-time polymerase chain reaction (PCR) and immunohistochemistry, Nakagawa et al. found ATP7B mRNA and protein expression levels were significantly higher in human NSCLC cisplatin-resistant xenografts than those in the cisplatin-sensitive xenografts [19]. The expression of hCtr1 was not investigated in these studies. Our finding showing that no correlation between ATP7B overexpression and cisplatin response in NSCLC patients differs from those published in these studies.
Despite reports suggesting that ATP7A and ATP7B expressions are associated with cisplatin resistance in clinical samples as mentioned above, however, convincing evidence that these Cu-ATPases are involved in cisplatin resistance remains needed to reconcile with the following observations. (i) It has been observed that no correlation between the levels of ATP7B expression and the resistance of cells to cisplatin in normal cells [22] and in cisplatin-resistant cells [23]. (ii) It was reported that although expressions of ATP7A and ATP7B are elevated in some cisplatin-resistant ovarian cancer cells, however, silencing these transporters by siRNA did not always confer cisplatin sensitivities [24]. (iii) It has been reported that increased expression of these Cu-ATPase transporters does not always reduce intracellular cisplatin contents [25]. Both ATP7A and ATF7B are located at the trans-Golgi network (TGN) to transport copper into the lumen to facilitate efflw of excess Cu by sequestering Cu into exocytic vesicles [26]. It has been proposed that these transporters may sequester and store cisplatin into intracellular vesicles [25]. Therefore, elevated expressions of ATP7A and ATP7B per se do not always result in increased cisplatin resistance. Taken together, our present results and those published in the literature suggest that the roles of ATP7A and ATP7B in clinical cisplatin resistance are complex and may depend on, for example, patient population, tumor types, drug resistance mechanisms, treatment modalities and methods of analyses. More studies are needed to clarify these issues. We note that humans have another CU transporter, hCtr2, which is mainly located in the late endosomal and Iysosomal vesicles [27,28] for Cu storage [29]. While it has been reported that expression of hCtr2 in cultured cells is correlated with cisplatin resistance [30], the clinical significance of hCtr2 in platinum drug cancer chemotherapy is also needed.
From drug development point of view, the demonstration that hCtr1 expression, rather than ATP7A and ATP7B, exhibits prognostic value in cisplatin cancer chemotherapy may provide a rationale for the development of individualized hctr1-based cancer treatment using platinum-based drugs. This study, in combination with the development of hCtr1 modulators may eventually lead to the improvement of treament efficacy of platinum-based cancer chemotherapy. We previously reported that expression of hCtr1 is transcriptionally upregulated under copper depleted conditions [12], and increased expression of physiological copper-depletor, glutathione, upregulates hCtr1 expression thereby enhanced cellular sensitivity to cisplatin toxicity [31]. Although glutathione can interact with platinum, the formation of glutathionated platinum is a very slow process [32,33]. These results provide a mechanistic basis for the development of copper chelators as enhancers for sensitization of cancer cells to cisplatin chemotherapy.
In this regard, the present study using stage III NSCLC patients who were previously untreated and subsequently underwent first-line cisplatin/carboplatin-containing doublets chemotherapy may be of particular value for clinical investigations of the predictive and prognostic value of hCtr1. These patients had relatively good performance status and locally advanced confined in the chest. Response assessments of the change in size of measurable and evaluable lesions after neoadjuvant chemotherapy could be performed objectively without biases. This study may shed a new light in the development of copper chelators as chemosensitizers for treating hCtr1-related platinum-resistant patients.
In conclusion, this is the first report that human copper transporter 1 (hCtr1) is an independent predictor for good responses of cisplatin/carboplatin-based doublet chemotherapy in stage IIIA and IIIB NSCLC patients. Overexpression of hCtr1 is also a prognostic factor for PFS and OS in stage IIIA and IllB NSCLC patients who received platinum-containing chemotherapy.
Acknowledgements
This work was supported by National Cheng Kung University Hospital, Tainan, Taiwan [grant number 9904005]; National Science Council, Taiwan [grant number 97-2314-B-006-043 and 99-2314-B-006-038-MY3]; and Department of Health, Taiwan [grant numbers 99-TD-B-111-002 and 99-TD-B-111-003]. The authors would like to thank the Cancer Registry at the Cancer Cen ter of National Cheng Kung University Hospital, Tainan, Taiwan for providing data.
Footnotes
Conflict of interest statement The authors have declared no conflicts of interest.
References
- [1].Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- [2].Blinman P, Alam M, Duric V, McLichlan SA, Stockler MR. Patient's preferences for chemotherapy in non-small-cell lung cancer: a systematic review. Lung Cancer. 2010;69:141–7. doi: 10.1016/j.lungcan.2010.05.001. [DOI] [PubMed] [Google Scholar]
- [3].Lustberg MB, Edelman MJ. Optimal duration of chemotherapy in advanced non-small cell lung cancer. Curr Treat Options Oncol. 2007;8:38–46. doi: 10.1007/s11864-007-0020-6. [DOI] [PubMed] [Google Scholar]
- [4].NCCN . Non-small cell lung cancer: NCCN clinical practice guidelines in oncology. v.2 2010. [Google Scholar]
- [5].Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- [6].Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer; the TAX 326 study group. J Clin Oncol. 2003;21:3016–24. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- [7].Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- [8].Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- [9].KUO MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The toles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- [10].Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, et al. Role of human copper transporter Ctrl in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther. 2004;3:1543–9. [PubMed] [Google Scholar]
- [11].Ho1zer AK, Varki NM, Le QT, Gibson MA, Naredi P, Howell SB. Expression of the human copper influx transporter 1 in normal and malignant human tissues. J Histochem Cytochem. 2006;54:1041–9. doi: 10.1369/jhc.6A6970.2006. [DOI] [PubMed] [Google Scholar]
- [12].Song IS, Chen HH, Aiba I, Hossain A, Liang ZD, Klomp LW, et al. Transcription factor Sp1 plays an important role in the regulation of copper homeostasis in mammalian cells. Mol Pharmacol. 2008;74:705–13. doi: 10.1124/mol.108.046771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol chem. 2003;278:9639–46. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- [14].Holzer AK, Howell SB. The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res. 2006;66:10944–52. doi: 10.1158/0008-5472.CAN-06-1710. [DOI] [PubMed] [Google Scholar]
- [15].Aida T, Takebayashi Y, Shimizu T, Okamura C, Higasimoto M, Kanzaki A, et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) as a prognostic factor in human endometrial carcinoma. Gynecol Oncol. 2005;97:41–5. doi: 10.1016/j.ygyno.2004.12.042. [DOI] [PubMed] [Google Scholar]
- [16].Nakayama K, Kanzaki A, Terada K, Mutoh M, Ogawa K, Sugiyama T, et al. Prognostic value of the Cu-transporting ATPase in ovarian carcinoma patients receiving cisplatin-based chemotherapy. Clin Cancer Res. 2004;10:2804–11. doi: 10.1158/1078-0432.ccr-03-0454. [DOI] [PubMed] [Google Scholar]
- [17].Ishida S, McCotmick F, Smith-McCune K, Hanahan D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell. 2010;17:574–83. doi: 10.1016/j.ccr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Therasse P, Arhuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstem L, et al. New guidelines to evaluate the response to treahnent in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer lnst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- [19].Nakagawa T, lnoue Y, Kodama H, Yamazalu H, Kawai K, Suemizu H, et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) correlates with cisplatin resistance in human non-small cell lung cancer xenografts. Oncol Rep. 2008;20:265–70. [PubMed] [Google Scholar]
- [20].Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol. 2008;48:495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426. [DOI] [PubMed] [Google Scholar]
- [21].Miyashita H, Nitta Y, Mori S, Kanzaki A, Nakayama K, Terada K, et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) as a chemoresistance marker in human oral squamous cell carcinoma treated with cisplatin. Oral Oncol. 2003;39:157–62. doi: 10.1016/s1368-8375(02)00038-6. [DOI] [PubMed] [Google Scholar]
- [22].Leonbrdt K, Gebhardt R, Mossner J, Lutsenko S, Huster D. Functional interactions of Cu-ATPase ATP7B with cisplatin and the tole of ATP7B in the resistance of cells to the drug. J Biol Chem. 2009;284:7793–802. doi: 10.1074/jbc.M805145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zisowsky J, Koegel S, Leyers S, Devarakonda K, Kassack MU, Osmak M, et al. Relevance of drug uptake and efflux for cisplatin sensitivity of tumor cells. Biochem Pharmacol. 2007;73:298–307. doi: 10.1016/j.bcp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- [24].Mangala LS, Zuzel V, Schmandt R, Leshane ES, Halder JB, Armaiz-Pena GN, et al. Therapeutic targeting of ATP7B In ovarian carcinoma. Clin Cancer Res. 2009;15:3770–80. doi: 10.1158/1078-0432.CCR-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gupta A, Lutsenko S. Human copper transporters: mechanmsm, role in human diseases and theapeutic potential. Future Med Chem. 2009;1:1125–42. doi: 10.4155/fmc.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation Of human copper-transporting ATPases. Physiol Rev. 2007;87:1011–46. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- [27].Bertinato J, Swist E, Plouffe LJ, Brooks SP, L'Abbe MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J. 2008;409:731–40. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- [28].van den Berghe PV, Folmer DE, Malingre HE, van Beurden E, Klomp AE, van der Sluis B, et al. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J. 2007;407:49–59. doi: 10.1042/BJ20070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–85. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- [30].Blair BG, Larson CA, Safaei R, Howell SB. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of cisplatin and carboplatin. Clin Cancer Res. 2009;15:4312–21. doi: 10.1158/1078-0432.CCR-09-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen HH, Song IS, Hossain A, Choi MK, Yamane Y, Liang ZD, et al. Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1. Mol Pharmacol. 2008;74:697–704. doi: 10.1124/mol.108.047969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen HH, Kuo MT. Role of glutathione in the regulation of cisplatin resistance in cancer chemotherapy. Metal-Based Drugs. 2010:1–7. doi: 10.1155/2010/430939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ishikawa T, Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993;268:20116–25. [PubMed] [Google Scholar]