Synopsis
Bitter taste-sensing receptors (T2Rs) are expressed in the oral cavity to prevent ingestion of dietary toxins through taste avoidance. They are also expressed in other cell-types including gut enteroendocrine cells where their physiological role is enigmatic. Previously, we proposed T2R dependent cholecystokinin (CCK) secretion from enteroendocrine cells limits absorption of dietary toxins but an active mechanism was lacking. Here we show T2R signaling activates ATP-binding cassette B1 (ABCB1) in intestinal cells through a CCK signaling mechanism. Phenylthiocarbamide (PTC), an agonist for the T2R38 bitter receptor, increased ABCB1 expression in both intestinal cells and mouse intestine. PTC induction of ABCB1 was decreased by either T2R38 siRNA or treatment with YM022, a gastrin receptor antagonist. Thus, gut ABCB1 is regulated through signaling by CCK/gastrin released in response to PTC stimulation of the T2R38 receptor on enteroendocrine cells. We also show that PTC increases the efflux activity of ABCB1 suggesting T2R signaling limits absorption of bitter tasting/toxic substances through modulation of gut efflux membrane transporters.
Keywords: Bitter taste receptors (T2Rs), ABCB1 Efflux transporter, CCK
Introduction
Because many dietary toxins are sensed by mammals as tasting “bitter”, a taste aversion to bitterness has evolved to prevent the ingestion of potentially toxic substances from the diet. A family of single-exon GPCRs called type 2 receptors (T2Rs) are expressed in taste buds of the tongue and adjacent oral epithelium where they “sense” bitter food components as agonists [1]. The expression of T2Rs has also been localized to the gastric and intestinal mucosa where their physiological function is less clear [2].
T2R signaling in cultured enteroendocrine cells results in secretion of gut polypeptide hormones such as cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1) [3]. Indeed, we have previously shown that T2R expression and signaling in cultured murine enteroendocrine cells and in the mouse intestine are regulated by the cholesterol sensing sterol regulatory element binding protein-2 (SREBP-2) protein which results in cholesterol regulated secretion of CCK [4]. These observations suggested that intestinal T2R activity and CCK secretion are regulated by dietary composition and based on the known actions of CCK in limiting intestinal motility and food intake we proposed that T2R mediated CCK secretion is important in a gut sensing mechanism to help limit the absorption of dietary derived bitter tasting toxins that escape taste aversion in the mouth [4]. We further hypothesized a more direct role for CCK in limiting absorption of bitter tasting toxic-substances through actively limiting their cellular uptake through gut efflux membrane transporters.
Here we report the T2R38 bitter taste receptor is a functional modulator of the ABCB1 efflux transporter in both Caco-2 human intestinal cells and mouse intestine. ABCB1 is a member of the ATP-binding cassette (ABC) transporters and is strategically expressed on the apical membrane of intestinal epithelial cells and is thus positioned in the expected location to limit absorption of toxic substrates contained in food. Known ABCB1 substrates include xenobiotics and many chemotherapeutic agents, [5] and increased ABCB1 activity explains why many cancer cells become resistant to chemotherapy [6].
Our results indicate that T2R signaling in enteroendocrine cells results in the secretion of CCK that stimulates enterocytes to produce more ABCB1. We further show that the T2R38 bitter agonist PTC is a substrate for ABCB1. Taken together, these results suggest a paracrine signaling mechanism where T2R stimulated CCK secretion from enteroendocrine cells increases ABCB1 mediated efflux from the neighboring intestinal epithelial cells.
Experimental
Cells
Caco-2 cells provided from Dr. Said, HM (Irvine, CA, U.S.A.) and AGS-E gastric adenocarcinoma cells (stably expressing the CCK-2 receptor) provided from Dr. Wang, TC (New York, NY, U.S.A.) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 % heat-inactivated fetal calf serum (FCS) and antibiotics in an atmosphere of 5 % CO2 at 37 °C. Puromycin (2 μg/ml) was used for selection in AGS-E cells [7].
Plasmids
The human ABCB1 promoter fragment (−1780 to +119) was amplified by PCR using human genomic DNA as template, and cloned into the pGL3-Basic firefly luciferase reporter vector (Promega Corp). The construct was verified by DNA sequencing.
siRNA Transfection in Caco-2 Cells
The siRNA targeting human T2R38 (GenBank accession number NM_176817) and non-targeting siRNA control were purchased from Dharmacon. The cells were transfected for 48 h with 100 nM of each siRNA by using X-tremeGENE siRNA transfection reagent (Roche) according to the manufacturer’s instructions, and then cells were incubated with PTC (Sigma-Aldrich) for 9 h.
RNA Analysis
Total RNA was extracted from Caco-2 cells and intestine with Trizol (Invitrogen) and QIAGEN RNeasy isolation kits (Qiagen) and used for RT-qPCR with a iQ5 Real-Time PCR Detection System (Bio-Rad). Primer sequences used in this study were shown in Table S1. mRNA levels were normalized for expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA as control and calculated by the comparative threshold cycle method.
Immunoblotting
Cells and intestine homogenates were centrifuged at 1000 g for 5 min at 4°C. Pellets were resuspended in extraction buffer containing 10 mM Tris-HCl at pH 8.6, 0.14 M NaCl, 1.6 mM MgCl2, 1% Nonidet P-40, and protease inhibitor cocktail and incubated on ice 5 min, followed by centrifugation at 20,000 g for 30 min at 4 °C. The supernatants were separated by 6.5 % SDS-PAGE and then transferred to nitrocellulose membranes and subjected to immunoblotting using monoclonal antibodies against ABCB1 (C219, Covance), and β-actin (Sigma-Aldrich). The blots were visualized using ECL detection reagents (GE Healthcare).
CCK Secretion
Cells were rinsed with Hanks’ balanced salt solution (HBSS, Invitrogen) supplemented with 20 mM HEPES and 0.1 % BSA, and 10 mM PTC dissolved in HBSS were then added to the culture. Cells were then incubated at 37 °C for 45 min. The medium was collected and centrifuged at 4 °C for 5 min at 1000 g to remove cell debris and the supernatants were stored at −20 °C. CCK was measured by ELISA (Phoenix Pharmaceuticals, Inc).
Transient Transfection in AGS-E Cells
AGS-E cells (2×105 cells/well) seeded in 24-well plate were transfected with luciferase reporter for human ABCB1 promoter (−1780 to +119) using Lipofectamine 2000 reagent (Invitrogen). A pCMV-β-gal expression construct was included in every transfection as a normalization control. 24 h post-transfection, gastrin (1 μM) was added to the cells. After 3 h, cells were harvested and assayed for luciferase and β-gal activities.
Rhodamine 123 Accumulation
Caco-2 cells (1×106 cells/well) seeded into 6-well plates with sterile coverslips were preincubated for 9 h with 10 mM PTC, and then rhodamine 123 (10 μM) dissolved in HBSS was added to the cells after washing. After incubation for 1 h, the cells were thoroughly washed with HBSS. Coverslips were mounted by VECTASHIELD (Vector laboratories), and images were acquired by an inverted microscope Axioskop using the AxioVision camera and software (Carl Zeiss). Fluorescence intensity of rhodamine 123 in cell lysates was measured at 500 nm (excitation) and 550 nm (emission) using a fluorescence microplate reader Gemini XPS (Molecualr Devices).
Flow Cytometric Analysis
FACS analysis was performed with MDR1 shift assay kit (Millipore). Briefly, cells were incubated with PTC or VIN at 37 °C for 10 min, followed by addition of IgG2a or UIC2 monoclonal ABCB1 antibody. After 15 min, cells were analyzed using a FACSCalibur (BD Biosciences). Flow cytometric data were analyzed with FlowJo software (Tree Star).
Gavage Administration of PTC
Six week old male FVB mice were purchased from Taconic Farms and fed a normal rodent chow diet and allowed to adapt for two weeks to a 12 h light/12 h dark cycle. A mixture of T2R agonists/bitter compounds (10 mM PTC, 10 mM denatoneum benzoate, 1.5 mM quinine, and 5 mM D-[-]salicin) in sterilized distilled water (DW) was administered by oral gavage to mice and DW was delivered to control group. CCK receptor antagonist YM022 (250 μM) was administered by intraperitoneal injection 30 min before oral gavage of bitter compounds mixture. Blood samples were collected from the heart after 1 h gavage and plasma CCK concentration was measured by ELISA (Phoenix Pharmaceuticals Inc.). For RNA and protein isolation, intestines were collected after 16 h gavage.
Statistical Analysis
The data are presented as mean ± SEM. Differences between the means of the individual groups were assessed by 1-way ANOVA with Dunnet’s multiple comparison test and Student’s t-test; differences were considered significant at p-value < 0.05. The statistical software package, Prism 5.0 (GraphPad Software), was used for these analyses.
Results and Discussion
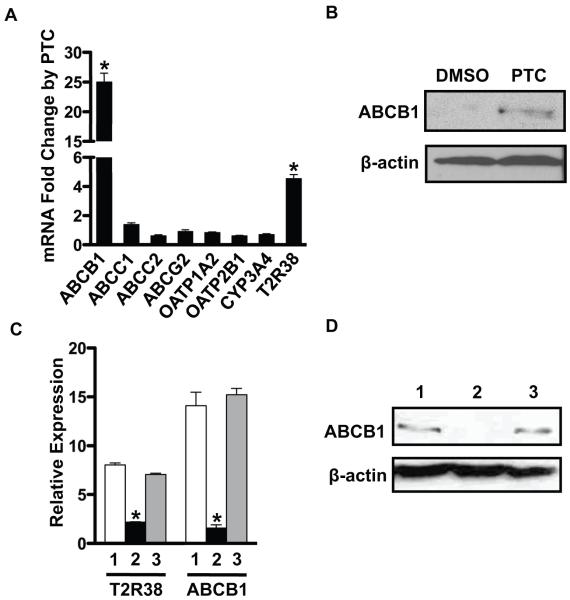
Although previous studies suggest bitter taste receptor signaling in the gut may limit absorption of bitter (and potentially toxic) dietary constituents, a mechanism for this protective response was lacking. We hypothesized that it might involve membrane transporters that either limit influx or enhance efflux of dietary toxins. To evaluate this, we first surveyed several intestinal derived cell lines and showed that the human epithelial colorectal cell line Caco-2 expresses both bitter taste receptors and several intestinal efflux transporters. Therefore, we treated Caco-2 cells with PTC, a bitter tastant that is an agonist for the T2R38 receptor [8]. Both the efflux transporter ABCB1 mRNA and protein levels were dramatically elevated by addition of PTC in the Caco-2 cells. (Figure 1A and 1B). ABCB1, a member of the ABC type membrane transporters, is well known to limit the absorption of xenobiotics [9] and expression of other ABC efflux transporters known to be expressed in the intestine including ABCC1 and ABCG2 were not affected by PTC (Figure 1A). There are two possible explanations for these findings: ABCB1 gene expression is activated through xenobiotic agonists for the pregnane X receptor (PXR) nuclear receptor or T2R38 signaling. However, it is unlikely that the PTC induction of ABCB1 is through PXR because CYP3A4, another PXR target gene involved in xenobiotic detoxification [10], was not induced. In contrast, PTC induction of ABCB1 is likely through bitter taste receptor signaling in Caco-2 cells because induction was prevented when cells were pre-treated with an siRNA against the T2R38 receptor (Figure 1C and 1D). In addition, Caco-2 cells expressed calcium signaling components (α-gustducin, PLCβ2, and TRPM5) (Supplementary Figure S1).
Figure 1. ABCB1 is induced by PTC through T2R38 in Caco-2 cells.
(A) Caco-2 cells were incubated with PTC (10 mM) for 9 h, and mRNA levels for intestinal transporters and T2R38 were analyzed by RT-qPCR. (B) ABCB1 protein was analyzed by immunoblotting. (C and D) Caco-2 cells were transfected with a siRNA targeting human T2R38 or negative control siRNA (100 nM). 48 h post-transfection, cells were treated with PTC for 9 h. (C) mRNA levels of T2R38 and ABCB1 were analyzed by RT-qPCR. (D) ABCB1 protein was analyzed by immunoblotting. Data are mean ± SEM, n=3 for three separate experiments. *p-value < 0.05. OATP, organic anion-transporting polypeptide; 1, control; 2, siRNA T2R38; 3, siRNA negative control.
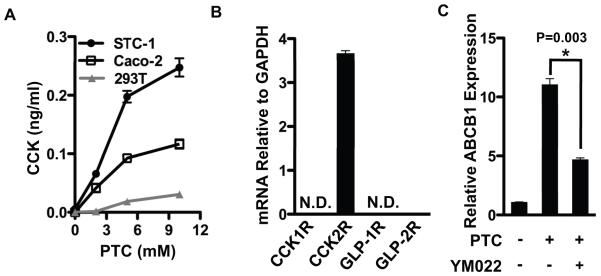
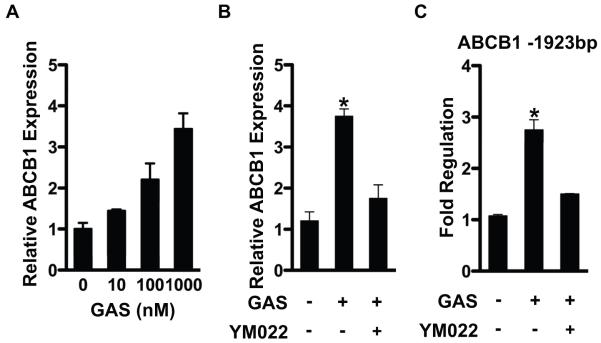
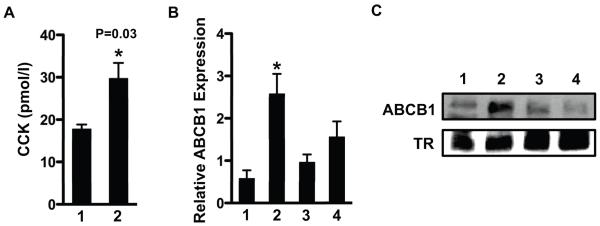
Because bitter agonist signaling increases intracellular Ca2+ levels and stimulates CCK and GLP-1 secretion from enteroendocrine cells [3, 4] and intestinal efflux transporters are expressed in the enterocytes [11, 12], we hypothesized that gut peptides secreted by bitter taste receptor signaling might regulate ABCB1 through a paracrine mechanism involving induction of a either CCK or GLP. This hypothesis predicts that Caco-2 is a heterogenous cell line with some cells that secrete gut peptides in response to bitter agonist signaling and some cells that express the functional peptide receptor. To address this, we first showed that CCK secretion from Caco-2 cells was stimulated by PTC treatment (Figure 2A). STC-1 and 293-T cells were analyzed as positive and negative controls respectively [3]. This result suggests Caco-2 cells possess at least some properties attributed to enteroendocrine cells and in support of this hypothesis we showed that Caco-2 also expresses chromogranin A, a well-established marker for enteroendocrine cells [13] (Supplementary Figure S2). We also analyzed expression of mRNAs encoding known receptors for both CCK and GLP in Caco2 cells by RT-qPCR. mRNA for the CCK2 receptor (CCK2R) was expressed in Caco-2 cells whereas mRNAs for other receptors were at near background levels (Figure 2B). Thus, Caco2 cells might secrete and respond to a CCK2 receptor agonist such as CCK itself or the related peptide hormone gastrin [14]. To determine whether PTC activation of ABCB1 might be mediated by CCK2R signaling, we analyzed the effects of YM022, a CCK2R antagonist on the PTC dependent induction of ABCB1. This inhibitor blunted the induction of ABCB1 by PTC (Figure 2C). Moreover, we demonstrated that gastrin directly induced expression of ABCB1 mRNA through its transcriptional activation (Figure 3). In order to determine whether ABCB1 is also regulated by T2R signaling in vivo, plasma CCK level and intestinal ABCB1 expression was measured after delivery of bitter compounds mixture (BCM) by oral gavage. Delivery of the BCM significantly increased plasma levels of CCK as well as intestinal expression of ABCB1 mRNA and protein (Figure 4). Importantly, inclusion of the CCK receptor antagonist YM022 completely blocked the induction of intestinal ABCB1 expression by BCM. Taken together, these observations suggest that the intestinal efflux transporter ABCB1 is regulated by bitter tasting agonists through a signaling mechanism mediated by CCK release from enteroendocrine cells in response to T2R38 signaling.
Figure 2. PTC induction of ABCB1 is mediated by CCK2R in Caco-2 cells.
(A) Three cell lines were treated with different concentrations of PTC for 45 min at 37 °C. The media was collected and analyzed for CCK by EIA assay. (B) mRNA level for receptors of CCKs and GLPs in Caco-2 cells was analyzed by RT-qPCR. (C) Caco-2 cells were pretreated with CCK2R antagonist, YM022 (10 μM) for 2 h, and then PTC was added to the cultured cells for 9 h. mRNA level for ABCB1 was analyzed by RT-qPCR. Data are mean ± SEM, n=3 for three separate experiments.
Figure 3. Gastrin induces ABCB1 through its promoter activation.
(A) Caco-2 cells were incubated with different concentration of gastrin as indicated in figure. (B) Caco-2 cells were incubated with gastrin (1 μM) in the presence or absence of YM022 (10 μM), and mRNA level for ABCB1 was analyzed by RT-qPCR. (C) AGS-E cells were transfected with -1923 ABCB1 reporter, and then gastrin (1 μM) was added to the cells in the presence or absence of YM022. Luciferase activities were normalized to β-gal activity. Data are mean ± SEM, n=3 for three separate experiments. *p-value < 0.05. GAS, gastrin.
Figure 4. Bitter agonists increase plasma CCK level and ABCB1 in mouse intestine.
Bitter compounds mixture (10 mM PTC, 10 mM denatoneum benzoate, 1.5 mM quinine, and 5 mM D-[-]salicin) was introduced to FVB mice through oral gavage. CCK receptor antagonist YM022 (250 μM) was administered 30 min before oral gavage of bitter compounds mixture. After 1 h gavage plasma CCK concentration was measured by EIA assay (A). Intestine was collected 16 h later, and both mRNA and protein levels for ABCB1 was analyzed by RT-qPCR (B) and immunoblotting (C), respectively. Immunoblotting data are shown for 3 individual mice of each group. Data are mean ± SEM, n=4 for three separate experiments. 1, control; 2, bitter compounds mixture; 3, YM022; 4, YM022 + bitter compounds mixture; TR, transferrin receptor.
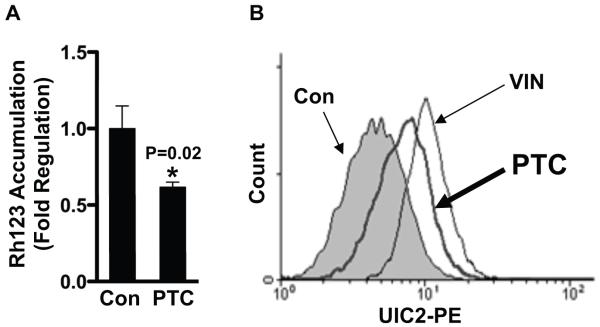
If T2R signaling increases the functional activity of the ABCB1 transporter we reasoned that Caco-2 cells treated with PTC would accumulate less intracellular rhodamine 123 which is a fluorescent dye and is a known efflux substrate for ABCB1 [15]. Consistent with this prediction, PTC addition to Caco-2 cells significantly decreased rhodamine 123 accumulation (Figure 5A and Supplementary Figure S3). Next, we sought to determine whether PTC is also a substrate for ABCB1. We treated cells with the UIC2 antibody which only interacts with ABCB1 when it is in the ATP bound active conformation [16]. The results of the FACS assay in Figure 5B show that ABCB1 shifts into its active conformation when cells are treated with either vinblastine, a known substrate for ABCB1 [17], or PTC. Thus, PTC is a functional modulator of ABCB1 activity in vivo.
Figure 5. PTC decreases rhodamine 123 accumulation and is a substrate of ABCB1.
(A) PTC-treated cells were exposed with rhodamine 123 for 1 h, and fluorescence intensity in cell lysates was analyzed by microplate reader. (B) Cells were incubated with UIC2 antibody in the presence and absence (filled histogram) of PTC (thick line) or VIN (dotted line) as described in Materials and Methods. UIC2 reactivity for ABCB1 was analyzed by flow cytometry. Rh123, rhodamine 123; Con, control; VIN, vinblastine.
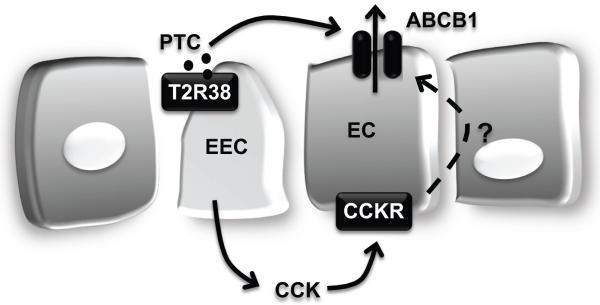
Our results indicate that T2Rs and ABCB1 play important interconnected roles in intestinal host defense: potentially toxic bitter-food substances are “sensed” by T2Rs expressed in enteroendocrine cells, which secrete CCK in response. Then, CCK acts on enterocytes to limit absorption of the dietary derived T2R agonist by increasing ABCB1 efflux activity. It is likely that a similar protection is afforded to limit many other orally ingested drug/toxic substances that are both agonists for other T2Rs and substrates for ABCB1 (Figure 6).
Figure 6. Model of intestinal host defense system by bitter taste receptor and efflux transporter.
Toxic substance (i.e. PTC) activates bitter taste receptor, T2R38 leading to release of gut hormones. CCK released from the basal surface of the enteroendocrine cells, increases through paracrine signaling expression of efflux transporter, ABCB1 in the enterocytes, resulting in rapid excretion of bitter or dietary toxin. EEC, enteroendocrine cells; EC, enterocytes.
Acknowledgements
We thank Helen Hobbs and Jonathan Cohen for helpful suggestions.
Funding
This work was supported in part by grants from the NIH to T.O. (HL48044, DK71021)
Abbreviations footnote
- ABCB1
ATP-binding cassette B1
- CCK
cholecystokinin
- CCKR
cholecystokinin receptor
- GLP-1
glucagon-like peptide-1
- PLCβ2
phospholipase C, beta 2
- PTC
phenylthiocarbamide
- T2Rs
bitter taste-sensing receptors
- TRPM5
transient receptor potential cation channel subfamily M member 5
References
- 1.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 2.Hofer D, Drenckhahn D. Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol. 1998;110:303–309. doi: 10.1007/s004180050292. [DOI] [PubMed] [Google Scholar]
- 3.Chen MC, Wu SV, Reeve JR, Jr., Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol. 2006;291:C726–739. doi: 10.1152/ajpcell.00003.2006. [DOI] [PubMed] [Google Scholar]
- 4.Jeon TI, Zhu B, Larson JL, Osborne TF. SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J Clin Invest. 2008;118:3693–3700. doi: 10.1172/JCI36461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 6.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 7.Tu S, Chi AL, Lim S, Cui G, Dubeykovskaya Z, Ai W, Fleming JV, Takaishi S, Wang TC. Gastrin regulates the TFF2 promoter through gastrin-responsive cis-acting elements and multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1726–1737. doi: 10.1152/ajpgi.00348.2006. [DOI] [PubMed] [Google Scholar]
- 8.Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221, 1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 9.Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sueyoshi T, Negishi M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol. 2001;41:123–143. doi: 10.1146/annurev.pharmtox.41.1.123. [DOI] [PubMed] [Google Scholar]
- 11.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, Kim RB. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–370. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 13.Facer P, Bishop AE, Lloyd RV, Wilson BS, Hennessy RJ, Polak JM. Chromogranin: a newly recognized marker for endocrine cells of the human gastrointestinal tract. Gastroenterology. 1985;89:1366–1373. doi: 10.1016/0016-5085(85)90657-2. [DOI] [PubMed] [Google Scholar]
- 14.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 15.Yumoto R, Murakami T, Nakamoto Y, Hasegawa R, Nagai J, Takano M. Transport of rhodamine 123, a P-glycoprotein substrate, across rat intestine and Caco-2 cell monolayers in the presence of cytochrome P-450 3A-related compounds. J Pharmacol Exp Ther. 1999;289:149–155. [PubMed] [Google Scholar]
- 16.Maki N, Hafkemeyer P, Dey S. Allosteric modulation of human P-glycoprotein. Inhibition of transport by preventing substrate translocation and dissociation. J Biol Chem. 2003;278:18132–18139. doi: 10.1074/jbc.M210413200. [DOI] [PubMed] [Google Scholar]
- 17.Ueda K, Cardarelli C, Gottesman MM, Pastan I. Expression of a full-length cDNA for the human “MDR1” gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987;84:3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]