Abstract
Objective
The objective of this pilot study was to evaluate possible differences in insulin sensitivity, food intake, and cravings between the follicular and luteal phases of the menstrual cycle in women with premenstrual syndrome (PMS).
Methods
Subjects were screened for PMS using the Penn Daily Symptom Rating (DSR) scale. Each subject had two overnight admissions (once in each cycle phase) to the Hospital of the University of Pennsylvania. They performed 3-day diet histories prior to each hospitalization. After admission, subjects received dinner and a snack, then were fasted until morning, when they underwent a frequently sampled intravenous glucose tolerance test (FSIGT). Insulin sensitivity was determined by Minimal Model analysis. Blinded analysis of diet histories and inpatient food intake was performed by a registered dietitian.
Results
There was no difference found in insulin sensitivity between cycle phases (n = 7). There were also no differences in proportions of macronutrients or total kilocalories by cycle phase, despite a marked difference in food cravings between cycle phase, with increased food cravings noted in the luteal phase (p = 0.002). Total DSR symptom scores decreased from a mean of 186 (± 29.0) in the luteal phase to 16.6 (± 14.2) in the follicular phase. Women in this study consumed relatively high proportions of carbohydrates (55%–64%) in both cycle phases measured.
Conclusions
These findings reinforce the suggestion that although the symptom complaints of PMS are primarily confined to the luteal phase, the neuroendocrine background for this disorder may be consistent across menstrual cycle phases.
INTRODUCTION
Premenstrual syndrome (PMS) is a disorder with a broad array of physiological and psychological symptoms that can include food cravings. As there is no definitive test for identifying PMS, a diagnosis relies on the perception of symptoms by the woman who experiences them.1–4 These symptoms must occur in a cyclical pattern, increasing during the luteal (postovulatory) phase and abating in the follicular phase of the menstrual cycle. There is some controversy as to whether these symptoms are merely an exacerbation of luteal phase symptoms that are typical for most women or there is something inherently different about women with PMS.5 The precise etiology of PMS is uncertain, but it is suspected that it is related to dysregulation of the serotonergic system.6 In fact, a more severe form of PMS, premenstrual dysphoric disorder (PMDD), is often successfully treated with serotonergic reuptake inhibitors.7,8 The goal of PMS treatment is the effective management of symptoms so that they do not interfere with a woman's social and work-related functioning.
Food cravings and binge eating of specific food items are reported more frequently by women in the luteal phase.9–11 Many10–19 but not all20–22 studies of food intake have shown increased caloric intake in the luteal phase, although only a few of these studies have controlled for PMS.16,17,19,22 When reported, the specific macronutrient composition of the increased calories consumed in the luteal phase varies but most often results from either increased fat13,16,19 or carbohydrate intake.16,17,19 Based on animal studies showing that carbohydrate ingestion increases brain serotonin levels,23,24 some investigators have suggested that in an attempt to relieve symptoms, women with PMS unconsciously self-medicate by increasing their carbohydrate intake.25
The reported effects of dietary carbohydrates on increasing brain levels of serotonin are dependent on the mechanism regulating amino acid transport across the blood-brain barrier (BBB). As the rate-limiting enzyme for serotonin synthesis is unsaturated at physiological concentrations of tryptophan, increasing levels of brain tryptophan will increase brain serotonin.24 For entry into the brain, tryptophan must compete for transport with other large neutral amino acids. Following carbohydrate ingestion, insulin is released and stimulates the uptake of competing branched-chain amino acids (leucine, isoleucine, and valine) into muscle. Consequently, circulating competing amino acids are lowered and, therefore, allow more tryptophan to be transported into the brain. The physiological relevance of this mechanism in humans is uncertain, as even small amounts of concurrent protein ingestion have been shown to inhibit this effect.26 Despite the limited human data demonstrating that dietary carbohydrate intake can increase functional active serotonin,26 however, the question of menstrual cycle-related increases in carbohydrate consumption in women is still an area of great controversy.
As the effect of dietary carbohydrates on brain serotonin levels is partially dependent on insulin, changes in the efficacy of insulin to promote nutrient uptake have the potential to influence brain tryptophan and brain serotonin levels. Insulin sensitivity may exhibit menstrual cycle-related changes in women with PMS,27–29 as decreased insulin sensitivity has been reported during the luteal phase of the menstrual cycle in healthy women. In susceptible women, a decline in insulin sensitivity could be a contributing factor to PMS symptomatology, particularly food cravings and macronutrient selection. Menstrual cycle changes in insulin sensitivity have not been investigated in women with PMS using standardized and sensitive methods. The aims of this pilot study were to determine if differences exist in insulin sensitivity, nutrient intake, and food cravings between the two phases of the menstrual cycle in nondiabetic women with PMS. It was hypothesized that there would be a decrease in insulin sensitivity and an increase in carbohydrate intake and food cravings in the luteal phase of the menstrual cycle in women with PMS.
MATERIALS AND METHODS
Subjects
A convenience sample of regularly menstruating adult women (aged 18–45) was recruited primarily through newspaper advertisements. Excluded from the study were women on any form of hormonal contraception or replacement therapy or current use of triptan medications, selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOs), or other antidepressant medications during the previous 2 months. Also excluded from the study were women who significantly altered their activity levels on a daily basis or women with chronic metabolic or psychiatric disease. Women were excluded from the study if they were pregnant, planning to become pregnant, or lactating. Screening history and physical examinations were performed by the nurse practitioner of the General Clinical Research Center (GCRC) at the Hospital of the University of Pennsylvania (HUP) to ensure that participants were in general good health. This study was approved by the Institutional Review Board at the University of Pennsylvania, and all subjects gave their written informed consent prior to active participation in the study.
Clinical procedures
Once subjects were accepted into the study, PMS status was confirmed by 2 months’ prospective symptom charting with the use of the Penn Daily Symptom Rating scale (DSR), a reliable and valid tool for this purpose.30 Subjects were required for two consecutive cycles to have at least a 30% increase in total symptoms reported in the luteal phase vs. the follicular phase, with a minimum luteal phase score of 80 for confirmation of PMS.
Subjects were scheduled for two overnight hospital admissions to the GCRC at the HUP, once in the follicular phase and once in the luteal phase. Each subject served as her own control, as all measurements were obtained under both cycle phase conditions. Depending on the subject's menstrual cycle pattern, overall schedule, and admission availability at the GCRC, the first admission could be either a follicular or luteal phase admission. Follicular phase was determined by the presence of menstrual bleeding, and follicular phase admissions were scheduled during days 4–8, with the onset of menses designated as day 1. Luteal phase determination was established with the use of a urinary luteinizing hormone (LH) surge detection kit (Carter-Wallace, Inc., New York, NY). Subjects were instructed in the use of the LH surge detection kits and told to call the investigator with the LH peak. Luteal phase admissions were scheduled to coincide with approximately day 7–9 after the LH peak was observed. Cycle phase was confirmed with plasma assays of estradiol and progesterone.
For each admission, subjects arrived at the GCRC in the evening and had dinner and an 8:00pm snack. Subjects were then fasted until the following morning. At 8:00 am, a frequently sampled intravenous glucose tolerance test (FSIGT) was administered to evaluate insulin sensitivity. After completion of the FSIGT and a meal, subjects were allowed to leave the hospital.
Frequently sampled intravenous glucose tolerance test (FSIGT)
Insulin sensitivity was determined using the FSIGT, a standardized methodology31,32 that has been described extensively in other studies.32–34
On the morning of the test (after an overnight fast), venipuncture was performed, and catheters were inserted into the antecubital veins of each arm for intravenous (i.v.) solution infusion and for obtaining blood specimens from the contralateral arm. The blood sampling catheter was kept patent by a slow infusion of saline, and the other catheter was kept patent with a saline flush. Subjects sat quietly for 30–60 minutes after insertion to acclimate to the insertion of the catheters. Baseline blood specimens for estradiol, progesterone, cortisol, epinephrine, norepinephrine, glucose, and insulin were obtained 30–60 minutes after i.v. placement. Glucose and insulin samples for the FSIGT were collected at t = –5 and t = 0. At t = 0, an i.v. injection of glucose (0.3 g/kg body weight as a 50% solution in water) was given over a 1-minute period. At t = 20 minutes, insulin was injected as an i.v. bolus (0.03 U/kg). Blood samples were obtained at t = 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 18, 20, 22, 25, 30, 40, 50, 70, 100, 140, and 180 minutes after the glucose injection. The protease inhibitors trasylol and leupeptin were added to the samples immediately postcollection. Samples were placed in a cold centrifuge, and the resultant plasma was aliquoted and frozen at –80° C within 1 hour of collection. Whole blood glucose measurements were also obtained at bedside as needed (at the beginning and end of the test and additionally, if clinically indicated).
Biochemical analysis procedures
Plasma was used for the measurement of estradiol, progesterone, glucose, insulin, cortisol, epinephrine, and norepinephrine. Plasma estradiol, progesterone, cortisol, and insulin were measured by radioimmunoassays at the Penn Diabetes Endocrinology Research Center. Epinephrine and norepinephrine were measured at the Core Lab of the GCRC. Plasma glucose was measured in duplicate by the glucose oxidase method using an automated glucose analyzer (YSI 2300, Yellow Springs Instruments, Yellow Springs, OH) at the Monell Chemical Senses Center.
Dietary analysis
Two approaches were taken to evaluate dietary intake and macronutrient selection during the two phases of the menstrual cycle. The first was to conduct a prospective 3-day diet history (in which subjects were asked to write down everything that they ate and drank) in the 3 full days immediately prior to each hospital admission. The second approach was to evaluate dietary intake during each subject's stay in the hospital. This period included 2 meals (dinner and snack in the evening before the FSIGT and a meal following the FSIGT). Subjects were not aware that their food intake was being monitored during their hospital stay. All research participants received three times the normal portion sizes of each item they had selected from a menu on the day of admission so that they would be able to eat to satiety. Subjects were able to select from a wide variety of foods that were freshly prepared in the GCRC's special metabolic kitchen. Food intake was determined by precise measurements of the weight of all food offered to the subjects and then, following ingestion, weighing of any leftover portions. Analysis of total calories and the macronutrient composition of the diets (carbohydrate, protein, and fat) for the 3-day prospective diet histories and the inpatient stay were analyzed by the registered dietitian of the GCRC using ESHA Food Processor for Windows, version 8.22 (Salem, OR). The dietitian was blinded to the menstrual cycle phase in which measurements were obtained. Individual results were provided to the principal investigator for group analysis.
Data and statistical analysis
Insulin and glucose values obtained during the FSIGT were entered into the MINMOD (Millenium Edition) computer program, which models insulin-dependent glucose use and determines a variety of parameters, including insulin sensitivity (SI, a measure of insulin's ability to stimulate glucose uptake into tissue), glucose effectiveness (SG, the ability of glucose to stimulate its own transport into tissue), acute insulin response to glucose (AIRg), and the product of AIRg and SI, termed the disposition index. All other statistical analyses were performed using the Statistica (Tulsa, OK) and Origin (Northampton, MA) computer software programs. Comparison of mean SI, SG, AIRg, disposition index, hormone assay results, proportions of macronutrients, and DSR scores by cycle phase were done with two-tailed, Student's paired t tests. The association of variables was also evaluated with Pearson's product-moment correlation, yielding a product-moment correlation coefficient. For all results, statistically significant differences were accepted as p < 0.05.
RESULTS
Subject characteristics
A total of 9 subjects with verified PMS completed the study. One subject's data were excluded from the final data analysis because of a faulty i.v. line during the FSIGT, and another subject was excluded because of a follicular phase SI value that was > 2 standard deviations (SD) from the mean of the rest of the group. Therefore, the reported results for the FSIGT data and the biochemical data are based on a total of 7 completed subjects. Table 1 describes the characteristics of these 7 subjects. For the nutritional analyses, 1 subject's inpatient nutritional data was lost (her food tray had been inadvertently disposed of by hospital staff before her food intake could be measured). Therefore, for nutritional data, the total n = 6. All results reported as means include ± SD.
Table 1.
Characteristics of Study Participants, n = 7
| Measure | Mean | Range | SD |
|---|---|---|---|
| Age, years | 25 | 18–31 | 4.5 |
| BMI, kg/m2 | 24.7 | 18–34.9 | 5.4 |
| No. of cycle day, Fa admission | 6.3 | 4–8 | 1.9 |
| No. of days post-LH surge, L admission | 7.3 | 4–10 | 1.9 |
| Estradiol, F, pg/mL | 53.7 | 33.3–92.9 | 21.1 |
| Estradiol, L, pg/mL | 116.5 | 27–189 | 60.5 |
| Prog, F, ng/mL | 0.2 | 0.15–0.4 | 0.08 |
| Prog, L, ng/mL | 13.8 | 6.1–22.7 | 5.7 |
F, follicular phase; L, luteal phase; Prog, progesterone.
Penn DSR
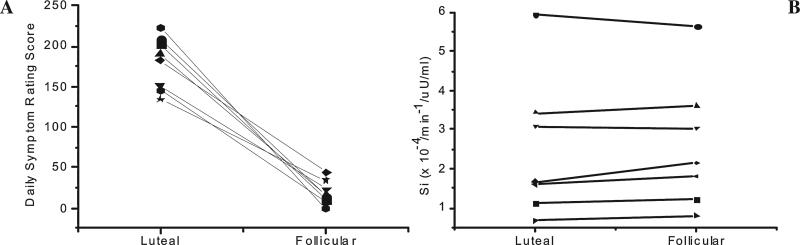
Mean premenstrual (luteal phase) score on the Penn DSR was 186 (±29.0), and the mean post-menstrual (follicular phase) score was 16.6 (±14.2). Individual DSR scores by cycle phase are shown in Figure 1. The one DSR symptom item of “craving foods, increased appetite, overeating” received a mean score of 14.2 (±5.4) in the luteal phase and decreased to a mean score of 0.3 (±0.5) in the follicular phase. Accurate cycle phase determination was confirmed by estradiol and progesterone assay results (Table 1).
FIG. 1.
Decrease in individual daily symptom rating (DSR) scores during the follicular phase of the menstrual cycle compared with the luteal phase (A). Individual insulin sensitivity values (SI) derived from the FSIGT during follicular and luteal phases (B). Each symbol designates an individual subject.
FSIGT and endocrine
Mean SI in the follicular phase was 2.6 (±1.65) × 10–4/min–1/μU/mL vs. a mean SI of 2.5 (±1.8) × 10–4/min–1/μU/mL in the luteal phase (p = 0.27). No significant differences were found in insulin sensitivity between the two phases of the menstrual cycle. Figure 1 illustrates individual subject SI values by cycle phase. There were also no statistically significant differences with other Minimal Model parameters (data not shown), such as SG, AIRg, and the disposition index. Notably, mean SI for subjects with body mass index (BMI) < 25 was 3.3 × 10–4/min–1/μU/mL in the follicular phase and 3.16 × 10–4/min–1/μU/mL in the luteal phase (n = 4), and mean SI for subjects with BMI > 25 was 1.69 × 10–4/min–1/μU/mL in the follicular phase and 1.63 × 10–4/min–1/μU/mL in the luteal phase (n = 3). Although not statistically significant, the decreased SI across both cycle phases in subjects with increased BMI was expected, as overweight and obesity are well-known risk factors for insulin resistance.35
Mean baseline plasma cortisol was 17.45 μg/dL (±4.3) in the follicular phase and 16.7 μg/dL (±4.6 in the luteal phase (p = 0.48). Mean plasma epinephrine levels were 39.9 pg/mL (±8.8) in the follicular phase and 38.0 pg/mL (±10.2) in the luteal phase (p = 0.77). Norepinephine levels were 94.28 pg/mL (±52.9) in the follicular phase and 96.89 (±36.2) in the luteal phase (p = 0.9).
Caloric and nutrient intake
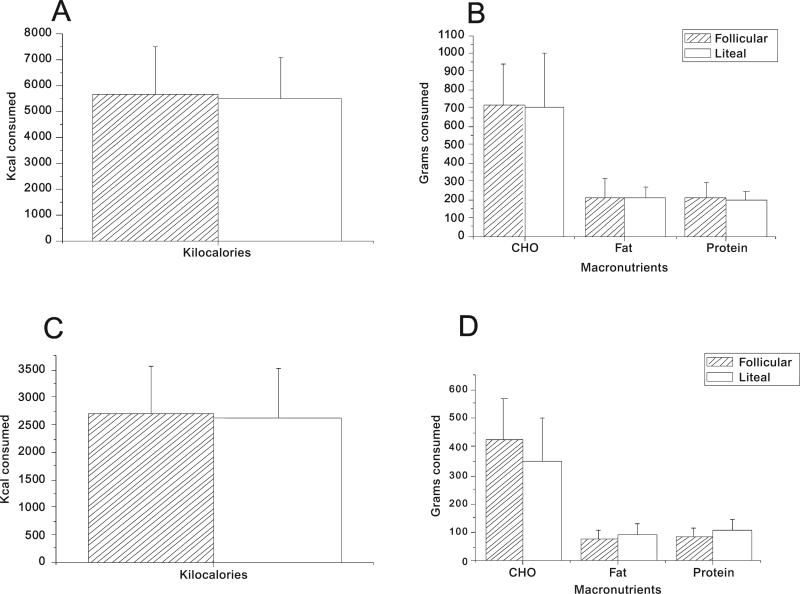
There was a significant correlation of dietary recall information of proportions of macronutrients from the prospectively recorded 3-day diet history with the actual measured inpatient intake of proportions of macronutrients during the hospital admission (R = 0.96, p = 0.002). There were no significant differences noted in mean carbohydrate, fat, protein, or calorie intake by cycle phase on analysis of subject dietary recall or with actual patient intake during hospital admissions (Fig. 2).
FIG. 2.
Mean (±SD) kilocalorie intake (A) and macronutrient intake (B) derived from 3-day diet records prior to hospital admission. Mean kilocalorie intake (C) and macronutrient intake (D) during the inpatient hospital stay. No significant differences were found between the two phases of the menstrual cycle.
Diet history information revealed that carbohydrate intake constituted 55% of total calories in the follicular phase vs. 56% in the luteal phase. Fat intake constituted 32% of total calories in the follicular phase vs. 31.6% in the luteal phase. Protein intake constituted 14% of total calories in the follicular phase vs. 13.4% in the luteal phase.
Analysis of inpatient nutrient intake for the two meals and one snack revealed that subjects consumed a mean of 473 g (±118.3) of carbohydrates in the follicular phase, constituting 64% of total caloric intake, compared with 327 g (±163.3) of carbohydrates in the luteal phase, constituting 55% of total caloric intake in the luteal phase (p = 0.08). There was a mean consumption of 78 g (±25.9) of fat in the follicular phase, constituting 23.7% of total caloric intake, as compared with 79 g (±19.4) of fat in the luteal phase, constituting 29% of total caloric intake in the luteal phase (p = 0.9). Mean protein consumption was 93 g (±23.7) in the follicular phase, constituting 12.6% of total caloric intake, compared with 102 g (±35.5) of protein in the luteal phase, constituting 17% of total caloric intake in the luteal phase (p = 0.4).
DISCUSSION
This small pilot study aimed to investigate, for the first time, differences in insulin sensitivity by menstrual cycle phase in women with PMS. As women with PMS have marked affective and behavioral symptomatology in the luteal phase, we had hypothesized that women with PMS would exhibit luteal phase differences in their insulin sensitivity and endocrine responses to the glucose challenge of the FSIGT. Despite careful documentation and demonstration of phase-dependent symptomatology, we found no significant differences in insulin sensitivity and endocrine responses between the luteal and follicular phases.
Previous studies have reported differences in insulin sensitivity during the menstrual cycle in nondiabetic women using the same methodology as the present study. However, these studies did not screen for the presence or absence of PMS, and it is not known if the absence of this variable contributed to the reported differences in insulin sensitivity. Valdes and Elkind-Hirsh28 found significant differences in insulin sensitivity between the follicular and luteal phases in healthy women (not screened for PMS) with a sample size of n = 8. In that study, there was a mean SI of 6.20 × 10–4/min–1/μU/mL in the follicular phase compared with a mean SI of 3.20 × 10–4/min–1/μU/mL in the luteal phase (p = 0.007). Our laboratory has also examined the effect of the menstrual cycle on insulin sensitivity in a small group of type 1 diabetic women documented not to have PMS, and in this group, 3 of 5 subjects exhibited a decline in insulin sensitivity during the luteal phase.36 Thus, it is notable that in the present study, absolutely no change in insulin sensitivity between menstrual cycle phases was observed even on an individual level in women with PMS. It is possible that the unidentified variables that contribute to changes in insulin sensitivity during the menstrual cycle may be static, that is, either constantly elevated or constantly depressed in women with PMS. As would be expected, overweight women (BMI> 25) exhibited a trend toward decreased insulin sensitivity compared with women of normal weight (BMI < 25), supporting the well-described inverse relationship between body adiposity and insulin sensitivity.35 The documentation of a decrease in SI in the over-weight women and the small intraindividual variability of SI measured in the participants indicate the reliability of the FSIGT results of this study.
In contrast to several prior studies reporting increased carbohydrate intake by women in the luteal phase,16,17,19 we found no significant differences in kilocalorie or carbohydrate intake either during the 3-day dietary recall or during the hospital stay. What was notable about the group of women with PMS in this study was that they consumed a relatively high proportion of dietary carbohydrates in both phases of the menstrual cycle (between 55% and 64%). In several previous studies12,13,22 where macronutrients as a percentage of total caloric intake by menstrual cycle phase were analyzed, the ranges of carbohydrate intake were typically lower than the percentages found in this study. Johnson et al.13 found that carbohydrate intake as a percentage of total energy intake remained relatively constant over the phases of the menstrual cycle in food records of women (n = 26) who were not screened for PMS, with carbohydrate intake varying from 47.5% to 47.9%. In contrast, using a 24-hour dietary recall method, Dalvit-McPhillips12 found that subjects without PMS consumed 40%–55% of their calories as carbohydrates in the follicular phase, but there was a significant increase to 60%–80% carbohydrates as the portion of total caloric intake in the luteal phase. Our results support the recent findings of Bryant et al.,22 who found no differences in total energy or carbohydrate consumption between the follicular and luteal phases in a group of women with documented PMS who prospectively recorded 3-day diet histories.
Assessment of dietary intake is fraught with limitations and difficulties. Although monitoring of food intake in a laboratory setting provides precision and accuracy, the type and quantity of food ingested may not reflect normal intake. Alternatively, evaluation of food intake in a natural setting is associated with imprecision and dependent on participants’ recall and estimation of the quantity of the food they have ingested. The 3-day dietary recall method is the most common tool for assessing food intake in a natural setting, and although there has been criticism of this method,37 in the present study the correlation between inpatient dietary recall and actual measured inpatient intake (laboratory setting) was very strong (R = 0.96), supporting the validity of the dietary recall results. Analysis of the DSR symptom item “craving foods, increased appetite, overeating” revealed that the mean score for this one symptom item went from 14.2 in the luteal (premenstrual) phase to 0.3 in the follicular phase (p = 0.002), indicating that craving for carbohydrates was significantly greater in the luteal phase. However, despite the decrease in reported food cravings during the follicular phase, macronutrient and kilocalorie consumption remained consistent throughout the menstrual cycle. Thus, as has been previously reported,38–40 cravings alone are not always predictive of actual food consumption. No measures were used in this study to determine if subjects were restrained vs. unrestrained eaters.
The question remains as to whether the lack of cycle phase differences seen with insulin sensitivity and carbohydrate intake in the present study is the result of some unique neuroendocrine effects of PMS that affect whole body glucose disposal and may, in fact, represent a functional adaptation of PMS. Girdler et al.41 found that whereas there were cycle phase-dependent differences in the affective and behavioral symptoms of PMDD, there was no evidence of phase-dependent differences in neuroendocrine measures that were evaluated in that study. Women with PMDD were compared with healthy controls and were shown to have consistently elevated norepinephrine levels and increased total peripheral resistance, with decreased stroke volume and cardiac output. This contrasted with results in a control group of healthy women in which statistically significant cycle phase differences were found in total peripheral resistance and cardiac output.41
CONCLUSIONS
This pilot study did not demonstrate a difference in insulin sensitivity or carbohydrate intake across menstrual cycle phases in women with PMS. There was consistently high carbohydrate intake in both the luteal and follicular phases as measured both by diet history and inpatient food intake. There was a significant difference in food craving between cycle phases, with increased food craving noted in the luteal phase. A clear limitation of this study was the small number of participants involved; therefore, this precludes drawing definite conclusions. Nonetheless, the findings help support the mounting evidence that although most of the symptoms of PMS are confined to the luteal phase, the neuroendocrine background for this disorder may be consistent across menstrual cycle phases and, as suggested previously, may represent a functional adaptation of this disorder. Future studies should focus on larger numbers of participants and use the same behavioral and neuroendocrine measures as used in this pilot study in women with documented PMS and compared with a control group of women documented not to have PMS. Accurate cycle phase determination and less variability in cycle phase measurement days (than in the present study) would also add strength to any future studies. A greater understanding of any of the physiological and psychological variables that contribute to PMS could allow researchers to develop further strategies to help women cope with PMS symptom management.
ACKNOWLEDGMENTS
We thank the women who participated in this study, the expert nursing staff at the HUP, Dr. Heather Collins of the University of Pennsylvania Diabetes Center, Dr. Shiv Kapoor and Dr. Andrew J. Cucchiara of the Clinical and Translational Research Center, University of Pennsylvania, Dr. Raymond C. Boston of the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania School of Medicine, and Huong-Lan Nguyen, B.S., of the Monell Chemical Senses Center for laboratory and technical assistance.
We also thank Dr. Suzanne Smeltzer, FAAN, Director of the Center for Nursing Research at Villanova University, for her editorial assistance in the preparation of this paper.
This work was supported by a grant from the American Diabetes Association (to K.L.T.). This work was also supported by the National Institutes of Health, National Institute of Nursing Research (1-F31-NR008179 to K.K.T.), and by Public Health Services Research Grants DK-19525 and M01 RR00040 from the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT
No competing financial interests exist.
REFERENCES
- 1.Ugarriza DN, Klingner S, O'Brien S. Premenstrual syndrome: Diagnosis and intervention. Nurse Pract. 1998;23:40, 45, 49–52. [PubMed] [Google Scholar]
- 2.Endicott J. History, evolution, and diagnosis of premenstrual dysphoric disorder. J Clin Psychiatry. 2000;61(Suppl 12):5. [PubMed] [Google Scholar]
- 3.Ling FW. Recognizing and treating premenstrual dysphoric disorder in the obstetric, gynecologic, and primary care practices. J Clin Psychiatry. 2000;61(Suppl 12):9. [PubMed] [Google Scholar]
- 4.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 5.Dye L, Blundell J. Menstrual cycle and appetite control: Implications for weight regulation. Hum Reprod. 1997;12:1142. doi: 10.1093/humrep/12.6.1142. [DOI] [PubMed] [Google Scholar]
- 6.Steiner M, Pearlstein T. Premenstrual dysphoria and the serotonin system: Pathophysiology and treatment. J Clin Psychiatry. 2000;61(Suppl 12):17. [PubMed] [Google Scholar]
- 7.Freeman EW, Rickels K, Arredondo F, Kao LC, Pollack SE, Sondheimer SJ. Full- or half-cycle treatment of severe premenstrual syndrome with a serotonergic antidepressant. J Clin Psychopharmacol. 1999;19:3. doi: 10.1097/00004714-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. N Engl J Med. 1995;332:1529. doi: 10.1056/NEJM199506083322301. [DOI] [PubMed] [Google Scholar]
- 9.Hill AJ, Heaton-Brown L. The experience of food craving: A prospective investigation in healthy women. J Psychosom Res. 1994;38:801. doi: 10.1016/0022-3999(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 10.Bowen DJ, Grunberg NE. Variations in food preference and consumption across the menstrual cycle. Physiol Behav. 1990;47:287. doi: 10.1016/0031-9384(90)90144-s. [DOI] [PubMed] [Google Scholar]
- 11.Cohen IT, Sherwin BB, Fleming AS. Food cravings, mood, and the menstrual cycle. Horm Behav. 1987;21:457. doi: 10.1016/0018-506x(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 12.Dalvit-McPhillips SP. The effect of the human menstrual cycle on nutrient intake. Physiol Behav. 1983;31:209. doi: 10.1016/0031-9384(83)90120-8. [DOI] [PubMed] [Google Scholar]
- 13.Johnson WG, Corrigan SA, Lemmon CR, Bergeron KB, Crusco AH. Energy regulation over the menstrual cycle. Physiol Behav. 1994;56:523. doi: 10.1016/0031-9384(94)90296-8. [DOI] [PubMed] [Google Scholar]
- 14.Barr SI, Janelle KC, Prior JC. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am J Clin Nutr. 1995;61:39. doi: 10.1093/ajcn/61.1.39. [DOI] [PubMed] [Google Scholar]
- 15.Martini MC, Lampe JW, Slavin JL, Kurzer MS. Effect of the menstrual cycle on energy and nutrient intake. Am J Clin Nutr. 1994;60:895. doi: 10.1093/ajcn/60.6.895. [DOI] [PubMed] [Google Scholar]
- 16.Cross GB, Marley J, Miles H, Willson K. Changes in nutrient intake during the menstrual cycle of over-weight women with premenstrual syndrome. Br J Nutr. 2001;85:475. doi: 10.1079/bjn2000283. [DOI] [PubMed] [Google Scholar]
- 17.Wurtman JJ, Brzezinski A, Wurtman RJ, Laferrere B. Effect of nutrient intake on premenstrual depression. Am J Obstet Gynecol. 1989;161:1228. doi: 10.1016/0002-9378(89)90671-6. [DOI] [PubMed] [Google Scholar]
- 18.Pliner P, Fleming AS. Food intake, body weight, and sweetness preferences over the menstrual cycle in humans. Physiol Behav. 1983;30:663. doi: 10.1016/0031-9384(83)90240-8. [DOI] [PubMed] [Google Scholar]
- 19.Brzezinski AA, Wurtman JJ, Wurtman RJ, Gleason R, Greenfield J, Nader T. d-Fenfluramine suppresses the increased calorie and carbohydrate intakes and improves the mood of women with premenstrual depression. Obstet Gynecol. 1990;76:296. [PubMed] [Google Scholar]
- 20.Lundman B, Asplund K, Norberg A. Metabolic control, food intake and mood during the menstrual cycle in patients with insulin-dependent diabetes. Int J Nurs Stud. 1994;31:391. doi: 10.1016/0020-7489(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 21.Piers LS, Diggavi SN, Rijskamp J, vanRaajj JM, Shetty PS, Hautvast JG. Resting metabolic rate and thermic effect of a meal in the follicular and luteal phases of the menstrual cycle in well-nourished Indian women. Am J Clin Nutr. 1995;61:296. doi: 10.1093/ajcn/61.2.296. [DOI] [PubMed] [Google Scholar]
- 22.Bryant M, Truesdale KP, Dye L. Modest changes in dietary intake across the menstrual cycle: Implications for food intake research. Br J Nutr. 2006;96:888. doi: 10.1017/bjn20061931. [DOI] [PubMed] [Google Scholar]
- 23.Fernstrom JD, Wurtman RJ. Brain serotonin content: Increase following ingestion of carbohydrate diet. Science. 1971;174:1023. doi: 10.1126/science.174.4013.1023. [DOI] [PubMed] [Google Scholar]
- 24.Teff KL, Young SN. Effects of carbohydrate and protein administration on rat tryptophan and 5-hydroxytryptamine: Differential effects on the brain, intestine, pineal, and pancreas. Can J Physiol Pharmacol. 1988;66:683. doi: 10.1139/y88-108. [DOI] [PubMed] [Google Scholar]
- 25.Wurtman RJ, Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res. 1995;3(Suppl 4):477S. doi: 10.1002/j.1550-8528.1995.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 26.Teff KL, Young SN, Marchand L, Botez MI. Acute effect of protein or carbohydrate breakfasts on human cerebrospinal fluid monoamine precursor and metabolite levels. J Neurochem. 1989;52:235. doi: 10.1111/j.1471-4159.1989.tb10922.x. [DOI] [PubMed] [Google Scholar]
- 27.Pulido JME, Salazar MA. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30:19. doi: 10.1016/s0188-0128(98)00008-6. [DOI] [PubMed] [Google Scholar]
- 28.Valdes CT, Elkind-Hirsch KE. Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab. 1991;72:642. doi: 10.1210/jcem-72-3-642. [DOI] [PubMed] [Google Scholar]
- 29.Ezenwaka EC, Akanji AO, Adejuwon CA, Abbiyesuku FM, Akinlade KS. Insulin responses following glucose administration in menstruating women. Int J Gynaecol Obstet. 1993;42:155. doi: 10.1016/0020-7292(93)90630-f. [DOI] [PubMed] [Google Scholar]
- 30.Freeman EW, DeRubeis RJ, Rickels K. Reliability and validity of a daily diary for premenstrual syndrome. Psychiatry Res. 1996;65:97. doi: 10.1016/s0165-1781(96)02929-0. [DOI] [PubMed] [Google Scholar]
- 31.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 32.Bergman RN, Lovejoy JC, editors. The minimal model approach and determinants of glucose tolerance. Pennington Center Nutrition Series. Vol. 7. Louisiana State University Press; Baton Rouge: 1997. [Google Scholar]
- 33.Prigeon RL, Roder ME, Porte DY, Kahn SE. The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique: Evidence for saturable insulin transport in humans. J Clin Invest. 1996;97:501. doi: 10.1172/JCI118441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steil GM, Murray J, Bergman RN, Buchanan TA. Repeatability of insulin sensitivity and glucose effectiveness from the minimal model: Implications for study design. Diabetes. 1994;43:1365. doi: 10.2337/diab.43.11.1365. [DOI] [PubMed] [Google Scholar]
- 35.Greco AV, Mingrone G, Giancaterini A, et al. Insulin resistance in morbid obesity: reversal with intramyo-cellular fat depletion. Diabetes. 2002;51:144. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- 36.Trout KK, Rickels MR, Schutta MH, et al. Menstrual cycle effects on insulin sensitivity in women with type 1 diabetes: A pilot study. Diabetes Technol Ther. 2007;9:176. doi: 10.1089/dia.2006.0004. [DOI] [PubMed] [Google Scholar]
- 37.Hill RJ, Davies PSW. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr. 2001;85:415. doi: 10.1079/bjn2000281. [DOI] [PubMed] [Google Scholar]
- 38.Hill AJ, Weaver CF, Blundell JE. Food craving, dietary restraint and mood. Appetite. 1991;17:187. doi: 10.1016/0195-6663(91)90021-j. [DOI] [PubMed] [Google Scholar]
- 39.Polivy JJ, Coleman J, Herman CP. The effect of deprivation on food cravings and eating behavior in restrained and unrestrained eaters. Int J Eat Disord. 2005;38:301. doi: 10.1002/eat.20195. [DOI] [PubMed] [Google Scholar]
- 40.Federoff I, Polivy J, Herman CP. The specificity of restrained versus unrestrained eaters’ responses to food cues: General desire to eat, or craving for the cued food? Appetite. 2003;41:7. doi: 10.1016/s0195-6663(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 41.Girdler SS, Pederson CA, Straneva PA, et al. Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Res. 1998;81:163. doi: 10.1016/s0165-1781(98)00074-2. [DOI] [PubMed] [Google Scholar]