Abstract
Introduction
An alcohol bolus causes the blood alcohol level (BAL) to peak at 1-2 hours post ingestion. The ethanol elimination rate is regulated by alcohol metabolizing enzymes, primarily alcohol dehydrogenase (ADH1), acetaldehyde dehydrogenase (ALDH), and cytochrome P450 (CYP2E1). Recently, S-adenosylmethionine (SAMe) was found to reduce acute BALs 3h after an alcohol bolus. The question, then, was: what is the mechanism involved in this reduction of BAL by feeding SAMe? To answer this question, we investigated the changes in ethanol metabolizing enzymes and the epigenetic changes that regulate the expression of these enzymes during acute binge drinking and chronic drinking.
Methods
Rats were fed a bolus of ethanol with or without SAMe, and were sacrificed at 3h or 12 h after the bolus.
Results
RT-PCR and Western blot analyses showed that SAMe significantly induced ADH1 levels in the 3h liver samples. However, SAMe did not affect the changes in ADH1 protein levels 12h post bolus. Since SAMe is a methyl donor, it was postulated that the ADH1 gene expression up regulation at 3h was due to a histone modification induced by methylation from methyl transferases. Dimethylated histone 3 lysine 4 (H3K4me2), a modification responsible for gene expression activation, was found to be significantly increased by SAMe at 3h post bolus.
Conclusion
These results correlated with the low BAL found at 3h post bolus, and support the concept that SAMe increased the gene expression to increase the elimination rate of ethanol in binge drinking by increasing H3K4me2.
INTRODUCTION
In the present study, the effects of acute alcohol consumption were investigated, particularly the epigenetic modifications that occurred following the exposure to high levels of ethanol. Previous studies showed that an acute bolus of alcohol consumption affects global gene expression (Li et al., 2009). 3h post ethanol bolus caused a marked change in gene expression compared to the 12h post bolus, and the genes that were affected differed. S-adenosylmethionine (SAMe) supplementation significantly changed the gene expression caused by acute alcohol feeding. The main observation in the study was the marked effect of SAMe supplementation in reducing the blood and urine ethanol levels (BAL and UAL) 3h post bolus. In the blood alcohol level cycling model of ethanol metabolism during chronic ethanol intragastric feeding, it was shown that there were a large number of changes in gene expression at high blood ethanol levels (peak), when compared to low blood ethanol levels (trough) and to the controls (Bardag-Gorce et al., 2006, French et al., 2005). When SAMe was added to the diet with ethanol feeding, the blood alcohol levels were decreased and the up regulation of gene expression was down regulated (Li et al., 2009). The question, then, is: by what mechanism did SAMe affect the metabolism of ethanol in the acute alcohol bolus model. Since SAMe is a major methyl donor, it is postulated that SAMe supplementation changes the gene expression of enzymes involved in the metabolism of ethanol by changing histone methylation levels and the histone methyltransferase modifying enzymes that induce the changes in gene expression. SAMe plays a key role in epigenetic gene regulation because it donates methyl groups to histone lysine residues, which regulate the expression of genes.
BAL is controlled by the enzymes alcohol dehydrogenase (ADH1), aldehyde dehydrogenase (ALDH) and cytochrome P450 2E1 (CYP2E1). Therefore, the objective was to study the effects of SAMe on the expression of these genes. The investigation of changes in ethanol metabolizing enzymes and the epigenetic changes that regulate these changes in acute binge drinking and chronic drinking were done. A reduction in BAL could potentially prevent the adverse effects of binge drinking. It was shown that the rate of alcohol metabolism was limited by the amount of alcohol metabolizing enzyme available (Zakhari 2006). Therefore, we hypothesized that the reduction of BAL in the liver of rats fed a bolus of ethanol and SAMe, was the result of histones methylated by SAMe. This would increase the rate of alcohol metabolism by up regulating the expression of the proteins ADH1, ALDH, and CYP2E1.
To test this hypothesis, Western blot and PCR analyses were performed at 3h and 12h after a bolus of alcohol. The results were compared with the group of animals given SAMe to investigate the role of SAMe in reducing blood and urine alcohol levels.
MATERIALS AND METHODS
Animals
Male Wistar rats from Harleco (Hollister, CA) weighing 250 g to 300 g were used. The livers used were from the previously published study (Li et al., 2009). Three or 4 rats per group were fed an acute bolus. Group 1 was fed a bolus of dextrose isocaloric to ethanol, by stomach gavage as control. Group 2 was fed an ethanol bolus (6 g/kg body weight, 20% solution of ethanol) by gavage. Group 3 was fed a dextrose bolus isocaloric to ethanol plus SAMe, 1 g/kg body weight. Group 4 was given an ethanol bolus with SAMe. The rats were then sacrificed 3h and 12h post ethanol, dextrose, and SAMe bolus. At sacrifice under isofluorane anesthesia, the liver was removed and weighed. A portion of the liver was quick frozen and stored in isopentane in liquid nitrogen followed by storage at -80°C. Urine and blood were collected at sacrifice to measure alcohol levels. The urine was collected under toluene using metabolic cages, one rat/cage. The urinary alcohol level was measured using a kit (QED Saliva Alcohol test kit A 150, STC Technologies, Bethlehem, PA). Blood ALTs and alcohol levels were also measured by a clinical analyzer (Bardag-Gorce et al., 2009).
The rats were maintained according to the Guidelines of Animal Care, as described by the National Academy of Sciences and published by the National Institute of Health (1996).
Western blot
Frozen liver samples were homogenized and prepared for Western blotting. Samples were homogenized using an ultraturrax T25 homogenizer in 50 mM Tris-HCl (pH8), 10% glycerol, 1 mM EDTA, 1 mM EGTA, protease inhibitors (Sigma, St. Louis, MO), and phosphatase inhibitor (Thermo Scientific Waltham, MA). Protein measurements were performed according to the Bradford method (1976), using BioRad reagent (BioRad, Hercules, CA). Proteins were denatured with Laemmli buffer, and were stored at -20°C. 15 μg of each sample was used the next day for Western blotting. They were separated by a 12% SDS-PAGE gel electrophoresis. Proteins were then transferred to a polyvinyl difluoride (PVDF) membrane (Bio-Rad, Hercules, CA) for 1h in 2.5 mM Tris-HCl (pH 8.3), 192 mM glycine and 20% methanol. Antibodies against mouse ADH class 1 (Santa Cruz Biotechnology, Inc. Santa Cruz, CA.), CYP2E1 (Enzo life Science, Plymouth Meeting, PA), ALDH1/2 (Santa Cruz, CA), H3k4me2 and H3K9me2 (Active Motif, Carlsbad, CA) were used. Immunologic stains were detected using an enhanced chemiluminescence kit (Pierce, Rockford, IL). The membranes were then stripped and stained with a second antibody to beta-actin as a loading control to correct for protein loading differences.
Quantitative real-time RT-PCR assay
Total liver RNAs were extracted with Trizol Plus RNA Purification Kit (Invitrogen, Carlsbad, CA). Synthesis of cDNAs was performed with 5 μg total RNA, and 50 ng random hexamer primers using SuperSriptIII RNase H-Reverse Transcriptase (Invitrogen, Carlsbad, CA). PCR primers were designed with the assistance of the Primer Express software (Applied Biosystems, Foster City, CA).
Quantitative PCR was achieved using the SYBR Green JumpStart™ Tag ReadyMix (Sigma, St. Louis, MO) on an ABI PRISM 7700 Sequence Detector System (Applied Biosystems, Foster City, CA). The thermal cycling consisted of an initial step at 50°C for 2 min, followed by a denaturation step at 95°C for 10 min, then 40 cycles at 95°C for 15 s and 60°C for 1 min. Single PCR product was confirmed with the heat dissociation protocol at the end of the PCR cycles. Quantitative values were obtained from the threshold PCR cycle number (Ct) at which point the increase in signal associated with an exponential growth for PCR product starts to be detected. The target mRNA abundance in each sample was normalized to its 18S level as ΔCt=Cttarget gene−Ct18S. For each target gene, the highest ΔCt was assigned as ΔCtmax.
List of primers sequences:
| Gene | Forward | Reverse | |
| ADH1 | NM_019286.3 | CCTTCACCATCGAGGACATAGAAG | GCCACCATCTTAATGCGAACTT |
| CYP2E1 | NM_031543.1 | TGACTTTGGCCGACCTGTTC | TGAGGATCAGGAGCCCATATCT |
| ALDH1a4 | NM_017272 | CGCACCATGGATGCTTCA | CGCGATCTCTCTCCATTAAGTCA |
Statistical Analysis
Tissues from 3-4 rats/group (control, control+SAMe, ethanol, ethanol+SAMe) were compared. P values were determined by ANOVA and Bonferroni for multiple group comparisons (Sigma Software, San Francisco, CA).
RESULTS
In a previous study, we showed that an ethanol bolus supplemented with SAMe significantly decreased the BAL and UAL after 3h compared to the rats given alcohol only (Li et al., 2009). Therefore, to determine the role that SAMe plays in reducing the blood ethanol levels, we analyzed the effects of SAMe on the protein levels of the enzymes responsible for ethanol metabolism, such as ADH1, CYP2E1 and ALDH. The results (Figure 1A) showed that SAMe up regulated ADH1 in the liver of both the ethanol-fed and dextrose controls 3h post bolus, suggesting that SAMe supplementation may regulate ethanol metabolism by increasing the protein levels of ADH1.
Figure 1.
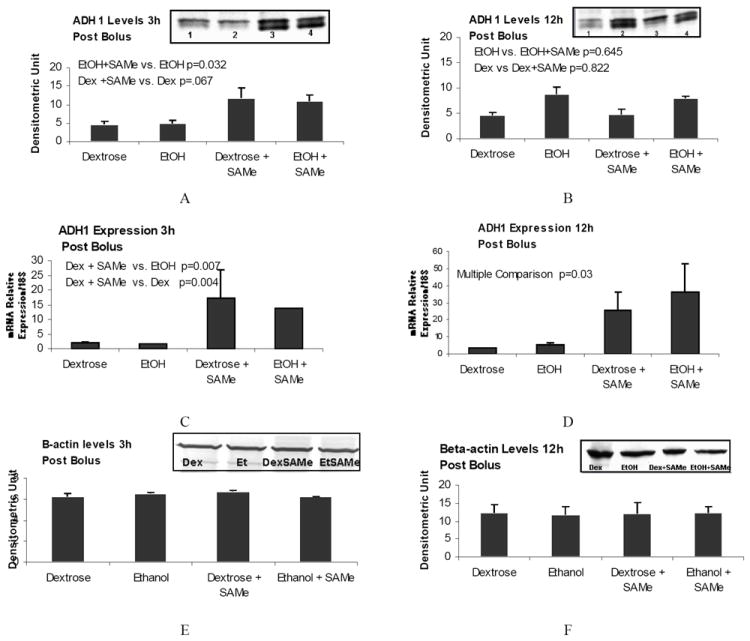
ADH1 was up regulated in the liver of rats given SAMe 3 h and 12 h post treatment. Western blot analysis of the protein levels 3h (A) and 12 (B) post bolus. Note that SAMe induced ADH1 in the control and ethanol fed rats 3h post bolus. C and D: ADH1 mRNA levels. SAMe significantly induced ADH1 mRNA in the control and ethanol fed rats, at both 3h and 12h post bolus (Mean ± SEM, n=3-4). E and F are beta-actin staining of the same samples used as a loading control. (Mean ± SEM, n=3-4).
However, 12h post ethanol bolus, ADH1 protein levels were up regulated by ethanol rather than by SAMe (Figure 1B). There was a slight, but not significant, increase of ADH1 in the control rats given SAMe. The effects of SAMe on ADH1 protein levels were absent at 12h post bolus. However, ADH1 mRNA levels were still increased (Figure 1 C and D).
We also analyzed the effects of SAMe on the levels of CYP2E1. SAMe did not change CYP2E1 protein levels (Figure 2 A and B). However SAMe increased the mRNA levels in the control, as well as in the liver of the ethanol fed rat, in both 3h and 12h post bolus (Figure 2 C and D). B-actin stain was performed to normalize the results of ADH1 at 3h (E) and 12 h (F).
Figure 2.
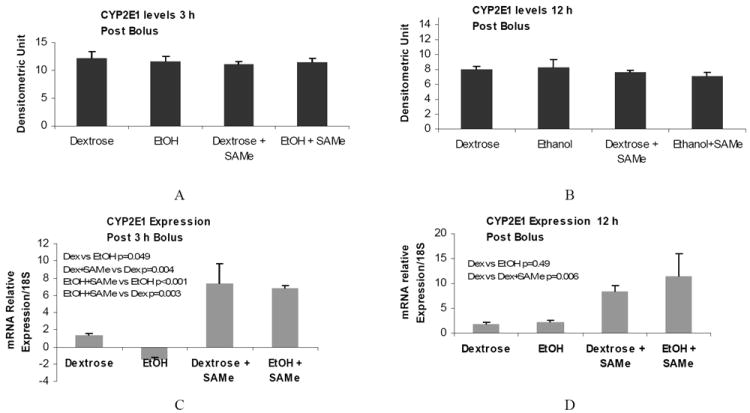
A and B: CYP2E1 protein levels in the liver of rats given SAMe 3h and 12h post treatment. C and D: CYP2E1 mRNA levels 3h and 12h post bolus C and D. Note that SAMe induced CYP2E1 gene expression in the control and ethanol fed rats 3h and 12h post bolus. (Mean ± SEM, n=3).
Similar to the CYP2E1 enzyme, ALDH1a4, which metabolizes acetaldehyde, was also up regulated at the mRNA levels at 3h and 12h post bolus by SAMe supplementation (Figure 3 C and D). However the protein levels did not change 3h and 12h post bolus (Figure 3 A and B)
Figure 3.
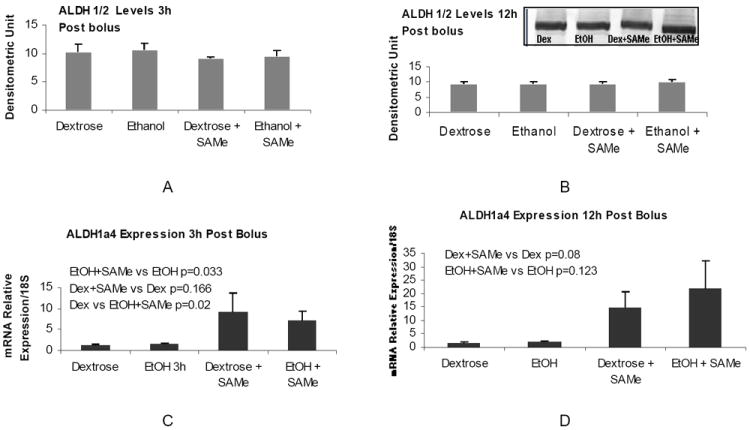
Gene expression of ALDH1a4 in the liver of rats fed an ethanol bolus 3h (A) and 12h (B). SAMe supplementation increased the ALDH1a4 gene expression at both time intervals (Mean ± SEM, n=3-4).
To investigate the mechanism by which SAMe supplementation induced an increase in the gene expression of ADH1, CYP2E1 and ALDHa4, we analyzed the levels of histone methylation. The results showed that SAMe induced the dimethylation of histone 3 lysine 4 (H3K4) and tended to induce dimethylation of histone 3 lysine 9 (H3K9), 3h post SAMe (Figure 4). It is possible that SAMe supplementation caused the up regulation of ethanol metabolizing enzymes via an epigenetic mechanism that involves increases in H3K4 methylation and the regulation of gene expression of the ethanol metabolizing enzymes.
Figure 4.
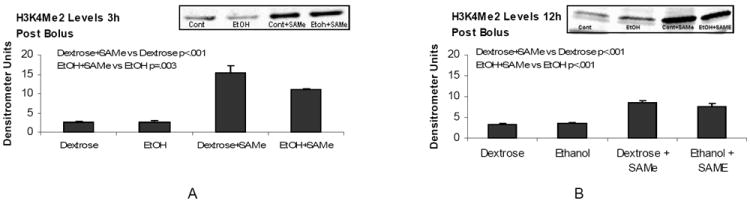
Western blot analysis of histone 3 lysine 4 and lysine 9 dimethylation (H3K4me2 and H3K9me2). Dimethylation of histone 3 lysine 4 was significantly increased in the liver of rats given SAMe and sacrificed 3h and 12h post bolus (A and B). Dimethylation of histone 3 lysine 9 was decreased by ethanol at 3h, and increased by SAMe at 3h and 12h (C and D) post bolus. (Mean ± SEM, n=3).
DISCUSSION
In previous studies, high levels of blood alcohol were found to induce global changes in gene expression (Bardag-Gorce et al., 2007; Bardag-Gorce et al., 2010; Li et al., 2009). The fact that high ethanol levels affected gene expression led to the idea that epigenetic mechanisms are also temporarily changed by high ethanol levels. This also suggested that SAMe, a major methyl donor, could alter the pattern of gene expression caused by ethanol by increasing the methylation of this histones (Li et al., 2009).
Data from these studies showed that SAMe not only prevented oxidative stress and inflammation, but also reduced the ethanol levels in blood and urine of the animals fed ethanol (Bardag-Gorce et al., 2010) (Li et al., 2009). This phenomenon was reported for both the acute and chronic model (Bardag-Gorce et al., 2010). Powell et al. (2010) reported that the dietary methyl donors increased the ethanol elimination rate by 35% (betaine 15g/kg diet, choline 13.2 g/kg diet, folic acid 13.6 mg/kg diet, 7.5 g L-methionine/kg diet, and 0.13 mg B12/kg). This diet, rich in methyl donor components, reduced the blood alcohol level in mice fed ethanol intragastrically for 1 month. The levels of ALDH and catalase activity were increased, while CYP2E1 activity was decreased and ADH activity was unchanged.
In our acute model, we found that alcohol dehydrogenase was significantly induced at the level of mRNA and protein, which may have contributed to the accelerated ethanol metabolism. This was observed 3h after ethanol bolus, and positively correlated with the reduced BAL levels by SAMe. However, the mRNA for ADH-1 was increased at 3h and 12h by SAMe. CYP2E1 protein levels as well as ALDH and catalase protein levels were unchanged by SAMe (data not shown). However, CYP2E1 and ALDH mRNA levels were increased at 3h and 12h by SAMe.
In order to explain how feeding SAMe accelerated the rate of BAL and UAL clearance, specific histone modifications were analyzed. SAMe supplements increased H3K4me2 levels in both 3h and 12h samples. Histone methylation is involved in the regulation of gene expression (Pal-Bahdra et al., 2007), and SAMe could be a powerful mediator of this regulation by increasing the methylation of histones. As previously reported (Bardag-Gorce et al., 2007), histone methylation of H3K4me2 was not changed by ethanol at 3 h and 12 h post bolus. However, SAMe supplementation significantly increased H3k4me2 at 3h and 12h post bolus, which could be the mechanism of the activation of ADH1 gene expression. An increase in histone H3K4me2 activates gene expression (Li et al., 2009b). We found that the gene expression of ADH, CYP2E1, ALDH were all up regulated by feeding SAMe. We did not find any changes in H3K4me2, when ethanol was fed without SAMe, which confirmed our previous results (Bardag-Gorce et al., 2009). In summary, the role of SAMe, as a major methyl donor, up regulated ADH1 expression at 3h, increasing histone 3 lysine 4 and 9 dimethylation by increasing the expression of ADH1.
Acknowledgments
This study was reported in part at the Experimental Biology Meeting 2010 (Bardag-Gorce et al, 2010). The grant NIH/NIAAA 8116 supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardag-Gorce F, French BA, Joyce M, Baires M, Montgomery RO, Li J, French S. Histone acetyltransferase p300 modulates gene expression in an epigenetic manner at high blood alcohol levels. Exp Mol Pathol. 2007;82(2):197–202. doi: 10.1016/j.yexmp.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, Oliva J, Dedes J, Li J, French BA, French SW. Chronic ethanol feeding alters hepatocyte memory which is not altered by acute feeding. Alcohol Clin Exp Res. 2009;33(4):684–692. doi: 10.1111/j.1530-0277.2008.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, Li J, Oliva J, Lu SC, French BA, French SW. The cyclic pattern of blood alcohol levels during continuous ethanol feeding in rats: the effect of feeding S-adenosylmethionine. Exp Mol Pathol. 2010a;88(3):380–7. doi: 10.1016/j.yexmp.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, Li J, Oliva J, Lu SC, French BA, French SW. S-adenosylmethionine down regulates blood alcohol levels. FASEB. 2010b doi: 10.1016/j.yexmp.2010.03.004. Suppl. Abst. 236.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, French BA, Dedes J, Li J, French SW. Gene expression patterns of the liver in response to alcohol: in vivo and in vitro models compared. Exp Mol Pathol. 2006;80(3):241–51. doi: 10.1016/j.yexmp.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Choudhury M, Shukla SD. Surrogate alcohols and their metabolites modify histone H3 acetylation: involvement of histone acetyl transferase and histone deacetylase. Alcohol Clin Exp Res. 2008;32(5):829–839. doi: 10.1111/j.1530-0277.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- French BA, Dedes J, Bardag-Gorce F, Li J, Wilson L, Fu P, Nan L, French SW. Microarray analysis of gene expression in the liver during the urinary ethanol cycle in rats fed ethanol intragastrically at a constant rate. Exp Mol Pathol. 2005;79(2):87–94. doi: 10.1016/j.yexmp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Li J, Bardag-Gorce F, Oliva J, Dedes J, French BA, French SW. Gene expression modifications in the liver caused by binge drinking and S-adenosylmethionine feeding. The role of epigenetic changes. Genes Nutr. 2009 doi: 10.1007/s12263-009-0158-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cui Y, Hart SN, Klaassen CD, Zhong XB. Dynamic Patterns of Histone Methylation Are Associated with Ontogenic Expression of the Cyp3a Genes during Mouse Liver Maturation. Mol Pharmacol. 2009b;75(5):1171–9. doi: 10.1124/mol.108.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Bhadra M, Bhadra U, Jackson DE, Mamatha L, Park PH, Shukla SD. Distinct methylation patterns in histone H3 at Lys-4 and Lys-9 correlate with up- & down-regulation of genes by ethanol in hepatocytes. Life Sci. 2007;81(12):979–987. doi: 10.1016/j.lfs.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CL, Bradford BU, Craig CP, Tsuchiya M, Uehara T, O’Connell TM, Pogribny IP, Melnyk S, Koop DR, Bleyle L, Threadgill DW, Rusyn I. Mechanism for Prevention of Alcohol-Induced Liver Injury by Dietary Methyl Donors. Toxicol Sci. 2010 doi: 10.1093/toxsci/kfq031. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CL, Bradford BU, Craig CP, Tsuchiya M, Uehara T, O’Connell TM, Pogribny IP, Melnyk S, Koop DR, Bleyle L, Threadgill DW, Rusyn I. Mechanism for prevention of alcohol-induced liver injury by dietary methyl donors. Toxicol Sci. 2010;115(1):131–139. doi: 10.1093/toxsci/kfq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SD, Velazquez J, French SW, Lu SC, Ticku MK, Zakhari S. Emerging role of epigenetics in the actions of alcohol. Alcohol Clin Exp Res. 2008;32(9):1525–34. doi: 10.1111/j.1530-0277.2008.00729.x. Review. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, French SW, Reidelberger RD, Largman C. Cyclic pattern of blood alcohol levels during continuous intragastric ethanol infusion in rats. Alcohol Clin Exp Res. 1985;9(1):31–37. doi: 10.1111/j.1530-0277.1985.tb05046.x. [DOI] [PubMed] [Google Scholar]
- Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29(4):245–54. [PMC free article] [PubMed] [Google Scholar]


