Abstract
The role of complement and complement-fixing IgG isotypes at mucosal surfaces is ill defined. Previous data have demonstrated that survival of an infection with the attaching and effacing pathogen Citrobacter rodentium requires production of systemic and CD4+ T cell-dependent IgG. We have found that both complement and complement-fixing IgG isotypes are needed to survive a C. rodentium infection. Our results indicate that both IgG and complement C3b enter the gut lumen and bind epithelially adherent, and fecally shed C. rodentium. Furthermore, C3-deficient mice demonstrate a profound survival defect, though means to replenish C3 in systemic or mucosal sites restores the protective capacity of complement in the host. Our data provide evidence that both IgG and complement interact constructively on both sides of the epithelium to fight colonizing mucosal infections.
Keywords: C. rodentium, complement, mucosal infection
Introduction
Citrobacter rodentium causes an attaching and effacing (AE) infection of the distal colon in mice [1–3]. Mouse infections with C. rodentium are used as a model system for human gastrointestinal infections with the enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC) that cause of approximately 2 million deaths every year [4]. These organisms use a type III secretion system to introduce translocated intimin receptor (Tir), and extracellularly secreted proteins (esp), into target epithelial cells [5–7]. Membrane-associated Tir is tethered to the actin cytoskeleton and binds the bacterially associated intimin, to form attaching and effacing lesions [8, 9]. To get more insight in the human defense mechanism against pathogenic pedestal forming E. coli, the mouse C. rodentium colitis mode is used in this study.
Resolution of infection relies on a variety of innate and adaptive responses. Pro-inflammatory cascades resulting in local secretion of IFNg, IL-8/KC, IL6, and TNFa-member cytokines are believed to mediate protection through recruitment of inflammatory infiltrates, and stimulation of anti-microbial peptides, from infected epithelium [10–13]. Surprisingly, mucosal IgA has negligible impact in C. rodentium infection, where the primary infection occurs over the luminal surface of the gut [14, 15]. Prior studies have demonstrated that systemic pathogen-specific IgG and CD4+ T cell responses are required for survival and resolution of this colonizing infection of the gut epithelium [15–17].
IgG’s mechanism(s) of action during a colonizing infection of the luminal epithelial surface remains ill defined. However, this question is critical not only for the study of attaching and effacing pathogens, but for the development of systemic/IgG-based vaccines that serve to protect the host prior to, or during early phases of infections involving mucosal contact. Studies by Masuda et al. [18] have previously shown the importance of Fc-receptor-bearing cells in facilitating survival and clearance of C. rodentium, suggesting that opsonization and uptake of the pathogen, or pathogen antigens by macrophages and DCs, contribute to host defense.
The protective host IgG response to a C. rodentium infection consists largely of complement-fixing isotypes, namely IgG2c and IgG2b [15]. IgG’s entry into the gut lumen during this infection likely occurs through a variety of mechanisms, including active uptake and release, and more passive entry through damaged mucosa. Active uptake and release of IgG by gut epithelium, particularly within the small bowel, is mediated by the neonatal Fc receptor [19, 20]. The receptor also transports IgG into the gut lumen before, and during, C. rodentium infection [21]. FcRn-deficient mice demonstrated decreased survival with C. rodentium infection. Absence of the receptor results in poor uptake of antigen-antibody complexes from the gut lumen during infection, a defect that can be circumvented by expressing the receptor solely in gut epithelium in FcRn–/– mice [21]. While intestinal expression may promote local immune responses, extra-intestinal expression of the receptor likely also contributes to host defense by prolonging the half-life of pathogen-specific IgG during infection. Infection also impacts normal barrier function of the epithelium, allowing passive leakage of not only macromolecules such as IgG, but other serum proteins including those of the complement cascade as well as cellular component [22].
IgG commonly combats pathogens through a combination of complement-dependent, opsonization, and phagocytic mechanisms, as well as direct inhibitory effects on microbial growth or neutralization of adhesins [23–25]. Binding of IgG to microbial adhesins can prevent epithelial adherence of enteric pathogens, as has been shown with polyclonal antibody against EPEC intimin [26]. Subsequent antimicrobial effects include lysis through activation of the classical arm of the complement cascade, and opsonization of bacteria with IgG and/or C3, which allows efficient uptake and killing by macrophages and neutrophils.
In addition to hepatic and myeloid sources of complement, other tissues, including gut epithelium, express components of each pathway. C3, C4, factor B and MBL have been demonstrated in luminal secretions from healthy stomach, small intestine, pancreatic, and biliary secretions [27–31]. As an acute-phase reactant, local inflammation up-regulates complement expression, particularly in response to IL-1, IL-6, IFNg, and TNF-a in intestinal epithelial cell lines Caco2 and T84 [27]. While components of the membrane attack complex (C7–C9) have been shown to be produced in myocardium, renal tubular epithelium, and other sites under inflammatory conditions, or after ischemia/reperfusion [31, 32]; this type of expression has not been demonstrated to date in gut epithelium.
While effector functions of IgG and complement at locations basolateral to mucosal surfaces are well defined, mechanisms of action across infected epithelium and within the gut lumen are not. The presented experiments indicating complement binds to, and acts upon luminal C. rodentium, support the hypothesis that IgG interacts constructively with complement on both sides of the epithelium to combat infection.
Results
IgG2b and complement C3b bind luminally adherent C. rodentium
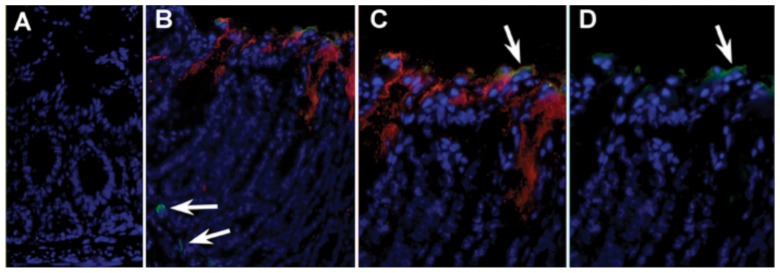
To determine if both IgG and complement were interacting at the gut surface, we stained for IgG2b and cleaved C3b in sections of infected distal colon from wildtype mice (Fig. 1). The immunofluorescent staining indicates the binding capability of secreted IgG and C3b to C. rodentium at the epithelial surface. Fecal FACS of GFP-tagged C. rodentium, shed in feces, was also used to evaluate the isotypes binding to bacteria. Bacteria were first analyzed by flow cytometry by gating on GFP+ events and analyzing signal in FL-2 for C3b, C4 and MBL-C bound to the bacterial cell surface. Figure 2 shows C3-opsonization of bacteria shed in the feces at day 15 of infection.
Fig. 1.
In vivo association of IgG and C3 with apically adherent C. rodentium: (A) Section of mouse colon (×200) stained with Hoescht dye donkey anti-rabbit-CY3 and rat-anti-mouse IgG-FITC. (B) Section from infected mouse (×200) showing host nuclei in blue, prominent C3 staining of apically adherent C. rodentium (red), faint IgG straining at the luminal surface (green), as well as IgG+ B cells in the lamina propria (arrows). (C) ×400 magnification of previous image showing distribution of C3+ C. rodentium and co-localization of IgG at the luminal surface. (D) Composite of previous image showing only IgG and Hoescht staining
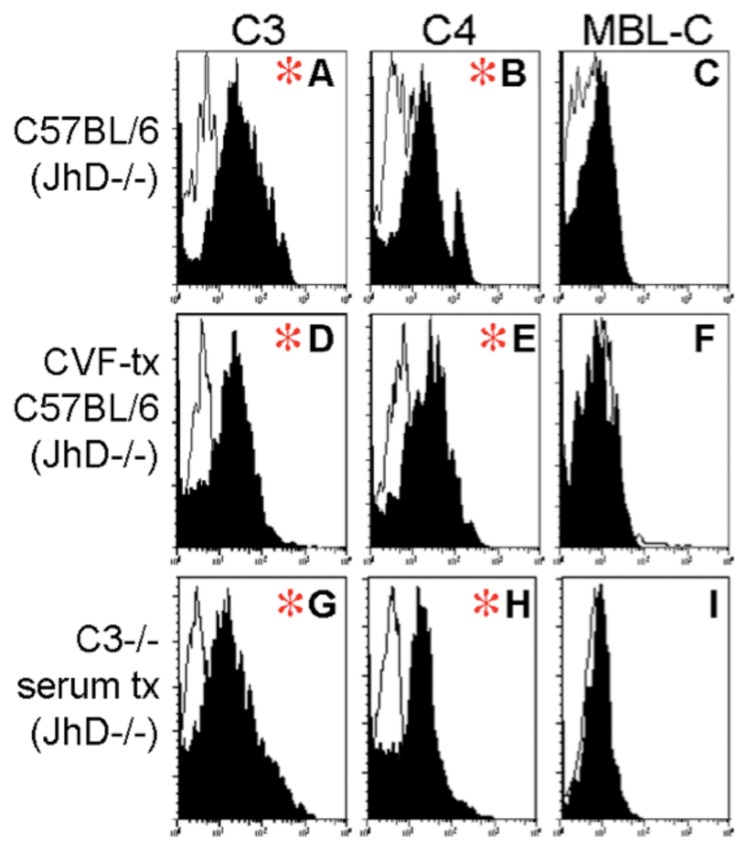
Fig. 2.
Fixation of C3 on fecally shed C. rodentium: Representative histograms from flow cytometry of GFP+ events in fecal bacterial isolated from mice at 15 days of infection with C. rodentium. X-axis indicates fluorescence intensity in FL-2 (log10 scale); Y-axis indicates number of events. Panels A–C demonstrate staining in a representative wildtype mouse. (A) C3 and (B) C4, but not (C) MBL-C to shed GFP+CR (black plots). Staining in control antibody-deficient JhD–/– mice (white tracings) are shown. Red asterisk indicates populations where the mean fluorescence intensity between wildtype and JhD–/– mice was significant. Similar plots are shown from a representative CVF-treated mouse showing (D) C3, (E) C4, and (F) MBL-C. The bottom row of plots shows representative histograms from a C3–/– mouse reconstituted intravenously with wildtype serum containing C3. (G) C3, (H) C4, and (I) MBL-C
Cobra venom factor (CVF) rapidly depletes circulating C3 when administered IP or IV but does not enter the gut lumen to degrade mucosally secreted C3. CVF treatment of wildtype mice was thus used to assess effects of systemic depletion of C3. Mice treated with CVF (Fig. 2D–F) and C3-deficient mice systemically reconstituted with C3-sufficient serum (Fig. 2G–I) show similar patterns to the wildtype mice. This result indicates the presence and importance of luminal-secreted complement and its binding capability there.
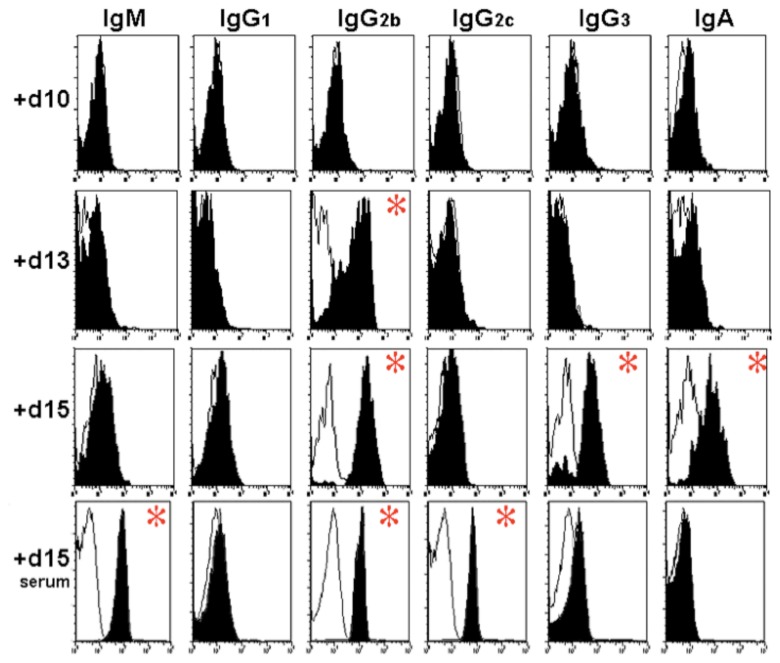
Serum antibody is also capable of entering the gut lumen to bind apically adherent C. rodentium during acute infection (Fig. 3). The amount of specific IgG2b isotype increased in the feces 15 days after infection. Figure 3 shows antibody levels in serum and on bacteria shed in the feces. Serum levels of IgM, IgG2c, and IgG2b all rice during infection. Increased levels of IgA, IgG2b, and IgG3 are detected in the feces during acute infection. This is suggestive of sufficient deterioration of epithelial barrier function to enable diffusion through the mucosal barrier.
Fig. 3.
Ig Binding to fecal bacteria during infection: Representative histograms from flow cytometry of GFP+ events in fecal bacteria isolated from mice at 10, 13, and 15 days of infection with C. rodentium. X-axis indicates fluorescence intensity in FL-2 (log10 scale); Y-axis indicates number of events. Panels: Staining in control antibody-deficient JhD–/– mice mice (white tracings) are shown. From left to right, the different antibodies are shown (IgM, IgG1, IgG2b, IgG2c, IgG3, and IgA). The three top rows indicate levels in the fecal pellets 10, 13, and 15 days after infection. The bottom row shows antibody levels in serum 15 days after infection. Asterisks indicate a significant difference between wildtype and immune compromised mice
Complement-dependent lysis of C. rodentium
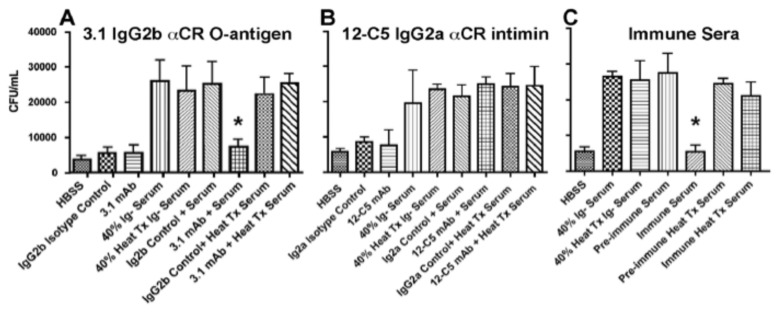
Our data suggest that both reactive IgG and C3 enter the gut lumen and bind apically adherent C. rodentium. Next the ability of these immune molecules to mediate complement-dependent lyses was analysed. Combinations of pre-immune or specific serum IgG against C. rodentium with or without the presence of serum complement were added to 106 CFU of mid-log phase C. rodentium cultured in vitro. Bacteria were plated after 2 hours of incubation to assess effect on growth. Growth inhibition of C. rodentium occurred only in the presence of immune IgG and complement. Pre-immune serum, lacking specific IgG and immune serum treated with CVF failed to inhibit growth (Fig. 4). These findings demonstrate that the polyclonal serum antibody response, and monoclonal antibodies against C. rodentium O-antigen, can inhibit actively dividing bacteria in the presence of serum complement.
Fig. 4.
Anti-bacterial activities of mAbs with complement-sufficient serum: Assays for complement-dependent lysis after 2-h incubation with (A) 3.1 mAb against C. rodentium O-antigen; asterisk indicates significant growth inhibition of bacteria in the presence of serum and 5 µg of 3.1 mAb, (B) 12-C5 mAb against bacterial intimin, or (C) effects of immune and pre-immune serum on bacterial growth inhibition. Asterisk indicates growth inhibition in the presence immune serum, as compared with adjacent peaks measuring growth in pre-immune serum or in heat-treated sera
Complement is required for effective host defense against C. rodentium
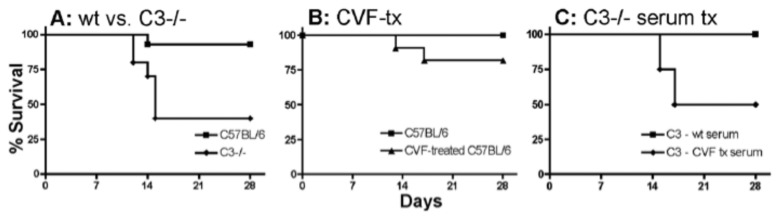
Complement has key protective functions in a variety of infections. To evaluate if complement is important in the defense against the intestinal pathogen, C. rodentium, we first performed mouse survival studies with complement C3-defficiant mice. Infection demonstrated a significant survival defect as 40% of C3–/– mice survived while >90% of wildtype mice survived (Fig. 5A). These studies demonstrate the need for complement in surviving C. rodentium infection. Subsequent experiments dissected the functions of mucosal vs. systemic stores of C3 in infection. CVF treatment of wildtype mice was thus used to assess effects of systemic depletion of C3, as shown in Fig. 5. To evaluate effects of systemic C3, in the absence of mucosally secreted C3, we reconstituted C3–/– mice with wildtype or CVF-treated serum administered intravenously. Systemic reconstitution with C3-sufficient serum was 100% protective, while 50% of mice receiving CVF-depleted serum failed to survive infection (Fig. 5C). Treatment of wildtype mouse with CVF does not affect their survival (Fig. 5B). Two CVF-treated mice succumbed to infection while wildtype controls survived, though differences failed to attain significance (p = 0.2605). These data suggest that systemic complement, as well as complement released from mucosal compartments, can provide protection.
Fig. 5.
Survival studies in C3–/– and CVF-treated mice infected with C. rodentium: (A) Comparison of survival in wildtype (11 of 12 mice survived) and C3–/– mice (4 of 10 mice survived) infected with C. rodentium at 21 days of age. (B) Wildtype CVF (n = 10) or saline (n = 10) mice. (C) Infection in C3–/– mice treated with wildtype serum (n = 5) or CVF-treated serum (n = 4 mice)
Impact of C3-deficiency on the development of pathogen-specific antibody responses
C3-deficiency has previously been shown to impact the development of antigen-specific IgG responses in the host [33–36]. Prior studies by our group have shown that mice surviving C. rodentium infection produce detectable pathogen-specific titers of IgG2b and IgG2c by 15 days of infection, whereas mice succumbing to infection do not. Adoptive transfer of purified IgG from day 15 serum of infected mice can rescue CD4-deficient mice that otherwise succumb to infection, demonstrating the protective capacity of IgG.
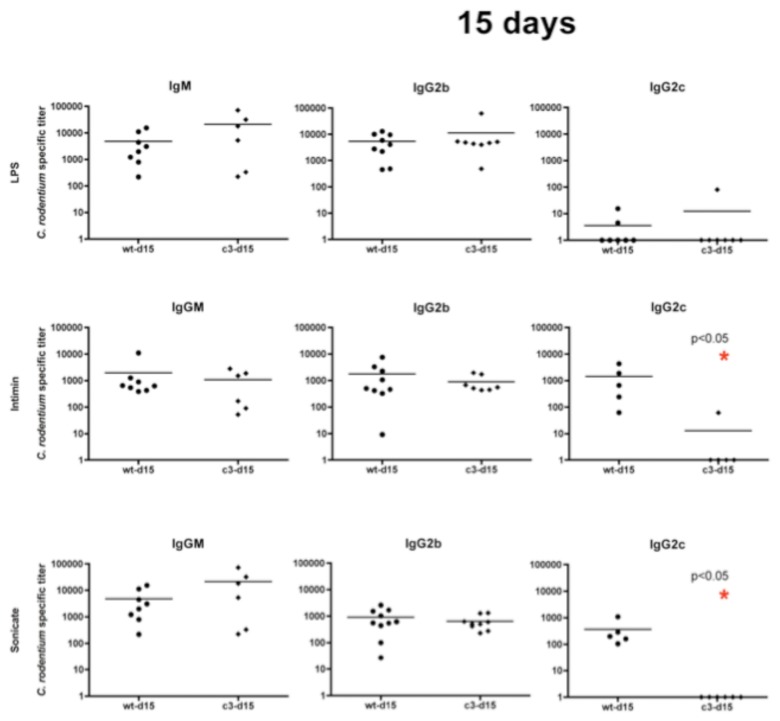
We evaluated the profile of pathogen-specific IgG isotypes in wildtype and C3-deficient mice prior to infection and at 15 days post-challenge to determine if lack of C3 prevented development of specific IgG. IgG2b and IgG2c IgM were assayed by ELISA to quantify titers against the following C. rodentium antigens: (1) purified LPS which contains the carbohydrate O-antigen, (2) his-tagged intimin, a primary virulence factor and protein antigen, and (3) sonicates of cultured C. rodentium which contain a mix of antigens and have served as capture material in prior studies [15, 16].
In C3–/– mice, IgM and IgG2b titers against all antigens are comparable to those of the wildtype. However, the IgG2c antibody response was reduced in C3–/– mice both against intimin (–1628 mean relative endpoint titer) and whole cells (–388 mean relative endpoint titer ) at day 15 during the infection (Fig. 6).
Fig. 6.
Complement and antibody responses: Demonstrating antibody titer against C. rodentium Intimin, LPS and sonicate in wildtype (circles) and C3–/– (diamonds) 14 days post-inoculation with C. rodentium. From left to right: the different antibodies that were tested (IgM, IgG2b and IgG2c). From top to bottom: the three antigens (Intimin, LPS and sonicate). Asterisk indicates a significant difference between wildtype and C3–/–
Discussion
The C. rodentium mouse model has helped our understanding of innate and adaptive immunity against A/E pathogens pathogens, which are the cause of worldwide morbidity and mortality [4]. The adaptive immune response involves CD4+ T cells and B cells via IgG secretion [14, 16]. Here we demonstrate that mucosal IgG and complement can act in the gut lumen and impact the course of C. rodentium infection in means of antimicrobial activity and stimulation of protective adaptive responses. Our data support the hypothesis that the secreted IgG together with complement acts across infected epithelium, and within the gut lumen.
As demonstrated in sepsis models, complement affects the development of Th1 IgG responses against protein antigens [34]. Herein, we have demonstrated that complement also affects development of Th1-depdendent antibody responses against a protein antigen from a mucosal pathogen. C3–/– mice demonstrated significantly lower titers of IgG2c against bacterial intimin, while deficiency had no effect on development of responses against LPS. The fact that C3–/– mice have delayed IgG2c responses against protein antigens is supported by other studies [37] demonstrating delay or absence of antibody responses against protein-antigen, but not carbohydrate antigens such as LPS O-antigen. Our results clearly demonstrate the importance of complement in development of responses against a known vaccine target for A/E pathogens. Further study is needed to determine whether complement-dependent IgG2c responses occur in the organized GALT, draining mesenteric lymph nodes or more systemic sites.
The importance of specific IgG in the resolution of C. rodentium infection is strengthened by a recent study using a using Lactobacillus casei-Intcv vaccine against C. rodentium infection. It was demonstrated that subcutaneous immunization with purified intcv induced concentration of ant-Intcv IgG1 ans IgG2a in sera, indicating a balanced Th1:Th2 profile [38]. In contrast, protection elicited by L. casei-Intcv vaccines was observed in conditions where no significant induction of anti-Intcv IgG in sera was detected [38]. These data implicate that other mechanisms are involved in the protection elicited by L. casei-Intcv oral vaccines as compared to subcutaneous immunization.
The overall importance of complement coming from mice bearing a deficiency in complement C3, C4, or CD21/CD35 is described and reviewed [36]. Our data are complementary and show that C3 complement is essential for mice surviving a C. rodentium infection. C3–/– mice could be rescued with C3-sufficient serum from uninfected mice, but not CVF-treated serum. As wildtype mice that were systemically treated with CVF show no survival defect during the infection, these findings suggest that mucosal stores of C3 may be protective in the absence of systemic protein. Alternatively, CVF-treatment may not be 100% effective at depleting systemically released C3 that had circulated into mucosal locations, or whether locally produced C3 from epithelial cells or myeloid cells, mediated protection.
Our data clearly demonstrate the protective role of complement, specifically C3, in the survival and resolution of mucosal infection with C. rodentium. Interestingly, we have demonstrated that both cleaved C3b and IgG isotypes are present on shed bacteria from a wildtype murine host, demonstrating that these factors enter the gut lumen in sufficient quantities to interact with luminal bacteria. This opsonization lead to growth inhibition of C. rodentium, and should be efficient for stimulation of cells of the adaptive immune system [39, 40].
In conclusion, the occurrence of multiple antibiotic resistance strains like EPEC favors the vaccine approach rather than conventional antibiotics treatment. Our data strengthen the finding that complement is important in humoral immunity and, thus, support the potential of development of specific vaccine against the emerging antibiotic resistant A/E pathogens.
Experimental procedures
GFP-tagged C. rodentium
GFP-CR was created by electroporating ClonTech’s eGFP plasmid into C. rodentium. Plasmid selection was on 100 µg/ml carbenicillin to overcome any induced expression of C. rodentium’s chromosomal ß-lactamase [41]. Mice were then infected with 5X108 CFU of CR-GFP. In vivo stability of GFP expression was first determined by plating fecal homogenates to MacConkey agar and replica plating colonies to LB agar to assess GFP in isolates. The plasmid GFP is stably expressed for a period of 2 weeks in vivo without antibiotic selection. Beyond 2 weeks, <90% of isolated C. rodentium maintain the tag. In the presence of ß-lactam selection, C. rodentium can de-repress its chromosomal lactamase, rendering useless in vivo antibiotic selection of the plasmid-expressed ß-lactamase (data not shown).
Survival studies
C3–/– mice were infected with C. rodentium and followed to define roles of complement in infection. Twenty-one-day-old wildtype and C3–/– C57BL/6 mice were orally infected with 5×108 CFU of C. rodentium and followed for 28 days. All studies performed under an IACUC protocol.
Cobra venom factor (CVF) treatment of wildtype mice
Wildtype mice received 2 doses of CVF IP, one day before infection, and maintenance IP doses on days 4, 8, 12, 16, and 20 after infection with C. rodentium. Control mice received saline only. To test CVF depletion of C3, serum from CVF, or saline-treated mice were collected at d15 of infection and incubated with 10 µl of rabbit RBCs (Pelfreez) for 30 min at 37 °C. After centrifugation, supernatants were analyzed at OD 405 to measure hemolysis. Mouse serum lyses rabbit RBCs through alternative pathway activation, while CVF-treated serum does not.
Reconstitution of C3–/– mice with systemic C3
Mice received 250 µl of serum on the day of infection with C. rodentium and on days 5, 10, 15, and 20 post-inoculation.
Fecal FACS
Fecal bacteria were stained for surface-bound C3 in mice in the following manner: (1) wildtype, (2) CVF-treated C57BL/6, or (3) control antibody-deficient JhD–/– Balb/c, which lack B cells, were infected with GFP-CR as described. Fecal pellets were collected on days 3, 7, 10, 13, and 15 post-inoculation and vortexed in 1ml PBS to obtain homogenates. A 3-min low-speed spin pelleted larger material. Supernatants, enriched for fecal bacteria, were blocked at 4 °C with 5% heat-treated donkey serum in PBS, followed by staining with rat anti-mouse C3, sheep anti-mouse C4 (Cell Sciences) or isotype control. After washing, bacterial cells were stained with donkey anti-rat-PE or donkey anti-sheep-PE antibody, washed and fixed in 2% paraformaldehyde. Bacteria were analyzed by flow cytometry by gating on GFP+ events and analyzing signal in FL-2 for C3, C4, and MBL-C bound to the bacterial cell surface. Lack of available mouse reagents for alternative pathway components prevents analysis of alternative activation with this method.
Localization of C3 and IgG in infected colon
Frozen sections of acutely infected mouse colon stained with Hoescht nuclear dye (blue), C3 antibody (red) and IgG (green).
Complement-dependent lysis
Mid-log phase C. rodentium (5×103) were added to 100 µl of Hanks buffer salt solution (HBSS)+2 mM CaCl2+2 mM MgCl2. Aliquots were incubated alone, with 5 µg specific or isotype control antibody, or with normal or heat-treated antibody-deficient mouse serum as a source of complement (40% final volume of serum in assays). Serum heated at 56 °C for 1 h inactivated complement proteins. Bacteria were also incubated in immune or pre-immune serum from C57BL/6 mice (Fig. 5C). Bacteria were incubated for 30 min at 37 °C to measure lytic effects in the absence of bacterial growth, and for two hours to assess effects on dividing cultures in 40% serum. After incubation, reactions were serially diluted and plated to LB agar to quantify remaining CFU. Experiments were repeated in triplicate and findings subjected to Kruskal-Wallis non-parametric test to identify significant differences among groups.
Purified his-tagged beta-intimin of C. rodentium
A vector expressing the his-tagged C-terminus of C. rodentium beta-intimin was kindly provided by Dr. Jim Sinclair (USAMRIID) [42]. Intimin’s C-terminus is surface exposed and facilitates bacterial binding to TIr. Expression in E. coli was induced by incubating cells in 2 mM IPTG at 37 °C for 6 h, binding of his-tagged protein to nickel columns (Qiagen), subsequent dialysis of eluate against PBS to obtain purified his-intimin for use in ELISAs.
Sonicates from C. rodentium
Stationary phase C. rodentium were washed with PBS+5 mM EDTA, pelleted, and resuspended in PBS, sonicated and centrifuged pellets were filter sterilized over 0.22-µM filters prior to use in ELISAs.
LPS purification of C. rodentium
LPS was isolated by the hot phenol method. A 100 ml of C. rodentium stationary phase were washed and resuspended in 10 ml 2:1 Ccl3: MetOH for overnight incubation. Hereafter, cells were pelleted and incubated for 20 min with 68 C phenol. Hereafter, the aquatious phase was re-extracted with hot phenol twice and thereafter dialyzed against water, followed by RNAse, DNAse, and proteinase treatment. LPS was detected using a Limulus amebocyte lysate (LAL) assay (Sigma-Aldrich) and visualized by silver staining after acrylamide gel electrophoreses.
ELISA
Polystyrene microtiter plates (Immunoplate Maxisorb; Nunc) were coated o.n. with either C. rodentium LPS, C. rodentium Intimin (1 mg/ml) or C. rodentium sonicate (1 mg/ml protein) as antigen. The next day plates were washed twice with PBS+0.5% Tween (PBST). Serum was added to the plates in serial dilutions and incubated for 2 h. Hereafter the plates were washed five times with PBST and the specific immunoglobuline conjungated to AP (Zymed) was added to the plates in a 1:1000 dilution and incubated for another hour. Plates were washed five more times with PBST and the reaction was developed with Alkaline Phosphatase Yellow (pNPP) (Sigma).
Acknowledgments
We want to thank Colleen Cavanaugh for her critical reading of the manuscript and the many helpful comments. Work supported by a pilot feasibility grant from the Harvard Digestive Diseases Center (NIH Award P30-DK034854; CMC, LB), R01-HD061916 (LB, CB).
Contributor Information
C. Belzer, 1Department of Pathology, Harvard University Medical School/Brigham and Women’s Hospital, Boston, MA 02115, USA.
Q. Liu, 1Department of Pathology, Harvard University Medical School/Brigham and Women’s Hospital, Boston, MA 02115, USA.
M. C. Carroll, 2Department of Pediatrics (Pathology), Immune Disease Institute Harvard Medical School, Boston, MA 02115, USA.
L. Bry, 1Department of Pathology, Harvard University Medical School/Brigham and Women’s Hospital, Boston, MA 02115, USA.
References
- 1.Wales AD, Woodward MJ, Pearson GR. Attaching-effacing bacteria in animals. J Comp Pathol. 2005 Jan;132(1):1–26. doi: 10.1016/j.jcpa.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schauer DB, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun. 1993 Jun;61(6):2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luperchio SA, Schauer DB. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 2001 Apr;3(4):333–340. doi: 10.1016/s1286-4579(01)01387-9. [DOI] [PubMed] [Google Scholar]
- 4.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010 Jun 5;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 5.Campellone KG. Cytoskeleton-modulating effectors of enteropathogenic and enterohaemorrhagic Escherichia coli: Tir, EspFU and actin pedestal assembly. FEBS J. 2010 Jun;277(11):2390–2402. doi: 10.1111/j.1742-4658.2010.07653.x. [DOI] [PubMed] [Google Scholar]
- 6.Hartland EL, Batchelor M, Delahay RM, Hale C, Matthews S, Dougan G, Knutton S, Connerton I, Frankel G. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol Microbiol. 1999 Apr;32(1):151–158. doi: 10.1046/j.1365-2958.1999.01338.x. [DOI] [PubMed] [Google Scholar]
- 7.Brady MJ, Campellone KG, Ghildiyal M, Leong JM. Enterohaemorrhagic and enteropathogenic Escherichia coli Tir proteins trigger a common Nck-independent actin assembly pathway. Cell Microbiol. 2007 Sep;9(9):2242–2253. doi: 10.1111/j.1462-5822.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- 8.Cleary J, Lai LC, Shaw RK, Straatman-Iwanowska A, Donnenberg MS, Frankel G, Knutton S. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology. 2004 Mar;150(Pt 3):527–538. doi: 10.1099/mic.0.26740-0. [DOI] [PubMed] [Google Scholar]
- 9.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005 Dec;7(12):1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 10.Simmons CP, Clare S, Ghaem-Maghami M, Uren TK, Rankin J, Huett A, Goldin R, Lewis DJ, MacDonald TT, Strugnell RA, Frankel G, Dougan G. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2003 Sep;71(9):5077–5086. doi: 10.1128/IAI.71.9.5077-5086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonçalves NS, Ghaem-Maghami M, Monteleone G, Frankel G, Dougan G, Lewis DJ, Simmons CP, MacDonald TT. Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis. Infect Immun. 2001 Nov;69(11):6651–6659. doi: 10.1128/IAI.69.11.6651-6659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dann SM, Spehlmann ME, Hammond DC, Iimura M, Hase K, Choi LJ, Hanson E, Eckmann L. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J Immunol. 2008 May 15;180(10):6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiomi H, Masuda A, Nishiumi S, Nishida M, Takagawa T, Shiomi Y, Kutsumi H, Blumberg RS, Azuma T, Yoshida M. Gamma interferon produced by antigen-specific CD4+ T cells regulates the mucosal immune responses to Citrobacter rodentium infection. Infect Immun. 2010 Jun;78(6):2653–2666. doi: 10.1128/IAI.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, Finlay BB, Schreiber JR, Varki NM, Kagnoff MF, Eckmann L. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect Immun. 2004 Jun;72(6):3315–3324. doi: 10.1128/IAI.72.6.3315-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bry L, Brigl M, Brenner MB. CD4+-T-cell effector functions and costimulatory requirements essential for surviving mucosal infection with Citrobacter rodentium. Infect Immun. 2006 Jan;74(1):673–681. doi: 10.1128/IAI.74.1.673-681.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bry L, Brenner MB. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J Immunol. 2004 Jan 1;172(1):433–441. doi: 10.4049/jimmunol.172.1.433. [DOI] [PubMed] [Google Scholar]
- 17.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011 Jan 28;34(1):122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda A, Yoshida M, Shiomi H, Ikezawa S, Takagawa T, Tanaka H, Chinzei R, Ishida T, Morita Y, Kutsumi H, Inokuchi H, Wang S, Kobayashi K, Mizuno S, Nakamura A, Takai T, Blumberg RS, Azuma T. Fcgamma receptor regulation of Citrobacter rodentium infection. Infect Immun. 2008 Apr;76(4):1728–1737. doi: 10.1128/IAI.01493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004 Jun;20(6):769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003 Apr 1;170(7):3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, Wakatsuki Y, Roopenian DC, Mizoguchi A, Lencer WI, Blumberg RS. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006 Aug;116(8):2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003 Dec 1;171(11):6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 23.Kochi SK, Johnson RC, Dalmasso AP. Facilitation of complement-dependent killing of the Lyme disease spirochete, Borrelia burgdorferi, by specific immunoglobulin G Fab antibody fragments. Infect Immun. 1993 Jun;61(6):2532–2536. doi: 10.1128/iai.61.6.2532-2536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan R, Clynes R, Oh J, Ravetch JV, Scharff MD. Antibody-mediated modulation of Cryptococcus neoformans infection is dependent on distinct Fc receptor functions and IgG subclasses. J Exp Med. 1998 Feb 16;187(4):641–648. doi: 10.1084/jem.187.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro S, Beenhouwer DO, Feldmesser M, Taborda C, Carroll MC, Casadevall A, Scharff MD. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun. 2002 May;70(5):2598–25604. doi: 10.1128/IAI.70.5.2598-2604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho HM, Teel LD, Kokai-Kun JF, O'Brien AD. Antibody against the carboxyl terminus of intimin alpha reduces enteropathogenic Escherichia coli adherence to tissue culture cells and subsequent induction of actin polymerization. Infect Immun. 2005 Apr;73(4):2541–2546. doi: 10.1128/IAI.73.4.2541-2546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molmenti EP, Ziambaras T, Perlmutter DH. Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem. 1993 Jul 5;268(19):14116–14124. [PubMed] [Google Scholar]
- 28.Passwell JH, Schreiner GF, Wetsel RA, Colten HR. Complement gene expression in hepatic and extrahepatic tissues of NZB and NZB x W (F1) mouse strains. Immunology. 1990 Oct;71(2):290–294. [PMC free article] [PubMed] [Google Scholar]
- 29.Uemura K, Saka M, Nakagawa T, Kawasaki N, Thiel S, Jensenius JC, Kawasaki T. L-MBP is expressed in epithelial cells of mouse small intestine. J Immunol. 2002 Dec 15;169(12):6945–6950. doi: 10.4049/jimmunol.169.12.6945. [DOI] [PubMed] [Google Scholar]
- 30.Andoh A, Fujiyama Y, Sakumoto H, Uchihara H, Kimura T, Koyama S, Bamba T. Detection of complement C3 and factor B gene expression in normal colorectal mucosa, adenomas and carcinomas. Clin Exp Immunol. 1998 Mar;111(3):477–483. doi: 10.1046/j.1365-2249.1998.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernet-Camard MF, Coconnier MH, Hudault S, Servin AL. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut. 1996 Feb;38(2):248–253. doi: 10.1136/gut.38.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasojima K, Kilgore KS, Washington RA, Lucchesi BR, McGeer PL. Complement gene expression by rabbit heart: upregulation by ischemia and reperfusion. Circ Res. 1998 Jun 15;82(11):1224–1230. doi: 10.1161/01.res.82.11.1224. [DOI] [PubMed] [Google Scholar]
- 33.Verschoor A, Brockman MA, Gadjeva M, Knipe DM, Carroll MC. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J Immunol. 2003 Nov 15;171(10):5363–5371. doi: 10.4049/jimmunol.171.10.5363. [DOI] [PubMed] [Google Scholar]
- 34.Mold C, Rodic-Polic B, Du Clos TW. Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fc gamma receptors. J Immunol. 2002 Jun 15;168(12):6375–6381. doi: 10.4049/jimmunol.168.12.6375. [DOI] [PubMed] [Google Scholar]
- 35.Díaz de Ståhl T, Dahlstrom J, Carroll MC, Heyman B. A role for complement in feedback enhancement of antibody responses by IgG3. J Exp Med. 2003 May 5;197(9):1183–1190. doi: 10.1084/jem.20022232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll MC. The complement system in B cell regulation. Mol Immunol. 2004 Jun;41(2-3):141–146. doi: 10.1016/j.molimm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Marsh JE, Farmer CK, Jurcevic S, Wang Y, Carroll MC, Sacks SH. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation. 2001 Oct 15;72(7):1310–1318. doi: 10.1097/00007890-200110150-00022. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira PC, da Silva JB, Piazza RM, et al. Immunization of mice with Lactobacillus casei expressing a beta-intimin fragment reduces intestinal colonization by Citrobacter rodentium. Clin Vaccine Immunol. 2011 doi: 10.1128/CVI.05262-11. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carroll MC. Complement and humoral immunity. Vaccine. 2008;26(Suppl 8):I28–I33. doi: 10.1016/j.vaccine.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pangburn MK, Ferreira VP, Cortes C. Discrimination between host and pathogens by the complement system. Vaccine. 2008;26(Suppl 8):I15–I21. doi: 10.1016/j.vaccine.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrella S, Clermont D, Casin I, Jarlier V, Sougakoff W. Novel class A beta-lactamase Sed-1 from Citrobacter sedlakii: genetic diversity of beta-lactamases within the Citrobacter genus. Antimicrob Agents Chemother. 2001 Aug;45(8):2287–2298. doi: 10.1128/AAC.45.8.2287-2298.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinclair JF, O'Brien AD. Intimin types alpha, beta, and gamma bind to nucleolin with equivalent affinity but lower avidity than to the translocated intimin receptor. J Biol Chem. 2004 Aug 6;279(32):33751–33758. doi: 10.1074/jbc.M401616200. [DOI] [PubMed] [Google Scholar]