Abstract
Chronic inflammation is an important component of the fibroproliferative changes that characterise pulmonary hypertensive vasculopathy. Fibrocytes contribute to tissue remodelling in settings of chronic inflammation, including animal models of pulmonary hypertension (PH). We sought to determine whether circulating fibrocytes were increased in children and young adults with PH.
26 individuals with PH and 10 with normal cardiac anatomy were studied. Fresh blood was analysed by flow cytometry for fibrocytes expressing CD45 and procollagen. Fibrocyte numbers were correlated to clinical and haemodynamic parameters, and circulating CC chemokine ligand (CCL)2 and CXC chemokine ligand (CXCL)12 levels.
We found an enrichment of circulating fibrocytes among those with PH. No differences in fibrocytes were observed among those with idiopathic versus secondary PH. Higher fibrocytes correlated to increasing mean pulmonary artery pressure and age, but not to length or type of treatment. Immunofluorescence analysis confirmed flow sorting specificity. Differences in plasma levels of CCL2 or CXCL12, which could mobilise fibrocytes from the bone marrow, were not found.
We conclude that circulating fibrocytes are significantly increased in individuals with PH compared with controls. We speculate that these cells might play important roles in vascular remodelling in children and young adults with pulmonary hypertension.
Keywords: Fluorescence-activated cell sorting, inflammation, mononuclear phagocyte, progenitor cells, vascular remodelling
Chronic pulmonary hypertension (PH) is distinguished by cellular and structural changes in the walls of pulmonary arteries. Intimal thickening and fibrosis, medial hypertrophy and fibroproliferative changes in the adventitia are common [1]. In adults and children, virtually all of these changes are characterised by increased numbers of cells expressing smooth muscle α-actin [2] and by perivascular accumulation of inflammatory cells [3, 4]. The source of these cells remains unclear but an origin from circulating progenitors has been postulated [4, 5]. In adults, the number and differentiation capability of endothelial progenitor cells (EPCs) has been examined, though no consensus has been reached as to a role for these cells in the pathogenesis of the disease [6, 7]. There are fewer data available regarding circulating cells of any kind in children and young adults with PH. Recently, it was reported that while no significant differences in early or late EPC endothelial colony-forming units exists between reversible and irreversible PH [8], late EPCs were found to be increased in children receiving treprostinil compared with those on endothelin receptor antagonists or phosphodiesterase inhibitors [6]. These cells had increased angiogenic and hyperproliferative potential, which was hypothesised to partly explain the clinical benefits of prostanoids in pulmonary arterial hypertension (PAH). Nevertheless, severe PH is confoundingly associated with vascular pruning in the periphery, with concomitant inflammation, hypertrophy and hyperplasia in larger bronchovascular structures [1]. Periarterial infiltrates of macrophages and T-lymphocytes have been documented in the setting of PH [9]. Within similar inflammatory infiltrates in hypoxic animals, c-kit+ progenitor cells have also been identified [10, 11]. The origin of inflammatory/progenitor cells and the mechanisms by which they contribute to the pulmonary vascular remodelling and inflammation observed in children and young adults with PH have not been thoroughly investigated.
Emerging experimental evidence indicates that circulating mesenchymal precursors may contribute significantly to vascular remodelling processes [12]. The development of vascular remodelling probably requires lung infiltration of mononuclear phagocytic cells. Depletion of circulating monocytic cells using clodronate-containing liposomes prevents PH in large and small animal models [13]. Recently, several studies have pointed to the importance of fibrocytes, a subpopulation of mononuclear phagocytes, in the development of cancer and inflammatory disorders [14]. These cells derive from haematopoietic precursor cells and promote fibrogenic and chronic inflammatory processes [15]. Fibrocytes are characterised by expression of CD45 and collagen, and are associated with poor prognosis in patients with idiopathic pulmonary fibrosis [16]. In the lung, they are recruited to sites of injury where they participate directly (by producing cytokines) or indirectly (by inducing resident cells to elicit cytokines) in persistent inflammatory remodelling and proangiogenic signalling [17]. Interestingly, use of the stable prostacyclin analogue treprostinil significantly reduced the lung recruitment of fibrocytes in hypoxic mice compared with normoxic mice [18]. Treprostinil also reduced right ventricular systolic pressure and slightly reduced vascular remodelling, but failed to reverse the right ventricular hypertrophy in these mice with hypoxic PH. This important study highlights the possibility that measurement and therapeutic targeting of circulating fibrocytes might represent a promising frontier in clinical management of PH.
Since circulating fibrocytes are elevated in adults with chronic inflammation and since chronic inflammation is associated with PH, we hypothesised that children and young adults with PH would manifest increased numbers of blood fibrocytes. The continual influx of these cells might thereby sustain a cycle of perpetual inflammation and vascular remodelling in the lung. To begin to test this, we used flow cytometry of peripheral blood to quantify the number of fibrocytes in individuals with PH undergoing treatment with a variety of agents, including prostacyclin analogues, compared with controls. We found significant increases in fibrocytes in PH blood that correlated with increasing mean pulmonary artery pressure (P̄pa) and age. It is concluded that PH is associated with elevated circulating fibrocytes that might play important roles in vascular remodelling.
METHODS
Subjects and blood collection
The Colorado Multiple Institutional Review Board (University of Colorado Denver, Denver, CO, USA) approved the study, and consent and assent was obtained from all patients where appropriate. The demographic data for all subjects are presented in tables 1 and 2. 5 mL blood was drawn from the femoral vein during initial cardiac catheterisation in all patients. The PAH group consisted of children and young adults who were <18 yrs of age at time of diagnosis with PH, from World Health Organization classification group I, defined as either idiopathic PAH (IPAH), heritable PAH or PAH associated with congenital heart disease (APAH-CHD) [2]. The control group included children <18 yrs of age with a structurally normal heart undergoing heart arrhythmia ablation before the onset of the procedure. All patients undergoing ablation were in normal sinus rhythm at the start of the catheterisation, when the blood was drawn, and had a normal echocardiogram.
TABLE 1.
Pulmonary hypertension (PH) subject data
| Sex | PH, WHO class |
Age yrs |
Date of diagnosis day/month/yr |
P̄pa at diagnosis mmHg |
Date of sample day/ month/yr |
P̄pa at date of sample mmHg |
Fibrocytes | MCP-1 pg·mL−1 |
SDF-1 pg·mL−1 |
Medications at transition |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | × 106 cells·mL−1 | |||||||||||
| 1 | F | IPAH, 1 | 20 | 7/2/2007 | 32 | 14/7/2010 | 30 | 0.9 | 0.21 | 218 | 2360 | Ca2+ blocker |
| 2 | M | IPAH, 2 | 15 | 25/9/1995 | NA | 14/7/2010 | 37 | 7.05 | 0.7 | 376.3 | 1394.4 | Sildenafil+treprostinil (i.v.)+ ambrisentan |
| 3 | F | IPAH, 2 | 9 | 29/6/2005 | 90 | 14/7/2010 | 28 | 4.23 | 0.55 | 201.5 | 2004 | Epoprostenol (i.v.)+ ambrisentan |
| 4 | F | SPAH, 2 | 15 | 21/5/1999 | NA | 15/7/2010 | 54 | 3.8 | 0.42 | 242.6 | 1370.1 | Tadalafil+treprostinil (i.v.)+ ambrisentan |
| 5 | M | SPAH, 4 | 19 | 21/03/2002 | 54 | 15/7/2010 | 55 | 7.49 | 0.75 | 271.7 | 1826.8 | Tadalafil+epoprostenol (i.v.)+ ambrisentan |
| 6 | F | SPAH, 2 | 15 | 15/05/2002 | 71 | 21/7/2010 | 51 | 6.74 | 0.65 | 243 | 2022 | Tadalafil+treprostinil (inhaled) |
| 7 | F | IPAH, 2 | 10 | 7/10/2008 | 58 | 21/7/2010 | 35 | 4.54 | 0.56 | 396.2 | 1909.8 | Tadalafil+ambrisentan |
| 8 | M | SPAH, 3 | 17 | 6/10/1992 | NA | 11/8/2010 | 43 | 1.13 | 0.26 | 322.1 | 2260 | Tadalafil+treprostinil (inhaled) |
| 9 | F | SPAH, 1 | 12 | 21/11/1001 | 19 | 11/8/2010 | 23 | 6.3 | 0.43 | 353.4 | 2272 | Ca2+ blocker |
| 10 | F | SPAH, 1 | 9 | 1/2/2007 | 56 | 11/8/2010 | 18 | 1.3 | 0.19 | 202.5 | 1530.6 | Sildenafil+ambrisentan+ Ca2+ blocker |
| 11 | M | IPAH, 2 | 2 | 20/08/2008 | NA | 11/8/2010 | 17 | 0.15 | 0.11 | 305.5 | 1175 | Sildenafil+treprostinil (i.v.) |
| 12 | M | IPAH, 1 | 15 | 1/8/2000 | NA | 11/8/2010 | 20 | 1.9 | 0.16 | 350 | 2764 | Tadalafil+iloprost+ ambrisentan |
| 13 | F | SPAH, 2 | 12 | 19/5/2005 | NA | 11/8/2010 | 37 | 1.65 | 0.17 | 202.6 | 2408 | Sildenafil+bosentan |
| 14 | F | SPAH, 1 | 5 | 26/8/2009 | 38 | 25/8/2010 | 26 | 5.68 | 0.49 | 343.8 | 1732 | Ca2+ blocker |
| 15 | F | IPAH, 1 | 3 | 20/9/2007 | NA | 25/8/2010 | 24 | 3.8 | 0.36 | 376.9 | 1886.2 | Sildenafil |
| 16 | M | SPAH, 2 | 13 | 11/10/2001 | 73 | 25/8/2010 | 56 | 14.2 | 0.85 | 377.3 | 1963.2 | Sildenafil+treprostinil (i.v.)+ambrisentan |
| 17 | F | SPAH, 3 | 18 | 1/3/1996 | NA | 8/9/2010 | 61 | 7.66 | 0.72 | 330.3 | 2254 | Sildenafil+treprostini (i.v.)+ bosentan |
| 18 | M | IPAH, 1 | 15 | 26/10/2005 | 70 | 8/9/2010 | 44 | 6.2 | 0.46 | 399.5 | 2414 | Tadalafil+ambrisentan |
| 19 | M | SPAH, 4 | 17 | 19/2/1997 | NA | 4/10/2010 | 80 | 4.5 | 0.39 | 343 | 2692 | Tadalafil+epoprostenol (i.v.)+bosentan |
| 20 | M | SPAH, 2 | 22 | 19/12/1995 | NA | 13/10/2010 | 32 | 4.5 | 0.46 | 240.2 | 1957.2 | Ambrisentan |
| 21 | M | IPAH, 2 | 10 | 17/12/2004 | 106 | 13/10/2010 | 74 | 6.5 | 0.66 | 327.1 | 1791.4 | Treprostinil (i.v.)+ tadalafil |
| 22 | M | SPAH, 2 | 4 | 29/05/2007 | 20 | 13/10/2010 | 14 | 0.4 | 0.13 | 319.6 | 1904 | None |
| 23 | M | SPAH, 2 | 2 | 29/10/2009 | 28 | 21/10/2010 | 28 | 2.2 | 0.24 | 316.6 | 1495 | Sildenafil |
| 24 | M | SPAH, 1 | 1 | 22/12/2008 | NA | 10/11/2010 | 19 | 0.6 | 0.16 | 212 | 1429.8 | Sildenafil |
| 25 | M | SPAH, 1 | 3 | 8/3/2010 | NA | 10/11/2010 | 32 | 3 | 0.37 | 382.4 | 1933.6 | Sildenafil |
| 26 | F | IPAH, 1 | 11 | 10/10/2005 | 48 | 10/11/2010 | 41 | 4 | 0.46 | 293.2 | 1785.4 | Tadalafil+bosentan |
WHO: World Health Organization; P̄pa mean pulmonary artery pressure; MCP: monocyte chemoattractant protein; SDF: stromal derived factor; F: female; M: male; IPAH: idiopathic pulmonary arterial hypertension; SPAH: secondary pulmonary arterial hypertension; NA: not available (patient not catheterised at the time).
TABLE 2.
Control subject data
| Sex | Diagnosis | Age yrs | Date of diagnosis day/month/yr |
Date of sample day/month/yr |
Fibrocytes | MCP-1 pg·mL−1 | SDF-1 pg·mL−1 | ||
|---|---|---|---|---|---|---|---|---|---|
| % | × 106 cells·mL−1 | ||||||||
| 1 | M | WPW | 8 | 7/2/2007 | 14/7/1010 | 1 | 0.13 | 195.2 | 1447.6 |
| 2 | M | WPW, closing VSD, no PH | 13 | 25/9/1995 | 14/7/1010 | 0.6 | 0.25 | 272.4 | 2236 |
| 3 | F | Arrhythmia (SVT) | 10 | 29/6/2005 | 14/7/1010 | 0.9 | 0.1 | 267 | 1672.8 |
| 4 | F | Arrhythmia (SVT) | 17 | 21/5/1999 | 15/7/2010 | 0.8 | 0.3 | 293.1 | 1702.4 |
| 5 | M | Arrhythmia (SVT) | 14 | 21/3/2002 | 15/7/2010 | 0.9 | 0.13 | 242.8 | 1880.2 |
| 6 | F | WPW | 9 | 15/5/2002 | 21/7/2010 | 1 | 0.32 | 318.7 | 2088 |
| 7 | M | Arrhythmia (SVT) | 13 | 7/10/2008 | 21/7/2010 | 1 | 0.15 | 273.5 | 2460 |
| 8 | M | WPW | 14 | 6/10/1992 | 11/8/2010 | 0.9 | 0.1 | 346.5 | 2496 |
| 9 | F | Arrhythmia (SVT) | 17 | 21/11/2001 | 11/8/2010 | 1 | 0.32 | 259.4 | 1933.6 |
| 10 | M | Arrhythmia (SVT) | 14 | 1/2/2007 | 11/8/2010 | 0.3 | 0.33 | 302 | 3084 |
MCP: monocyte chemoattractant protein; SDF: stromal derived factor; M: male; F: female; WPW:Wolff–Parkinson–White syndrome; VSD: ventricular septal defect; PH: pulmonary hypertension; SVT: supraventricular tachycardia.
Haemodynamic parameters and echocardiographic data were obtained at the time of initial catheterisation, then later at therapeutic assessment. Fresh, never-frozen blood (5 mL) was prepared without a Ficoll gradient, as described by Moeller et al. [16]. Buffy coat cells were removed from blood centrifuged for 10 min at 450 × g (1,200 rpm) at room temperature. Following red blood cell lysis by addition of ammonium chloride (0.15 M, pH 7.3), leukocytes were washed, verified for viability by trypan blue exclusion and prepared to a concentration of 1 × 106 cells·mL−1 for antibody labelling and flow cytometry.
Flow cytometric analysis
Cells were labelled for flow cytometry using a Pacific Blue-conjugated antibody against CD45 (PB986; BD Biosciences, Franklin Lakes, NJ, USA), fixed and permeabilised according to the manufacturer’s protocol (Fix/Perm Solution 554714; BD Biosciences), and then cells were labelled with rabbit anti-human procollagen I (MAB1913; Millipore, Billerica, MA, USA). Secondary antibody (fluorescein isothiocyanate (FITC)-conjugated goat antirabbit immunoglobulin G; AP132F; Millipore) was applied according to the manufacturer’s protocols. The negative thresholds for CD45–Pacific Blue and the FITC isotype control were set using isotype control-labelled cells from both normal and PH subjects exactly as described by Mathai et al. [19]. All subsequent samples were gated for the CD45+ region. Data were recorded as fibrocyte numbers per millilitre of blood and as a percentage of total leukocyte (CD45+) counts. A minimum of 200,000 events was used to generate each histogram. Flow cytometry was performed on an FC500 flow cytometer using CXP software (Beckman Coulter Inc., Brea, CA, USA).
Cytospin and immunofluorescence
In separate experiments, cells were resuspended to 0.5 × 106 cells·mL−1 and 200 µL was centrifuged at 250 × g (800 rpm) for 3 min. Cytospins were fixed in ice cold 1/1 acetone/methanol for 10 min then rehydrated in phosphate buffer prior to addition of antibody. Prepared cells were stained with antibodies exactly as described earlier for flow cytometry. Antibody isotype negative controls were included with each sample group, as was pre-adsorption of antibody to its cognate antigen, which was supplied by the manufacturers. Slides were mounted in Vectashield plus 4′,6-diamidino-2-phenylindole (DAPI) medium (Vector Laboratories Ltd, Burlingame, CA, USA). Images were acquired at room temperature using a Zeiss Axiovert S100 microscope fitted with Zeiss 20 × 0.4na and 10 × 0.3na objective lenses and an Axiocam camera (Carl Zeiss Inc., Thornwood, NY, USA). Acquisition of images was performed using Axiovision 4.6 software (Carl Zeiss Inc.). Immunopositive cells (green) were counted in five fields from three cytospin preparations each from three control preparations and three PH preparations, then divided by the total number of DAPI+ cells.
Clinical data
Charts were reviewed, and data on demographics, haemodynamics and the 6-min walk test were extracted. All information describing the patient populations is presented in tables 1 and 2.
Statistical analysis
All values were expressed as mean±sem. Comparison between groups was performed using GraphPad software (GraphPad Software for Science Inc., San Diego, CA, USA). An unpaired t-test was performed for two-group comparisons while a Pearson r correlation coefficient was used to correlate fibrocyte number to clinical and haemodynamic parameters. Statistical significance was defined as p<0.05.
RESULTS
Fibrocyte quantification
Circulating fibrocytes are increased in several chronic inflammatory disorders, including fibrotic lung disease in adults [12]. Furthermore, higher numbers of these cells correlate with disease severity and poor outcome [16]. We hypothesised that children and young adults with severe PH would harbour higher numbers of circulating fibrocytes. Indeed, we found that those with PH had significantly higher numbers and percentages of fibrocytes in peripheral blood compared with controls (mean±sd 0.42 × 106±0.21 × 106 CD45+ procollagen I+ cells per millilitre (4.24±3.06%) (n=26) versus 0.21 × 106±0.10 × 106 CD45+ procollagen I+ cells per millilitre (0.84±0.23%) (n=10), respectively) (figs 1, and 2a and b). No statistically significant differences were observed in fibrocyte numbers among those with IPAH compared with secondary PH. No differences were observed in fibrocyte numbers among females and males with PH. Individuals untreated or undergoing one treatment (Ca2+ channel blockers, sildenafil or ambrisentan) had a mean fibrocyte/CD45+ count of 3.35%, while for patients treated with two drugs it was 3.90%, and for patients undergoing three or more treatments it was 5.99%. Patients with the most severe disease were those receiving three or more medications.
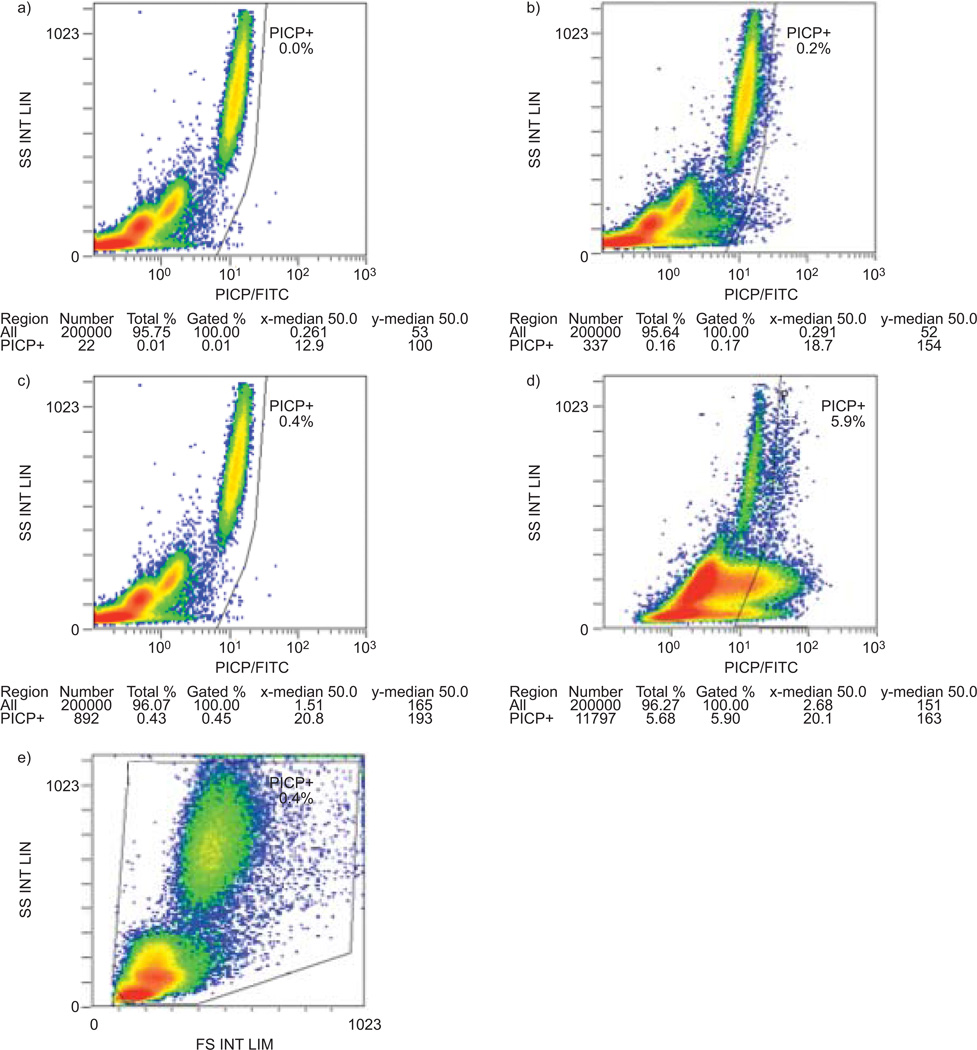
FIGURE 1.
Representative flow cytometric analysis for circulating CD45+/procollagen I C-terminal peptide (PICP)+ cells in controls and pulmonary hypertension (PH) patients. a) CD45/Pacific Blue (PB) versus intracellular isotype control/fluorescein isothiocyanate (FITC) analysis on blood from a representative normal subject. This control was used to set the negative gates. b) CD45+/PICP+ cells in normal blood. x axis: procollagen stained with goat anti-rabbit-FITC secondary antibody. c) CD45/PB versus intracellular isotype control/FITC analysis on blood from a patient with PH. This control was used to set the negative gates for the PH sample, same axes as in panel a. d) CD45+/PICP+ cells in PH blood, same axes as in panel b. e) Side scatter (SS) versus forward scatter (FS) analysis of buffy coat cells from the sample in panel d).
FIGURE 2.
Circulating fibrocytes are enriched in pulmonary hypertension (PH) compared with controls and correlate to mean pulmonary artery pressure (P̄pa) and age. Fibrocyte counts for 10 controls and 26 PH patients were mean±sd a) 0.84±0.23% versus 4.24±3.06%, and b) 0.21 × 106±0.10 × 106 versus 0.42 × 106±0.21 × 106 CD45+ procollagen I+ cells per millilitre, respectively. Analysis of correlation of fibrocyte c and e) differential and d and f) absolute cell counts in patients with PH to c and d) P̄pa and e and f) age. e) Note that the correlation between age and fibrocytes expressed as a percentage did not reach significance at α=0.05. c) r=0.575, p<0.05; d) r=0.695, p<0.05; e) r=0.354, p>0.05; f) r=0.401, p<0.05. *: p<0.05 versus control.
Fibrocyte correlation to clinical and haemodynamic parameters
Fibrocyte numbers showed a statistically significant correlation to P̄pa (r=0.575, p<0.05) and increasing age (r=0.354, p<0.05) (fig. 2c–f). No correlations were found between fibrocyte numbers and either sex, 6-min walk distance, plasma brain natriuretic peptide (BNP), N-terminal pro-BNP or duration of treatment (data not shown). We found no statistically significant differences in plasma levels of monocyte chemoattractant protein-1 (305.3±64.7 versus 276.7±39.7 pg·mL−1) or stromal derived factor (SDF)-1 (1,943.6±398.5 versus 2,100.1±459.9 pg·mL−1) between those with PH versus controls, respectively.
Immunophenotypic confirmation of fibrocytes
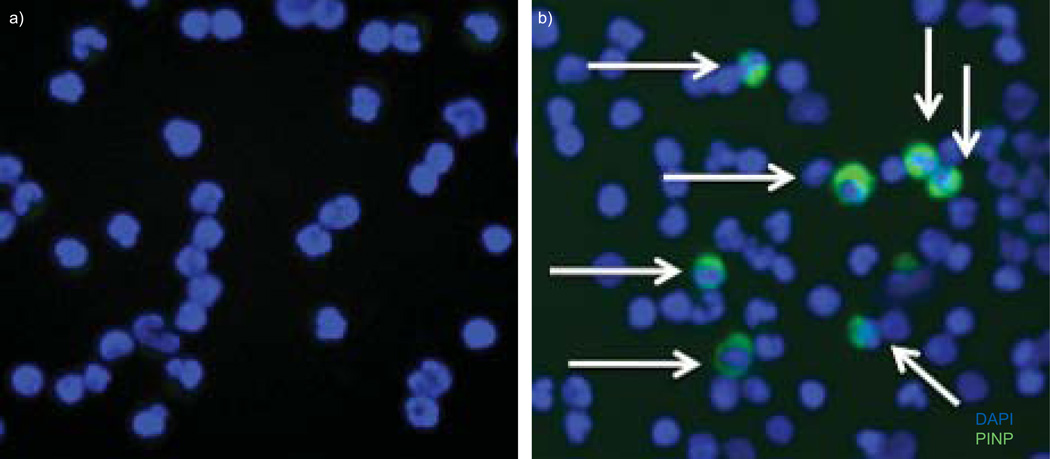
We sought to confirm the flow sorting results for fibrocytes from control and PH subjects. Using the same procedures as for flow cytometry, we verified their phenotype by immunofluorescence. As expected, a percentage of cells from PH patients, but not controls, strongly expressed procollagen, thus confirming the efficacy of the cytometry protocol (fig. 3).
FIGURE 3.
Immunofluorescent analysis of circulating fibrocytes. Equivalent numbers of cytospun buffy coat cells were examined from a) controls and b) individuals with pulmonary hypertension for expression of procollagen I N-terminal peptide (PINP) stained with Alexa488 (Invitrogen, Carlsbad, CA, USA) (arrows). Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
DISCUSSION
Based on animal models that implicate fibrocytes in the pathobiology of PH and on human studies demonstrating increased fibrocyte numbers in patients with interstitial lung disease, we tested the hypothesis that fibrocytes would be increased in the circulation of patients with PH. We found a significant enrichment of circulating fibrocytes (defined as CD45+ procollagen+ cells) in patients with PH compared with control individuals undergoing catheterisation for arrhythmia. In addition, we found a correlation between fibrocyte counts and P̄pa as well as age. Taken together with previous findings from our group and others of exuberant decoration of fibrocytes in and around pulmonary vessels in a neonatal calf model of hypoxic PH [18, 20], our data here support the notion that the presence of these progenitor cells may contribute to pathological pulmonary vascular remodelling in the setting of inflammation. This is the first demonstration that individuals with PH harbour increased numbers of circulating fibrocytes, a cell type that has been identified in adults with chronic inflammatory disorders and is associated with poor prognosis, inflammation and tissue remodelling in the lung. Our study might, therefore, represent the first step in understanding the putative pathological roles of fibrocytes in the course of paediatric PH.
The biology of fibrocytes is rapidly coming into sharper focus. Originally described as a leukocyte subpopulation that effected tissue repair [21], fibrocytes are critically involved in normal wound healing and fibrosis [22]. Their differentiation from precursor cells is complex but may be inhibited by bacterial infection through Toll-like receptor 2 activation [23]. In other contexts, fibrocytes can be differentiated to myofibroblast and adipocyte phenotypes, by transforming growth factor-β [24] and peroxisome proliferator-activated receptor-γ [25], respectively. Apart from tissue remodelling, fibrocytes have been negatively implicated in chronic inflammatory conditions. Fibrocytes are mobilised from the bone marrow and accumulate in regions of chronic inflammation, perhaps through the SDF-1/CXC chemokine receptor 4 signalling axis [26]. In vivo, fibrocytes have been studied in animal models of PH, and are associated with vascular remodelling, elevated pulmonary artery pressures and accumulation in bronchovascular regions [4, 18]. Although the extent of monocytic cell influx into the lung has been well documented in adults with PH [27], ours is the first study to quantitatively assess circulating fibrocytes in children and young adults with PH. The present study has confirmed that fibrocytes are present in the blood of patients with PH. How (or whether) fibrocytes contribute to the remodelling of pulmonary vessels in the human condition remains to be determined. A more precise functional characterisation of fibrocytes, at least in the clinical setting, is currently hampered by the seeming requirement of (intracellular) collagen as the “gold standard” for fibrocyte identification. Magnetic bead or flow sorting using extracellular markers as surrogates for the expression of collagen would obviate the need for fixation and permeabilisation of putative fibrocytes, a process incompatible with many downstream functional assays. Recently, fibrocytes isolated from patients with chronic inflammation have been shown to display an activated phenotype [28]. Additionally, the expression profile for cultured fibrocytes is distinct from monocytes, macrophages and fibroblasts [29]. These findings highlight the complexity of fibrocyte differentiation, the details of which (stem cell, process, temporal mechanics, tissue influence, etc.) remain shrouded in obscurity. Identification of cell surface markers with a high specificity for fibrocytes will facilitate studies designed to understand the functional roles of fibrocytes in vivo, as has already been established for serumamyloid P [30]. Our group has previously shown that vascular remodelling and inflammation of the rat pulmonary vasculature during hypoxia requires circulating cells of a monocytic/macrophage lineage [13]. Use of fibrocyte-specific agonists and antagonists, based on in vitro and in vivo immunophenotyping of surface markers, will more sharply define the cell type most responsible for the remodelling.
Correlation analysis between fibrocyte numbers and P̄pa and increasing age showed a weak but positive relationship. Fibrocyte counts did not correlate with other patient demographic data. The lack of a stronger correlative relationship between fibrocyte count and P̄pa is not altogether surprising for several reasons. First, the age range of our PH patient cohort spanned 22 yrs and there was a large standard deviation (6.18 yrs). There are no studies available that define a link between fibrocyte counts and age. We assumed a priori that (young) individuals in the control group would have low fibrocyte numbers (≤1% of total white blood cells), as is observed in control adults. However, this is an assumption that needs additional testing. In any case, we found low numbers of fibrocytes in peripheral blood of controls, numbers similar to those documented in older controls [16]. Secondly, many of the PH patients in our study are on significantly long-standing and varied treatment regimens, including calcium channel blockers, endothelin receptor antagonists, phosphodiesterase inhibitors and prostanoids. In addition, some of the children responded to acute vasodilator challenge and others did not. As such, the variable clinical presentation and underlying pathobiology (e.g. IPAH versus APAH-CHD) of our study group probably reduced the power of fibrocyte number to more closely correlate with severity of PH as measured by P̄pa. In fact, it remains possible that, since treatments with prostacyclin agonists appear to increase the number of late EPCs, such treatment could also lead tomobilisation of fibrocytes into the circulation. In future studies, restricting the analysis of circulating fibrocytes to exclude patients with PH not associated with extensive lung inflammation and remodelling will probably prove fruitful, as will studying patients at diagnosis (untreated patients). Fibrocytes are involved in both normal wound healing and inflammation but at present we are unable to say which of these mechanisms is involved in these patients. We speculate that the mechanism is pro-inflammatory and, perhaps, the data that the patients with the most severe disease (on triple therapy) also have the highest fibrocyte numbers offers a clue in support of this assumption. This is in contrast to EPCs, which are thought to be decreased with increased severity of PH [4, 5]. It is thus plausible that the collective contributions of distinct progenitor cell populations govern lung homeostasis, and by extension, deviation from homeostasis during pathogenesis.
In conclusion, we have shown quantitative differences in circulating fibrocytes between individuals with PH and those without PH. Fibrocyte count correlated with increased P̄pa and age. This study suggests a potential role for these cells in the pathogenesis of PH, but more studies are needed to confirm our findings. A great deal of renewed research emphasis over the past decade has been placed on mechanisms of lung inflammation and vascular remodelling in PH. Our study points to the potential contributions of circulating mesenchymal progenitors to those inflammatory processes.
ACKNOWLEDGEMENTS
We would like to especially acknowledge the generosity and kindness of R. Strieter (Robert M. Byrne Cardiovascular Research Center, University of Virgina, Charlottesville, VA, USA) in whose laboratory we learned the techniques used in this report. We also wish to thank M. Burdick from the Strieter laboratory for teaching and graciously hosting us. We thank the University of Colorado Cancer Center Flow Cytometry Core (Denver, CO, USA) for technical assistance.
SUPPORT STATEMENT
This study was funded by National Institutes of Health (NIH) Specialized Centers of Clinically Oriented Research grant HL-084923-02, NIH Program Project Grant HL-014985-35, NIH/NCRR Colorado CTSI grant number UL1 RR025780, the Jayden DeLuca Foundation and the Leah Bult Pulmonary Hypertension Research Fund.
Footnotes
STATEMENT OF INTEREST
A statement of interest for M.E. Yeager can be found at www.erj.ersjournals.com/site/misc/statements.xhtml
REFERENCES
- 1.Humbert M. Update in pulmonary hypertension 2008. Am J Respir Crit Care Med. 2009;179:650–656. doi: 10.1164/rccm.200901-0136UP. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ, Ertel SI, Beghetti M, et al. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J. 2011;37:1–13. doi: 10.1183/09031936.00056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrell NW, Adnot S, Archer SL, et al. Cellular andmolecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toshner M, Voswinckel R, Southwood M, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;180:780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Junhui Z, Xingxiang W, Guosheng F, et al. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med. 2008;102:1073–1079. doi: 10.1016/j.rmed.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Smadja DM, Mauge L, Gaussem P, et al. Treprostinil increases the number and angiogenic potential of endothelial progenitor cells in children with pulmonary hypertension. Angiogenesis. 2010;14:17–27. doi: 10.1007/s10456-010-9192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asosingh K, Aldred MA, Vasanji A, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol. 2008;172:615–627. doi: 10.2353/ajpath.2008.070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diller GP, van Eijl S, Okonko DO, et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008;117:3020–3030. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 9.Hall S, Brogan P, Haworth SG, et al. Contribution of inflammation to the pathology of idiopathic pulmonary arterial hypertension in children. Thorax. 2009;64:778–783. doi: 10.1136/thx.2008.106435. [DOI] [PubMed] [Google Scholar]
- 10.Gambaryan N, Perros F, Montani D, et al. Targeting of c-kit+ hematopoietic progenitor cells prevents hypoxic pulmonary hypertension. Eur Respir J. 2011;37:1392–1399. doi: 10.1183/09031936.00045710. [DOI] [PubMed] [Google Scholar]
- 11.Davie NJ, Crossno JT, Jr, Frid MG, et al. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L668–L678. doi: 10.1152/ajplung.00108.2003. [DOI] [PubMed] [Google Scholar]
- 12.Stenmark KR, Frid MG, Yeager ME. Fibrocytes: potential new therapeutic targets for pulmonary hypertension? Eur Respir J. 2010;36:1232–1235. doi: 10.1183/09031936.00137410. [DOI] [PubMed] [Google Scholar]
- 13.Frid MG, Brunetti JA, Burke DL, et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006;168:659–669. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L, Louie MC, Vannella KM, et al. New concepts of IL-10 induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol. 2010;300:L341–L353. doi: 10.1152/ajplung.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeley EC, Mehrad B, Strieter RM. Fibrocytes: bringing new insights into mechanisms of inflammation and fibrosis. Int J Biochem Cell Biol. 2010;42:535–542. doi: 10.1016/j.biocel.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 17.Lama VN, Phan SH. The extrapulmonary origin of fibroblasts: stem/progenitor cells and beyond. Proc Am Thorac Soc. 2006;3:373–376. doi: 10.1513/pats.200512-133TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikam VS, Schermuly RT, Dumitrascu R, et al. Treprostinil inhibits the recruitment of bone marrow-derived circulating fibrocytes in chronic hypoxic pulmonary hypertension. Eur Respir J. 2010;36:1302–1314. doi: 10.1183/09031936.00028009. [DOI] [PubMed] [Google Scholar]
- 19.Mathai SK, Gulati M, Peng X, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 2010;90:812–823. doi: 10.1038/labinvest.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frid MG, Li M, Gnanasekharan M, et al. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1059–L1072. doi: 10.1152/ajplung.90611.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 22.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 23.Maharjan AS, Pilling D, Gomer RH. Toll-like receptor 2 agonists inhibit human fibrocyte differentiation. Fibrogenesis Tissue Repair. 2010;3:23. doi: 10.1186/1755-1536-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang CH, Huang CD, Lin HC, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178:583–591. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 25.Hong KM, Belperio JA, Keane MP, et al. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-β and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 26.Song JS, Kang CM, Kang HH, et al. Inhibitory effect of CXC chemokine receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary fibrosis. Exp Mol Med. 2010;42:465–472. doi: 10.3858/emm.2010.42.6.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenmark KR, Davie NJ, Reeves JT, et al. Hypoxia, leukocytes, and the pulmonary circulation. J Appl Physiol. 2005;98:715–721. doi: 10.1152/japplphysiol.00840.2004. [DOI] [PubMed] [Google Scholar]
- 28.Galligan CL, Siminovitch KA, Keystone EC, et al. Fibrocyte activation in rheumatoid arthritis. Rheumatology (Oxford) 2010;49:640–651. doi: 10.1093/rheumatology/kep265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilling D, Fan T, Huang D, et al. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilling D, Roife D, Wang M, et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]