Abstract
Intraperitoneal (IP) chemotherapy is an effective way of treating local and regional malignancies confined in the peritoneal cavity such as ovarian cancer. However, a persistent major challenge in IP chemotherapy is the need to provide effective drug concentrations in the peritoneal cavity for an extended period of time. We hypothesized that hyaluronic acid (HA)-based in-situ crosslinkable hydrogel would serve as a carrier of paclitaxel (PTX) particles to improve their IP retention and therapeutic effects. In-vitro gel degradation and release kinetics studies demonstrated that HA gels could entrap microparticulate PTX (>100 μm) and release the drug over 10 days, gradually degraded by hyaluronidase, but had limited effect on retention of Taxol, a 14-nm micelle form of PTX. When administered IP to tumor-bearing nude mice, PTX was best retained in the peritoneal cavity as PTX-gel (microparticulate PTX entrapped in the HA gel), whereas Taxol-gel and other Taxol-based formulations left negligible amount of PTX in the cavity after 14 days. Despite the increase in IP retention of PTX, PTX-gel did not further decrease the tumor burdens than Taxol-based formulations, presumably due to the limited dissolution of PTX. This result indicates that spatial availability of a drug does not necessarily translate to the enhanced anti-tumor effect unless it is accompanied by the temporal availability.
Keywords: Paclitaxel, intraperitoneal, hydrogel, particles, drug delivery
1. INTRODUCTION
Ovarian cancer is the second most common and the most lethal gynecologic cancer [1]. Due to the lack of early-stage symptoms and reliable detection methods, >70% of patients present with advanced disease at diagnosis, at which point the 5-year survival rate is only 20–30% [2]. Current standard treatment of ovarian cancer is cytoreductive surgery, followed by intravenous (IV) administration of a combination of platinum and taxane analogues for removal of residual microscopic tumors [3–6]. While most patients respond to initial chemotherapy, the majority ultimately develop disease recurrence [7]. Therefore, more effective post-surgical therapy is desperately needed [7].
Meanwhile, a growing number of preclinical and clinical studies have shown that intraperitoneal (IP) chemotherapy is effective in post-surgical therapy of ovarian cancer [3]. IP administration of drugs can provide an effective local concentration of drug in the peritoneal cavity, facilitate treatment of the local (confined to the ovaries) and regional (extended to surrounding organs or tissues) malignancies, and attenuate systemic exposure of chemotherapy drugs [8–10]. Accordingly, the National Cancer Institute has recommended IP chemotherapy for patients with optimally debulked ovarian cancer [11]. Moreover, recent studies show that sustained delivery of chemotherapy reduces the occurrence of multidrug resistance [12, 13], one of the main causes of treatment failure and low survival rates in ovarian cancer. However, a persistent major challenge in IP chemotherapy is the need to provide effective drug concentration in the peritoneal cavity for an extended period of time. IP-administered low molecular weight (MW) drugs such as docetaxel or paclitaxel (PTX) are rapidly absorbed to the systemic circulation and cleared from the peritoneal cavity in less than 24 hours [14–16]. The short IP residence time necessitates frequent or continuous dosing, which leads to high risk of infection, pain, toxicity, or other catheter-related problems [4, 17, 18].
In light of this challenge in IP drug delivery, particulate formulations have been used to prevent rapid clearance of low MW drugs from the peritoneal cavity [4, 12, 16, 19, 20]. A clinical study showed that systemic absorption of IP-administered Taxol, a surfactant-based formulation of PTX that forms micelles in the peritoneal cavity, was slower than that of surfactant-free PTX [21]. Gelatin nanoparticles (NPs) (600 nm) [16] or polymeric NPs (265 nm) [22] were also considered for IP drug delivery, although their abilities to control drug release and persist in the peritoneal cavity were quite limited [16, 22]. In this regard, larger particles (4–6 μm [16, 23] or 47 μm [24]) were found to be superior to NPs in their retention in the peritoneal cavity and maintenance of drug level [16]. Similarly, 1 μm liposomes were better retained in the peritoneal cavity than 100 nm liposomes [25]. However, larger particles tend to induce inflammatory responses and peritoneal adhesions [23, 26], which interfere with IP therapy. To maximize the regional effect of particulate formulations, their IP delivery remains to be improved.
We hypothesize that a biocompatible hydrogel can be used as a carrier of particulate formulations to enhance their spatial availability and therapeutic effects on IP tumors. Due to the ability to form a sustainable depot in the peritoneal cavity, hydrogels have been explored as a standalone drug delivery system for IP chemotherapy. They include an implantable polymer film, which swells in the presence of ascites fluid [12, 27–30], and in-situ forming hydrogels, which are injected IP as liquid but form a gel via interactions between the components [13, 31, 32] or temperature change [33, 34]. These hydrogels were used in animal models with peritoneal malignancies and achieved promising anti-tumor effects [13, 29, 32–34]. Hydrogels can retain drug precipitates [35] or NPs [20] for a prolonged period and thus are ideal for their local delivery. In-situ crosslinkable hydrogels are particularly useful for delivery of drug precipitates or NPs, because the gel precursor solutions are prepared as aqueous solutions, and the particles can be easily incorporated as a suspension.
Here we used a hyaluronic acid (HA)-based in-situ crosslinkable hydrogel as a carrier of two different particulate formulations of PTX (Taxol and PTX precipitates) to improve their IP retention and effectiveness of the therapy. Taxol is a PTX formulation based on Cremophor EL (polyethoxylated castor oil), which forms micelles at concentrations that can be reached by IV and IP administrations [36]. Microparticulate PTX precipitates were prepared by diluting a concentrated organic solution of PTX in aqueous solutions. Taxol and PTX precipitates were administered IP, using HA gel as a delivery medium, in a murine model of human ovarian cancer. Their anti-tumor effects were compared with gel-free formulations as well as intermittently applied Taxol to observe the ability of HA gel to improve IP availability of PTX and effectiveness of the applications.
2. MATERIALS AND METHODS
2.1. Materials
Paclitaxel (PTX) was a gift of Samyang Genex Corp (Seoul, Korea). Hyaluronic acid (HA, 35 kDa and 357.4 kDa) was purchased from Lifecore Biomedical, LLC (Chaska, MN, USA). Cremophor EL was a gift from BASF (New York, NY, USA). Carbamazepine was purchased from Enzo life sciences (Plymouth meeting, PA, USA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Preparation of HA derivatives and crosslinked HA gel
In-situ crosslinkable HA derivatives and a crosslinked HA gel were prepared as previously described [37]. HA–adipic acid dihydrazide (HA-ADH) was synthesized by conjugating adipic dihydrazide to carboxyl groups in HA (35 kDa). HA was reacted with 30-fold molar excess of adipic dihydrazide in the presence of 1-ethyl-3-carbodiimide (EDC) and 1-hydroxybenzotriazole (HOBt) at pH 6.8 and room temperature. HA–aldehyde (HA-CHO) was produced by reacting HA (357.4 kDa) with equimolar sodium periodate for 2 hours at room temperature in darkness. The reaction was terminated by adding ethylene glycol. HA-ADH and HA-CHO were purified by dialysis, filter-sterilized, lyophilized and stored at 4°C until use. For formation of HA gels, the HA derivatives (HA-ADH and HA-CHO) were prepared as 40 mg/mL solution in phosphate buffered saline (PBS, pH 7.4) and extruded though a common outlet using a double-barreled syringe. The gel formed in less than 1 min after the extrusion.
2.3. Preparation of PTX formulations
Taxol and a suspension of microparticulate PTX precipitates (‘PTX-susp’) were prepared by diluting “Taxol concentrate” (6 mg PTX dissolved in 1 mL of 1:1 mixture of Cremophor EL and ethanol) and 30 mg/mL PTX-dimethyl sulfoxide (DMSO) solution, respectively, in PBS. For preparation of HA gels containing Taxol (‘Taxol-gel’) or PTX precipitates (‘PTX-gel’), the Taxol concentrate and PTX-DMSO solution were diluted in solutions of HA derivatives and combined in the same manner as HA gels (sec. 2.2). After the dilution, PTX concentration in each solution was 0.69 mg/mL. When PTX-DMSO solution was diluted in aqueous solutions, white precipitates of PTX formed immediately. The suspensions of PTX precipitates were sonicated for 2 sec at 80% amplitude using a Sonics Vibra-Cell Ultrasonic Processor (Sonics & Materials Inc. Newtown, CT) prior to use. Particle sizes of Taxol and PTX-susp were measured with a Zetasizer Nano-ZS90 and Mastersizer 2000 (Malvern instruments, Westborough, MA, USA), respectively.
2.4. In-vitro release kinetics
Taxol-gel and PTX-gel were prepared in a small disc shape for the study of release kinetics. Solutions of HA-ADH and HA-CHO, which contained Taxol or PTX precipitates, were mixed in equal volume (150 μL each) in a cylindrical rubber mold (diameter × height: 8 mm × 3.5 mm) sandwiched between two rubber sheets. The resulting disc-shape gel was separated from the mold, weighed, and enclosed in a plastic histology cassette. The cassette was placed in 20 mL of release medium (PBS containing 25% of fetal bovine serum (FBS) and 10 U/mL hyaluronidase) and incubated at 37°C for 10 days under constant agitation. At predetermined time points, 2 mL of the release medium was sampled for analysis and replaced with fresh medium. At the end of the study, precipitates remaining in the container, if existed, were collected, suspended in a smaller volume of release medium, and prepared for analysis as described in the following section. The container was washed with a 50:50 mixture of acetonitrile and water.
2.5. PTX analysis
PTX in the release medium or peritoneal lavage fluid was extracted with ethyl acetate and analyzed with HPLC. Release medium or peritoneal lavage fluid of untreated tumor-bearing mice were spiked with a known amount of PTX and used for calibration. Briefly, 1 mL of a sample or standard was put in a 15 mL tube, to which carbamazepine was added to 5 μg/mL as an internal standard. After vortex-mixing, ethyl acetate 3 mL was added to the 1 mL sample, and the mixture was shaken on a rotating shaker for 30 minutes. The mixture was then centrifuged at 4000 rpm for 10 minutes to separate an organic layer, which was transferred to a new glass vial and dried in ambient air. The dried sample was resuspended in 250 μL of HPLC mobile phase and analyzed by HPLC. The acetonitrile/water washes of containers and cassettes were directly analyzed with HPLC, comparing with a PTX standard calibration curve prepared in acetonitrile/water.
PTX was analyzed with HPLC equipped with a UV detector (1100 series, Agilent Technologies, Palo Alto, CA) and an Ascentis C18 column (25 cm × 4.6 mm, particle size 5 μm) (Supelco, St. Louis, MO, USA). A mixture of acetonitrile and water (50:50, v/v) was run in the isocratic mode at a flow rate of 1 mL/min. PTX was detected at 227 nm.
2.6. Gel degradation kinetics
Disc-shape HA gels (without PTX) were prepared as described in sec. 2.4. The gel was weighed and placed in a Transwell (Corning), which was immersed in 5 mL of PBS containing 25% FBS and 10 U/mL hyaluronidase. The Transwell was perforated with fifteen 21-gauge needle holes to facilitate the medium exchange between the well and the receptor compartment. The gel was incubated at 37°C on an orbital shaker for 10 days. At regular time points, the remaining HA gel was recovered and weighed after blotting. The weight of remaining HA gel at each time point was expressed as percentage of the original wet mass.
2.7. Development of an intraperitoneal tumor model
A murine model of IP tumor was developed with SKOV-3 human ovarian cancer cells and 4–5 week-old female athymic (nu/nu) mice (National Cancer Institute, Frederick, MD, USA) according to a method described in the literature [38]. The procedure was approved by Purdue Animal Care and Use Committee, in conformity with the NIH guidelines for the care and use of laboratory animals. SKOV-3 cells were maintained in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin. For tumor inoculation, 107 SKOV-3 cells (suspended in RPMI) were injected IP to the nude mice. Animals were observed throughout the study for the signs of weight loss. In a preliminary study, animals were randomly chosen and euthanized on 14, 28, 42 and 56 days after inoculation to determine the take rate of SKOV-3 cells.
2.8. In-vivo efficacy studies
The treatment schedule is summarized in Supporting Fig. 1. Fourteen days after the IP injection of cancer cells, the animals were anesthetized with subcutaneous injection of ketamine 50 mg/kg and xylazine 10 mg/kg. A 0.5 cm skin incision was made in the skin 0.5 cm above the costal margin, and the peritoneum was nicked with a 24-gauge catheter. One milliliter of PTX-susp (n=10), PTX-gel (n=10), Taxol (n=9), or Taxol-gel (n=9) (all equivalent to 30 mg/kg of PTX) was injected into the peritoneal cavity through the catheter, and the skin was closed with suture. One milliliter of PBS (n=6) or HA gel (n=6) was injected for control groups. In another control group, Taxol was IP injected at 10 mg/kg on the q4d × 3 schedule (Taxol-multiple) (n=6). Animals receiving no treatment was also included as a control group (n=15). Animals were allowed to recover from the procedure with access to water and food ad libitum, and their body weights were monitored every other day.
Animals were sacrificed by CO2 asphyxiation 14 days after the treatment (for the Taxol-multiple group, 14 days after the initial treatment) to record the weights, numbers, and locations of the tumor nodules. Blood was collected by post-mortem cardiac puncture and submitted to Animal Disease and Diagnostic Laboratory for hematology and blood chemistry analysis. For selected animals, peritoneal lavage samples were collected for quantitative determination of the PTX remaining in the peritoneal cavity. Three milliliters of PBS was injected IP prior to necropsy. Approximately 2 mL of lavage fluid was collected and stored in −80°C until analysis. PTX was extracted from the lavage and analyzed by HPLC as described in sec. 2.5.
2.9. Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 (La Jolla, CA) or OriginPro 8 (Northampton, MA). Unless specified otherwise, one-way ANOVA was used to determine difference among the groups, and pairs were compared using Tukey’s multicomparison test or Dunnett’s test when compared with control. A value of p<0.05 was considered statistically significant.
3. RESULTS
3.1. Characterization of PTX formulations
Taxol appeared clear to the naked eyes. The average particle size of Taxol (Cremophor micelles entrapping PTX) was 14 ± 0.1 nm (Supporting Fig. 2). PTX solution in DMSO started to form precipitates as soon as it was added to PBS or solutions of HA derivatives (Supporting Fig. 3). PTX-susp or PTX suspensions in HA solutions were therefore turbid. PTX-susp showed bimodal size distribution with peaks at ~5 μm and ~1000 μm (Supporting Fig. 2). Upon brief sonication, PTX-susp showed additional size peaks at 91 μm, indicating that the precipitates were loose agglomerates of smaller particles. None of the components (Cremophor EL, ethanol, DMSO, and PTX) interfered with the gel formation. Solutions of HA derivatives that contained Taxol concentrate or PTX-DMSO formed a gel upon contact, typically in 1 min, just as quickly as drug-free HA solutions.
3.2. Degradation of HA gel
Gel degradation was monitored in PBS containing FBS and hyaluronidase. We used 25% FBS to approximate the ratio of protein content in the peritoneal fluid to that of serum [39]. The hyaluronidase level in tumor-bearing peritoneal cavity was unknown, but we chose 10 U/mL since we previously observed that this level of hyaluronidase degraded an HA gel in vitro at a similar rate as that in vivo [40]. The HA gel swelled initially, with the wet gel mass reaching 191.1 ± 12.8 % of the original gel mass in 1 day (Supporting Fig. 4). The gel then started to lose weight and continuously degraded over the next 9 days. The time for the wet gel mass to reach 50% of the original mass was 7 days, and the gel disappeared completely in 10 days. This in-vitro gel degradation rate appeared to be faster than that in vivo, where the degrading gel was still observed 14 days after the treatment (sec. 3.4). The in-vitro gel degradation may have been expedited due to the relatively large volume of the medium (0.3 mL gel per 10 mL medium) compared to the peritoneal fluid in vivo (1 mL gel per 0.1 ± 0.05 mL [41]).
3.3. In vitro PTX release kinetics from PTX-gel and Taxol-gel
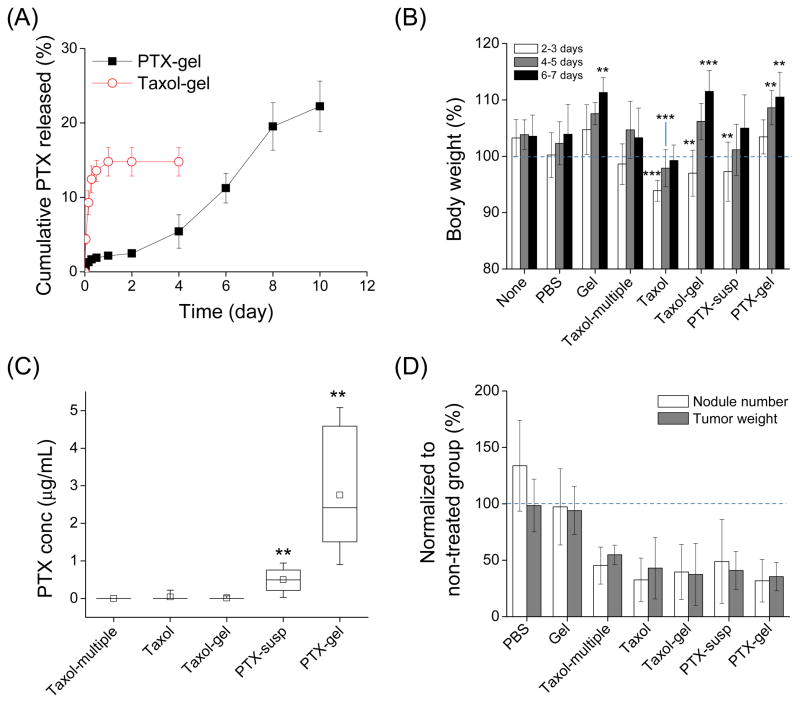
The release kinetics of PTX from PTX-gel and Taxol-gel were examined with the PBS containing FBS and hyaluronidase (Fig. 1A). Taxol-gel completed PTX release in 24 hours, followed by no further release. 14.8±1.9% of total loaded PTX was released from Taxol-gel in 24 hours, and 0.8±0.0% of the loaded PTX was found to be adsorbed to the container. On the other hand, PTX-gel continuously released 22.2±3.4% of the loaded PTX over a period of 10 days. By then the PTX-gel became small pieces of white mass, which accounted for 15.4±4.4% of the loaded PTX. PTX adsorbed to the container was 0.9±0.2% of the loaded PTX. Taken together, the total recovery of PTX was 15.6% for Taxol-gel and 38.5% for PTX-gel, far less than the drug loaded in each gel.
Fig. 1.
(A) In-vitro PTX release kinetics from HA-gel (40 mg/mL). % Cumulative PTX released = (PTX released in the medium by the specified time)/(PTX loaded in the gel). Results shown are averages of two independently and identically prepared samples. (B) Body weight changes after treatments. Body weights were measured every other day (2, 4, and 6 days) after each treatment (after initial treatment for the Taxol-multiple group) and normalized to the weight measured shortly prior to the treatment (indicated as a dotted line). *: p<0.05; **: p<0.01; ***: p<0.001, as compared to the no treatment group of each time point (Dunnett test). (C) PTX level in peritoneal lavage fluid after each treatment. **: p<0.01, as compared to Taxol-multiple (Mann-Whitney test). (D) Anti-tumor efficacy of each treatment. Data represent numbers and weights of tumor nodules observed in the peritoneal cavity, as normalized to those of untreated mice (indicated as a dotted line). Any PTX treatments were significantly different from PBS or Gel (p<0.001 Tukey test).
3.4. IP tumor development and application of treatments
The anti-tumor effects of the PTX formulations were evaluated in a murine model of IP tumors. Seventy five percent of animals developed tumors widespread in the peritoneal cavity in 14 days. Tumor nodules were readily visible in the peritoneal cavity by this time, and the weight and number of the tumor nodules subsequently increased (Supporting Fig. 5). According to this result, treatments were given on day 14 after the tumor inoculation, either as one time instillation of gels (or gel-free formulations) or as multiple administrations of Taxol, providing 30 mg/kg PTX in total. We chose 30 mg/kg because a prior study found a comparable dose to be effective in reducing tumor burden [32]. IP Taxol exceeding this dose was found to be fatal [42]. In our hands, animals receiving >30 mg/kg Taxol IP did not die, but their recovery from anesthesia was increasingly delayed with the escalation of dose.
Mice receiving Taxol, Taxol-gel, and PTX-susp lost weights in 2–3 days after the treatment as compared with the no treatment group (Fig. 1B). Interestingly, mice receiving gel-based formulations (Gel, Taxol-gel, and PTX-gel) showed significant weight gain in 6–7 days after the treatment as compared with the no treatment group. When the peritoneal cavities were observed 14 days after the treatment, the IP organs of mice treated with Gel (HA-gel with no drug) looked more hydrated than those of non-treated mice, due to the viscous liquid covering the organs (Supporting Fig. 6). The mice injected with PTX-susp had white solid materials, which were thought to be PTX precipitates. In the mice injected with PTX-gel, white gel mass was present in the peritoneal cavity, indicating the presence of PTX entrapped in the gel.
3.5. PTX level in the peritoneal fluid
To quantify PTX remaining in the peritoneal cavity 14 days after the treatment, peritoneal lavage was performed prior to dissection (Fig. 1C). The highest IP level of PTX was obtained with PTX-gel (0.9 – 5.1 μg/mL), followed by PTX-susp (0.02 – 0.94 μg/mL). No PTX was detected in 5 out of 6 mice treated with Taxol or Taxol-gel. The remaining 1 mouse in each group showed low PTX concentration (0.22 μg/mL in the Taxol group and 0.07 μg/mL in the Taxol-gel group). No PTX was detected in mice treated with Taxol-multiple.
3.6. Anti-tumor efficacy of the PTX formulations
The anti-tumor effects were estimated from the weight and number of tumor nodules. This study was performed over different sets of experiments due to the large number of animals. Since the tumor burden varied from experiment to experiment, as also experienced by the other group [29], the tumor weights and nodule numbers of treatment groups were expressed as normalized to those of the no treatment group in each experiment.
In the control groups which were not provided with PTX (no treatment, PBS, or Gel), a big tumor mass was usually present in the upper left quadrant of the peritoneal region, and a large number of nodules were present close to the liver, spleen, stomach, and on the mesentery. In contrast, animals treated with PTX formulations (PTX, PTX-gel, Taxol, Taxol-gel, or Taxol-multiple) showed significant reduction in both numbers and weights of tumors (Fig. 1D). However, there was no statistical difference among these groups.
3.7. Blood analysis
To evaluate the systemic effect of each treatment, blood samples were analyzed with respect to the levels of glucose, creatinine, alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), and the complete blood count (white blood cells (WBC), red blood cells (RBC), hemoglobin). No clinical abnormalities in blood chemistry and RBC indices were observed in any treatment groups (Supporting Tables). Taxol-multiple and Taxol groups showed lower WBC and neutrophil counts than the no-treatment group (p<0.05 by Mann-Whitney test), indicating the effect of systemically absorbed PTX on the bone marrow. Taxol-gel, PTX-susp, and PTX-gel groups showed a similar trend, but no statistical difference was observed.
4. DISCUSSION
Biomaterials for IP application require extreme caution. The peritoneal cavity is particularly sensitive to foreign insults, which is relevant to the fact that the peritoneum is a primary protection mechanism against breaches in the integrity of the gut [43]. Peritoneal mesothelial cells, polymorphonuclear neutrophils, and the resident peritoneal-associated lymphoid tissues work together in the insulted peritoneal cavity, playing an active role in inflammatory responses to the foreign materials [43]. Due to this sensitivity, some biomaterials known to be compatible with other anatomical locations often induce significant tissue reactions in IP applications [44, 45]. However, HA, a naturally occurring non-sulfated glycosaminoglycan, is well tolerated in the peritoneal cavity and has been used as physical barriers [20, 46] and drug carriers [35, 40] for preventing peritoneal adhesion formation. Due to the well-established biocompatibility in the peritoneal cavity, we chose a HA-based gel as a delivery medium for IP application of PTX particles.
The HA gel is produced by mixing solutions of HA derivatives, HA–ADH and HA–CHO, which crosslink to form a hydrazone link that is relatively stable at the physiological pH. An advantage of this system is that the gel precursor solutions can be applied as liquid and form a gel in situ. Since the gel precursor solutions are prepared as aqueous solutions, a drug can be readily incorporated in the gel as long as it is can be dissolved or suspended in the aqueous or water-miscible solutions. Since it is injected as a solution and forms a gel as the solution flows, the gel can provide broad coverage over the most areas in the peritoneal cavity while keeping the drug in place. This flexibility is particularly beneficial for the treatment of IP tumors, which grow in various locations throughout the peritoneal cavity. The HA gel degrades by hyaluronidase, an enzyme prevalent in the body in various concentrations. The degradation rate of a HA gel varies with the concentration and MW of HA, which determines crosslinking density of the gel. In our previous in-vitro study, 10 or 20 mg/mL HA gels (HA-ADH, 551 kDa; HA-CHO, 188 kDa) completely degraded in 4–6 days in 10 U/mL hyaluronidase, whereas it took 9 days for 60–75 mg/mL HA gel (HA-ADH, 141 kDa; HA-CHO, 188 kDa) to degrade completely [40]. In-vivo gel degradation is further affected by the enzyme levels in tissues, volume of the body fluid, and the extent of shear stress the gel is exposed to. In the present study, 40 mg/mL gel (HA-ADH, 35 kDa; HA-CHO, 357.4 kDa) degraded over 2 weeks to viscous liquid in the mouse peritoneal cavity.
Due to the high water content, the HA gels are usually poor at delaying the release of low molecular-weight drugs [40] but can retain precipitates of hydrophobic drugs [35] or NPs [20] relatively well. In the present study, Taxol was not well entrapped in the gel, whereas PTX precipitates remained with the gel, evident from the difference in PTX release kinetics. The persistent white color of PTX-gel during the release study also supported the retention of PTX precipitates in the gel. This difference may be attributable to the particle size of Taxol and PTX precipitates. Taxol micelles (14 nm) appear to be too small to remain in the degrading gel, but PTX precipitates in micrometer ranges are sufficiently large. It is worth mentioning that the total recovery of PTX in the release studies was far less than the loaded amount for both gels. This may be partly explained by the potential loss of gel fragments during repeated samplings, but a significant fraction of released PTX appeared to have degraded in the release medium. The instability of PTX in serum-containing media [47] and plasma [48] has been previously reported, and we also confirmed the gradual loss of PTX in the release medium with HPLC (Supporting Fig. 7). Given the instability of PTX in the release medium, the relatively high total recovery of PTX from PTX-gel indicates that HA gel had a protective effect on the entrapped PTX.
PTX was best retained in the peritoneal cavity as PTX-gel, followed by PTX-susp. According to the degradation kinetics of HA gel and the in-vitro release kinetics of PTX-gel, the IP retention of the PTX-gel is primarily attributable to the ability of HA gel to form a depot in the peritoneal cavity entrapping the microparticulate PTX precipitates. In addition, the large size of PTX particles (be they by themselves or bound to the degrading gel) is likely to have further contributed to the IP retention of PTX. In general, water-soluble low molecular drugs are known to be readily absorbed to the systemic circulation through the peritoneal capillaries [49, 50], and relatively small particles (<1 μm) are trafficked to the lymphatic system and subsequently enter the systemic circulation via the lymph duct [50]. Neither systemic nor lymphatic mechanism should be able to remove PTX precipitates because of the large size (>100 μm). IP retention of PTX-susp, although not as effective as PTX-gel, is mainly explained by the large particle size. Conversely, the lack of detectable PTX in groups treated with Taxol or Taxol-multiple is attributable to the small size of Taxol (14 nm). Taxol was readily removed from the peritoneal cavity in the first few days, leaving negligible PTX level in the peritoneal cavity after 14 days. Since HA-gel did not delay the release of Taxol, IP retention of Taxol-gel was not superior to Taxol.
Interestingly, the clear difference in the IP PTX level did not translate to significant difference in anti-tumor effects. Neither PTX-gel nor PTX-susp excelled the Taxol-based formulations in number and weight of the tumor nodules. The slow and incomplete PTX release from the PTX-gel in vitro and the presence of visible PTX precipitates after 14 days in vivo suggest that this is due to the limited dissolution of PTX. For a water-insoluble drug like PTX, a small particle size is critical to accelerating the dissolution rate [51, 52]. In the absence of a surfactant stabilizing the surface, however, the PTX particles tend to aggregate, limiting the continuous supply of PTX to tumors. Apparently, the HA gel did not prevent particle aggregation.
Taken together, the limitations of Taxol-gel and PTX-gel are attributable to opposite reasons. Taxol-gel was not superior to Taxol due to the small size of Taxol micelles, which prevented their retention in the gel and the peritoneal cavity. On the other hand, PTX-gel had limited effects because of the formation of large PTX aggregates, which were not dissolved readily. In this context, one may expect that the gel would be more effective in enhancing the regional effect of PTX if used with PTX particles of an intermediate size, which is large enough to remain entrapped in the gel but small enough to allow continuous and unhindered supply of PTX. Nanocrystal formation [53] or encapsulation of PTX in polymeric NPs could be potential ways of controlling the particle size. Alternatively, a new gel system that can prevent aggregation of the PTX particles would be also useful.
Aside from the pharmacological effect, HA gel may contribute to reducing the side effect of Taxol-based chemotherapy. Cremophor EL is implicated with hypersensitivity reactions, hyperlipidemia, and neurotoxicity [54, 55]. In recent studies with mouse models of IP cancer, IP-injected Taxol (20 mg/kg PTX, 4 times over 37 days [56] or 20 mg/kg q7d × 3 [29]) induced significant loss of body weight during the treatment period. Similarly we observed a significant weight loss with a bolus IP administration of Taxol (30 mg/kg PTX) and Taxol-gel in 2–3 days after the treatment, as compared with the no treatment group. On the other hand, mice receiving Taxol-gel recovered the weight quickly, reaching the same level as the no treatment group in 4–5 days. Given that all three groups receiving gel formulations gained weight in 6–7 days, the fast weight recovery observed in the Taxol-gel group is likely to be a unique function of HA gel, although the mechanism is yet unclear.
5. CONCLUSIONS
HA-based in-situ crosslinkable hydrogel was used as a carrier of PTX particles for the regional delivery of PTX to the IP tumors. PTX was best retained in the peritoneal cavity as PTX-gel (microparticulate PTX entrapped in the HA gel) followed by PTX-susp (microparticulate PTX particles). Taxol-based formulations (Taxol-gel, Taxol, and Taxol-multiple) were cleared from the peritoneal cavity early on and were not detected after 14 days. Despite the relatively good IP retention, PTX-gel and PTX-susp did not further enhance the therapeutic effects against the IP tumors than the rapidly clearing Taxol formulations (Taxol-gel, Taxol, and Taxol-multiple) in 14 days due to the limited dissolution of PTX. This result indicates that spatial availability of a drug does not necessarily translate to the enhanced anti-tumor effect unless it is accompanied by the temporal availability. HA gel may enhance the regional effect of a drug as desired if paired with particles of an optimal size that allow for continuous and unhindered supply of PTX.
Supplementary Material
Acknowledgments
The authors thank Travis Huber and Joonyoung Park for technical assistance with PTX analysis, Dr. Michael Tsifansky for reading the blood data, and Drs. James Litster and Lynne Taylor for allowing us to use the Mastersizer and microscope, respectively. This work was supported by NIH R21 CA135130, AAPS new investigator award, and AAPS travel award. This work was also partially supported by a grant from the Lilly Endowment, Inc., to the College of Pharmacy, Purdue University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts & Figures 2010. 2010 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf.
- 2.Poveda AA, Salazar RR, del Campo JJM, Mendiola CC, Cassinello JJ, Ojeda BB, Arranz JJA, Oaknin AA, García-Foncillas JJ, Rubio MMJ, González Martín AA. Update in the management of ovarian and cervical carcinoma. Clin Transl Oncol. 2007;9(7):443–451. doi: 10.1007/s12094-007-0083-7. [DOI] [PubMed] [Google Scholar]
- 3.Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335(26):1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj G, Yeo Y. Drug delivery systems for intraperitoneal therapy. Pharm Res. 2010;27(5):735–738. doi: 10.1007/s11095-009-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 6.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 7.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61(3):183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dedrick RL, Myers CE, Bungay PM, DeVita VT., Jr Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62(1):1–11. [PubMed] [Google Scholar]
- 9.Markman M. Intraperitoneal drug delivery of antineoplastics. Drugs. 2001;61(8):1057–1065. doi: 10.2165/00003495-200161080-00003. [DOI] [PubMed] [Google Scholar]
- 10.Aviram N, Alexander S, Alfredo G, Jesus E, Pompiliu P. Evidence-based medicine in the treatment of peritoneal carcinomatosis: Past, present, and future. J Surg Oncol. 2009;100(4):335–344. doi: 10.1002/jso.21323. [DOI] [PubMed] [Google Scholar]
- 11.NCI. NCI Clinical Announcement on Intraperitoneal Therapy for Ovarian Cancer. 2006 http://ctep.cancer.gov/highlights/docs/clin_annc_010506.pdf.
- 12.Ho EA, Soo PL, Allen C, Piquette-Miller M. Impact of intraperitoneal, sustained delivery of paclitaxel on the expression of P-glycoprotein in ovarian tumors. J Control Release. 2007;117(1):20–27. doi: 10.1016/j.jconrel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Zahedi P, De Souza R, Huynh L, Piquette-Miller M, Allen C. Combination drug delivery strategy for the treatment of multidrug resistant ovarian cancer. Mol Pharmaceutics. 2011;8(1):260–269. doi: 10.1021/mp100323z. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed F, Marchettini P, Stuart OA, Sugarbaker PH. Pharmacokinetics and tissue distribution of intraperitoneal paclitaxel with different carrier solutions. Cancer Chemother Pharmacol. 2003;52(5):405–410. doi: 10.1007/s00280-003-0680-2. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed F, Stuart OA, Sugarbaker PH. Pharmacokinetics and tissue distribution of intraperitoneal docetaxel with different carrier solutions. J Surg Res. 2003;113(1):114–120. doi: 10.1016/s0022-4804(03)00162-8. [DOI] [PubMed] [Google Scholar]
- 16.Tsai M, Lu Z, Wang J, Yeh T-K, Wientjes M, Au J. Effects of carrier on disposition and antitumor activity of intraperitoneal paclitaxel. Pharm Res. 2007;24(9):1691–1701. doi: 10.1007/s11095-007-9298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozols RF. Challenges for chemotherapy in ovarian cancer. Ann of Oncol. 2006;17(Suppl 5):v181–v187. doi: 10.1093/annonc/mdj978. [DOI] [PubMed] [Google Scholar]
- 18.Markman M, Walker JL. Intraperitoneal chemotherapy of ovarian cancer: a review, with a focus on practical aspects of treatment. J Clin Oncol. 2006;24(6):988–994. doi: 10.1200/JCO.2005.05.2456. [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Li B, Kang Y, Jiang W, Huang Q, Chen Q, Li L, Xu C. Paclitaxel nanoparticle inhibits growth of ovarian cancer xenografts and enhances lymphatic targeting. Cancer Chemother Pharmacol. 2007;59(2):175–181. doi: 10.1007/s00280-006-0256-z. [DOI] [PubMed] [Google Scholar]
- 20.Yeo Y, Ito T, Bellas E, Highley CB, Marini R, Kohane DS. In situ cross-linkable hyaluronan hydrogels containing polymeric nanoparticles for preventing postsurgical adhesions. Ann Surg. 2007;245(5):819–824. doi: 10.1097/01.sla.0000251519.49405.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelderblom H, Verweij J, van Zomeren DeM, Buijs D, Ouwens L, Nooter K, Stoter G, Sparreboom A. Influence of Cremophor EL on the bioavailability of intraperitoneal paclitaxel. Clin Cancer Res. 2002;8(4):1237–1241. [PubMed] [Google Scholar]
- 22.Kohane DS, Tse JY, Yeo Y, Padera R, Shubina M, Langer R. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. J Biomed Mater Res A. 2006;77(2):351–361. doi: 10.1002/jbm.a.30654. [DOI] [PubMed] [Google Scholar]
- 23.Lu Z, Tsai M, Lu D, Wang J, Wientjes MG, Au JLS. Tumor-penetrating microparticles for intraperitoneal therapy of ovarian cancer. J Pharmacol Exp Ther. 2008;327(3):673–682. doi: 10.1124/jpet.108.140095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura T, Imai J, Matsumoto A, Tanimoto M, Suzuki A, Horikiri Y, Suzuki T, Yoshino H, Ike O. Organ distribution of cisplatin after intraperitoneal administration of cisplatin-loaded microspheres. Eur J Pharm Biopharm. 2002;54(1):1–7. doi: 10.1016/s0939-6411(02)00037-1. [DOI] [PubMed] [Google Scholar]
- 25.Dadashzadeh S, Mirahmadi N, Babaei MH, Vali AM. Peritoneal retention of liposomes: Effects of lipid composition, PEG coating and liposome charge. J Control Release. 2010;148:177–186. doi: 10.1016/j.jconrel.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Yeo Y, Kohane DS. Polymers in the prevention of peritoneal adhesions. Eur J Pharm Biopharm. 2008;68(1):57–66. doi: 10.1016/j.ejpb.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho EA, Vassileva V, Allen C, Piquette-Miller M. In vitro and in vivo characterization of a novel biocompatible polymer-lipid implant system for the sustained delivery of paclitaxel. J Control Release. 2005;104(1):181–191. doi: 10.1016/j.jconrel.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Soo PL, Cho J, Grant J, Ho E, Piquette-Miller M, Allen C. Drug release mechanism of paclitaxel from a chitosan-lipid implant system: Effect of swelling, degradation and morphology. Eur J Pharm Biopharm. 2008;69(1):149–157. doi: 10.1016/j.ejpb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Vassileva V, Grant J, De Souza R, Allen C, Piquette-Miller M. Novel biocompatible intraperitoneal drug delivery system increases tolerability and therapeutic efficacy of paclitaxel in a human ovarian cancer xenograft model. Cancer Chemother Pharmacol. 2007;60(6):907–914. doi: 10.1007/s00280-007-0449-0. [DOI] [PubMed] [Google Scholar]
- 30.Hyoudou K, Nishikawa M, Ikemura M, Kobayashi Y, Mendelsohn A, Miyazaki N, Tabata Y, Yamashita F, Hashida M. Cationized catalase-loaded hydrogel for growth inhibition of peritoneally disseminated tumor cells. J Control Release. 2007;122:151–158. doi: 10.1016/j.jconrel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 31.De Souza R, Zahedi P, Allen CJ, Piquette-Miller M. Biocompatibility of injectable chitosan-phospholipid implant systems. Biomaterials. 2009;30(23–24):3818–3824. doi: 10.1016/j.biomaterials.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Zahedi P, De Souza R, Piquette-Miller M, Allen C. Chitosan-phospholipid blend for sustained and localized delivery of docetaxel to the peritoneal cavity. Int J Pharm. 2009;377(1–2):76–84. doi: 10.1016/j.ijpharm.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Yu J, Lee HJ, Hur K, Kwak MK, Han TS, Kim WH, Song SC, Yanagihara K, Yang HK. The antitumor effect of a thermosensitive polymeric hydrogel containing paclitaxel in a peritoneal carcinomatosis model. Invest New Drugs. doi: 10.1007/s10637-010-9499-y. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Gong C, Yang L, Wu Q, Shi S, Shi H, Qian Z, Wei Y. 5-FU-hydrogel inhibits colorectal peritoneal carcinomatosis and tumor growth in mice. BMC Cancer. 2010;10:402. doi: 10.1186/1471-2407-10-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeo Y, Adil M, Bellas E, Astashkina A, Chaudhary N, Kohane DS. Prevention of peritoneal adhesions with an in situ cross-linkable hyaluronan hydrogel delivering budesonide. J Control Release. 2007;120(3):178–185. doi: 10.1016/j.jconrel.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Sparreboom A, van Zuylen L, Brouwer E, Loos WJ, de Bruijn P, Gelderblom H, Pillay M, Nooter K, Stoter G, Verweij J. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999;59(7):1454–1457. [PubMed] [Google Scholar]
- 37.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47(2):152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 38.Satpathy M, Cao L, Pincheira R, Emerson R, Bigsby R, Nakshatri H, Matei D. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007;67(15):7194–7202. doi: 10.1158/0008-5472.CAN-07-0307. [DOI] [PubMed] [Google Scholar]
- 39.Bouckaert PX, Evers JL, Doesburg WH, Schellekens LA, Brombacher PH, Rolland R. Patterns of changes in proteins in the peritoneal fluid of women during the periovulatory phase of the menstrual cycle. J Reprod Fertil. 1986;77(2):329–336. doi: 10.1530/jrf.0.0770329. [DOI] [PubMed] [Google Scholar]
- 40.Yeo Y, Bellas E, Highley CB, Langer R, Kohane DS. Peritoneal adhesion prevention with an in situ cross-linkable hyaluronan gel containing tissue-type plasminogen activator in a rabbit repeated injury model. Biomaterials. 2007;28(25):3704–3713. doi: 10.1016/j.biomaterials.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 41.Hartveit F, Thunold S. Peritoneal fluid volume and the oestrus cycle in mice. Nature. 1966;210(5041):1123–1125. doi: 10.1038/2101123a0. [DOI] [PubMed] [Google Scholar]
- 42.Auzenne E, Ghosh SC, Khodadadian M, Rivera B, Farquhar D, Price RE, Ravoori M, Kundra V, Freedman RS, Klostergaard J. Hyaluronic acid-paclitaxel: Antitumor efficacy against CD44(+) human ovarian carcinoma xenografts. Neoplasia. 2007;9(6):479–486. doi: 10.1593/neo.07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall JC, Heel KA, Papadimitriou JM, Platell C. The pathobiology of peritonitis. Gastroenterology. 1998;114(1):185–196. doi: 10.1016/s0016-5085(98)70646-8. [DOI] [PubMed] [Google Scholar]
- 44.Dufrane D, Steenberghe Mv, Goebbels R-M, Saliez A, Guiot Y, Gianello P. The influence of implantation site on the biocompatibility and survival of alginate encapsulated pig islets in rats. Biomaterials. 2006;27(17):3201–3208. doi: 10.1016/j.biomaterials.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 45.Yeo Y, Burdick JA, Highley CB, Marini R, Langer R, Kohane DS. Peritoneal application of chitosan and UV-cross-linkable chitosan. J Biomed Mater Res A. 2006;78(4):668–675. doi: 10.1002/jbm.a.30740. [DOI] [PubMed] [Google Scholar]
- 46.Yeo Y, Highley CB, Bellas E, Ito T, Marini R, Langer R, Kohane DS. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials. 2006;27(27):4698–4705. doi: 10.1016/j.biomaterials.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 47.Ringel I, Horwitz SB. Taxol is converted to 7-epitaxol, a biologically active isomer, in cell culture medium. J Pharmcol Exp Ther. 1987;242(2):692–698. [PubMed] [Google Scholar]
- 48.Willey TA, Bekos EJ, Gaver RC, Duncan GF, Tay LK, Beijnen JH, Farmen RH. High-performance liquid chromatographic procedure for the quantitative determination of paclitaxel (Taxol) in human plasma. J Chromatogr. 1993;621(2):231–238. doi: 10.1016/0378-4347(93)80100-i. [DOI] [PubMed] [Google Scholar]
- 49.Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther. 1971;178(3):562–566. [PubMed] [Google Scholar]
- 50.Hirano K, Hunt CA. Lymphatic transport of liposome-encapsulated agents: effects of liposome size following intraperitoneal administration. J Pharm Sci. 1985;74(9):915–921. doi: 10.1002/jps.2600740902. [DOI] [PubMed] [Google Scholar]
- 51.Kipp JE. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int J Pharm. 2004;284(1–2):109–122. doi: 10.1016/j.ijpharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 52.Liversidge GG, Cundy KC. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int J Pharm. 1995;125(1):91–97. [Google Scholar]
- 53.Roby KF, Niu F, Rajewski RA, Decedue C, Subramaniam B, Terranova PF. Syngeneic mouse model of epithelial ovarian cancer: effects of nanoparticulate paclitaxel, Nanotax. Adv Exp Med Biol. 2008;622:169–181. doi: 10.1007/978-0-387-68969-2_14. [DOI] [PubMed] [Google Scholar]
- 54.Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR, Van Echo DA, Von Hoff DD, Leyland–Jones B. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8(7):1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 55.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 56.De Stefano I, Battaglia A, Zannoni G, Prisco M, Fattorossi A, Travaglia D, Baroni S, Renier D, Scambia G, Ferlini C, Gallo D. Hyaluronic acid-paclitaxel: effects of intraperitoneal administration against CD44(+) human ovarian cancer xenografts. Cancer Chemother Pharmacol. 2011;68(1):107–116. doi: 10.1007/s00280-010-1462-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.