Abstract
Emerging evidence support a role of purinergic P2X3 receptors in modulating nociceptive signaling in sensory neurons. Previously we showed that DRG neurons (L1-S1) express both ERα and ERβ receptors. In this study we investigated the expression of P2X3 receptors and the effect of 17β-estradiol (E2) on ATP-induced [Ca2+]i increase in DRG neurons collected from C57Bl/6J, ERαKO and ERβKO mice. Our data showed a significant decrease for P2X3 in ERαKO (all levels) and ERβKO (mostly observed in L1, L2, L4, and L6). Furthermore, 17β-estradiol (100 nM) significantly attenuated the ATP (10 μM)-induced [Ca2+]i in C57Bl/6J mice. ERs antagonist ICI 182,780 (1μM) blocked this attenuation. Homomeric P2X3 receptors are plentifully expressed in DRG neurons and contribute to nociceptive signals. α,β-me ATP which is a specific agonist of P2X2/3 receptors showed similar responses to the ATP-induced calcium increase in knock-out mice. A membrane-impermeable E-6-BSA (1μM) had the same effect as E2 suggesting action on the membrane. In DRG neurons from ERβKO and WT miceE2 attenuated the ATP/α,β-me ATP-induced [Ca2+]i fluxes but in DRG neurons from ERαKO mice, this hormone had no effect suggesting that this attenuation depends on membrane-associated ERα receptors. Together our data indicate an interaction between P2X3 and membrane-associated ERα in primary sensory neurons that may represent a novel mechanism to explain sex differences observed in clinical presentation of visceral nociceptive syndromes.
Keywords: 17β-estradiol (E2), DRG, P2X3, ATP, Ca2+, ERα/ERβ
Introduction
Sex-related differences in pain processing and responsiveness have been observed in clinical studies [1] as well as in animal models of nociception [2]. Experts generally agree that in both population-based studies and patient-based studies, women are more likely affected by most chronic functional pain conditions, including painful bladder syndrome (PBS), irritable bowel syndrome (IBS), chronic pelvic pain (CPP) and fibromyalgia. The mechanisms underlying the greater vulnerability of women remains incompletely understood.
17β-estradiol (E2), the most common form of estrogen, interferes with pain transmission by regulating rapid changes of ion-channel opening and membrane-associated neurotransmitter receptors [3-4]. Estrogen-binding proteins have been associated with the plasma membraneand transfected chinese hamster ovary cells with the cDNAs for ERα and ERβ showed that the putative membrane-associated estrogen receptors are indistinguishable to the nuclear receptors [5]. Localization of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) in DRG neurons [6] suggests that estrogen may regulate nociceptive signaling at the level of primary afferents.
DRG neurons can be activated or modulated by the activation of chemosensitive receptors on peripheral terminals and ATP has been implicated in sensory transduction of noxious stimuli by activating purinergic P2X receptors [7]. Once ATP is released into the intercellular areas, P2X3 receptors are activated on primary afferent fibers and cell bodies within DRG. Activation of P2X3 receptors results in the depolarization and opening of voltage-gated Ca2+ channels (VGCC) [8]. A sensation of pain is produced by depolarization of the peripheral nerve terminals. ATP-sensitive P2X3 and α,β-methylene ATP-sensitive P2X2/3 receptors play an important role within the nociceptive systems triggering a nociceptive signaling.
Primary DRG neurons culture has been a useful model system for investigating sensory physiology and putative nociceptive signaling [9]. ATP-induced intracellular calcium concentration ([Ca2+]i) transients in cultured DRG neurons have been used to model the response of nociceptors to painful stimuli [10].
Previously we showed that E2, acting at the level of the plasma membrane, attenuates ATP-induced [Ca2+]i fluxes [11]. Within the context of our present hypothesis E2 modulation of visceral nociception and nociceptor sensitization appear to be regulated by both P2X3 and P2X2/3. Estrogen attenuation of DRG neurons response to ATP suggests that visceral afferent nociceptors can be modulated by sex steroids at a new site at the level of primary afferent neurons. In this manuscript we report that the expression of P2X3 depends on the expression of both ERs and that E2 mediates its effect through membrane-associated ERs.
Materials and Methods
Animals
We have used 6~8 week old female wild type (Wt, C57Bl/6J), ERαKO (B6.129P2-Esr1tm1Ksk/J), and ERβKO (B6.129P2-Esr2tm1Unc/J) mice (Jackson Laboratory, Bar Harbor, ME). Upon arrival mice were housed in microisolator caging and maintained on a 12-h light/dark cycle in a temperature-controlled environment with access to food and water ad libitum for two weeks. All studies were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Charles R. Drew University and the NIH Guide for the Care and Use of Laboratory Animals. In some experiments we used animals from our breeding colony.
Primary culture of DRG neurons
The isolation procedure and primary culture of mouse lumbosacral DRG has been published in detail [11]. DRG tissues were obtained from C57Bl/6J (30 g), ERαKO and ERβKO (Jackson Laboratory; 20 g) transgenic types. Briefly, lumbosacral adult DRGs (level L1-S1) were collected under sterile technique and placed in ice-cold medium Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma-Aldrich St. Louis, MO). Adhering fat and connective tissue were removed and each DRG was minced with scissors and placed immediately in a medium consisting of 5 ml of DMEM containing 0.5 mg/ml of trypsin (Sigma, type III), 1 mg/ml of collagenase (Sigma, type IA) and 0.1 mg/ml of DNAase (Sigma, type III) and kept at 37°C for 30 minutes with agitation. After dissociation of the cell ganglia, soybean trypsin inhibitor (Sigma, type III) was used to terminate cell dissociation. Cell suspensions were centrifuged for one minute at 1000 rpm and the cell pellet were resuspended in DMEM supplemented with 5% fetal bovine serum, 2 mM glutamine-penicillin-streptomycin mixture, 1 μg/ml DNAase and 5 ng/ml NGF (Sigma). Cells were placed on Matrigel® (Invitrogen, Carlsbad, CA)- coated 15-mm coverslips (Collaborative Research Co., Bedford, PA) and kept at 37° C in 5% CO2 incubator for 24h, given fresh media and maintained in primary culture until used for experimental procedures.
Western Blot Analysis
The expressions of P2X3 receptors in L1-S1 DRGs were studied by using Western blot analyses. Tissues from Wt (C57Bl/6J), ERαKO, and ERβKO mice were quick frozen in tubes on dry ice during collection. L1-S1 DRG were combined, homogenized by mechanical disruption on ice-cold RIPA buffer plus protease inhibitors and incubated on ice for 30 minutes. Homogenates were then spun at 5000g for 15 minutes and supernatants collected. Total protein was determined on the supernatants using the BCA microtiter method (Pierce, Rockford, IL, USA). Samples containing equal amounts of protein (40μg) were electrophoresed under denaturing conditions using Novex Mini-cell system (San Diego, CA, USA) and reagents (NuPage 4-12% Bis-Tris gel and MOPS running buffer). After electrophoretic transfer onto nitrocellulose membrane using the same system, the membrane was blocked with 5% non-fat dry milk (NFDM) in 25 mM TRIS buffered saline, pH 7.2, plus 0.1% Tween 20 (TBST) for 1 hour at room temperature, followed by incubation with polyclonal rabbit antibody against P2X3 receptor (1:1000, Neuromics, Edina, MN) for overnight at 4°C. The membrane was then washed in TBST plus NFDM, and proteins were visualized using a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, CA). Following a final wash in TBST without NFDM, the membrane was incubated with ECL+ (Amersham, Arlington Heights, Ill., USA) substrate for HRP. Membranes were probed with primary antibody and corresponding secondary antibodies, signals were scanned and quantified by Image J version 1.28U and NIH Image 1.60 scan software. Following enhanced chemiluminescence detection of proteins, the membranes were stripped with stripping buffer (Pierce, Rockford, IL, USA) and re-hybridized with β-actin antibody as a loading control. At least three independent cell preparations were used.
Immunohistochemistry
For tissue collection DRG from bilateral spinal levels L1-S2 were removed and fixed in 4% paraformaldehyde for overnight at 4°C. DRGs were rinsed in Delbecco’s Phosphate Buffered Saline (DPBS) and kept in sucrose (20%, 4°C) for cryoprotection (48 h), after which excess liquid was removed. Each DRG was mounted in Tissue-Tek® OCT embedding medium (Sakura Finetek, Torrance, CA), and sectioned at −20°C in a MICROM H505E (Viocompare, San Francisco, CA) cryostat. Sections were cut at 20μm and collected in PBS. Endogenous tissue peroxidase activity was quenched by soaking the sections for 10 min in 3% hydrogen peroxide solution in 0.01 M PBS. The specimens were washed and then treated for 60 min in blocking solution, 0.01 M PBS containing 0.5% Triton X-100 and 1% normal donkey serum (NDS) at room temperature. They were processed with P2X3 receptor antibody (1:5000, Neuromics, Edina, MN) for overnight at 4°C, washed in 0.01 M phosphate-buffered saline (PBS) and 0.01M Tris Buffered Saline (TBS), followed by incubation in solutions of donkey anti-rabbit fluorophore-conjugated secondary antibodies (1:200, Invitrogen) in 0.01M Tris Buffered Saline (TBS) for 3 hours at room temperature. Cells showing no apparent or only faint membrane / intracellular labeling were considered to be negative for P2X3. P2X3-positive neurons showed diffuse membrane/ intracellular labeling were mounted and coverslipped with Aqua Poly Mount (Polisciences, Warrington, PA). Images from at least three sections in each level were taken using Leica DMLB M130X microscope. The total numbers of DRG neurons expressing P2X3 were counted. Immunohistochemical signal percent was measured by computerized image analysis (Image Pro-Plus, Media Cybernetics, Silver Spring, MD, USA).
[Ca2+]i fluorescence imaging
Ca2+ fluorescence imaging was carried out as previously described [12-13]. DRG neurons were loaded with fluorescent dye 5 mM Fura-2 AM (Invitrogen, Carlsbad, CA) for 45 min at 37°C in HBSS supplemented with 20 mM HEPES, pH 7.4. The coverslips were mounted in a RC-26 recording chamber P-4 (Warner Instruments, Hamden, CT) and placed on a stage of Olympus IX51 inverted microscope (Olympus America, Center Valley, PA). Observations were made at room temperature (20-23°C) with 20X UApo/340 objective. Neurons were bathed and perfused with HBSS buffer using with using gravity at a rate of 1-2 ml/min. Fluorescence intensity at 505 nm with excitation at 334 nm and 380 nm were captured as digital images (sampling rates of 0.1-2 s). Regions of interest were identified within the soma from which quantitative measurements were made by re-analysis of stored image sequences using Slidebook® Digital Microscopy software. [Ca2+]i was determined by ratiometric method of Fura-2 fluorescence from calibration of series of buffered Ca2+ standards. E2 was applied acutely for five minutes onto the experimental chamber. Application of drugs was achieved by superfusion in a rapid mixing chamber and Perfusion Fast-Step system SF-77B (Warner Instruments) to add drugs in 100-500 ms interval.
We calculated actual [Ca2+]i in areas of interest in each neurons with the formula:
Where Kd is the indicator’s dissociation constant of the fluoroprobe; R is ratio of fluorescence intensity at two different wavelengths (340/380 nm for fura-2); Rmax and Rmin are the ratio at fura-2 with an saturated Ca2+ and free Ca2+. β is the ratio of the denominators of the minimum and maximum conditions.
Statistical analysis
The amplitude of [Ca2+]i response represents the difference between baseline concentration and the transient peak response to drug stimulation. Significant differences in response to chemical stimulation were obtained by comparing [Ca2+]i increases during the first stimulation with the second. All of the data are expressed as the mean ± SEM. Statistical analysis was performed using Statistical Package for the Social Sciences 15.0 (SPSS, Chicago, IL, USA). To assess the significance among different groups, data were analyzed with one-way ANOVA followed by Schéffe post hoc test. A p <0.05 was considered statistically significant.
Results
Expression of P2X3 in DRG neurons
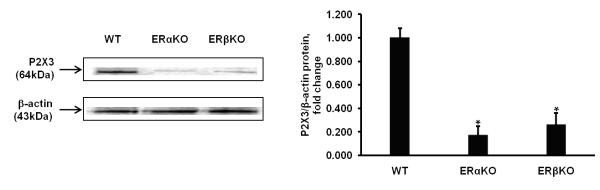
Previously we detected both ERα and ERβ in Wt mouse DRG neurons using mRNA RT-PCR [14]. In this study P2X3 receptors expression was examined by Western blot analysis of lysates from Wt, ERαKO, and ERβKO DRG tissues using a P2X3 specific primary antiserum. An intense band representing a ~64 kDa protein (P2X3) was seen in DRG lysates from Wt animals. In our experiments we found that there was a dramatic decrease in intensity of this band using lysates from the both knock out DRG tissues (>4 fold decrease of control). A representative result of P2X3 receptors is shown and the standardization ratio statistics of three tests is shown in Fig.1. The average intensities of the bands in both knock-out mice decreased significantly. When the density in the control group was standardized to 1.0, the average densities were 0.172 ± 0.08 of ERαKO and 0.262 ± 0.10 of ERβKO in P2X3 receptors suggesting that P2X3 protein decreased in DRG from knock-out mice p<0.05 (n=4).
Figure 1.
Western blot analysis of DRG lysates shows reduced expression of P2X3 in both knock-out mice. Quantification of signals from Western blots shows statistically significant difference between the intensity of the bands from both knock-out DRG neurons when compared with wild type animals.
Our data also show that DRG neurons from Wt, ERαKO, ERβKO express nociceptive ATP-sensitive P2X3 receptors by using immunohistochemistry. Representative neuronal profiles from each group (L2 level) presented in Fig 2 (a). The distributions of labeled dorsal root ganglion neurons represent the statistically significant difference between L1, L2, L4, and L6 levels for P2X3 receptors in both ERαKO and ERβKO DRG neurons when compared with Wt mice. In ERαKO mice P2X3 receptors expression was reduced in L1-L6 levels but not in S1 (Fig. 2 (b), P<0.05, n=4 in each group).
Figure 2.
(a) Expression of P2X3 receptors in DRG neurons from Wt, ERαKO, and ERβKO in vivo using fluorescent microscopy. DRG sections (L2 level) were incubated in P2X3 primary antibodies. (b) Percentage distribution of labeled dorsal root ganglion neurons in ERαKO and ERβKO as well as wild type mice with P2X3 through L1-S1 levels. * indicate statistically significant difference from control, P<0.05.
Effect of E2 and pharmacological profile of E2-mediated modulation on ATP-induced [Ca2+]i in DRG neurons
Our data suggest that ATP-induced [Ca2+]i transients in DRG neurons in mice, a result similar to that observed in rat DRG neurons [11]. Brief 10 second application of ATP (10μM) by fast superfusion produced equal [Ca2+]i spikes in 65% of tested neurons.. After a 5-min washout with HBSS, additional stimulation with ATP (10μM) induced a subsequent [Ca2+]i transients (Fig 3 (a)). Pretreatment with purinergic receptor antagonist PPADS (5 μM) blocked the ATP-induced [Ca2+]i transients (data not shown). Similarly, ATP stimulation in a Ca2+-free media with the Ca2+ chelator, BAPTA (10 mM), eliminated [Ca2+]i spikes indicating the necessity for P2X3 receptors and extracellular Ca2+(data not shown).
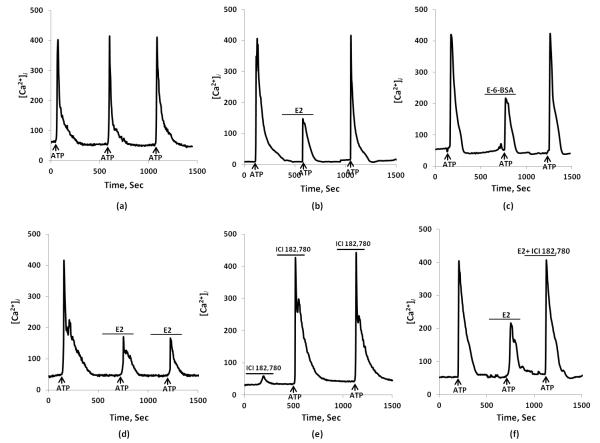
Figure 3.
17β-estradiol (E2) inhibits ATP-induced [Ca2+]i transients in wild type mice.
(a) Typical indication of equal [Ca2+]i responses to repeated ATP (10 μM) stimulation (indicated by arrow) with 10 min interval under control condition. (b) Second ATP-induced [Ca2+]i response rapidly attenuated by E2 (100 nM) in dorsal root ganglion cells. (c) E2-BSA (1 μM) inhibited the ATP-induced [Ca2+]i transient. After wash-out with experimental medium, ATP response on [Ca2+]i returned to initial (control) amplitude of stimulation. (d) The effect of E2 does not desensitize upon repeated application of ATP. (e) Effect of estrogen receptor antagonist ICI 182,780 alone and application of ATP. (f) ICI 182,780 (1 μM) blocked the E2 attenuation of ATP-induced [Ca2+]i transients.
E2 (100 nM) by itself had no effect on basal [Ca2+]i, but this hormone attenuated the ATP-induced [Ca2+]i transients (Figs 3 (b) & 4). The effect of E2 was reversible. After the initial ATP response, five minute incubation with E2 inhibited ATP-induced [Ca2+]i transient (440.3±58.3 vs. 280.4±48.8 nM, n=15, p < 0.05). To confirm the desensitization of E2, we administered application of repeated ATP, indicating that E2 does not desensitize upon of application of repeated ATP (Fig. 3 (d). The estrogen receptor antagonist ICI 182,780 (1 μM) blocked the 17β-estradiol effect on attenuated ATP-induced [Ca2+]i transients (Fig 3 (f) & Fig. 4) and ICI 182,780 demonstrates no effect by itself (Fig 3 (e)).
Figure 4.
Summary of ATP-induced [Ca2+]i influxes in control, in the presence of E2, E-6-BSA, and ICI 182,780. E2 significantly decreased [Ca2+]i response to ATP whereas estrogen receptor antagonist ICI 182,780 blocked E2 effect. Values are expressed as mean ± SEM. * indicate statistically significant difference from control, P<0.05.
Effect of E2-BSA on ATP-induced [Ca2+]i response in DRG neurons
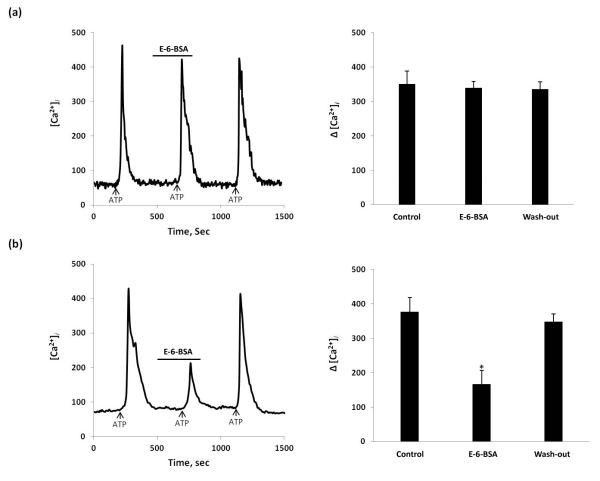
In the present study, the rapid time course and the higher doses required for ER activation suggest that E2 inhibition of ATP-induced [Ca2+]i flux was mediated through a membrane associated ERs. A membrane impermeable construct, E-6-BSA (17β-estradiol 6-(O-carboxymethyl) oxime-BSA, Sigma) was used to determine whether the E2 actions on ATP-induced [Ca2+]i were mediated at the membrane. E-6-BSA (1μM), filtered to remove any potentially unconjugated estradiol, mimicked the effect of E2 (Fig 3 (c) & Fig. 4). Our data indicate that diffusion of E2 into DRG neurons to act on nuclear ERs was not essential for the E2 inhibition of ATP-induced [Ca2+]i transients in DRG neurons in primary culture. To clarify theE-6-BSA diminution of ATP-induced Ca2+ response with ER subtypes, we compared E-6-BSA action mediating [Ca2+] flux in DRG neurons from Wt, and knockout mice. The effect of E-6-BSA mimicked in ERβKO mouse DRG neurons to that performed in Wt mice (n = 38 cells/3 mouse) (Fig 5 (b)). However, in ERαKO mice, E-6-BSA did not block ATP-induced [Ca2+]i fluxes (n=32 cells/3 mouse)(Fig 5 (a)), indicating that its diminution relies on ERα.
Figure 5.
The effect of E-6-BSA on ATP-induced [Ca2+]i fluxes in ERαKO and ERβKO mice. (a) In ERαKO mouse, E-6-BSA added for 5 min didn’t inhibit ATP-induced [Ca2+]i flux; (b) In ERβKO mouse, Effect of E-6-BSA mimicked that observed in Wt mouse. Summary data represented on the right bar graphs. * Statistically significant difference from control, P<0.05.
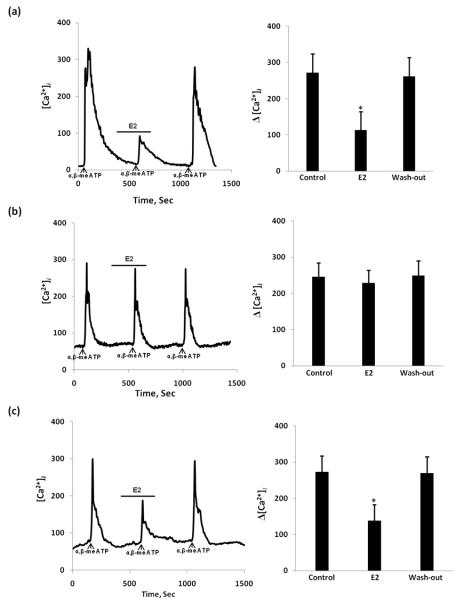
17β-estradiol attenuation of ATP and α,β-me ATP-induced [Ca2+]i influx in DRGs from ERαKO and ERβKO mice
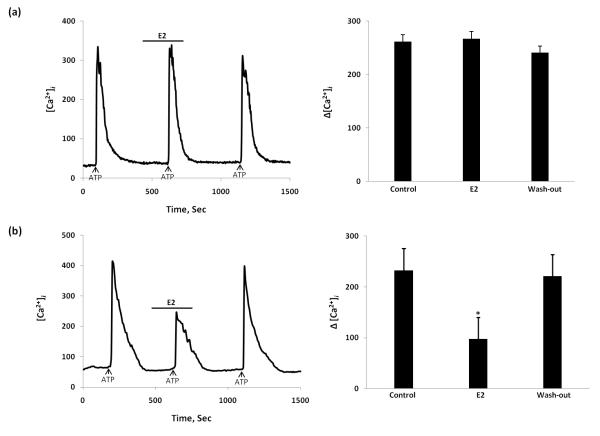
To confirm which ER subtype mediates the E2 attenuation of ATP-induced response, we compared estradiol action mediating Ca2+ signaling in DRG neurons from Wt, and knockout mice. The effect of E2 was similar in ERβKO mouse DRG neurons to that observed in Wt mice (n = 125 cells/5 mouse) (Fig 6 (b)). However, in DRG neurons from ERαKO mice, E2 did not block ATP-induced [Ca2+]i suggesting that its attenuation depends on ERα (n = 112 cells/5 mouse) (Fig. 6 (a)). 17α-Estradiol had no effect on Wt, ERαKO or ERβKO mice. We also used α,β-me ATP, a specific agonist of P2X2/3, to confirm the observations presented with ATP (n=105 cells/each group 6 mouse). Even the properties of P2X2/3 receptors are not similar to those of the P2X3 receptors both P2X3 and P2X2/3 receptors may be a target of action for estradiol (Fig 7). Our experiments used a combination of techniques to determine that DRG neurons in culture can be used to study the cellular response to a putative nociceptive signal, ATP. Our data suggest that in primary DRG neuronal cultures, E2 attenuates the ATP/α,β-meATP -induced [Ca2+]i responses and interferes with the membrane-associated ERα. In our experiments we noticed significant decrease in number of responsive neurons in both knock-out mice. Fewer than 20% of tested cells responded to ATP/α,β-meATP stimulation which correspond to the fact that that both ERKO and ERKO exhibit decrease in their expression of P2X3 receptors (Fig. 1).
Figure 6.
The effect of E2 on ATP-induced [Ca2+]i transients in estrogen receptor-α knockout (ERαKO) and estrogen receptor-β knockout (ERβKO) mice. (a) In ERαKO mouse, E2 added for 5 min didn’t inhibit ATP-induced [Ca2+]i transient; (b) In ERβKO mouse E2 stimulation significantly attenuated the ATP-stimulated [Ca2+]i transient similar to that observed in Wt mouse. Summary data represented on the right bar graphs. *Statistically significant difference from control, P<0.05.
Figure 7.
The effect of 17β-estradiol (E2) on α,β-meATP-induced [Ca2+]i transients in Wt, ERαKO, and ERβKO mice. (a) Wt mouse as a control, (b) ERαKO mouse, E2 added for 5min didn’t inhibit α,β-meATP-induced [Ca2+]i transient in control vs. after E2 treatment. (c) In ERβKO mouse, E2 stimulation significantly attenuated the α,β-meATP-stimulated [Ca2+]i transient similar to that observed in Wt mouse. Summary data represented on the right bar graphs. Values are expressed as mean±SEM. Δ[Ca2+]i were determined by subtracting the [Ca2+]i peak levels from the basal [Ca2+]i levels. *Statistically significant difference from control, p < 0.05.
Discussion
Visceral pain sensations associated with Irritable Bowel Syndrome, Pain Bladder Syndrome and Chronic Pelvic Pain are different from cutaneous pain based on clinical, neurophysiologic and pharmacological characteristics. The pathophysiology of visceral hyperalgesia is less well-known than its cutaneous counterpart, and our understanding of visceral hyperalgesia is colored by comparison to cutaneous hyperalgesia, which is believed to arise as a consequence of the sensitization of peripheral nociceptors due to long-lasting changes in the excitability of spinal neurons [15]. Many chronic functional syndromes characterized by recurring symptoms of abdominal discomfort or pain and pathophysiological alterations in the absence of detectable organic disease are more prevalent in women. In general, pain thresholds are lower in women than in men and pain symptoms vary with reproductive cycle. In women, estrogen may be a causative factor, inducing inflammatory diseases that may contribute to nociception associated with functional pain syndromes. Functional syndromes lack a specific pathology in the affected organ but may respond to a viscero-visceral cross-sensitization in which increased nociceptive input from an inflamed organ (i.e., uterus) sensitizes neurons that receive convergent input from an unaffected organ (i.e., colon) [16]. The site of visceral cross-sensitivity is unknown. One explanation is a central nervous system E2 modulation and convergence. Data from our laboratory and others suggest that E2-induced modulation of viscero-visceral cross-sensitization occurs in the dorsal root ganglion [11, 14, 17-18]. The localization of ERs in DRG neurons [6] and the attenuation of ATP-induced [Ca2+]i strongly suggest that E2 modulates visceral pain processing peripherally. Visceral nociception and nociceptor sensitization appear to be regulated by ATP. More than half the small diameter DRG neurons (presumably nociceptors) in culture respond to ATP and were estrogen-sensitive [13-14, 19]. ATP effect was blocked by PPADS, indicating an involvement of purinoreceptors. The involvement of the P2X3 receptor in the ATP response was proven by using selective P2X3 agonist α,β-meATP therefore ERα interacts with P2X3 in DRGs.
Sex hormones and E2, in particular, may directly influence the functions of primary afferent neurons. Both subtypes of estrogen receptors (ERα and ERβ) are present in small-diameter DRG neurons [20]. While several actions of E2 have been demonstrated in the nervous system, the mechanisms of E2 pain modulation remain unclear. ERs were traditionally envisioned as E2-activated transcription factors. However, E2 has a multiplicity of actions: membrane, cytoplasmic and nuclear (reviewed in [3]). As we expected, treatment with E2 was blocked by ICI 182,780 indicating the mechanism through ER. Most of the published reports in the area of sex and hormone-related differences in pain have addressed the modulatory effect of E2 on CNS mechanisms of nociception [21].
Recent studies demonstrate that E2 has a significant role in modulating visceral sensitivity, indicating that E2 alterations in sensory processing may underlie sex-based differences in functional pain symptoms [22]. However, reports of E2 modulation of visceral and somatic nociceptive sensitivity are inconsistent. For example, elevated E2 levels have been reported to increase the threshold to cutaneous stimuli but decrease the percentage of escape responses to ureteral calculosis [23]. However, nociceptive sensitivity appears to increase when E2 levels are elevated [24-25]. Indeed in most clinical studies, women report more severe pain levels, more frequent pain and longer its duration of pain than men [26-27]. To help resolve these inconsistencies we propose to study E2 actions on the primary afferents. Little is known about E2 -mediated mechanisms in peripheral nervous system, but the fact that DRG neurons express ER and respond to E2 treatment suggest that they are a potential target for mediating nociception.
Visceral nociception and nociceptor sensitization appear to be regulated by ATP [28]. Large body of literature supports the idea that E2 modulates nociceptive responses in pelvic pain syndromes, however, whether E2 is pro- or anti-nociceptive remains unresolved. Recent study by R. Traub laboratory showed that spinal ERα mediates the pronociceptive effect of E2 on visceral signal processing through activation of the MAPK pathway [29]. Recently, our data showed that that membrane-associated ERα-initiated signaling involves interaction with mGluRs [30]. Within the context of our hypothesis E2 modulation of nociceptive response depends on the type of pain, its durations and the involvement of other anti-nociceptive mechanisms. The P2X3 receptor subtype has been found to be involved in peripheral pain signal transduction but to date, changes in the expression and function of P2X3 receptors from DRG neurons in the varied gonadal hormone levels have not been well documented. E2 modulates DRG neurons response to ATP suggesting that visceral afferent nociceptors are modulated by E2 in the DRG.Although estradiol doses of 10-100 nM are commonly used in neuronal preparations are higher than those achieved during the preovulatory peak of estradiol (physiological concentrations of 100pM). Lower levels of estradiol typically provide homeostatic (negative) feedback, whereas during the female reproductive cycle, exposure to the sustained high level of estradiol in the circulation at the end of the follicular phase elicits a neurobiological switch to positive feedback action. In contrast to the marked inhibition of ATP-induced calcium signaling by 100 nM estradiol in nociceptors, low physiological doses of estradiol (100 pM) had no effects that may reveal differences between rapid effects (5-10 min) independent of gene transcription, and longer-term actions (24 - 48 hrs) that may suggest differences in transcription. Indeed, our data support the idea that estradiol can signal through both genomic mechanisms and rapid changes in signaling cascades. The short latency (<5 min) of responses and 100 nM dose strongly argues for a mechanism that does not rely on changes in gene expression that may involve different receptor subtype activation.
In this study we obtained the same effect with E-6-BSA as with E2 proving that rapid action of E2 occurs at the membrane site. The DRG is an important site of visceral afferent convergence and cross-sensitization. Mouse DRG neurons in culture retain the same responses to a rapid application of E2 as rat cultured DRG, providing investigators with an additional tool of ER ‘knock-outs’ for studying the effects of estradiol. In this study we showed that P2X3 receptors expression significantly decreased in ER knock-out mice and that E2 acts through an ERα in modulating the ATP-mediated [Ca2+]i response in DRG, since its effect was eliminated in ERαKO mouse and retained in ERβKO. This result shows an important non-reproductive role of ERα in modulating ATP-induced Ca2+ signaling at the level of the primary afferent neuron, thereby modulating the sensitivity to painful stimuli in the periphery. To test if E2 directly modulates [Ca2+]i responses in visceral nociceptors in a future experiments DRG from retrogradely labeled colonic afferents will be compared with retrogradely labeled cutaneous DRG neurons.
Nociceptive systems implicated in the etiology of functional disorders may be affected by E2 and often are complicated by co-morbid disorders, all of which may pose health risks. Treatment strategies for patients should consider this modulation in response to therapy. Sex steroids have been suggested to play an important role in pain regulation. E2 effects on visceral nociceptive signaling have been observed in clinical studies, but most of the research has been focused on the CNS. Our data reveal a new mechanism how E2 modulates primary sensory neurons response to ATP suggesting that visceral afferent nociceptors are modulated by E2 in the DRG. Therefore, our study supports the potential of the P2X3 receptor as a target for pain therapy in females.
Acknowledgement
Supported by NIH Grants NS 063939 from the National Institute of Neurological Disorders and Stroke
References
- 1.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123(5):1686–701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 2.Berkley KJ, Zalcman SS, Simon VR. Sex and gender differences in pain and inflammation: a rapidly maturing field. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R241–4. doi: 10.1152/ajpregu.00287.2006. [DOI] [PubMed] [Google Scholar]
- 3.Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News Physiol Sci. 2001;16:251–5. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- 4.Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67(6):471–5. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 5.Razandi M, et al. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23(5):1633–46. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papka RE, Mowa CN. Estrogen receptors in the spinal cord, sensory ganglia, and pelvic autonomic ganglia. Int Rev Cytol. 2003;231:91–127. doi: 10.1016/s0074-7696(03)31003-4. [DOI] [PubMed] [Google Scholar]
- 7.Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65(2):107–34. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 8.Koshimizu TA, et al. Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Mol Pharmacol. 2000;58(5):936–45. doi: 10.1124/mol.58.5.936. [DOI] [PubMed] [Google Scholar]
- 9.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16(11):1248–57. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci. 2002;22(1):93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaban VV, et al. Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118(4):941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 12.Chaban VV, et al. Nitric oxide synthase inhibitors enhance mechanosensitive Ca(2+) influx in cultured dorsal root ganglion neurons. Brain Res. 2001;903(1-2):74–85. doi: 10.1016/s0006-8993(01)02407-6. [DOI] [PubMed] [Google Scholar]
- 13.Chaban VV. Peripheral sensitization of sensory neurons. Ethn Dis. 2010;20(1 Suppl 1):S1–3. [PMC free article] [PubMed] [Google Scholar]
- 14.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81(1):31–7. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- 15.Mayer EA, et al. Functional GI disorders: from animal models to drug development. Gut. 2008;57(3):384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272–80. doi: 10.1016/j.physbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Xu S, et al. 17beta-estradiol activates estrogen receptor beta-signalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology. 2008;149(11):5540–8. doi: 10.1210/en.2008-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarajari S, Oblinger MM. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp Neurol. 2010;224(1):163–9. doi: 10.1016/j.expneurol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaban VV. Visceral sensory neurons that innervate both uterus and colon express nociceptive TRPv1 and P2X3 receptors in rats. Ethn Dis. 2008;18(2 Suppl 2):S2-20–4. [PubMed] [Google Scholar]
- 20.Papka RE, Storey-Workley M. Estrogen receptor-alpha and -beta coexist in a subpopulation of sensory neurons of female rat dorsal root ganglia. Neurosci Lett. 2002;319(2):71–4. doi: 10.1016/s0304-3940(01)02562-9. [DOI] [PubMed] [Google Scholar]
- 21.Aloisi AM, Ceccarelli I, Herdegen T. Gonadectomy and persistent pain differently affect hippocampal c-Fos expression in male and female rats. Neurosci Lett. 2000;281(1):29–32. doi: 10.1016/s0304-3940(00)00819-3. [DOI] [PubMed] [Google Scholar]
- 22.Al-Chaer ED, Traub RJ. Biological basis of visceral pain: recent developments. Pain. 2002;96(3):221–5. doi: 10.1016/S0304-3959(02)00046-5. [DOI] [PubMed] [Google Scholar]
- 23.Bradshaw HB, Berkley KJ. Estrogen replacement reverses ovariectomy-induced vaginal hyperalgesia in the rat. Maturitas. 2002;41(2):157–65. doi: 10.1016/s0378-5122(01)00261-4. [DOI] [PubMed] [Google Scholar]
- 24.Holdcroft A. Hormones and the gut. Br J Anaesth. 2000;85(1):58–68. doi: 10.1093/bja/85.1.58. [DOI] [PubMed] [Google Scholar]
- 25.Bereiter DA. Sex differences in brainstem neural activation after injury to the TMJ region. Cells Tissues Organs. 2001;169(3):226–37. doi: 10.1159/000047886. [DOI] [PubMed] [Google Scholar]
- 26.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371–80. doi: 10.1017/s0140525x97221485. discussion 435-513. [DOI] [PubMed] [Google Scholar]
- 27.Fillingim RB, Edwards RR. The association of hormone replacement therapy with experimental pain responses in postmenopausal women. Pain. 2001;92(1-2):229–34. doi: 10.1016/s0304-3959(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 28.Burnstock G. Purinergic receptors and pain. Curr Pharm Des. 2009;15(15):1717–35. doi: 10.2174/138161209788186335. [DOI] [PubMed] [Google Scholar]
- 29.Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain. 2011;152(5):1182–91. doi: 10.1016/j.pain.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaban V, et al. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. J Neurosci Res. 2011;89(11):1707–10. doi: 10.1002/jnr.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]