Abstract
Objectives
To determine whether haptolgobin phenotype is related to preeclampsia risk, or to plasma concentrations of soluble endoglin (sEng), soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF).
Study Design
Haptoglobin phenotype was retrospectively determined in primiparous women with uncomplicated pregnancies (n=309), gestational hypertension (n=215) and preeclampsia (n=249). Phenotype was assessed by peroxidase staining following native polyacrylamide gel electrophoresis of hemoglobin-supplemented serum.
Results
Compared to haptoglobin 1-1, haptoglobin 2-1 was associated with a significantly increased risk of preeclampsia (odds ratio (95% confidence interval) 2.11 (1.07, 4.18)) and term preeclampsia (2.45 (1.07, 5.83)) in Caucasian women. Haptoglobin phenotype was not associated with preeclampsia risk in African Americans. Preeclamptic women had higher plasma sEng and sFlt-1, and lower PlGF, than controls. sEng, sFlt-1 and PlGF did not differ among women of different haptoglobin phenotypes.
Conclusion
Haptoglobin 2-1 is associated with higher preeclampsia risk in primiparous Caucasian women.
Keywords: Haptoglobin phenotype, angiogenesis, preeclampsia, gestational hypertension
Introduction
Preeclampsia affects 2–7% of pregnancies,1, 2 and remains a substantial cause of maternal3 and fetal4 morbidity and mortality. Oxidative stress, high concentrations of the anti- angiogenic proteins soluble endoglin (sEng) and soluble fms-like tyrosine kinase 1 (sFlt-1), and low concentrations of the pro-angiogenic placental growth factor (PlGF), are proposed to contribute to the vascular dysfunction leading to maternal pathophysiology.5, 6
Haptoglobin (Hp) is an acute phase protein that acts as an antioxidant by binding free hemoglobin.7, 8 Hp also stimulates angiogenesis in vitro and in vivo.9 Hp has three common polymorphisms (Hp 1-1, 2-1, and 2-2), which are composed of a common beta allele, and two different alpha alleles (1 and 2).8 Phenotype can be used to infer genotype, except in cases where Hp is not produced (i.e. fetal/early life), or has been depleted (i.e. severe infection).8 Structural differences between the polymorphisms affect protein function.7 Antioxidant activity is strongest for Hp 1-1.7 Pro-angiogenic activity is strongest for Hp 2- 2.9 Hp phenotype predicts cardiovascular disease risk, and treatment response to antioxidant supplementation, in other populations.10, 11
Small European studies examining the relationship between Hp phenotype and preeclampsia risk have produced conflicting results.12–14 Larger studies, and North American studies that include black women, are needed. When we initiated this investigation, the largest published study showed that Hp 1-1 was associated with increased preeclampsia risk, higher blood pressure and greater proteinuria.12 We hypothesized that the reduced angiogenic capacity of Hp 1-1 might explain these effects. The mechanism through which Hp promotes angiogenesis is not known. One possibility is that Hp may effect concentrations of pro- and anti-angiogenic factors in a phenotype- dependant manner.
The study objectives were to determine whether Hp phenotype is associated with altered risk of preeclampsia or gestational hypertension in primiparous North American women, and to assess the relationship between Hp phenotype and plasma concentrations of sEng, sFlt-1 and PlGF in normotensive pregnant and preeclamptic women. We hypothesized that Hp 1-1 would be associated with higher preeclampsia risk, lower plasma concentrations of the pro-angiogenic factor PlGF, and higher concentrations of the anti-angiogenic factors sEng and sFlt-1.
Materials and Methods
Study Population
We used banked serum from women enrolled in the ongoing Pregnancy Exposures and Preeclampsia Prevention (PEPP) Study (1999–2010), which is approved by the University of Pittsburgh Institutional Review Board. All subjects provided written informed consent. This analysis was restricted to primiparous women with a singleton pregnancy. We included all African American women with an uncomplicated pregnancy (n = 106), gestational hypertension (n = 60), or preeclampsia (n = 60), all non-Hispanic Caucasian women with gestational hypertension (n = 155), and a subset of non-Hispanic Caucasian women with an uncomplicated pregnancy (n = 203) or preeclampsia (n = 189). Gestational hypertension was defined as persistent, de novo hypertension (systolic ≥140 mmHg and/or diastolic ≥90 mmHg) appearing after 20 weeks gestation. Preeclampsia was defined as gestational hypertension and proteinuria. Proteinuria was the excretion of ≥300 mg of protein/24 hours, a dipstick of 2+, a catheterized sample of 1+, or protein:creatinine ≥0.3. Hyperuricemia was defined as a plasma uric acid ≥1 standard deviation above the mean value at the gestational age when the sample was obtained.15 Among preeclamptic women, 80.3% of Caucasians and 83.3% of African Americans had hyperuricemia. Women with gestational hypertension did not have hyperuricemia. Birthweight centiles were based on growth curves derived from Magee-Womens Hospital, which account for race, gestational age at delivery and fetal sex.16
Hp Phenotyping
Hp phenotype was determined by native polyacrylamide gel electrophoresis (PAGE) using a modification of previously described methods.17, 18 Five μl of serum was supplemented with 3μl of 25μM human hemoglobin (Sigma-Aldrich, St. Louis, MO). The peroxidase activity of hemoglobin in the Hp-hemoglobin complex can be detected directly, eliminating the need for primary and secondary anti-bodies. Samples were run on 6% tris-glycine gels (Invitrogen, Carlsbad, CA) for 2 hours at 120 volts. Proteins were transferred to PVDF (Millipore, Billerica, MA).
Hemolyzed samples or samples with low Hp concentrations were phenotyped by SDS PAGE of 1.5μl of serum, using a modification of previously described methods.18 Serum was combined with 2μl of β-mercaptoethanol and heated for 7 minutes at 82°C. Samples were run on 12% tris-glycine gels (Invitrogen, Carlsbad, CA) at 120 volts for 75 minutes. Proteins were transferred to a PVDF membrane, and incubated with blocking solution (TBS containing 5% non-fat milk, 0.1% Tween 20), primary antibody (1:5,000, Polyclonal Rabbit Anti-Human Haptoglobin, DakoCytomation, Carpinteria, CA) and secondary antibody (1:25,000, Goat anti-Rabbit IgG horseradish peroxidase, Millipore, Billerica, MA) for one hour each at room temperature. Antibodies were dissolved in blocking solution. Membranes were washed between incubations in TBS containing 0.1% Tween 20.
Membranes were stained for peroxidase activity (SuperSignal West Pico Chemiluminescent Substrate, Fisher Scientific, Pittsburgh, PA), and imaged (FlouroChem Q System, Cell Biosciences, Santa Clara, CA). Hp phenotypes were identified by characteristic banding patterns (Figure 1).
Figure 1. Hp phenotypes by Native and SDS PAGE.
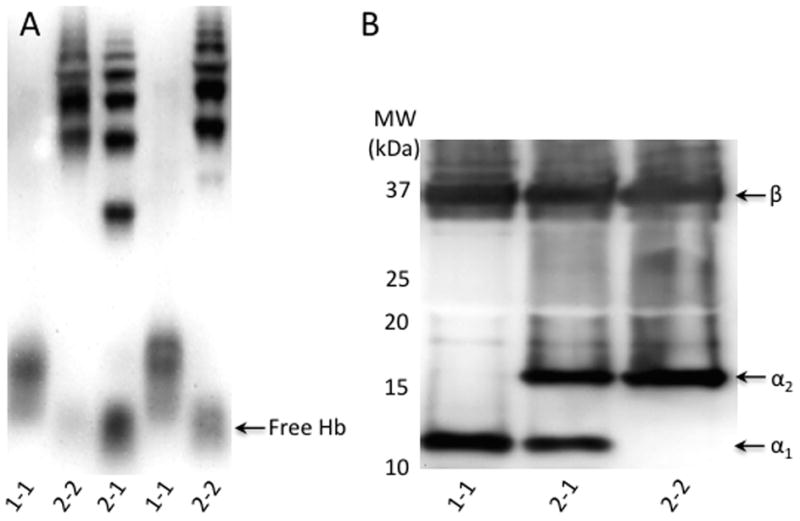
Panel A: Hp phenotyping of Hb-supplemented serum by Native PAGE on a 6% gel using Hb peroxidase detection. Hp 1-1 has a single fast moving band (Lanes 1, 4). Hp 2-2 has a series of slow moving bands (Lanes 2, 5). Hp 2-1 (Lane 3) has a band between the Hb/Hp 1-1 and Hb/Hp 2-2 bands, as well as a series of slow-moving bands in the same area as the Hb/Hp 2-2 bands.
Panel B: Hp phenotyping of serum by SDS PAGE on a 12% gel with Western blotting. The Hp β band is present in all three phenotypes. The α1 band denotes the presence of the Hp 1 allele, whereas the α2 band denotes the presence of the Hp 2 allele.
Plasma Concentrations of Angiogenic Factors
In a subset of controls (n = 247) and women with preeclampsia (n = 77), EDTA plasma was collected, aliquoted, and stored at −80°C. Samples from preeclamptic women were obtained less than 48 hours before delivery. Control samples were matched for gestational age at sample collection. sFlt-1 and PlGF concentrations were measured on the Abbott Architect i2000SR (Abbott Park, IL) using prototype reagents.19 sEng was quantified by ELISA (R&D Systems, Minneapolis, MN). The inter-assay variability was: sFlt-1, 3.1–4.5%; PlGF, 1.4–5.5%; sEng, 9%.
Statistical Analysis
Subject characteristics and indicators of disease severity were compared between women of different phenotypes. Continuous data were compared using the student’s t test or Wilcoxon rank-sum test. Categorical data, expressed as proportions, were compared using the chi-squared test or Fisher’s exact test. Logistic regression or the generalized logit model was used to evaluate the relationship between Hp phenotype and pregnancy outcome, stratified by race, after adjusting for age. A linear regression model was used to investigate the relationship between Hp phenotype, pregnancy outcome, and the log-transformed concentrations of PlGF, sFlt-1 and sEng, after adjusting for confounding variables. Analyses were performed using PASW 18 (IBM, New York, NY) or SAS 9.2 (SAS Institute Inc., Cary, NC) at a statistical significance level of 0.05.
Results
Subject Characteristics
The rare 2-1M phenotype was found in 3 African American women (Control, n=1; Gestational hypertension, n=2), and 2 Caucasian women (Preeclampsia, n=2). These women were excluded from statistical analyses due to small sample size.
Pre-pregnancy BMI, average systolic and diastolic blood pressure before 20 weeks, the percentage of smokers in the 3rd trimester, and infant sex did not differ between women of different phenotypes in the entire sample (Table 1), or when the analysis was stratified by pregnancy outcome (data not shown). Women with the Hp 2-2 phenotype were significantly older than women with the 1-1 and 2-1 phenotypes (p=0.001). When the analysis was stratified by pregnancy outcome, this difference remained significant in the control group (p=0.047), but not in women with gestational hypertension (p=0.094) or preeclampsia (=0.075). The effect of age was due to the significantly higher proportion of Caucasians in the Hp 2-2 group, as Caucasian women were older than African American women (25.5 ± 5.8 vs. 21.3 ± 4.0 years, p<0.001). When stratified by race, age did not differ between women of different Hp phenotypes (Caucasian, p=0.32; African American, p=0.86).
Table 1.
Subject Characteristics
| Subject Characteristics | Hp 1-1 (n = 130) | Hp 2-1 (n = 358) | Hp 2-2 (n = 280) | Hp 2-1M (n = 5) |
|---|---|---|---|---|
| Maternal age (years) | 23.4 ± 5.4 | 23.8 ± 5.3 | 25.2 ± 6.2a,b | 22.4 ± 5.2 |
| Pre-pregnancy BMI (kg/m2) | 25.4±5.4 | 26.1 ± 6.4 | 259±6.8 | 24.1 ± 8.0 |
| Average blood pressure before 20 weeks (mmHg) | ||||
| Systolic | 114 ± 9 | 114 ± 9 | 114 ± 9 | 111 ± 7 |
| Diastolic | 69 ± 6 | 69 ± 7 | 68 ± 8 | 67 ± 4 |
| Race (% Caucasian) | 52% | 69%a | 83%a,b | 40% |
| Smoked in the 3rd trimester (%) | 28% | 22% | 21% | 20% |
| Infant sex (% Female) | 45% | 46% | 50% | 20% |
Values represent mean ± SD unless otherwise indicated. Women with the 2-1M phenotype were excluded from statistical analyses due to small sample size.
Significant difference from: Hp 1-1,
p<0.01; Hp 2-1,
p<0.01.
Compared to controls, women with gestational hypertension had higher systolic and diastolic blood pressure during labor, and delivered smaller babies (Table 2). The lower birth weight was explained by a slightly earlier gestational age at delivery, as birth weight centile did not differ between groups (p=0.30). Preeclamptic women had higher systolic and diastolic blood pressure during labor, and delivered infants with lower birth weights and birth weight centiles at an earlier gestational age, than women in the other groups.
Table 2.
Pregnancy Outcome in Women with Uncomplicated Pregnancies, Gestational Hypertension and Preeclampsia
| Pregnancy Outcomes | Control (n = 309) | Gestational Hypertension (n = 215) | Preeclampsia (n = 249) |
|---|---|---|---|
| Blood pressure in labor (mmHg) | |||
| Systolic | 121 ± 11 | 139 ± 13a | 154 ± 15a,b |
| Diastolic | 73 ± 8 | 85 ± 8a | 92 ± 9a,b |
| Gestational age at delivery (weeks) | 40.0 (39.3, 40.9) | 39.4 (38.6, 40.4)a | 37.4 (34.1, 39.1)a,b |
| Birth weight (g) | 3415 (3075, 3738) | 3265 (2980, 3635)a | 2751 (1897, 3239)a,b |
| Birthweight centile (%) | 47 (27, 71) | 44 (24, 68) | 24 (11, 51)a,b |
Values represent mean ± SD or median (interquartile range).
Significant difference from: Control,
p<0.001; Gestational Hypertension,
p<0.001.
Relationship Between Hp Phenotype and Race
The Hp phenotype distribution in Caucasian and African American women was comparable to values reported for North Americans,20 and was in Hardy-Weinberg equilibrium. Compared to African Americans, the Hp 1 allele was significantly less common in Caucasians in the entire sample (Caucasians: 0.35, African Americans, 0.53, p<0.001), and when stratified by pregnancy outcome (p<0.001 for all comparisons). Hp phenotype differed according to race in the entire sample (p<0.001, Table 1), and when the analysis was stratified by pregnancy outcome (Control, p<0.001; Gestational Hypertension, p=0.004; Preeclampsia, p=0.001, data not shown). The percentage of Caucasians was lowest for Hp 1-1, and highest for Hp 2-2.
Relationship Beten Hp Phenotype and Pregnancy Outcome
When stratified by race, Hp 1 allele frequency did not differ between controls, women with gestational hypertension, and women with preeclampsia (Caucasians: Controls, 0.36, Gestational hypertension, 0.34, Preeclampsia, 0.34; African Americans: Controls, 0.55, Gestational hypertension, 0.52, Preeclampsia, 0.52). The Hp phenotype distribution for each pregnancy outcome group, stratified by race, is shown in Table 3. Overall, Hp phenotype was not significantly associated with gestational hypertension (Caucasian, p=0.82; African American, p=0.68) or preeclampsia (Caucasian, p=0.63; African American, p=0.79) after adjusting for age. However, when Hp 2-1 and 2-2 were compared directly with Hp 1-1, Caucasian women with the 2-1 phenotype were significantly more likely to develop preeclampsia than women with the 1-1 phenotype (adjusted OR (95% CI): 2.11 (1.07, 4.18), Table 4). Caucasian women with the Hp 2-2 phenotype were not more likely to develop preeclampsia than women with the Hp 1-1 phenotype (1.47 (0.74, 292), Tables 1S, 4). When Caucasian preeclamptic women were subdivided according to preterm (<37 weeks, p=0.084) and term delivery (p=0.025, Table 5), the increased risk associated with Hp 2-1 was only statistically significant in term preeclampsia. There was no association between Hp phenotype and the risk of preeclampsia or gestational hypertension among African Americans.
Table 3.
Incidence of Hp Phenotypes in Primiparous Women with Uncomplicated Pregnancies, Gestational Hypertension and Preeclampsia Subdivided by Race
| Race | Group | n | Hp 1-1 | Hp 2-1 | Hp 2-2 | Hp 2-1M |
|---|---|---|---|---|---|---|
| Caucasian | Control | 203 | 30 (15%) | 86 (42%) | 87 (43%) | 0 (0%) |
| Gestational Hypertension | 155 | 19 (12%) | 68 (44%) | 68 (44%) | 0 (0%) | |
| Preeclampsia | 189 | 18 (9%) | 92 (49%) | 77 (41%) | 2 (1%) | |
| African American | Control | 106 | 33 (31%) | 50 (47%) | 22 (21%) | 1 (1%) |
| Gestational Hypertension | 60 | 14 (23%) | 32 (53%) | 12 (20%) | 2 (3%) | |
| Preeclampsia | 60 | 16 (27%) | 30 (50%) | 14 (23%) | 0 (0%) |
Values are n (%).
Table 4.
Odds ratios for the development of gestational hypertension and preeclampsia, relative to the 1-1 phenotype
| Race | Outcome | Hp 2-1 | Hp 2-2, | ||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Caucasian | Gestational hypertensiona | 1.23 (0.64, 2.38) | 0.608 | 1.19 (0.62, 2.30) | 0.761 |
| Preeclampsiab | 2.11 (1.07, 4.18) | 0.019 | 1.47 (0.74, 2.92) | 0.960 | |
| African American | Gestational hypertensionc | 1.41 (0.65, 3.07) | 0.464 | 1.23 (0.47, 3.17) | 0.939 |
| Preeclampsiad | 1.25 (0.59, 2.64) | 0.811 | 1.33 (0.54, 3.27) | 0.657 | |
Abbreviations: OR, odds ratio; CI, confidence interval.
All models were adjusted for age.
Model includes controls (n=203), and women with gestational hypertension (n=155)
Model includes controls (n=203) and women with preeclampsia (n=187)
Model includes controls (n=105) and women with gestational hypertension (n=58)
Model includes controls (n=105) and women with preeclampsia (n=60)
Table 5.
Odds ratios for the development of preterm and term preeclampsia, relative to the 1-1 phenotype
| Race | Outcome | Hp 2-1 | Hp 2-2 | ||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Caucasiana | Preterm Preeclampsia | 1.87 (0.79, 4.39) | 0.084 | 1.24 (0.52, 2.94) | 0.751 |
| Term Preeclampsia | 2.45 (1.07, 5.83) | 0.025 | 1.73 (0.74, 4.05) | 0.757 | |
| African Americanb | Preterm Preeclampsia | 1.58 (0.55, 4.53) | 0.692 | 1.77 (0.52, 5.97) | 0.505 |
| Term Preeclampsia | 1.09 (0.44, 2.69) | 0.900 | 1.07 (0.35, 3.23) | 0.964 | |
A preterm control group was not included, as only 1 African American and 3 Caucasian controls delivered preterm. Abbreviations: OR, odds ratio; CI, confidence interval.
Model includes controls (n=200), and women with preterm (n=81) and term (n=106) preeclampsia. Adjusted for age.
Model includes controls (n=104), and women with preterm (n=27) and term (n=33) preeclampsia. Adjusted for age.
We hypothesized that Hp 1-1 would be associated with a two-fold greater preeclampsia risk, relative to Hp 2-1 or 2-2. However, our results showed that Hp 1-1 was associated with a two-fold reduction in preeclampsia risk in Caucasians, relative to Hp 2-1. A total of 176 Caucasian women and 140 African American women would be required to detect a 2- fold increase in the incidence of preeclampsia in Hp 2-1 or 2-2, relative to Hp 1-1 (two- sided Chi-square test, prevalence of preeclampsia of 30% in Hp 1-1, 80% power, alpha = 0.05). Therefore, our study was adequately powered to detect a two-fold greater frequency of preeclampsia in Hp 2:1 or 2:2 relative to Hp 1:1 in both Caucasians (n = 392) and African Americans (n = 166).
Hp phenotype was not associated with factors related to disease severity in women who developed gestational hypertension or preeclampsia (Table 3S).
Relationship Between Hp Phenotype, Pregnancy Outcome, and PlGF, sFlt-1, and sEng
In the subset of women in whom angiogenic factors were measured, age, pre-pregnancy BMI, gestational age at sample collection, blood pressure before 20 weeks, percentage of smokers in the 3rd trimester, and infant sex were not significantly different between women with different phenotypes (Table 2S, p > 0.05). As expected, Hp phenotype varied by race (p<0.001). PlGF was significantly lower, and sFlt-1 and sEng were significantly higher, in women with term preeclampsia compared to controls after adjusting for race, BMI, fetal sex, age, smoking in the third trimester and Hp phenotype (data not shown). Women with preterm preeclampsia had significantly lower PlGF, and significantly higher sEng and sFlt-1, than controls or women with term preeclampsia after adjusting for confounding variables (data not shown). Plasma concentrations of PlGF (p=0.97), sEng (p=0.86) and sFlt-1 (p=0.99) did not differ between women with different Hp phenotypes after adjusting for group (control, preterm preeclampsia, term preeclampsia), race, BMI, gestational age at sample collection, infant sex, age, and smoking in the 3rd trimester (Figure 2). The analysis of PlGF and Hp phenotype was also adjusted for the interaction between age and group, as younger age was associated with higher PlGF in the control group, but not in the other groups. This interaction was not significant for sEng and sFlt-1.
Figure 2. Concentrations of serum angiogenic factors in controls and women with preeclampsia according to Hp phenotype.
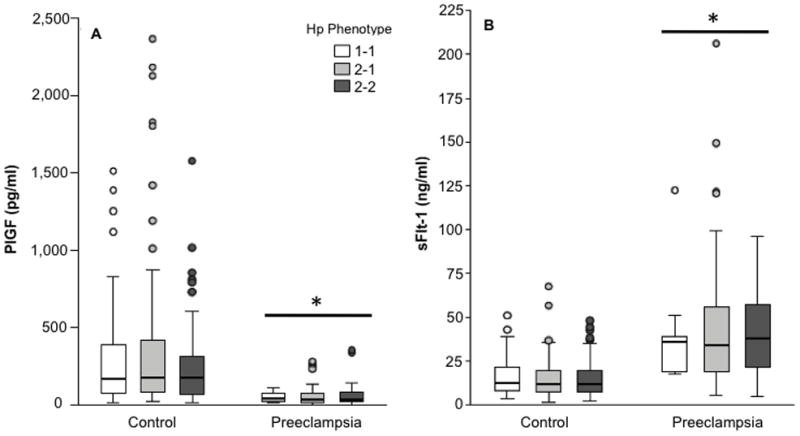
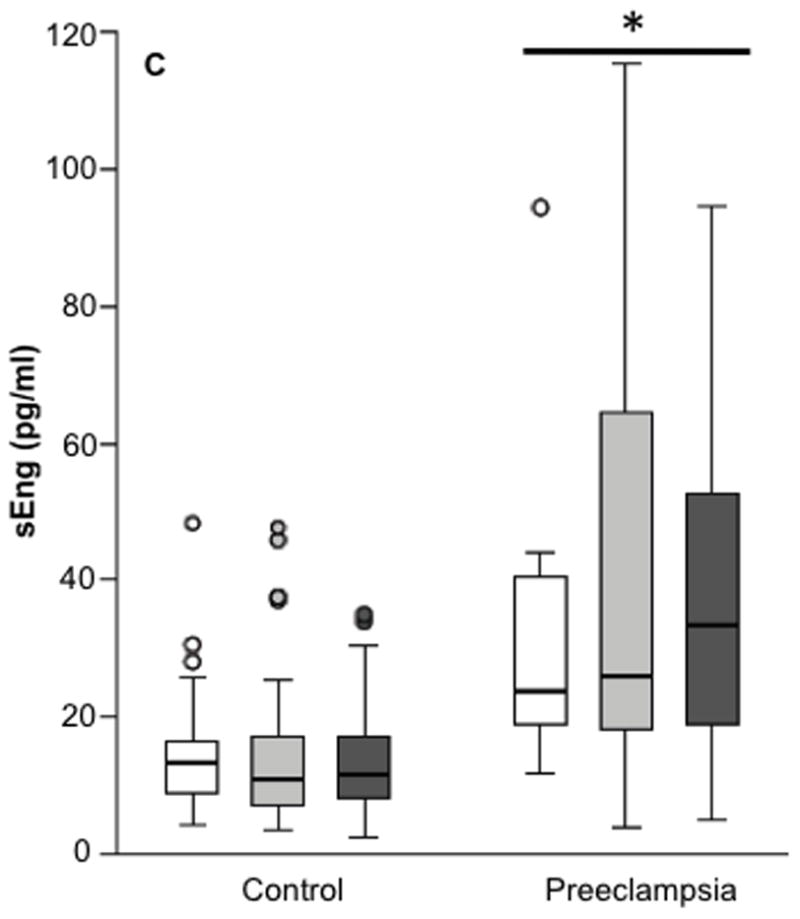
Concentrations of serum PlGF (Panel A), sFlt-1 (Panel B) and sEng (Panel C). Sample size for each group was a follows: Control (Hp 1-1, n = 55; Hp 2-1, n = 104; Hp 2-2, n = 90); Preeclampsia (Hp 1-1, n = 9; Hp 2-1, n = 49; Hp 2-2, n = 22). Compared to controls, PlGF concentrations were significantly lower, and sFlt-1 and sEng concentrations were significantly higher, in women with preeclampsia. PlGF, sFlt-1 and sEng did not differ between women of different Hp phenotypes.
Comment
This study examined the relationship between Hp phenotype, the risk of preeclampsia and gestational hypertension, and plasma concentrations of sFlt-1, sEng and PlGF. There were three findings. First, Hp 2-1 was associated with a doubling of preeclampsia risk compared to Hp 1-1 in primiparous non-Hispanic Caucasian, but not African American, women. Second, we found no association between Hp phenotype and the severity of disease or outcomes in women with gestational hypertension or preeclampsia. Third, we confirm our previous findings19 and those of others5, 6, 21 that preeclamptic women have higher plasma sFlt-1 and sEng, and lower PlGF, than controls. However, plasma sFlt-1, sEng and PlGF did not differ between women of different Hp phenotypes, irrespective of diagnosis.
Relationship between Hp phenotype and the risk of gestational hypertension and preeclampsia
Previous studies examining the relationship between Hp phenotype and preeclampsia risk are contradictory.12–14, 22 The largest previous study reported that Hp 1-1 was associated with lower preeclampsia risk among Isreali women (5.8% vs. 12.5%).14 The protective effect of Hp 1-1 was strongest in primiparous women (odds ratio 0.329, 95% confidence interval 0.1–1.1, p=0.053), and was not significant in multiparous women. All women in the present study were primiparous, however our results only partially agree. Hp 1-1 was protective when compared to Hp 2-1, but not Hp 2-2. Two smaller studies reported conflicting results. Hp 1-1 was associated with increased preeclampsia risk among Caucasian Belgian women (28% vs. 16%).12 Hp phenotype did not effect the risk of preeclampsia, or preeclampsia with HELLP, in Danish women.13 A Russian study also reported no relationship between Hp phenotype and preeclampsia risk.22 However, preeclampsia was diagnosed using the gestosis index,22 which includes edema and allows a diagnosis of preeclampsia with lower blood pressure and less proteinuria than current criteria.
Hp phenotype distribution depends on race and geographic location.20 Other authors hypothesized that the divergent study results could reflect genetic differences between populations.14 We observed racial differences in our study population, as Hp 2-1 was associated with an increased preeclampsia risk in Caucasians, but not African Americans. Our study included fewer African Americans than Caucasians. Nonetheless, our sample size was adequate to detect a two-fold greater preeclampsia risk among Hp 2-1 or 2-2, relative to Hp 1-1, in either race. However, we cannot exclude a smaller difference between 2-1 and 1-1 in African Americans, or between 2-2 and 1-1 in either race. Differing results between our study and others could also reflect Type II error due to the small sample size of previous studies. Our study included more than twice as many preeclamptic women (n = 249) as the largest previous study (n = 120).14 The smaller European studies included only 2513 and 6012 preeclamptic women.
Relationship between Hp phenotype and pregnancy outcome in women with preeclampsia and gestational hypertension
We did not find an association between Hp phenotype and disease severity. In Caucasians, the increased preeclampsia risk among women with the 2-1 phenotype was statistically significant for term preeclampsia (OR 2.45, p=0.025), but not for preterm preeclampsia (OR 1.87, p=0.084). This could reflect a stronger relationship between Hp 2-1 and term preeclampsia. However, it might also be due to the smaller sample size of the preterm preeclampsia group (81 preterm vs. 106 term preeclampsia). Sammour et al. reported no differences in phenotype distribution when preeclamptic women were subdivided according to disease severity (mild vs. severe) or timing of delivery (before or after 34 weeks gestation).14 In contrast, Depypere et al.12 reported higher blood pressure and proteinuria in preeclamptic women with the Hp 1-1 phenotype. However, this study included only 60 preeclamptic women, of whom 17 were Hp 1-1.12
Relationship of Hp phenotype on plasma PlGF, sFlt-1 and sEng
Hp stimulates vascular tube formation in vitro, and vascularization of a plug inserted into mice, through an unknown mechanism.9 These angiogenic effects are strongest for Hp 2-2, and weakest for Hp 1-1.9 At the time we initiated this investigation, the largest published study reported that women with the Hp 1-1 phenotype had an increased preeclampsia risk, higher blood pressure, and greater proteinuria.12 We hypothesized that the reduced angiogenic capacity of Hp 1-1 might explain these effects. However, concentrations of sFlt-1, sEng and PlGF do not differ between women of different Hp phenotypes, irrespective of diagnosis. The mechanism regulating phenotype dependent differences in the angiogenic activity of Hp remains to be determined.
Conclusions
Hp 2-1 is associated with increased preeclampsia risk in Caucasian, but not African American women. Hp phenotype was not associated with differences in preeclampsia severity. Phenotype-dependant differences in the angiogenic activity of Hp do not appear to be related to PlGF, sFlt-1 or sEng. Future studies should determine how Hp phenotype may predispose some women to develop preeclampsia. One possibility is that Hp 2-1 provides neither the potent anti-oxidant activity of Hp 1-1, nor the strong angiogenic capacity of Hp 2-2. However, this remains to be tested. Although the different molecular configuration of Hp 2-1 is believed to result in functional differences from Hp 1-1 and 2-2,23 detailed functional studies of Hp 2-1 have not been conducted. Isolated Hp 2-1 is not commercially available, and animal models for Hp 2-1 have not been created. Future research should investigate racial differences in any proposed mechanism.
Acknowledgments
Financial support: This work was supported by NIH P01 HD030367. Measurement of sFlt- 1 and PlGF was funded by Abbott Laboratories. Dr. Tracey Weissgerber was supported by a Canadian Institute of Health Research Fellowship, and the Amy Roberts Health Promotion Research Award.
We thank Drs. Dominick L. Pucci, Don M. Laird, and David C. Sogin at Abbott Laboratories for their contributions to the measurement of sFlt-1 and PlGF using the Abbott Architect i2000SR.
Footnotes
Disclosure: Saul Datwyler is an employee of Abbott Laboratories. The remaining authors have no conflict on interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hauth JC, Ewell MG, Levine RJ, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24–8. doi: 10.1016/s0029-7844(99)00462-7. [DOI] [PubMed] [Google Scholar]
- 2.Knuist M, Bonsel GJ, Zondervan HA, Treffers PE. Intensification of fetal and maternal surveillance in pregnant women with hypertensive disorders. Int J Gynaecol Obstet. 1998;61:127–33. doi: 10.1016/s0020-7292(98)00024-1. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. World Health Report: Make Every Mother, and Child Count. Geneva: WHO; 2005. [Google Scholar]
- 4.Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo- controlled trial. Lancet. 2002;359:1877–90. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 5.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell LC, Seed PT, Briley A, et al. A longitudinal study of biochemical variables in women at risk of preeclampsia. Am J Obstet Gynecol. 2002;187:127–36. doi: 10.1067/mob.2002.122969. [DOI] [PubMed] [Google Scholar]
- 7.Levy AP, Asleh R, Blum S, et al. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 12:293–304. doi: 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- 8.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42:1589–600. [PubMed] [Google Scholar]
- 9.Cid MC, Grant DS, Hoffman GS, Auerbach R, Fauci AS, Kleinman HK. Identification of haptoglobin as an angiogenic factor in sera from patients with systemic vasculitis. J Clin Invest. 1993;91:977–85. doi: 10.1172/JCI116319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy AP, Friedenberg P, Lotan R, et al. The effect of vitamin therapy on the progression of coronary artery atherosclerosis varies by haptoglobin type in postmenopausal women. Diabetes Care. 2004;27:925–30. doi: 10.2337/diacare.27.4.925. [DOI] [PubMed] [Google Scholar]
- 11.Milman U, Blum S, Shapira C, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28:341–7. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- 12.Depypere HT, Langlois MR, Delanghe JR, Temmerman M, Dhont M. Haptoglobin polymorphism in patients with preeclampsia. Clin Chem Lab Med. 2006;44:924–8. doi: 10.1515/CCLM.2006.182. [DOI] [PubMed] [Google Scholar]
- 13.Raijmakers MT, Roes EM, te Morsche RH, Steegers EA, Peters WH. Haptoglobin and its association with the HELLP syndrome. J Med Genet. 2003;40:214–6. doi: 10.1136/jmg.40.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sammour RN, Nakhoul FM, Levy AP, et al. Haptoglobin phenotype in women with preeclampsia. Endocrine. 38:303–8. doi: 10.1007/s12020-010-9392-7. [DOI] [PubMed] [Google Scholar]
- 15.Lind T, Godfrey KA, Otun H, Philips PR. Changes in serum uric acid concentrations during normal pregnancy. Br J Obstet Gynaecol. 1984;91:128–32. doi: 10.1111/j.1471-0528.1984.tb05895.x. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JM, Bodnar LM, Lain KY, et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46:1263–9. doi: 10.1161/01.HYP.0000188703.27002.14. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg I, Roguin A, Nikolsky E, Chanderashekhar PV, Cohen S, Levy AP. Haptoglobin phenotype and coronary artery collaterals in diabetic patients. Atherosclerosis. 2002;161:441–6. doi: 10.1016/s0021-9150(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 18.Kapralov A, Vlasova, Feng W, et al. Peroxidase activity of hemoglobin-haptoglobin complexes: covalent aggregation and oxidative stress in plasma and macrophages. J Biol Chem. 2009;284:30395–407. doi: 10.1074/jbc.M109.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers RW, Roberts JM, Plymire DA, et al. Low maternal PLGF across pregnancy identifies a subset of women with preterm preeclampsia; Type 1 vs. Type 2 preeclampsia? doi: 10.1161/HYPERTENSIONAHA.112.191213. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter K, Worwood M. Haptoglobin: a review of the major allele frequencies worldwide and their association with diseases. Int J Lab Hematol. 2007;29:92–110. doi: 10.1111/j.1751-553X.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 21.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122:478–87. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 22.Mekbeb T. The association of serum proteins with preeclampsia. Ethiop Med J. 1990;28:9–14. [PubMed] [Google Scholar]
- 23.Napolioni V. Regarding “haptoglobin 2-1 phenotype predicts rapid growth of abdominal aortic aneurysms”. J Vasc Surg. 2011;53:266–7. doi: 10.1016/j.jvs.2010.07.072. author reply 267. [DOI] [PubMed] [Google Scholar]


