Abstract
This study sought to examine the safety of percutaneous coronary intervention (PCI) before and during the de novo establishment of a transradial (TR) program at a teaching hospital. TR access remains underused in the United States, where cardiology fellowship programs continue to produce cardiologists with little TR experience. The establishment of TR programs at teaching hospitals may affect PCI safety. Starting in July of 2009, a TR program was established at teaching hospital. PCI-related data for the 2008–2009 (Y1) and 2009–2010 (Y2) academic years were prospectively collected and retrospectively analyzed. Of 1,366 PCIs performed over two years, 0.1% in Y1 and 28.7% in Y2 were performed via TR access. No major complications were identified in 194 consecutive patients undergoing TR PCI, and combined bleeding and vascular complication rates were lower in Y2 vs. Y1 (0.7 vs. 2.0%, p = 0.05). Patients treated in Y2 vs. Y1 and by the TR vs. transfemoral approach required slightly more fluoroscopy but similar contrast volumes and had similar procedural durations, lengths of stay, and pre-discharge mortality rates. PCI success rates were 97% in Y1, 97% in Y2, and 98% in TR cases. TR PCIs were performed by 13 cardiology fellows and 9 attending physicians, none of whom routinely performed TR PCI previously. In conclusion, the de novo establishment of a TR program improved PCI safety at a teaching hospital. TR programs are likely to improve PCI safety at other teaching hospitals and should be established in all cardiology fellowship training programs.
Keywords: coronary angioplasty, radial artery, bleeding, pseudoaneurysm
INTRODUCTION
Since the first description of transradial (TR) coronary intervention in 19931, several advantages of the TR approach have been described. Reduced bleeding2–6 and vascular complications2,7,8, reduced procedural cost9–12, reduced length of stay8, reduced nursing needs9, and earlier ambulation with improved patient comfort and satisfaction10,13 have driven an increase in its global popularity. Despite these advantages, the TR approach was used in only 1.3% of all PCIs performed in the United States between 2004 and 20076. Reasons for the reluctance of many United States cardiologists to adopt the TR approach are not well established, but likely contributors include familiarity with the transfemoral (TF) approach and concern for longer procedure times as well as increased radiation exposure14,15. The substantial learning curve16,17 for TR procedures is another deterrent, one that is perhaps best overcome by formal TR training during fellowship. While such training programs should ultimately improve PCI safety, the TR learning curve may reduce PCI safety as training programs are established. The effects of the de novo establishment of TR training programs on PCI safety at teaching hospitals are unknown. The purpose of this study was to assess the impact of a newly established TR training program on PCI safety at a previously femoral-only teaching hospital.
MATERIALS AND METHODS
In July of 2009 a physician-initiated, programmatic transition toward routine TR coronary arteriography and PCI was initiated at the Medical University of South Carolina, where cardiology fellows are the primary operators for almost all cardiac catheterizations and are assisted by attending cardiologists. None of the involved physicians had any significant, prior experience with TR procedures. Formal TR training was not part of the transition; rather, physicians’ learning was self-directed. Staff were actively involved in the establishment of the program including the management of its effect on lab workflow and patient preparation and recovery.
Attending cardiologists were encouraged at the same time (the beginning of the TR study period) to start performing TR procedures, but there was not a formal, stepwise transition to TR access. The TR approach was recommended as the default approach except in patients with inadequate Allen tests and/or Barbeau grade D perfusion by plethysmography; the TF approach remained the default approach for these patients. The use of the TR approach was especially encouraged in patients who were thought to be at high risk of bleeding and vascular complications. However, the TR approach was not mandated: the final decision to use a TR or a TF approach was made by the attending cardiologist.
Decisions regarding peri-procedural treatment with anti-thrombotic therapy were made by the attending cardiologist. An intravenous bolus of 3,000 to 5,000 units of unfractionated heparin was usually given at the initiation of TR procedures. Bivalirudin or additional heparin was administered if PCI was performed. The radial sheath was flushed at the time of all catheter exchanges with nicardipine solution or a “radial cocktail” including verapamil, nitroglycerin, and lidocaine. A TR Band™ (Terumo Medical Corporation, Somerset, NJ) was applied at the end of every TR procedure. Femoral arteriotomy closure devices were used at the discretion of the attending cardiologist. Dual anti-platelet therapy with aspirin and a thienopyridine was prescribed after nearly every PCI.
Data including baseline patient characteristics, procedural characteristics, and procedural outcomes were prospectively collected and retrospectively analyzed using the American College of Cardiology-National Cardiovascular Data Registry® (ACC-NCDR®) Cath Lab Module v3.04 (for procedures completed from July 15th, 2008 to June 30th, 2009; Year 1 or Y1) and CathPCI Registry® v4.3 (for procedures completed from July 1st, 2009 to June 30th, 2010; Year 2 or Y2). No cases were excluded from analysis. The primary outcome was the composite of bleeding and vascular complications. Secondary outcomes included the components of the primary outcome as well as procedural success, length of stay, and pre-discharge mortality. Definitions for outcomes are listed in Box 1. Procedural characteristics of interest included arterial access site(s), procedural duration, maximum sheath size, closure device use, fluoroscopy time, contrast volume, number of lesions treated, and treatment with anticoagulants and inhibitors of glycoprotein IIb/IIIa. Baseline patient characteristics of interest included age, sex, weight, prothrombin time, and platelet count; levels of hemoglobin, blood urea nitrogen, and creatinine; the presence or absence of a history of diabetes, hypertension, or dyslipidemia; and indications for PCI.
Procedural success was defined by the passage of any interventional device across a target lesion. In cases where PCI was attempted on more than one lesion, the procedure as a whole was considered successful if at least one lesion was crossed successfully. In transradial cases, conversion to a transfemoral approach was counted as procedural failure. Pre-discharge mortality was defined as death prior to discharge from the hospitalization with which the PCI was associated. In ACC-NCDR® Cath Lab Module v3.04 and in the ACC-NCDR® CathPCI Registry® v4.3, bleeding from the access site or retroperitoneal, gastrointestinal, genitourinary, or other sources constituted a complication when associated with a hematocrit drop of ≥10% or a hemoglobin drop of ≥3 g/dL, transfusion of whole blood or packed red blood cells, or the need for a procedural intervention at the bleeding site. Acute anemia with a drop in hemoglobin of ≥ 3 g/dL attributable to procedure-related blood loss without an obvious, alternative source was considered bleeding at the access site. In ACC-NCDR® Cath Lab Module v3.04, vascular complications were defined as any access site arterial occlusion, peripheral embolization, dissection, pseudoaneurysm, or arteriovenous fistula. For the ACC-NCDR® CathPCI Registry® v4.3, the definition of vascular complications was clarified to include only those complications that required a procedural intervention.
Continuous variables were summarized as means, and dichotomous variables were summarized in absolute numbers as percentages. Generalized mixed models were used to evaluate for differences in procedural outcomes, procedural characteristics, and baseline patient characteristics in Y2 vs. Y1 and with TR vs. TF PCI. These models account for correlated observations. For comparisons with a zero cell, Fisher’s exact test was used. Fisher’s exact test does not take into account correlated observations; however, models with a zero cell did not converge. Significance was set at a two-sided alpha level of 0.05, and all analyses were completed using SAS® 9.2 (Cary, North Carolina).
RESULTS
A total of 1,366 PCIs were performed in 1,249 patients at the Medical University of South Carolina between July 15th, 2008 and June 30th, 2010. 693 PCIs were performed in Y1 and 673 in Y2. TR access was used in 1 PCI in Y1 and 193 PCIs (28.7%) in Y2. The TR PCIs in Y2 were performed by a total of 13 different cardiology fellows and 9 different attending cardiologists, none of whom routinely performed TR PCI previously.
Baseline patient characteristics and indications for PCI are summarized in Table 1. Patients treated in Y2 vs. Y1 were more likely to have a history of diabetes or hypertension, and patients undergoing TR vs. TF PCI were heavier and had minimally lower blood urea nitrogen and creatinine levels. Patients treated via the TR vs. TF approach presented less commonly with ST-elevation myocardial infarction (STEMI). Patients treated in Y2 vs. Y1 and via the TR vs. TF approach presented less commonly with non-STEMI and more commonly with angina.
Table 1.
Baseline Patient Characteristics and Indications for Percutaneous Coronary Intervention
| Characteristic | Y2 (n = 673) | Y1 (n = 693) | p Value* | TR (n = 194) | TF (n = 1,172) | p Value* |
|---|---|---|---|---|---|---|
| Baseline Characteristics | ||||||
| Age (years) | 63.2 (11.9) | 63.6 (11.9) | 0.4 | 62.0 (11.0) | 63.6 (12.0) | 0.1 |
| Weight (kg) | 88.1 (20.7) | 86.6 (19.6) | 0.2 | 91.1 (23.6) | 86.7 (19.5) | 0.01 |
| Men | 434 (64%) | 447 (65%) | >0.9 | 119 (61%) | 762 (65%) | 0.3 |
| Diabetes mellitus | 284 (43%) | 254 (37%) | 0.04 | 69 (36%) | 469 (40%) | 0.3 |
| Hypertension | 619 (92%) | 581 (84%) | <0.001 | 176 (91%) | 1,024 (87%) | 0.2 |
| Dyslipidemia | 587 (88%) | 602 (87%) | 0.6 | 173 (91%) | 1,016 (87%) | 0.2 |
| Hemoglobin (g/dL) | 12.8 (2.0) | 13.0 (1.9) | 0.2 | 13.2 (1.9) | 12.9 (2.0) | 0.1 |
| Platelet Count (K/cmm) | 214 (67.4) | 218 (63.7) | 0.4 | 216 (65.0) | 216 (65.7) | >0.9 |
| Blood Urea Nitrogen (mg/dL) | 18.8 (13.3) | 17.7 (10.9) | 0.1 | 16.5 (10.4) | 18.6 (12.4) | 0.05 |
| Creatinine (mg/dL) | 1.3 (1.4) | 1.3 (1.4) | 0.4 | 1.1 (0.5) | 1.4 (1.5) | 0.01 |
| Prothrombin Time (s) | 14.8 (2.3) | 14.6 (2.0) | 0.2 | 14.5 (1.8) | 14.7 (2.2) | 0.4 |
| Indications for Percutaneous Coronary Intervention | ||||||
| STEMI | 72 (11%) | 58 (8%) | 0.1 | 8 (4%) | 122 (10%) | 0.008 |
| NSTEMI | 113 (17%) | 149 (22%) | 0.03 | 25 (13%) | 237 (20%) | 0.02 |
| Unstable Angina Pectoris | 234 (35%) | 209 (30%) | 0.07 | 75 (39%) | 368 (32%) | 0.05 |
| Stable Angina Pectoris | 161 (24%) | 103 (15%) | <0.001 | 65 (34%) | 199 (17%) | <0.001 |
| Atypical Chest Pain | 20 (3%) | 19 (3%) | 0.8 | 7 (4%) | 32 (3%) | 0.5 |
| Other | 73 (11%) | 149 (22%) | <0.001 | 14 (7%) | 208 (18%) | <0.001 |
Data are presented as means (standard deviations) and n (%), which are raw values and do not account for multiple observations per individual patient. NSTEMI = non-ST-elevation myocardial infarction; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction; TF = transfemoral; TR = transradial; Y1 = year 1; Y2 = year 2. Hypertension, dyslipidemia, and diabetes were defined according to American College of Cardiology-National Cardiovascular Data Registry® guidelines. “Other” indications for percutaneous coronary intervention included exertional dyspnea, cardiac allograft vasculopathy, spontaneous or traumatic coronary artery dissection, and ventricular tachycardia/fibrillation.
P-values take into consideration the fact that some observations were made on the same individuals.
Procedural characteristics are shown in Tables 2 and 3. Nearly all TR procedures were completed via the right radial artery. Patients with left internal mammary arterial bypass grafts or acute STEMI were more likely to be treated from a TF approach. Procedures completed in Y2 vs. Y1 and via the TR vs. TF approach were similar in total duration, required slightly more fluoroscopy time, and required similar volumes of iodinated contrast. Unfractionated heparin was used in over 80% of all procedures. Glycoprotein IIb/IIIa inhibitors were used less commonly and bivalirudin was used more commonly for procedures completed in Y2 vs. Y1 and via the TR vs. TF approach. Enoxaparin and fondaparinux were used infrequently. Sheath sizes >6 Fr were used only for TF PCIs, and femoral arteriotomy closure devices were used less frequently in Y2 vs. Y1.
Table 2.
Procedural Characteristics
| Characteristic | Y2 (n = 673) | Y1 (n = 693) | p Value* | TR (n = 194) | TF (n = 1,172) | p Value* |
|---|---|---|---|---|---|---|
| Duration (min) | 76.7 (39.7) | 72.8 (44.6) | 0.1 | 75.8 (43.3) | 74.5 (42.1) | 0.7 |
| Lesions Treated (number) | 1.4 (0.7) | 1.4 (0.7) | 0.2 | 1.4 (0.7) | 1.4 (0.7) | >0.9 |
| Contrast Volume (mL) | 156 (68.3) | 150 (68.4) | 0.1 | 159 (66.8) | 151 (68.6) | 0.2 |
| Fluoroscopy (min) | 18.6 (12.9) | 17.2 (12.8) | 0.05 | 20.4 (12.9) | 17.5 (12.8) | 0.01 |
| Largest Sheath (Fr) | 6.3 (0.7) | 6.4 (0.8) | 0.1 | 6.0 (0.4) | 6.4 (0.8) | <0.001 |
| Treatment with Glycoprotein IIb/IIIa Inhibitors | ||||||
| Any IIb/IIIa Inhibitor | 16 (2%) | 107 (15%) | <0.001 | 1 (0.5%) | 122 (10%) | 0.002 |
| Abciximab | 13 (2%) | 38 (5%) | 0.001 | 1 (0.5%) | 50 (4%) | 0.03 |
| Eptifibatide | 3 (0.5%) | 69 (10%) | <0.001 | 0 (0%) | 72 (6%) | <0.001† |
| Treatment with Anticoagulants | ||||||
| Bivalirudin | 189 (28.1%) | 140 (20.2%) | <0.001 | 61 (31.4%) | 268 (22.9%) | 0.01 |
| Enoxaparin | 2 (0.3%) | 29 (4.2%) | <0.001 | 2 (1.0%) | 29 (2.5%) | 0.2 |
| Fondaparinux | 12 (1.8%) | 1 (0.1%) | 0.02 | 1 (0.5%) | 12 (1.0%) | 0.5 |
| Unfractionated Heparin | 588 (87.4%) | 616 (88.9%) | 0.4 | 175 (90.2%) | 1029 (87.8%) | 0.3 |
Data are presented as means (standard deviations) and n (%), which are raw values and do not account for multiple observations per individual patient. Abbreviations as in Table 1.
P-values take into consideration the fact that some observations were made on the same individuals.
For comparisons with a zero cell, Fisher’s exact tests were used.
Table 3.
Femoral Arteriotomy Closure
| Closure Method | Y2 (n = 481) | Y1 (n = 691) | p Value* |
|---|---|---|---|
| Manual Pressure | 277 (58%) | 221 (32%) | <0.001 |
| Perclose® ProGlide™ | 151 (31%) | 334 (48%) | <0.001 |
| Angioseal™ | 7 (1%) | 15 (2%) | 0.4 |
| Mynx™ | 46 (10%) | 121 (18%) | <0.001 |
| Any Closure Device | 204 (42%) | 470 (68%) | <0.001 |
Data are presented as n (%), which are raw values and do not account for multiple observations per individual patient. Abbreviations as in Table 3.
P-values take into consideration the fact that some observations were made on the same individuals.
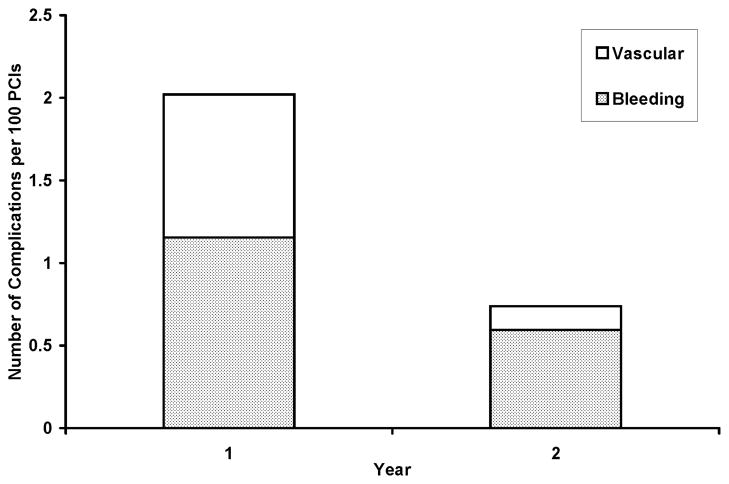
Outcomes are summarized in Table 4. Major bleeding and vascular complications over the 2-year study period were confined to TF procedures. No bleeding or vascular complications were identified in 194 consecutive TR cases, and the combined incidence of bleeding and vascular complications was significantly lower in Y2 vs. Y1 (0.7 vs. 2.0%, p = 0.05; Figure 1 and Table 4). There were not any cases of symptomatic radial arterial occlusion that required post-procedural imaging or intervention. Several cases of radial arterial spasm did occur peri-procedurally, but all of them resolved with vasodilators and/or additional sedative medication. Combined bleeding and vascular complication rates were similar with the TF approach in Y2 vs. Y1 (2.0 vs. 1.0%, p = 0.2). Pre-discharge mortality was statistically similar in Y2 vs. Y1 and with the TR vs. the TF approach. PCI was successfully accomplished in 97% of patients in Y1, in 97% of patients in Y2, and in 98% of all TR cases. Thus, procedural success rates were similar in Y2 vs. Y1 and with the TR vs. TF approach. Of 194 TR PCIs, 3 (1.5%) were converted to the TF approach and were counted as failures for TR PCI. Mean length of stay after TR vs. TF PCI was shorter (2.5 vs. 3.1 days), but the difference was not statistically significant.
Table 4.
Outcomes
| Outcome | Y2 (n = 673) | Y1 (n = 693) | p Value* | TR (n = 194) | TF (n = 1,172) | p Value* |
|---|---|---|---|---|---|---|
| Bleeding or Vascular Complications | 5 (0.7%) | 14 (2.0%) | 0.05 | 0 (0.0%) | 19 (1.6%) | 0.1† |
| Bleeding Complications | 4 (0.6%) | 8 (1.2%) | 0.3 | 0 (0.0%) | 12 (1.0%) | 0.2† |
| Access Site Bleeding or Hematoma | 2 (0.3%) | 3 (0.4%) | - | 0 (0.0%) | 5 (0.4%) | - |
| Retroperitoneal Bleeding | 2 (0.3%) | 3 (0.4%) | - | 0 (0.0%) | 5 (0.4%) | - |
| Gastrointestinal Bleeding | 0 (0.0%) | 2 (0.3%) | - | 0 (0.0%) | 2 (0.2%) | - |
| Vascular Complications | 1 (0.1%) | 6 (0.9%) | 0.1 | 0 (0.0%) | 7 (0.6%) | 0.6† |
| Pseudoaneurysm | 0 (0.0%) | 5 (0.7%) | - | 0 (0.0%) | 5 (0.4%) | - |
| Arteriovenous Fistula | 0 (0.0%) | 1 (0.1%) | - | 0 (0.0%) | 1 (0.1%) | - |
| Arterial Dissection | 1 (0.1%) | 0 (0.0%) | - | 0 (0.0%) | 1 (0.1%) | - |
| Pre-discharge Mortality | 7 (1.0%) | 8 (1.2%) | 0.8 | 1 (0.5%) | 14 (1.2%) | 0.4 |
| Procedural Success | 653 (97.0%) | 670 (96.7%) | 0.7 | 190 (97.9%) | 1,133 (96.7%) | 0.4 |
| Length of Stay (days) | 3.1 (12.5) | 2.9 (5.8) | 0.6 | 2.5 (4.0) | 3.1 (10.3) | 0.4 |
Data are presented as means (standard deviations) and n (%), which are raw values and do not account for multiple observations per individual patient. Abbreviations as in Table 1.
P-values take into consideration the fact that some observations were made on the same individuals.
For comparisons with a zero cell, Fisher’s exact tests were used.
Figure 1. Combined Bleeding and Vascular Complication Rates in Y1 and Y2.
Segmented bar chart comparing combined bleeding (shaded segments) and vascular (open segments) complication rates for years 1 and 2 (p = 0.05). Abbreviations as in Table 1.
DISCUSSION
These prospectively collected data describe the establishment of a TR PCI training program at a teaching hospital in the United States, where consecutive patients were treated by 9 different attending cardiologists and 13 cardiology fellows. None of the involved physicians was routinely performing TR procedures prior to the start of the study period, yet there were no procedural complications attributable to the TR learning curve. In fact, the TR program’s establishment was associated with a statistically significant 1.3% absolute reduction in the combined incidence of PCI-related bleeding and vascular complications in its first year. The success rate for TR PCIs was high, and there were no clinically significant differences in total procedural durations, fluoroscopy times, or contrast requirements for patients treated with TR vs. TF PCI.
No major vascular or bleeding complications were identified in our first 194 TR PCI patients, which is consistent with published data from a university hospital with a newly established TR program in Germany18. There were no major complications at the access site in their first 160 TR patients, 61 (38%) of whom were treated with TR PCI. In a larger analysis of their first 784 TR patients, 290 (37%) of whom were treated with TR PCI, 6 patients (0.8%) developed radial artery closure and 3 patients (0.4%) had other access site complications. Their TR PCI-treated patients required similar total fluoroscopy times and contrast volumes when compared to a contemporary cohort of 842 TF PCI-treated patients.
Several cases of radial arterial spasm did occur peri-procedurally in our patients, but all of them resolved with vasodilators and/or additional sedative medication; these events were not catalogued as complications. There were no cases of symptomatic radial arterial occlusion in our patients. The clinical significance of asymptomatic radial arterial occlusion after TR PCI is unknown, and post-procedural screening examinations are not performed at our center. Therefore, the incidence of asymptomatic radial arterial occlusion in our patients is unknown. Serious complications such as limb ischemia and compartment syndrome are rare after TR procedures19, and none were observed in our patients.
Selection bias limits to some degree the validity of comparative data analyses for patients treated with TR vs. TF PCI in our study. However, selection bias should not be an issue for comparisons between patients treated in Y2 vs. Y1, all of whom underwent PCI. Thus, the observed reduction in bleeding and vascular complications in Y2 vs. Y1 cannot be attributed to selection bias. Patients who underwent PCI at our center in Y2 vs. Y1 and via the TR vs. TF approach were less likely to be treated with glycoprotein IIb/IIIa inhibitors and more likely to be treated with bivalirudin. Glycoprotein IIb/IIIa inhibitors have been associated with increased risk of bleeding, and anticoagulation with bivalirudin20,21 has been associated with reduced bleeding when compared to anticoagulation with unfractionated heparin or enoxaparin. Also, it should be noted that 2 gastrointestinal bleeding events occurred in patients treated via TF access in Y1, and none occurred in patients treated via TR access or in Y2. TF PCI patients in Y2 were less likely to be treated with femoral arteriotomy closure devices, which may22,23 or may not24 be associated with increased bleeding risk as compared to manual compression. Thus, treatment differences other than in the arterial approach may have affected complication rates in our patients. It should be noted that bleeding and vascular complications occurred at statistically similar rates with TF PCI in Y2 vs. Y1, suggesting that the observed reduction in bleeding and vascular complications in Y2 can be attributed to the establishment of the TR program and is not simply due to a lower incidence of bleeding and vascular complications with TF PCI in Y2 vs. Y1.
These data demonstrate that TR programs for coronary arteriography and PCI can be established at United States teaching hospitals with an immediate improvement in PCI safety and the potential for far-reaching improvement in PCI safety as graduating trainees continue to use the TR approach. To date, the major, American cardiovascular professional societies have only weakly encouraged the establishment of TR training during fellowship. The American College of Cardiology’s Core Cardiology Training Symposium (COCATS) guidelines for training in diagnostic and interventional cardiac catheterization were updated in 200225 and in 200826. Radial arterial access is mentioned for the first time in the 2008 update, but a requirement for a minimum number of TR procedures still does not exist. In partnership with the American Heart Association and the Society for Cardiovascular Angiography and Interventions, the American College of Cardiology updated its Guidelines on Percutaneous Coronary Intervention in 200727 and 200928. Nowhere in these guidelines does the word “radial” appear.
As physicians we are obligated to practice medicine in a manner that minimizes morbidity and mortality for our patients. As cardiologists we have the opportunity to do so by learning and teaching TR techniques for coronary angiography and intervention. Practicing cardiologists should pursue transradial experience, and cardiology fellowship training programs should incorporate TR training into their formal curricula. These data suggest that, even during the transition period, historically TF programs can achieve excellent outcomes with the TR approach, that cardiology fellows can safely learn TR techniques, and that patients will benefit from the change.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kiemeneij F, Laarman GJ. Percutaneous transradial artery approach for coronary stent implantation. Cathet Cardiovasc Diagn. 1993;30:173–178. doi: 10.1002/ccd.1810300220. [DOI] [PubMed] [Google Scholar]
- 2.Achenbach S, Ropers D, Kallert L, Turan N, Krahner R, Wolf T, Garlichs C, Flachskampf F, Daniel WG, Ludwig J. Transradial versus transfemoral approach for coronary angiography and intervention in patients above 75 years of age. Catheter Cardiovasc Interv. 2008;72:629–635. doi: 10.1002/ccd.21696. [DOI] [PubMed] [Google Scholar]
- 3.Agostoni P, Biondi-Zoccai GG, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, Vassanelli C, Zardini P, Louvard Y, Hamon M. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349–356. doi: 10.1016/j.jacc.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Chase AJ, Fretz EB, Warburton WP, Klinke WP, Carere RG, Pi D, Berry B, Hilton JD. Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A. L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg) Heart. 2008;94:1019–1025. doi: 10.1136/hrt.2007.136390. [DOI] [PubMed] [Google Scholar]
- 5.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–140. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Rao SV, Ou FS, Wang TY, Roe MT, Brindis R, Rumsfeld JS, Peterson ED. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–386. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 8.Eichhofer J, Horlick E, Ivanov J, Seidelin PH, Ross JR, Ing D, Daly P, Mackie K, Ridley B, Schwartz L, Barolet A, Dzavik V. Decreased complication rates using the transradial compared to the transfemoral approach in percutaneous coronary intervention in the era of routine stenting and glycoprotein platelet IIb/IIIa inhibitor use: a large single-center experience. Am Heart J. 2008;156:864–870. doi: 10.1016/j.ahj.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Amoroso G, Sarti M, Bellucci R, Puma FL, D’Alessandro S, Limbruno U, Canova A, Petronio AS. Clinical and procedural predictors of nurse workload during and after invasive coronary procedures: the potential benefit of a systematic radial access. Eur J Cardiovasc Nurs. 2005;4:234–241. doi: 10.1016/j.ejcnurse.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Cooper CJ, El-Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, Moore JA. Effect of transradial access on quality of life and cost of cardiac catheterization: A randomized comparison. Am Heart J. 1999;138:430–436. doi: 10.1016/s0002-8703(99)70143-2. [DOI] [PubMed] [Google Scholar]
- 11.Mann T, Cowper PA, Peterson ED, Cubeddu G, Bowen J, Giron L, Cantor WJ, Newman WN, Schneider JE, Jobe RL, Zellinger MJ, Rose GC. Transradial coronary stenting: comparison with femoral access closed with an arterial suture device. Catheter Cardiovasc Interv. 2000;49:150–156. doi: 10.1002/(sici)1522-726x(200002)49:2<150::aid-ccd7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Roussanov O, Wilson SJ, Henley K, Estacio G, Hill J, Dogan B, Henley WF, Jarmukli N. Cost-effectiveness of the radial versus femoral artery approach to diagnostic cardiac catheterization. J Invasive Cardiol. 2007;19:349–353. [PubMed] [Google Scholar]
- 13.Sciahbasi A, Romagnoli E, Burzotta F, Trani C, Sarandrea A, Summaria F, Pendenza G, Tommasino A, Patrizi R, Mazzari M, Mongiardo R, Lioy E. Transradial approach (left vs right) and procedural times during percutaneous coronary procedures: TALENT study. Am Heart J. 2011;161:172–179. doi: 10.1016/j.ahj.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Brasselet C, Blanpain T, Tassan-Mangina S, Deschildre A, Duval S, Vitry F, Gaillot-Petit N, Clement JP, Metz D. Comparison of operator radiation exposure with optimized radiation protection devices during coronary angiograms and ad hoc percutaneous coronary interventions by radial and femoral routes. Eur Heart J. 2008;29:63–70. doi: 10.1093/eurheartj/ehm508. [DOI] [PubMed] [Google Scholar]
- 15.Lange HW, von Boetticher H. Randomized comparison of operator radiation exposure during coronary angiography and intervention by radial or femoral approach. Catheter Cardiovasc Interv. 2006;67:12–16. doi: 10.1002/ccd.20451. [DOI] [PubMed] [Google Scholar]
- 16.Ball WT, Sharieff W, Jolly SS, Hong T, Kutryk MJ, Graham JJ, Fam NP, Chisholm RJ, Cheema AN. Characterization of operator learning curve for transradial coronary interventions. Circ Cardiovasc Interv. 2011;4:336–341. doi: 10.1161/CIRCINTERVENTIONS.110.960864. [DOI] [PubMed] [Google Scholar]
- 17.Looi JL, Cave A, El-Jack S. Learning curve in transradial coronary angiography. Am J Cardiol. 2011;108:1092–1095. doi: 10.1016/j.amjcard.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann R, Ehrlich JR, Weber V, SDER, Gotarda MN, Schachinger V, Zeiher AM, Fichtlscherer S. Implementation of the Transradial Approach for Coronary Procedures is Not Associated with an Elevated Complication Rate and Elevated Radiation Patient Exposure. J Interv Cardiol. 2011;24:56–64. doi: 10.1111/j.1540-8183.2010.00603.x. [DOI] [PubMed] [Google Scholar]
- 19.Tizon-Marcos H, Barbeau GR. Incidence of compartment syndrome of the arm in a large series of transradial approach for coronary procedures. J Interv Cardiol. 2008;21:380–384. doi: 10.1111/j.1540-8183.2008.00361.x. [DOI] [PubMed] [Google Scholar]
- 20.Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–2216. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 21.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–2230. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 22.Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004;291:350–357. doi: 10.1001/jama.291.3.350. [DOI] [PubMed] [Google Scholar]
- 23.Nikolsky E, Mehran R, Halkin A, Aymong ED, Mintz GS, Lasic Z, Negoita M, Fahy M, Krieger S, Moussa I, Moses JW, Stone GW, Leon MB, Pocock SJ, Dangas G. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004;44:1200–1209. doi: 10.1016/j.jacc.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 24.Sanborn TA, Ebrahimi R, Manoukian SV, McLaurin BT, Cox DA, Feit F, Hamon M, Mehran R, Stone GW. Impact of femoral vascular closure devices and antithrombotic therapy on access site bleeding in acute coronary syndromes: The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circ Cardiovasc Interv. 2010;3:57–62. doi: 10.1161/CIRCINTERVENTIONS.109.896704. [DOI] [PubMed] [Google Scholar]
- 25.Beller GA, Bonow RO, Fuster V. ACC revised recommendations for training in adult cardiovascular medicine. Core Cardiology Training II (COCATS 2) (Revision of the 1995 COCATS training statement) J Am Coll Cardiol. 2002;39:1242–1246. doi: 10.1016/s0735-1097(02)01795-3. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs AK, Babb JD, Hirshfeld JW, Jr, Holmes DR., Jr Task force 3: training in diagnostic and interventional cardiac catheterization endorsed by the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2008;51:355–361. doi: 10.1016/j.jacc.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 27.King SB, 3rd, Smith SC, Jr, Hirshfeld JW, Jr, Jacobs AK, Morrison DA, Williams DO, Feldman TE, Kern MJ, O’Neill WW, Schaff HV, Whitlow PL, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209. doi: 10.1016/j.jacc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]