Abstract
Perceived discrimination remains a salient and significant environmental stressor for ethnic and racial minority youth. Although many studies have examined the impact of racial/ethnic discrimination on mental health symptomatology and physical health, little is known of the potential physiological processes underlying such experiences, especially during adolescence. In an attempt to understand how varying perceptions of discrimination relate to functioning of the hypothalamic-pituitary-adrenal axis (HPA axis), the current study examined the relation between Mexican American adolescents’ (N= 100, Mage = 15.3 years old) perceptions of discrimination and aspects of their diurnal cortisol profiles. Three salivary samples (wakeup, +30 waking, bedtime) were collected across three days (total of 9 samples). Utilizing multi-level modeling, results revealed that adolescents’ perceived discrimination related to greater overall cortisol output (area under the curve; AUC) after controlling for other life stressors, depressive symptoms, family income, acculturation level, daily stress levels and daily behaviors. Findings also revealed that perceived discrimination was marginally related to a steeper cortisol awakening response (CAR). Together, these findings suggest that perceived discrimination is a salient and impactful stressor for Mexican American adolescents. Understanding the physiological correlates of discrimination can provide insight into larger health disparities among ethnic and racial minority individuals.
Keywords: HPA axis, diurnal cortisol, discrimination, Mexican American, adolescents
Discrimination is a commonly experienced stressor among ethnic and racial minority individuals in the U.S. (Williams and Mohammed, 2009). Nearly 30% of Mexican American adults and 50% of Mexican American adolescents report experiences of ethnic discrimination on a daily basis (Fisher, Wallace, and Fenton, 2000; Pérez, Fortuna, and Alegría, 2008). This frequency is alarming given that perceived ethnic/racial discrimination has been consistently linked to a variety of physical and mental health outcomes including hypertension, self-reported poor health, breast cancer, depression, and anxiety (for review see: Pascoe and Richman, 2009; Williams and Mohammed, 2009). Numerous theories posit that there are physiological pathways through which perceptions of discrimination affect health (Beauchaine, Neuhaus, Brenner, and Gatzke-Kopp, 2008; Cicchetti and Gunnar 2008; Clark, Anderson, Clark and Williams, 1999; Meyers, 2009; Pascoe and Richman, 2009). Specifically, such experiences set in motion a process of physiological responses that include cardiovascular activity and greater stress response. These responses are theorized to contribute to health deterioration (Geronimus, Hicken, Keene, and Bound, 2006) and over time, damaging changes in physiological functioning, often referred to as allostatic load (McEwen, 1998; 2002). Empirically, perceived racial/ethnic discrimination has been found to predict greater cardiovascular reactivity, which includes higher nocturnal blood pressure (Brondolo et al., 2008), and higher systolic and diastolic blood pressure throughout the day (Steffen, McNeilly, Anderson, and Sherwood, 2003); however, the link between perceived discrimination and other major stress response systems remains relatively unexplored, especially among adolescents.
To address the gap in the literature and extend our knowledge of the physiological mechanisms underlying the effects of perceived discrimination during adolescence, the current study examined the relation between Mexican American youths’ perceptions of ethnic discrimination and the main stress hormone of the hypothalamic-pituitary-adrenal (HPA) axis, cortisol (Johnson, Kamilaris, Chrousos, and Gold, 1992). Cortisol levels have been linked to day-to-day variation in daily stressors (Adam, 2006), more persistent, chronic life stressors (Miller, Chen and Zhou, 2007), and recently, perceived discrimination in adults (Kaholokula, Grandinetti, Keller, Nacapoy, Kingi, & Mau, 2011); however, the link between perceived ethnic discrimination and cortisol has yet to be examined in adolescents. Adolescence appears to be a particularly compelling developmental stage to examine given the increased awareness of perceived discrimination (Brown and Bigler, 2005) and the increased attention to allostatic load (Hasting et al., 2011). Further, a focus on Mexican American youth appears critical given that they make up the youngest and fastest growing ethnic minority populations in the U.S. (U.S. Census, 2006). Exploring the physiological correlates of these adolescents’ experiences has implications for our understanding of health disparities among the larger Latino population and other racial/ethnic minorities.
Perceived Discrimination as a Stressor
Discrimination is a reality for many ethnic and racial minority individuals living within the U.S. (Fisher, Wallace, and Fenton, 2000). For Latinos specifically, recent political attention to immigration in the U.S. has increased perceptions of such experiences with nearly 61% of Latino adults describing ethnic discrimination as a “major problem” in 2010 compared to 50% in 2004 (Lopez, Morin, and Taylor, 2010). Empirical evidence of the deleterious effects of perceived discrimination in ethnic and racial minorities including Mexican Americans is mounting. In a recent meta-analysis of 134 studies, a robust and strong relation emerged between perceptions of racial/ethnic discrimination and physical health outcomes that included cardiovascular disease, hypertension, diabetes, and respiratory conditions (Pascoe and Richman, 2010). An equally strong relation emerged between racial/ethnic discrimination and mental health conditions (e.g., depression, anxiety, posttraumatic stress disorder, and perceived quality of life). Although most of this work has focused on adult populations, researchers have begun to examine the effects of discrimination on adolescents’ development. Cross-sectional and longitudinal studies have consistently found that adolescents’ perceptions of ethnic discrimination relate to mental and physical health outcomes in Mexican American (e.g., Berkel et al., 2010; Delgado, Updegraff, Roosa, & Umaña-Taylor, 2011; Flores, Tschann, Dimas, Pasch, and de Groat, 2010) and other ethnic and racial minority youth (e.g., Clark, 2006; Galliher, Jones, and Dahl, 2011; Simons, Murray, McLoyd, Lin, Cutrona, and Conger, 2002). Together, such evidence underscores the seriousness of perceived discrimination in adolescents’ development.
Adolescence is a particularly compelling period of development to understand the impact of discriminatory experiences. Due to increased cognitive functioning and a greater sense of self-identity, theorists have posited that this developmental stage brings an increased understanding that societal attitudes of racial/ethnic biases are based upon opinions about and perspectives of its majority members (Brown and Bigler, 2005; Selman, 1976). Adolescents, in turn, develop a greater awareness of biases and discrimination at an interpersonal level, leading to increased perceptions of discrimination. Understanding the pathways linking discrimination to adolescents’ outcomes during a developmental period in which such events gain salience could provide researchers clues into the impact of discrimination over the life course.
Hypothalamic-pituitary-adrenal Axis Response to Stressors
The biopsychosocial model of minority health (Meyers, 2009) and other theoretical frameworks (Pascoe and Richman, 2009) posit that there are physiological pathways and mechanisms linking perceptions of racial/ethnic discrimination and health. One of those mechanisms is the HPA axis. As one of the bodies’ major stress-response systems, the HPA axis reacts to both physical and psychological environmental stressors and includes complex interactions between the hypothalamus, the pituitary gland, and the adrenal cortex (Johnson et al., 1992). Stated simply, when stressors arise, the limbic system activates the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) from the hypothalamus, which in turn interacts with receptors of the anterior pituitary, releasing adrenocorticotropin hormone (ACTH) into general circulation (Herman and Cullinan, 1997). ACTH circulates in the blood and binds to the receptors of the adrenal cortex, stimulating the release of cortisol (de Kloet and Derijk 2004). To help with self-containment, the HPA axis is equipped with important feedback mechanisms that inhibit further production of CRH, AVP, and ACTH, effectively turning off the HPA axis when individuals have recovered from the environmental stressor (Chrousos and Gold, 1992).
The entire process of the HPA axis responding to immediate stressors is often referred to as cortisol reactivity. Most of the early work on cortisol has focused on this area; researchers have used laboratory stressor tasks to elicit a cortisol response (for review see Dickerson and Kemeny, 2004) and more recently examined reactivity in naturalistic settings (Adam, 2006). Researchers have also begun to understand the importance of examining cortisol outside of the reactivity framework, focusing on the basal activity of the HPA axis (e.g., Adam, Doane, Zinbarg, Mineka, Craske, and Griffth, 2010; Shirtcliff and Essex, 2008). The HPA axis produces levels of cortisol that follow a strong diurnal rhythm; cortisol levels are high upon waking, increase by 50–60% in the first 30–40 minutes [known as cortisol awakening response (CAR)], and then rapidly drop off throughout the day, reaching nadir around midnight (Kirschbaum and Hellhammer, 2000; Pruessner, et al., 1997). Although the relation between cortisol reactivity and basal diurnal levels is not completely understood, theories of HPA axis activity posit that the periodic activation of the HPA axis and the release of cortisol are necessary to cope with acute stress; when the HPA axis response is frequent or persistent, however, chronically low or chronically high levels of cortisol can emerge, leading to changes in basal cortisol levels and possibly, damaging effects that include receptor desensitization and tissue damage and contributing to allostatic load (McEwen, 1998, 2001).
Although empirical studies have lagged behind theory, emerging research into diurnal cortisol rhythms suggests that flattened diurnal slopes, increased CAR, and/or low or high levels of overall cortisol output [typically referred to as area under the curve (AUC)] might be an indication of basal cortisol level changes and possibly, allostatic load (McEwen, 2002). For instance, prolonged or cumulative stressors have related to decreased morning cortisol levels and higher afternoon/evening cortisol levels, which results in a flatter slope or a less steep decline in cortisol across the day (for review see: Miller et al., 2007; Michaud, Mathenson, Kelly, and Anisman, 2008). Further, persistent environmental stressors have been linked to a flatter diurnal slopes (Do et al., 2011), steeper CAR (Schulz, Kirshbaum, Prubner, and Hellhammer, 1998; Pruessner, Hellhammer, and Kirschbaum, 1999; Pruessner, Hellhammer, Pruessner, and Lupien, 2003) and a greater AUC (Gustafsson, Gustafsson, and Nelson, 2006; Kirschbaum et al., 1995). Together, such evidence suggests that persistent or chronic environmental stressors have the potential of influencing diurnal cortisol patterns.
Further, studies suggest that activation of the HPA axis is sensitive and more prone to react to stressors that are socially evaluative and uncontrollable. In a meta-analysis of 208 adult laboratory studies, Dickerson and Kemeny (2004) found that adults had a strong cortisol response to stressors when exposed to threats in which an aspect of the self (e.g., trait, ability) were negatively judged by others or when stressors were deemed uncontrollable. Stressors with both characteristics evoked the strongest cortisol responses. Naturalistic studies corroborate these findings such that uncontrollable stressors and/or stressors that pose a threat to the individual’s social standing were related to flattened diurnal slopes lower morning values, and higher afternoon values (Michaud, et al., 2008, Miller et al., 2007). Experiences of ethnic discrimination could be considered both socially evaluative and uncontrollable; socially evaluative because such experiences threaten an individuals’ social standing in their peer group and their immediate context (e.g., school) and uncontrollable because discrimination has nothing to do with individuals’ actions, but rather their ethnic appearance or national origin. Guided by these findings and theory, we might expect perceptions of ethnic discrimination to be a particularly important stressor in activating the HPA axis.
The limited empirical examinations of the relations between discrimination and cortisol among adults, however, have yielded inconsistent findings. In a study examining psychosocial mediators of the relation between SES and diurnal cortisol among African Americans and European Americans, Cohen and colleagues (2006) found that discrimination based on SES, gender or ethnic/racial background was not related to diurnal cortisol. In contrast, Fuller-Rowell and colleagues (2012) focused on daily reports of perceived racial discrimination, finding that greater discrimination related to flatter diurnal cortisol slopes for European American adults, but steeper diurnal cortisol slopes for African American adults. They argued that the acknowledgment of and ability to perceive daily experiences of discrimination protected African American adults, resulting in healthier diurnal rhythms. Finally, Kaholokula and colleagues (2011) examine Hawaiian adults and found that racial discrimination related to lower levels of cortisol. It should be noted, however, that participants’ morning and evening samples were combined (a mean level of cortisol) and taken at a specified time rather than in relation to their wake time. Such a protocol would likely be masking the CAR (since this is captured 30 minutes after waking) and does not allow for the examination of diurnal slopes (the decline from wakeup to bedtime; Adam and Kumari, 2009). Given the limited studies and the inconsistencies of this literature, the current study drew from a more established chronic stress/HPA literature when hypothesizing about the relations between perceived discrimination and diurnal cortisol.
The Current Study
To address prior limitations in the literature and to advance our understanding of physiological processes underlying perceptions of discrimination during adolescence, the current study examined the relation between Mexican American youths’ diurnal cortisol levels and self-reported perceptions of ethnic discrimination in their naturalistic settings. We focused on three commonly used parameters in cortisol research: CAR, diurnal slopes, and AUC. We hypothesized that greater perceptions of discrimination would relate to steeper CARs, flatter diurnal slopes and greater AUCs, controlling for gender, socioeconomic status, life stressors, depressive symptomatology, acculturation level, daily stress level, and daily behaviors. This study contributed to the existing literature by being the first empirical study to examine the relation of discrimination and HPA axis diurnal functioning among male and female adolescents. Although focused on Mexican American adolescents, it contributes to our understanding of the physiological processes of discrimination in ethnic and racial minority males and females at large.
Method
Participants and Procedure
Data for the current study came from a longitudinal study of 749 Mexican-origin families focused on culture and context (Roosa et al., 2008). A subsample of these families (N = 131) were asked to participate in a cortisol sampling protocol during the third wave of data collection when adolescents were in tenth grade. To be eligible to participate, the families must have been scheduled for interviews in the larger project between February, 2010 and December, 2010. The selected families were contacted to schedule the in-home interview and asked if their adolescent was interested in participating in a 3-day cortisol sampling protocol. All study procedures were approved by the University’s Institutional Review Board (IRB) and informed consent/assent procedures were followed. In the home, bilingual (Spanish and English) trained interviewers obtained informed consent from the mother and/or father and assent from the adolescent, and distributed the cortisol “spit kit” to the adolescent. Interviewers briefed the adolescent on the materials contained in the kit and the sampling protocol and asked participants to start the sampling protocol the next day (if interviewed Sunday – Tuesday) or to start the following Monday to ensure sampling on three consecutive weekdays. Study personnel contacted participants each night of the sampling protocol to ensure proper cortisol sampling techniques and answer participants’ questions. Adolescents were also asked to respond to a series of questions about their daily behaviors/activities that included medication use (i.e., depression or asthma related medication), hours of exercise, alcohol consumption, cigarette use, caffeine consumption, and stress levels. After completion of the third day, study personnel picked up cortisol samples from each adolescent’s home.1 Adolescents were paid $55 for the in-home interview and $15 for their completion of the salivary cortisol protocol.
Of the 131 families approached, 113 adolescents (86.2%) agreed to participate. Thirteen adolescents were excluded from the current analyses because they did not complete the cortisol protocol or completed it incorrectly (n = 5), did not label cortisol samples (n =1), reported that they were on corticosteroid medication (n = 4), or did not have complete data on study variables (n = 3) resulting in a final sample of 100 adolescents. Participants (51.0% female) were approximately 15 years old (M = 15.3, SD = 0.50) and 86.0% reported being born in the U.S. Adolescents came from families in which 34.8 % of fathers and 48.0% of mothers reported being born in the U.S. Family income ranged from $5,000 to $99,000 with a mean range of $40,000 to $45,000. All adolescents completed the interview and cortisol materials in English, whereas 45.6% of father and 51.5% of mothers completed the in-home interviews in Spanish. Adolescents excluded from the current analyses (n = 13) did not differ from participating adolescents (n = 100) on age, gender, nativity (adolescent and parent), family income, or interview language; however, those in the current sample (n = 100) did differ from the larger longitudinal study sample (N = 649) on adolescent nativity (χ2[1] = 12.76, p < .001), maternal nativity (χ2[1] = 29.93, p < .001), paternal nativity (χ2[1] = 10.75, p < .01), family income (t [731] = 3.83, p < .001), mothers’ interview language (χ2[1] = 66.83, p < .001) and fathers’ interview language (χ2[1] = 41.22, p < .001). That is, family members in the larger study were more likely to be born in Mexico, have lower family income, and more likely to complete the interview in Spanish than those included in the current study.
Measures
Salivary cortisol
Salivary samples were gathered each day for three consecutive weekdays at wake up, 30 minutes after waking, and bedtime. To help with compliance of the second sample, participants were given a preset 30-minute timer. On average, adolescent reported their second sample 32 minutes after their waking sample (Range = 10 minutes after wakeup sample to 50 minutes after wakeup sample; SD = 4 minutes). Participants expelled saliva through a small straw into a 2-mL polypropylene tube and labeled tubes with the time and date. Participants were instructed not to eat, drink, or brush their teeth 30 minutes before sampling. Samples were picked up from participants’ homes, refrigerated at −20 degrees Celsius, and then sent on dry ice by courier to Biochemisches Labor, Trier, Germany to be assayed for cortisol. Cortisol levels are stable at room temperature for several weeks and are unaffected by shipping Clements and Parker, 1998). Assays were conducted using a time-resolved immunoassay with fluorometric detection DELFIA; (see Dressendorfer et al., 1992 for greater assay description). Intra-assay coefficients of variation (CVs) were between 4.0% and 6.7%, and inter-assay CVs ranged from 7.1% to 9.0%.
To compute the CAR, the difference between the waking cortisol level and the 30 min after waking cortisol level was calculated for each day ([wakeup +30 min cortisol] − [wakeup cortisol level]; Adam and Kumari, 2009). Daily diurnal slope was computed by taking the difference between waking cortisol level and bedtime cortisol level and dividing by the time between the samples ([bedtime cortisol] − [wakeup cortisol]/time between waking and bedtime sample). Finally, daily AUC (with respect to ground) was calculated using the trapezoid formula (Pruessner, Kirschbaum, Meinlschmid, and Hellhammer, 2003).
Perceived discrimination
Adolescents’ perceptions of discrimination were assessed during the in-home interview (before adolescents completed the diurnal cortisol protocol) using the Brief Perceived Ethnic Discrimination Questionnaire-Community Version (Brief PEDQ-CV; Brondolo et al., 2005). The original scale utilizes 17 items that assess experiences of ethnic discrimination within social or interpersonal contexts. In the original scale, each item begins with the phrase: “Because of your race or ethnicity … ” followed by a description of exposure in the following subscales: Exclusion/Rejection (3 items; e.g. “others ignored you or did not pay attention to you”), Stigmatization (4 items; e.g., “others hinted that you are dishonest or can’t be trusted”), Threat/Aggression (4 items; e.g., “others threatened to hurt you”), Discrimination at School (4 items; e.g., “been treated unfairly by teachers or other staff”) and Police attitudes (1 item; i.e., “policemen or security guards been unfair to you”). The scale was developed on Latino and African American adults and college students. To adapt for adolescents, a few alterations were made. First, the item “Has your boss been unfair to you?” was changed to “Other than at school, have adults been unfair to you?” An additional item was added to assess opinions of intelligence (i.e., “hinted that you are not very smart”). Finally, instead of beginning each item with the phrase “Because of your race or ethnicity” we added the phrase “because you are Mexican/Mexican American” to the end of each item. Participants were asked how often each of these experiences happened during the past year and responded using a Likert scale, ranging from 1 (never happened) to 5 (happened very often). The mean was calculated for the scale, with higher scores reflecting greater levels of perceived ethnic discrimination. The scale demonstrated good reliability for the current study (α = .93).
Day-level control variables
To account for the many daily behaviors known to be associated with diurnal cortisol, adolescents reported their daily consumption of caffeine, daily exercise, waking time, previous night’s hours of sleep, daily consumption of alcohol and cigarettes, oral contraceptives (females only), and/or other medication.
Individual-level control variables
To account for individual-level factors that have been found to relate to cortisol levels, the current study controlled for adolescent gender, family income, adolescents’ overall life stressors, acculturation level, and adolescents’ major depressive disorder (MDD) symptoms. For family income, mothers and fathers responded to an open ended question asking them their annual household income. Their responses were coded in $5,000 increments (e.g., 1 = < $5000, 2 = $5000–$10,000, etc.). For two parent families, the mean of mothers’ and fathers’ reports were used. For single parent families, only mothers’ reports were used. Adolescent overall stressors were examined using the Multicultural Events Scale for Adolescents (MESA; Gonzales, Tein, Sandler, and Friedman, 2001). The MESA is a life events scale used to assess stressors for adolescents that specifically fit the lifestyle and experiences of culturally diverse adolescents. Adolescents responded to items assessing family economic hassles (10 items; e.g, “Your parent lost a job”), peer conflict (14 items; e.g., “You had a disagreement or fight with a close friend.), family conflict (9 items; e.g., “You had a serious disagreement or fight with a parent”) and language hassles (7 items; e.g., “A teacher put you down for not speaking English or not speaking it well”). Adolescents responded to each item by indicating if the event happened or did not happen in the past three months. For the current study, a sum of all events experienced except the language hassles subscale, which might overlap with discriminatory experiences, were used. Adolescents’ acculturation was assessed using the Mexican American Cultural Values Scale (Knight, et al., 2010). The acculturation subscale utilizes 14 items to assess varying aspects of acculturation (i.e., self-reliance, material success, competition and personal achievement). For each item, adolescents are asked how much they believe in the statement and respond using a likert scale ranging from (1) not at all to (5) completely. Finally for MDD symptoms, parent and adolescents completed the Diagnostic Interview Schedule for Children (DISC-IV; Shaffer et al., 2000), a structured diagnostic instrument for use by nonclinicians, to assess indicators of mental health symptomatology. We scored the DISC using the combined DISC scoring algorithm (Shaffer et al., 2000).
Statistical Analysis
To examine the current study’s research questions, a series of multilevel model (MLM) regressions were conducted. MLM was utilized because of the nested structure of our data: daily cortisol samples were nested within individuals (Raudenbush & Bryk, 2002). This technique allows each daily cortisol value to be examined instead of taking an aggregate approach (e.g., taking the mean of values across the three days). Separate regressions were run for each cortisol parameter of interest (Equations presented below).
| Level 1 |
| Level 2 |
Before adding in predictors in the models, unconditional models were examined to understand the amount of variability in the cortisol parameter of interest across individuals. If the variability was significant we proceeded with our analyses by adding in day level control variables (i.e., caffeine, exercise, wake time, hours of sleep, and daily stress level) at level 1, and perceptions of discrimination along with individual level control variables (i.e., gender, life stressors, family income, MDD symptoms, and birth control) at level 2. Additional level 1 control variables were assessed (i.e., alcohol consumption, cigarette consumption, and medication) but were excluded from models because of the lack of variability. Only one participant reported drinking one alcohol beverage and only two adolescents reported smoking cigarettes. Less than 7% of adolescents reported medication use across the three days of sampling. For CAR analysis, the duration of time between sample 1 and sample 2 for each individual on each day was added in as an additional level 1 covariate. In line with recommendations, all level 1 and level 2 predictors were grand mean centered (Enders and Tofighi, 2007).
Results
Descriptive statistics and correlations among study variables are presented in Tables 1 and 2. Morning and evening cortisol values were highly skewed (2.46, 4.59, respectively) and kurtotic (11.42, 28.31, respectively). Consistent with prior literature (e.g., Adam et al., 2010; DeSantis et al., 2007), they were transformed using the natural log. Unconditional multi-level models for the cortisol parameters revealed significant variability across individuals on the CAR [τ00= .026 standard error ([SE] = .007), p < .001] and the AUC [τ00 = 2.74 [SE = .61], p < .001]. However, the variation across individuals was not significant for diurnal slopes [τ00 = .001 (SE = .001), p = .71] meaning we could not continue examining variation in diurnal slopes. Given that the diurnal slopes were computed using both waking levels of cortisol and bedtime levels of cortisol, we examined these two cortisol parameters separately to see if significant variability across individuals existed. Results revealed significant variability for waking cortisol [τ00= .25 (SE = .063), p < .001] and bedtime cortisol [τ00= .70 [SE = .14], p < .001]. We proceeded by treating the following cortisol parameters as our dependent variables: CAR, AUC, waking cortisol and bedtime cortisol.
Table 1.
Means, Standard Deviations, and Ranges for Study Variables
| Mean | SD | Range | |
|---|---|---|---|
| Discrimination | 1.22 | .36 | 1.00–02.67 |
| CAR (μg/dL) | .21 | .30 | −1.47–01.34 |
| Waking cortisol (μg/dL)* | .28 | .21 | .01–01.78 |
| Bedtime cortisol (μg/dL)* | .08 | .15 | .01–01.31 |
| Diurnal slope (μg/dL) | −.20 | .24 | −1.73–01.03 |
| Cortisol AUC (μg/dL) | 4.32 | 2.62 | .04–16.37 |
| Life stressors | 6.94 | 4.67 | .00–24.00 |
| Acculturation | 2.65 | .49 | 1.50–04.36 |
| Daily life stress | 1.83 | .91 | 1.00–04.00 |
| MDD symptoms | 3.32 | 2.96 | .00–15.00 |
Cortisol values indicated are raw scores: those used in MLM analyses were natural log transformed.
Table 2.
Correlation among study variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Discrimination | -- | |||||||||
| 2. | CAR | .13* | -- | ||||||||
| 3. | Waking cortisol | .08 | − .14* | -- | |||||||
| 4. | Bedtime cortisol | .10 | − .01 | .19** | -- | ||||||
| 5. | Diurnal slope | −.02 | .15* | − .64*** | .29*** | -- | |||||
| 6. | Cortisol AUC | .19** | .67*** | .40*** | .18** | −.28*** | -- | ||||
| 7. | Family income | .01 | .07 | .03 | .07 | − .03 | .07 | -- | |||
| 8. | Life stressors | .44*** | .13* | .03 | .07 | .01 | .11† | − .05 | -- | ||
| 9. | Acculturation | .16** | .15* | − .02 | .02 | .02 | .08 | .11† | .15* | -- | |
| 10. | Daily life stress | .20** | .12† | − .06 | − .04 | .01 | .07 | .02 | .20** | .09 | -- |
| 11. | MDD symptoms | .08 | .04 | − .12* | − .02 | .09 | −.03 | −.01 | .37*** | .15** | .28*** |
Note. All cortisol levels reflect μg/dL. Morning and evening cortisol values were log transformed. AUC = Area under the curve; CAR = Cortisol awakening response; MDD = major depressive disorder.
p < .10,
p < .05,
p < .01,
p < .001.
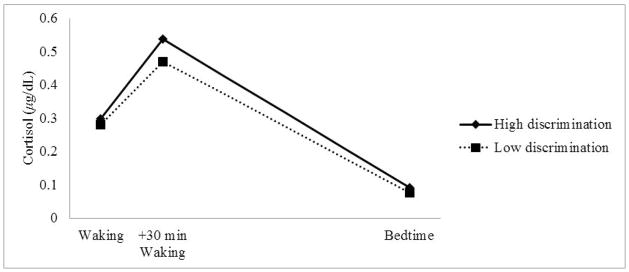
Next, discrimination and control variables were added into the regressions to predict individuals’ CAR, AUC, waking and evening cortisol levels (Table 3). For the CAR, discrimination emerged as a marginally significant predictor [B = .11 (Standard error (SE) = .06, p = .08]; for every 1 unit increase in discrimination, the CAR increased by 53 %), revealing that greater perceptions of discrimination were related to a steeper CAR (greater increase in cortisol from waking to 30 minutes after waking). For the AUC, both discrimination emerged as significant predictors, revealing that greater perceptions of discrimination were related to a greater AUC cortisol levels [B = 1.49 (SE = .61), p < .05]; every one unit increase in discrimination related to a 35% increase in the AUC). For waking and bedtime cortisol, discrimination was not significant. For descriptive purposes, Figure 1 presents adolescents’ diurnal cortisol profiles based on reported levels of perceived discrimination. As seen in the figure, adolescents who reported high discrimination (above the mean) have a greater jump in cortisol from waking to 30 minutes after waking (CAR) and greater overall cortisol levels compared to adolescents who reported low discrimination (below the mean).
Table 3.
Unstandardized Parameter Estimates (Standard Errors) for MLM Regressions Predicting Cortisol Parameters (N = 100)
| CAR | Cortisol AUC | Waking Cortisol | Bedtime Cortisol | |
|---|---|---|---|---|
| Intercept | 0.206 (.032)*** | 4.268 (0.185)*** | −1.560 (.066)*** | −3.284 (.100)*** |
| Discrimination (γ01) | 0.106 (.060)† | 1.493 (0.611)* | 0.201 (.221) | 0.427 (.331) |
| Gender (γ02) | 0.199 (.038)*** | 1.894 (0.405)*** | 0.064 (.145) | −0.198 (.219) |
| Control Variables | ||||
| Life stressors (γ03) | −0.001 (.005) | −0.023 (0.049) | 0.008 (.018) | 0.016 (.027) |
| Family income (γ04) | 0.001 (.003) | 0.007 (0.035) | 0.006 (.013) | 0.001 (.019) |
| MDD symptoms (γ05) | −0.008 (.007) | −0.068 (0.073) | −0.034 (.025) | −0.009 (.039) |
| Acculturation level (γ06) | 0.068 (.038)† | 0.352 (0.398) | 0.017 (.143) | 0.189 (.022) |
| Oral contraceptives(γ07) | −0.154 (.096) | −0.703 (1.025) | 0.368 (.360) | 0.881 (.553) |
| Daily caffeine use (β10) | 0.053 (.019)** | 0.255 (0.184) | −0.041 (.065) | 0.219 (.089)* |
| Daily hrs. of exercise (β20) | −0.007 (.019) | 0.067 (0.181) | 0.080 (.067) | 0.010 (.092) |
| Daily wake time (β30) | −0.042 (.019)* | −0.416 (0.184)* | 0.026 (.067) | 0.020 (.095) |
| Daily hours of sleep (β40) | −0.032 (.016)* | −0.284 (0.148)† | 0.045 (.055) | −0.021 (.076) |
| Daily stress level (β50) | −0.005 (.019) | −0.185 (0.177) | −0.014 (.064) | −0.076 (.087) |
| Variances | ||||
| Level 2 variance (τ00) | 0.013 (.005)** | 2.084 (0.528)*** | 0.245 (.075)** | 0.694 (.156)*** |
| Level 1 variance (σ2) | 0.043 (.005)*** | 3.152 (0.359)*** | 0.487 (.056)*** | 0.745 (.085)*** |
Note. AUC= Area under the curve; CAR = Cortisol awakening response. Gender coded 0 = male, 1 = female; Oral contraceptives coded 0 = no, 1 = yes. For CAR analysis, each individual’s time from Sample 1 to Sample 2 was added as an additional covariate. Waking cortisol and bedtime cortisol values were log transformed.
p ≤ .10,
p < .05,
p < .01,
p < .001. All cortisol levels reflect μg/dL.
Figure 1.
Observed cortisol levels for individuals with high (above the mean) and low levels (at or below the mean) of perceived discrimination (N = 100).
Discussion
The current study contributed to the extant literature by being the first empirical study to examine the relation between perceived discrimination and diurnal cortisol rhythms among adolescents. Although perceived discrimination has been consistently linked to physical and mental health outcomes cross-sectionally and longitudinally (Williams and Mohammed, 2009), the pathways and processes underlying the relation remain relatively unexplored. Numerous theories suggest that physiological processes mediate the link (e.g., Meyers, 2009; Pascoe and Richman, 2009); however, most empirical examinations have focused on cardiovascular reactivity, with little attention given to the stress response and the HPA axis in particular. Uncovering stress response processes underlying experiences of discrimination can start to provide researchers with a clearer understanding of the physiological mediators linking discriminatory experiences and health outcomes among ethnic and racial minorities in the U.S and abroad. The current study focused on Mexican American adolescents, one of the largest and fastest growing ethnic minority groups in the U.S. (U.S. Census, 2006), and examined the relation between aspects of diurnal cortisol and perceived discrimination. This examination appears especially relevant at a time when Mexican Americans perceive greater discrimination given the recent political focus on immigration in the U.S. (Lopez et al., 2010). Our findings suggest that there are indices of HPA axis functioning that are related to Mexican American adolescents’ perceptions of discrimination.
First, Mexican American youth who perceived greater discrimination had greater AUCs. The AUC is a measure of overall cortisol output across the sampling protocol, which for the current study was from wakeup to bedtime. These findings align with prior work that has demonstrated that increased levels of environmental stressors relate to greater overall cortisol output (e.g., Gustaffson et al., 2006). Further, the findings suggest that discrimination serves as a strong correlate for Mexican American adolescents; nearly a 35% increase in AUC was associated with a one unit increase in perceived discrimination. Our results suggest that discriminatory experiences may set in motion the HPA axis, eventually resulting in the release of cortisol. Repeated exposure to perceived discrimination could then lead to heightened levels of cortisol circulating in the body. Our findings align with prior literature that suggests that uncontrollable and socially evaluative stressors evoke a strong HPA axis response (Dickerson and Kemeny, 2004) consistent with numerous theories positing that discrimination is an important, potent stressor (Pachter and García Coll, 2010; Pascoe and Richman, 2009).
The current results also suggest that perceived discrimination was related to steeper CARs; however, these findings should be interpreted with caution given that they were only marginally significant (p = .08). Lack of significance could be due to the study’s lack in power, limited by the sample size and restricted range in discriminatory experiences. Despite this limitation, to place the current study’s findings in context, the CAR, defined as the increase in cortisol from waking to 30 minutes post-awakening, typically reflects a 50–60% change in cortisol levels (e.g., Kirschbaum and Hellhammer, 2000; Adam, 2006). For the current study, Mexican American youth demonstrated a similar increase (i.e., a 56% increase from waking to 30 minutes post-waking). Results revealed that the inclusion of discrimination further increased the CAR; for every one unit increase in discrimination, the CAR increased by nearly 53% above the average CAR of the study sample.
Our finding that greater perceptions of discrimination were related to greater/steeper CARs is consistent with prior work examining other environmental stressors (Schulz et al., 1998). Similar to the AUC, a steeper CAR could reflect an individuals’ overall greater activation of the HPA axis; however, it could also reflect an individuals’ preparation for such experience. Recent work has demonstrated that the CAR might be a unique component of the diurnal rhythm that is controlled by slightly different neurological processes (Clow, Hucklebridge, Stalder, Evans and Thorn, 2010). Further, the CAR might have an anticipatory mechanism activated by individuals’ preparing for an upcoming challenge (Fries, Dettenborn, and Kirschbaum, 2009). For instance, prior work has demonstrated that individuals facing social evaluative challenges the upcoming day, awake with a greater CAR than on days where challenges were not expected (Rohleder, Beulen, Chen, Wolf and Kirschbaum, 2007). Interestingly, this anticipatory reaction has been found in linking cardiovascular functioning and racial discrimination (Clark, Benkert, & Flack, 2006). Clark et al. refer to this process as racism-related vigilance and found that in African American males, racism-related vigilance predicted greater arterial elasticity, which in turn has implications for arterial and blood pressure. A similar argument could be made with the HPA axis; adolescents who anticipate experiences of discrimination could cope by exhibiting a boost in their morning cortisol levels as a way to prepare for the upcoming challenge. Over time, this preparation could take a toll on their HPA diurnal functioning resulting in a more lasting alteration of the CAR. Answering this question is beyond the scope of the current study; however, future research focused on linking day-to-day experiences of discrimination to day-today fluctuations in CAR could uncover the potential anticipatory function. Further, longitudinal work examining the HPA response to perceived discrimination over time could provide information into the long term alteration in the CAR.
While discrimination was related to individuals’ AUC and CAR, we found no relation to morning and evening levels of cortisol. Prior work among adults examining the link between chronic stressors and diurnal cortisol has often yielded evidence of blunted diurnal response (lower morning levels and higher evening levels; e.g., Miller et al., 2007; Michaud, Mathenson, Kelly, and Anisman, 2008). While unexpected, our lack of findings could have more to do with the developmental period under examination and the particular environmental stressor of interest. Theories of HPA axis dysregulation suggest that repeated exposure of chronic environmental stress have to potential of altering diurnal cortisol rhythms (Gunnar and Quevedo, 2007; McEwen, 2002). The time frame for when this happens, however, is not known. Indeed, studies among much younger age groups have found evidence of altered HPA axis functioning; however, these studies have usually examined serious childhood stressors that include maltreatment and chronic poverty (e.g., Gunnar, Morison, Chisholm and Schuder, 2001). As some have suggested, alterations in HPA axis functioning could differ based on the nature, duration, and characteristics of the environmental stressor (Chen and Peterson, 2006). Thus, given that adolescence is a time in which discriminatory experiences gain salience and relevance, it could be that we are uncovering only the initial effects of discrimination on the HPA axis (over-reactivity of the HPA axis as evidenced by greater AUCs). As individuals progress through adolescence and into adulthood and if their perceptions of discrimination remain constant, we might expect that continued over-reactivity of the HPA axis would then lead to changes in alterations in the diurnal rhythm, evidenced by changes in morning and evening levels. Longitudinal work focused on developmental changes in perceptions of discrimination and HPA axis functioning across the adolescent period into adulthood is needed to uncover such questions.
It should be noted that in the current sample significant variability across individuals was observed in overall cortisol levels, CAR, waking and bedtime. There was, however, little variability in adolescents’ diurnal slopes (or the rate of decline from waking to bedtime cortisol). Prior work among children and adolescents has demonstrated variability in diurnal slopes (e.g., DeSantis et al., 2007; Pendry and Adam, 2007), ruling out a developmental explanation. However, prior work has utilized non-Latino samples, or utilized multi-ethnic samples and compared Latino participants to individuals of other races/ethnicities. The current study utilized an ethnic homogenous design, with the interest of understanding diversity within the Mexican American adolescent population. Additionally, prior work predicting variability in slopes among older adolescents has tended to collect more than one evening sample (e.g., Adam et al., 2006; Doane and Adam, 2010) which could allow for more variability across the day. Our limited sampling protocol could be restricting the amount of variability in the decline of cortisol across the day.
Together, these results suggest that, indeed, perceptions of discrimination in Mexican American youth are associated with greater cortisol output above and beyond adolescents’ daily behaviors, economic conditions, other stressors, acculturation levels and depressive symptoms. The study provides an important first step in understanding the physiological mechanisms that may underlie youths’ experiences of discrimination and has the potential of propelling researchers into identifying the mechanisms underlying racial/ethnic health disparities in the U.S. These findings come as no surprise given that discrimination has long been theorized as a salient and impactful stressor for ethnic and racial minority youth living within the U.S (Pachter and García Coll, 2010).
Limitations and Directions for Future Research
Despite this study’s contributions, there are important limitations to consider. First, the current study utilized cross-sectional data, limiting our understanding of the direction of effects. Although theory suggests that environmental stressors activate the HPA axis and that chronic exposure can result in diurnal changes in cortisol secretion, it could also be that individuals with specific personality traits and/or childhood experiences share certain diurnal cortisol profiles that that relate to greater perceptions of discrimination. For example, prior work has found that individuals who experience severe adverse conditions in early life tend to have distinct diurnal cortisol rhythms that are related to psychopathology (Gunnar, Morison, Chisholm and Schuder, 2001). Other recent research has found that personality traits like neuroticism are associated with greater cortisol AUCs (Nater, Hoppmann, and Klumb, 2010) and indirectly associated with flatter cortisol slopes through levels of depressive symptoms (Doane et al., 2011). The current study did control for adolescents’ depressive symptoms, but did not account for early childhood trauma, stress or personality characteristics. Future longitudinal work uncovering how early life stress, personality, and perceptions of discrimination interact to influence diurnal cortisol could inform these questions.
Second, the study’s sample was limited in the representation of the Mexican American population. Compared to the larger longitudinal study from which the current sample was drawn (see Roosa et al., 2008), this sample contained Mexican American youth who were more likely to be born in the U.S., English speaking, higher income, and come from families where parents were more likely to be born in the U.S. These differences were likely restricting the current samples’ range in discriminatory experiences, cultural values, and acculturation levels. This is important given that restriction of range often has implications for power (e.g., Glass & Hopkins, 1996; Schmidt, Hunter, and Urry, 1976). Further, prior work examining the Mexican American population has found that cultural values and acculturation experiences are important when linking environment stressors and outcomes (Mangold, Wand, Javors and Mintz, 2010). Future studies focused on a more representative sample have the ability to examine the intersection of cultural values, discriminatory experiences, and diurnal cortisol in Mexican American youth.
Finally, the study’s protocol instructed adolescents to follow a strict schedule for salivary samples. To assist adolescents, nightly phone calls were made to remind participants of the protocol and a preset 30 minute timer was provided to assist with the timing of the 2nd sample. Further, in our analyses with the CAR, which are particularly sensitive to timing, we included each individual’s duration of time between their first and second sample as a covariate. However, electronic monitoring devices to track the exact timing of cortisol samples were not used. Future studies with compliance checks could contribute to greater power in detection of the relation between discrimination and cortisol.
Despite these limitations, the current study takes an important first step in understanding the physiological stress response in adolescents in general and, particularly, that underlying experiences of discrimination in Mexican American youth. These findings are important given the growth of the Mexican American population, the salience of discriminatory experiences to this population, and the health disparities experienced by this population. Further, our study provides a starting point for researchers interested in further investigating the physiological mechanisms underlying perceptions of discrimination and larger health disparities.
Acknowledgments
Work on this paper was supported, in part, by NIMH grants R01-MH68920, Arizona State University’s School of Social and Family Dynamics’ Cowden Fellowship and Graduate Student Association’s Graduate Dissertation Award. We gratefully acknowledge participating families, interviewers, and other project staff for their contributions to this project.
Footnotes
The first 28 participants were given prepaid envelopes to ship the samples back to the project and 23 participants complied. To increase completion rates, we changed the protocol for the remainder of participants (n = 85) by having study personnel pick up samples from participants’ homes.
References
- Adam EK. Transactions among adolescents’ trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendo. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depression disorder from cortisol awakening responses in adolescence. Psychoneuroendo. 2010;6:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendo 2009. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Dev Psychopathol. 2008;20:745–774. doi: 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkel C, Knight GP, Zeiders KH, Tein J, Roosa MW, Gonzales NA, Saenz D. Discrimination and adjustment for Mexican American adolescents: A prospective examination of the benefits of culturally related values. J Res Adolescence. 2010;20:893–915. doi: 10.1111/j.1532-7795.2010.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondolo E, Kelly KP, Coakley V, Gordon T, Thompson S, Levy E, Cassells A, Tobin JN, Sweeney M, Contrada RJ. The perceived ethnic discrimination questionnaire: Development and preliminary validation of community version. J App Soc Psychol. 2005;35:335–365. [Google Scholar]
- Brondolo E, Libby DJ, Denton E, Thompson S, Beatty DL, Schwartz J, et al. Racism and ambulatory blood pressure in a community sample. Psychosom Med. 2008;70:49–56. doi: 10.1097/PSY.0b013e31815ff3bd. [DOI] [PubMed] [Google Scholar]
- Brown CS, Bigler RS. Children’s perceptions of discrimination: A developmental model. Child Dev. 2005;76:533–553. doi: 10.1111/j.1467-8624.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Peterson LQ. Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psych. 2006;25:704–714. doi: 10.1037/0278-6133.25.6.704. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cicchetti D, Gunnar MR. Integrating biological measures into the design and evaluation of prevention interventions. Dev Psychopatho. 2008:737–743. doi: 10.1017/S0954579408000357. [DOI] [PubMed] [Google Scholar]
- Clark R. Perceived racism and vascular reactivity in Black college women: Moderating effects of seeking social support. Health Psychol. 2006;25:20–25. doi: 10.1037/0278-6133.25.1.20. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans. Am Psychol. 1999;54:805–816. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- Clark R, Benkert RA, Flack JM. Large arterial elasticity varies as a function of gender and racism-related vigilance in Black youth. Journal of Adolescent Health. 2006;39:562–569. doi: 10.1016/j.jadohealth.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendo. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci Biobehav R. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Cohen S, et al. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in young adults (CARDIA) study. Psychosomatic Medicine. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Derijk R. Signaling pathways in brain involved in predisposition and pathogenesis of stress-related disease: Genetic and kinetic factors affecting the MR/GR balance. Ann NY Acad Sci. 2004;1032:14–34. doi: 10.1196/annals.1314.003. [DOI] [PubMed] [Google Scholar]
- Delgado MY, Updegraff KA, Roosa MW, Umaña-Taylor AJ. Discrimination and Mexican-origin adolescents’ adjustment: The moderating roles of adolescents’, mothers’, and fathers’ cultural orientation and values. J Youth Adolescence. 2009 doi: 10.1007/s10964-009-9467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms, in community sample of adolescents. J Adolescent Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Do DP, Diez Roux AV, Hajat A, Auchincloss AH, Merkin SS, Ranjit N, Shea S, Seeman T. Circadian rhythm of cortisol an neighborhood characteristics in a population-based sample: The Multi-Ethnic Study of Atherosclerosis. Health & Place. 2011;17:625–632. doi: 10.1016/j.healthplace.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Adam EK. Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendo. 2010;35:430–441. doi: 10.1016/j.psyneuen.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Franz C, Prom-Wormley E, Eaves L, Hellhammer D, Lupien S, Lyons M, Mendoza S, Xian H, Kremen WS, Jacobson K. Negative emotionality, depressive symptoms and cortisol diurnal rhythms: Analysis of a community sample of middle-aged males. Hormones and Behavior. 2011;60:202–209. doi: 10.1016/j.yhbeh.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol–biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychol Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Fisher CB, Wallace SA, Fenton RE. Discrimination distress during adolescence. J Youth Adolescence. 2000;29:679–695. [Google Scholar]
- Flores E, Tschann JM, Dimas JM, Pasch LA, de Groat CL. Percieved racial/ethnic discrimination, posttraumatic stress symptoms, and health risk behaviors among Mexican American adolescents. Journal of Counseling Psychology. 2010;57:264–273. doi: 10.1037/a0020026. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response CAR: Facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Doan SN, Eccles JS. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendo. 2012;37:107–118. doi: 10.1016/j.psyneuen.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher RV, Jones MD, Dahl A. Concurrent and longitudinal effects of ethnic identity and experiences of discrimination on psychosocial adjustment in Navajo adolescents. Dev Psychol. 2011;47:509–526. doi: 10.1037/a0021061. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. Amercian J of Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass GV, Hopkins KD. Statistical methods in education and psychology. 3. Boston: Allyn and Bacon; 1996. pp. 121–123. [Google Scholar]
- Gonzales NA, Tein JY, Sandler IN, Friedman RJ. On the limits of coping. J Adolescent Res. 2001;16:372–395. [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Gustafsson PA, Nelson N. Cortisol levels and psychosocial factors in preadolescent children. Stress Health. 2006;22:3–9. [Google Scholar]
- Hasting PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, Derose L, Kendziora KT, Usher BA, Zahn-Waxler C. Allostasis and the development of internalizing and externalizing problems: Changing relations with physiological systems across adolescence. Develop and Psychopath. 2011;23:1149–1165. doi: 10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitryof stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Johnson Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav R. 1992;16:115–130. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Kaholokula JK, Grandinetti A, Keller S, Nacapoy AH, Kingi TK, Mau MK. Associations between perceived racism and phsyciological stress indices in Native Hawaiians. J Behav Med. 2011 doi: 10.1007/s10865-011-9330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pruessner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Helhammer D. Salivary cortisol. In: Fink G, editor. Encyclopedia of stress. Vol. 3. San Diego, CA: Academic Press; 2000. pp. 379–383. [Google Scholar]
- Knight GK, Gonzales NA, Saenz DS, Bonds DD, Germán M, Deardorff J, Roosa MW, Updegraff KA. The Mexican American Cultural values scale for adolescents and adults. J or Early Adolesc. 2010;30:444–481. doi: 10.1177/0272431609338178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MH, Morin R, Taylor P. Illegal immigration backlash worries, divides Latinos. Pew Hispanic Center; 2010. http://pewhispanic.org/files/reports/128.pdf. [Google Scholar]
- Mangold D, Wand G, Javors M, Mintz J. Acculturation, childhood trauma and the cortisol awakening response in Mexican-American adults. Horm Behav. 2010;58:637–646. doi: 10.1016/j.yhbeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New Engl J Med. 1998:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: Allostasis, allostatic load, and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Meyers HF. Ethnicity-and socio-economic status-related stresses in context: An integrative review and conceptual model. J Behav Med. 2009;32:9–19. doi: 10.1007/s10865-008-9181-4. [DOI] [PubMed] [Google Scholar]
- Michaud K, Matheson K, Kelly O, Anisman H. Impact of stressors in a natural context on release of cortisol in healthy adult humans: A meta-analysis. Stress. 2008;11:177–197. doi: 10.1080/10253890701727874. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psycholog Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Nater UM, Hoppmann C, Klumb PL. Neuroticism and conscientiousness are associated with cortisol diurnal profiles in adults-Role of positive and negative affect. Psychoneuroendo. 2010;35:1573–1577. doi: 10.1016/j.psyneuen.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Pachter LM, García Coll C. Racism and child health: A review of the literature and future directions. J Dev Behav Pediatr. 2010;30:255–263. doi: 10.1097/DBP.0b013e3181a7ed5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe EA, Richman LS. Perceived discrimination and health: A meta-analytic review. Psycholog Bull. 2009;135:531–554. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendry P, Adam EK. Associations between parents’ mariatal functioning, maternal parenting quality, maternal emotion, and child cortisol levels. Intern J of Behav Develop. 2007;31:218–231. [Google Scholar]
- Pérez DJ, Fortuna L, Alegría M. Prevalence and correlates of everyday discrimination among US Latinos. J Community Psychol. 2008;36:421–433. doi: 10.1002/jcop.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, et al. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and coritsol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom Med. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendo. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage publications; 2002. [Google Scholar]
- Rohleder N, Beulen SE, Chen E, Wolf JM, Kirschbaum C. Stress on the dance floor: the cortisol stress response to social-evaluative threat in competitive ballroom dancing. Pers Soc Psychol B. 2007;33:69–84. doi: 10.1177/0146167206293986. [DOI] [PubMed] [Google Scholar]
- Roosa MW, Liu F, Torres M, Gonzales N, Knight G, Saenz D. Sampling and recruitment in studies of cultural influences on adjustment: A case study with Mexican Americans. J Fam Psychol. 2008;22:293–302. doi: 10.1037/0893-3200.22.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FL, Hunter JE, Urry VW. Statistical power in criterion-related validation studies. J of App Psych. 1976;61:473–485. [Google Scholar]
- Schulz P, Kirshbaum C, Prubner J, Hellhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Medicine. 1998;14:91–97. [Google Scholar]
- Selman RL. Social-cognitive understanding: A guide to educational and clinical practice. In: Lickonia T, editor. Moral development and behavior: Theory, research, and social issues. New York: Holt, Rinehart, Winston; 1976. pp. 299–316. [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV NIMH DISC-IV: description, differences from previous versions, and reliability of some common diagnoses. J Am Child Adolesc Psychiatry. 2000;3991:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Murray V, McLoyd V, Lin K, Cutrona C, Conger RD. Discrimination, crime, ethnic identity, and parenting as correlates of depressive symptoms among African American children: A multilevel analysis. Dev Psychopathol. 2002;14:371–393. doi: 10.1017/s0954579402002109. [DOI] [PubMed] [Google Scholar]
- Steffen PR, McNeilly M, Anderson N, Sherwood A. Effects of perceived racism and anger inhibition on ambulatory blood pressure in African Americans. Psychosom Med. 2003;65:746–750. doi: 10.1097/01.psy.0000079380.95903.78. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Annual Social and Economic Supplement. Washington, DC: U.S. Department of Commerce; 2006. Current population survey. [Google Scholar]
- Williams DR, Mohammed SA. Discrimination and racial disparities in health: Evidence and needed research. J Behav Med. 2009;32:20–57. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]