Abstract
The discovery of apolipoprotein A-V (apoA-V) in 2001 has raised a number of intriguing questions about role in lipid transport and triglyceride (TG) homeostasis. Genome wide association studies (GWAS) have consistently identified APOA5 as a contributor to plasma TG levels. Single nucleotide polymorphisms (SNP) with-in the APOA5 gene locus have been shown to correlate with elevated plasma TG. Furthermore, transgenic and knockout mouse models support the view that apoA-V plays a critical role in maintenance of plasma TG levels. The present review describes recent concepts pertaining to apoA-V SNP analysis and their association with elevated plasma TG. The interaction of apoA-V with glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1) is discussed relative to its postulated role in TG-rich lipoprotein catabolism. The potential role of intracellular apoA-V in regulation of TG homeostasis, as a function of its ability to associate with cytosolic lipid droplets, is reviewed. While some answers are emerging, numerous mysteries remain with regard to this low abundance, yet potent, modulator of TG homeostasis. Given the strong correlation between elevated plasma TG and heart disease, there is great scientific and public interest in deciphering the numerous biological riddles presented by apoA-V. This article is part of a Special Issue entitled Triglyceride Metabolism and Disease.
Keywords: Apolipoprotein A-V, Triglyceride, Lipid droplet, Genome wide association study, Glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1, Nonalcoholic fatty liver disease
1. Introduction
Elevated triglyceride (TG) is strongly and positively correlated with increased risk of cardiovascular disease. Furthermore, hypertriglyceridemia (HTG) is associated with enhanced risk of metabolic syndrome and accompanying insulin resistance. Apolipoprotein (apo) A-V, a minor plasma apolipoprotein (100–250 ng/ml) [1], has been documented to play a major role in TG metabolism by enhancement of VLDL lipolysis and clearance. Studies with human APOA5 transgenic and apoa5 knockout mice revealed that apoA-V concentration is inversely associated with plasma TG levels. Transgenic mice overexpressing human apoA-V [2] or adenoviral vector-mediated gene transfer of apoa5 into mice [3] revealed a 60–70% decline in plasma TG, whereas apoa5 knockout mice manifest 4-fold higher plasma TG than control littermates [2]. These studies make a strong case that apoA-V plays an important role in regulating plasma TG levels.
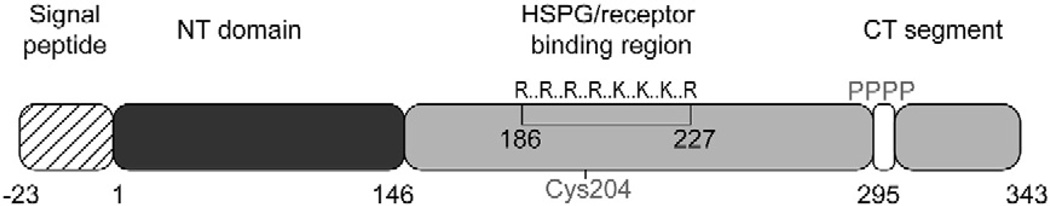
In humans, the APOA5 gene locus forms part of the APOA1/C3/A4/A5 gene cluster on chromosome 11q23. The sole site of apoA-V synthesis is the liver, where it is translated as a 366 amino acid preprotein. Following intracellular cleavage of a 23 amino acid signal peptide, mature apoA-V (343 amino acids) appears in plasma in association with VLDL and HDL. Despite considerable effort, the precise mechanism whereby apoA-V influences TG metabolism is not completely understood. Recent studies have demonstrated both intracellular, as well as extracellular, effects of apoA-V on TG metabolism [4]. The present review reports on recent genomic and functional studies of apoA-V that provide insight into the physiology of this unique apolipoprotein.
2. Genomic studies
Plasma TG is an example of a complex polygenic trait. In addition to environmental factors, gene variation is a major determinant of TG levels in individuals. Knowledge of the contribution of genetic variation to plasma TG has increased dramatically through Genome Wide Association Studies (GWAS), an approach that tests for associations between single nucleotide polymorphisms (SNPs) and specific traits. While GWAS provide an association, this method alone is not sufficient to define mechanism. Studies that have examined TG-associated loci in GWAS of population-based TG concentrations [5–8] provide compelling data linking apoA-V to TG metabolism. GWAS that identified loci harboring common genetic variants associated with TG levels have invariably identified apoA-V as a prominent player. As such, there is growing support for the notion that this minor protein exerts major effects on TG metabolism.
Common SNPs in the APOA5 locus have been identified in human population studies. Patients with severe HTG manifest an increase in allelic frequency of certain SNPs compared to random controls [9]. A summary of common APOA5 SNPs is presented in Table 1. These SNPs may be coding or noncoding and are located throughout the apoA-V gene and promoter region. The − 1131T>C SNP, located in the promoter region, is associated with HTG and its importance in TG modulation is well known [2,10]. The − 3A>G SNP, located in the Kozak sequence of APOA5, is postulated to reduce apoA-V expression by affecting translation initiation efficiency [11]. The coding SNP, c.56C>G, results in a substitution of the serine at position 19 with tryptophan (S19W) in the apoA-V signal peptide. This polymorphism has been shown to be functionally significant since this substitution reduces apoA-V translocation efficiency to the endoplasmic reticulum and hence, apoA-V secretion, in vitro [12]. Another coding SNP, c.553G>T, that is uniquely associated with Asian subjects, has been reported [13–15]. Carriers of this SNP have elevated plasma TG whereas homozygotes manifest HTG. The c.553G>T SNP introduces a cysteine for glycine substitution at position 162 in mature apoA-V. Introduction of a cysteine at this site raises the possibility that an intramolecular disulfide bond forms between the substituted cysteine and cysteine 204, the sole cysteine in wild type apoA-V (Fig. 1). Formation of a disulfide bond between these residues would be expected to alter the conformation of this region of the protein. Cysteine 204 is located within a positively charged sequence element in apoA-V that is involved in heparin binding [16] and cell surface receptor interactions [17,18]. An altered conformation in this key region of the apoA-V sequence may adversely affect TG metabolism by reducing lipolysis and/or VLDL remnant clearance. Clarification of the functionality of this SNP awaits future studies with engineered mouse models expressing this variant.
Table 1.
Frequency of single nucleotide polymorphisms (SNP) in apoA5.
| SNP | Allelic frequency |
Population group |
Plasma TGa |
Location/defect | Reference |
|---|---|---|---|---|---|
| −1131T>C | 0.12 | African American |
++ | Promoter region; defective transcription |
[10] |
| −3A>G | 0.15 | African American |
++ | Kozak sequence; defective translation |
[11] |
| c.56C>G | 0.07 | Caucasian | ++ | Signal sequence; defective secretion |
[12] |
| c.553G>T | 0.15 | Chinese American |
++ | Amino acid 162; putative disulfide bond formation |
[15] |
Relative level of plasma TG versus wild type apoA-V.
Fig. 1.
Diagram of apoA-V structural features. The 23 amino acid signal peptide (hatched box) is cleaved to give rise to mature apoA-V. The N-terminal helix bundle domain is shown in black while the C-terminal domain, containing a positively charged sequence element (single letter code is used), a tetraproline motif and terminal peptide, are in gray. The position of the lone cysteine in apoA-V is shown. HSPG; heparin sulfate proteoglycan.
3. Glycosylphosphatidylinositol-anchored high density lipoprotein binding protein 1 (GPIHBP1) and apoA-V
GPIHBP1, a unique glycosylphosphatidylinositol-anchored glycoprotein, is required for lipolytic processing of TG-rich lipoproteins. Indeed, mice lacking this protein have extremely high plasma TG. ApoA-V has been shown to bind GPIHBP1 in vitro and this interaction has been postulated to facilitate lipoprotein lipase (LPL) mediated hydrolysis of the TG component of chylomicrons (CM). The positively charged heparin-binding sequence within apoA-V (Fig. 1) and the acidic domain in GPIHBP1 are both required for binding [19]. These authors showed that a mutant apoA-V whose positively charged sequence element has been disrupted is unable to bind GPIHBP1. It has been proposed that GPIHBP1 dimerizes in such a manner that it presents a highly negatively charged surface capable of recognizing LPL and apoA-V, promoting efficient lipolytic processing of CM. Thus, it may be that GPIHBP1 facilitates interaction between LPL and apoA-V associated with the CM surface. Beigneux et al. [18] suggested that the GPI-anchored complexes might cluster in subdomains of the plasma membrane of endothelial cells, thereby bringing LPL into contact with CM. Our laboratory has recently shown that intravenous injection of apoA-V into apoa5 knockout mice induces an ~60% decrease in plasma TG within 4 h [20]. Similar studies carried out in gpihbp1 (−/−) mice showed that apoA-V injection fails to reduce plasma TG levels, suggesting that coordination between GPIHBP1, LPL and apoA-V is a prerequisite for efficient hydrolysis of plasma TG. Interestingly, apoA-V is cleared over time following injection into apoa5 −/− mice but this is not the case with gpihbp1 −/− mice. These data suggest that turnover of apoA-V forms part of a GPIHBP1-LPL-apoA-V axis. Severely elevated plasma TG and reduced clearance of apoA-V in GPIHBP1 deficiency are not specific to this metabolic aberration. Vaessen et al. [21] examined plasma TG and apoA-V levels in lpl −/− mice and noted a parallel elevation of TG and apoA-V. LPL gene transfer into LPL deficient mice resulted in a simultaneous decrease in TG and apoA-V. Thus, hydrolysis of TG-rich lipoproteins is necessary for normal clearance of apoA-V transported on CM and VLDL. While loss of apoA-V is likely to be offset by replenishment from a pool of newly secreted apoA-V, this supposition requires experimental verification. In this case, apoA-V sequestration in intracellular lipid droplets (see below), as opposed to its release into plasma, may serve to modulate LPL-dependent hydrolysis of TG-rich lipoproteins.
4. ApoA-V as a potential regulator of TG secretion via lipid droplet interaction
Early studies on apoA-V established its presence, albeit minor, in the plasma compartment and its critical role in lipolysis of TG-rich lipoproteins and clearance of their remnants. These physiological properties apparently relate to the ability of apoA-V to facilitate LPL activity and promote remnant particle clearance via binding to members of the low-density lipoprotein receptor family. In addition to its function(s) to modulate TG in plasma, apoA-V also appears to regulate TG secretion from liver. Early studies in mice exploring possible links between apoA-V and TG secretion from liver revealed that adenovirus-mediated gene transfer-induced overexpression of apoA-V lowers VLDL TG secretion but not apoB secretion [22] resulting in the formation of smaller VLDL particles without changing the number of apoB-containing particles. Current evidence suggests that apoA-V does not function to regulate apoB-100 production. By the same token, apoA-V may influence TG flux in hepatocytes such that lipid accrual by nascent VLDL is reduced.
Recent efforts have focused on understanding how apoA-V may regulate intracellular TG and, subsequently, influence plasma TG levels. Transient overexpression of apoA-V in Hep3B cells had no effect on apoB-100 particle secretion or lipidation state [23], consistent with the results of adenovirus-mediated overexpression of apoA-V in mice [22,24]. On the other hand, apoA-V was found in association with intracellular lipid droplets (LD) following transient transfection into Hep3B and McA-RH7777 cell lines [23,25]. Binding to LD requires the carboxy-terminal region of apoA-V (Fig. 1) [25]. Furthermore, expression of signal peptide-deficient apoA-V induced major morphological changes wherein cytoplasmic LD were replaced by amorphous lipid-staining entities. These studies support the view that intracellular apoA-V plays a role in LD assembly and/or stability as well as in storage and secretion of hepatocyte TG. Toward this end, it is interesting that apoA-V mRNA is elevated in hepatectomized rats [3] coincident with increased intracellular lipid accumulation [26,27]. One might argue that overexpression of apoA-V in hepatocytes is non-physiological and leads to aberrant trafficking of the protein to LD rather than to a lipid pool for export in the form of VLDL. Studies on LD isolated from livers of wild type mice and APOA5 transgenic mice have helped to clarify this issue [28]. Even in wild type mice, where apoA-V levels are extremely low, intracellular apoA-V is associated with LD, providing strong evidence that apoA-V plays a role in regulating intracellular TG storage and/or mobilization. TG accumulation in the liver is greater in human apoA-V transgenic mice that express higher levels of apoA-V than livers from wild type mice. Whether increased levels of intracellular apoA-V affects LD formation, stability or residence time remains to be determined. However, the observation of Pamir et al. [29] that APOA5 transgenic mice fed a diet high in fat and sucrose secrete lower amounts of TG is consistent with high apoA-V expression fostering cellular TG accumulation.
The seminal studies of Shu et al. [23,25] in cultured hepatocarcinoma cells transiently transfected with human APOA5 showed that a large proportion of apoA-V (>50%) is retained within these cells in association with LD. Recently Blade et al. [30] examined the effect of oleic acid supplementation on apoA-V and TG synthesis and secretion in McA-RH7777 cells stably transfected with APOA5. These authors observed that intracellular apoA-V content was increased upon oleate supplementation, along with cellular TG in LD. Experiments with a doxycycline (Dox) inducible promoter strengthened these observations by demonstrating that induction of apoA-V expression increased cellular TG and decreased TG secretion in a concentration dependent manner. Dynamic light scattering studies on VLDL1 and VLDL2 fractions isolated from media before and after Dox treatment indicated that VLDL1 particles are substantially decreased in size (~40%). Thus, it is plausible that a reduction in VLDL maturation is responsible for the decreased TG secretion observed in this system. Overall, studies employing in vitro cell culture models and vivo mouse studies provide compelling evidence that apoA-V association with LD is fundamentally involved in the regulation of TG secretion.
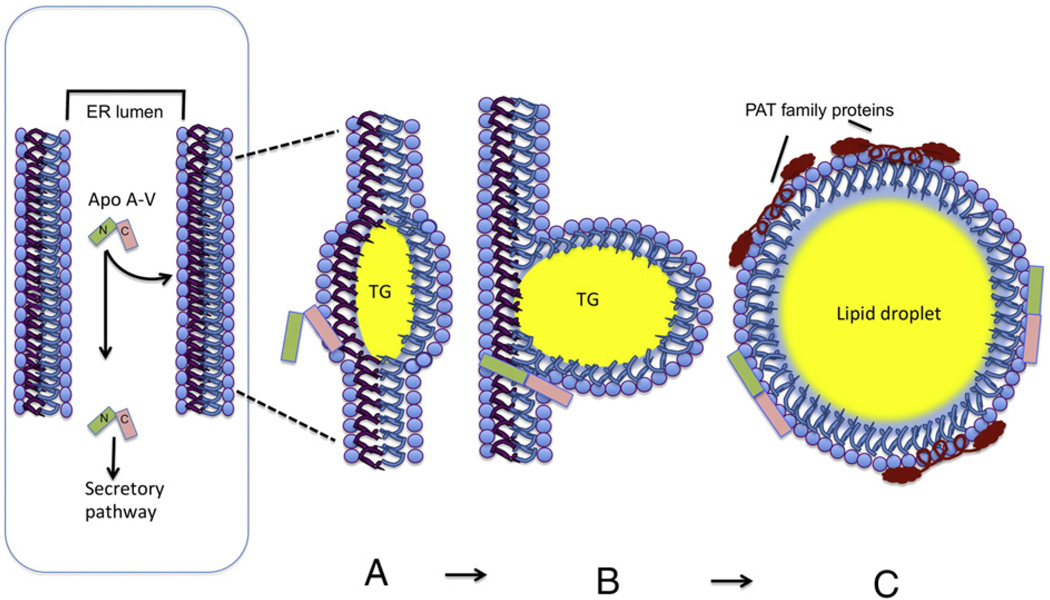
A salient question that emerges, however, is, “how does apoA-V associate with cytoplasmic lipid droplets since it is synthesized with an N-terminal signal peptide”? Such sequences are considered to predestine newly synthesized protein for export from the cell. The answer may come from a combination of the strong lipid surface seeking properties of apoA-V together with the spatial location LD assembly. LD are dynamic organelles that store excess neutral lipids for utilization when required. Nascent LD form between leaflets of the endoplasmic reticulum (ER) bilayer membrane [31,32]. As the primordial droplet increases in size it bulges in the direction of the cytosol with retention of the phospholipid complement from the outer leaflet of the ER membrane. Following cleavage of its signal peptide, given its intrinsic hydrophobicity, apoA-V may localize to the lumenal leaflet of the ER membrane, specifically binding to defects created by the nascent LD assembly process. ApoA-V may then translocate to the opposite bilayer leaflet and form a stable association with the budding LD, an interaction that is retained following “release” of the intact mature organelle (Fig. 2). Such an association may be facilitated by the lipid-binding, carboxy terminus of apoA-V [33], since it has been shown that deletion of this region decreases the protein's lipid binding affinity [25]. The net effect of this process is diversion some fraction of newly synthesized apoA-V from the ER–Golgi secretory pathway. Once in the cytoplasm, LD will assimilate other known LD associated proteins, such as the PAT family proteins (perilipin, adipophilin and TIP47). The latter protein has structural similarities to apoE, suggesting a common mode of lipid interaction [34]. While apoA-V is the only known hepatocyte protein to exist on LD and plasma lipoproteins, it is not unique in its apparent retrograde trafficking to LD. Studies with apoO, which also possesses a signal peptide, have shown that it traffics to LD in cardiomyocytes [35]. ApoA-V trafficking in the hepatocyte must, therefore, strike a balance between secretion and retention and how this is regulated remains a mystery. Furthermore, there is a lack of information on the nascent lipoprotein particle that transports apoA-V into the plasma. The work of Shu et al. [23] suggests that association of apoA-V with apoB-containing, TG-rich lipoproteins, is a post-secretory event, begging the question, “in what form does apoA-V exit the hepatocyte”?
Fig. 2.
Model of apoA-V's role in hepatic lipid droplet assembly. (Left) Newly synthesized apoA-V is translocated to the endoplasmic reticulum (ER) lumen with concomitant cleavage of its 23 amino acid signal peptide (N refers to the N-terminal helix bundle domain and C refers to the C-terminal domain; see Fig. 1). In the ER lumen, apoA-V has two possible fates, passage down the secretory pathway and release into the plasma or association with cytosolic lipid droplets. This latter process (see membrane expanded panels on the right) requires at least 3 steps including: A) adherence of apoA-V to ER membrane defects arising from nascent LD assembly; B) translocation of apoA-V to the cytosolic side of the ER membrane, most likely while maintaining contact with the protruding TG droplet and C) stable association of apoA-V with the surface monolayer of discrete LD, which accrue additional protein components, such as perilipin, adipophilin and TIP47 (collectively referred to as PAT family proteins).
5. ApoA-V and non-alcoholic fatty liver disease (NAFLD)
The term non-alcoholic fatty liver disease (NAFLD) refers to a spectrum of liver diseases ranging from simple steatosis (accumulation of hepatic intracellular lipids), to the more serious non-alcoholic steatohepatitis (NASH), a condition that is accompanied by inflammation and, finally, cirrhosis, liver failure and hepatocellular carcinoma [36–39]. NAFLD is closely associated with obesity, dyslipidemia and insulin resistance, hallmarks of the metabolic syndrome. Importantly, accumulation of TG-rich LD in hepatocytes is also a manifestation of metabolic syndrome. NAFLD is a major disease affecting up to 30% of the population, not unlike the incidence of obesity and Type 2 diabetes. Recent studies have shown that there is a link between NAFLD and apoA-V in obese subjects [39]. Prior to bariatric surgery, liver TG and apoA-V expression were elevated whereas, post surgery, hepatic steatosis improved and apoA-V expression was significantly reduced. In addition, Ress at al. [40] transfected human HepG2 cells with a small interfering RNA and noted that apoA-V knockdown was associated with both decreased apoA-V mRNA and lower intracellular TG accumulation. These observations are consistent with previous studies in APOA5 transgenic mice wherein overexpression of apoA-V was associated with increased liver TG [28]. On the other hand, wild type and apoA-V deficient mice have reduced TG. Based on these studies, it may be hypothesized that apoA-V is a player in the development of NAFLD via its effects on intrahepatic lipid metabolism. A conundrum that emerges is that adenovirus-mediated gene transfer of apoA-V in mice reduces secreted TG, an effect that should be beneficial since elevated plasma TG is associated with increased risk for atherosclerosis. However, if intracellular apoA-V drives increased LD assembly in lieu of TG secretion, lipotoxicity and NASH could ensue. At this juncture it is not clear whether apoA-V mediated LD retention is causal for NAFLD. Clearly, we are in the early stages of understanding the functional role of apoA-V in NAFLD. Further studies need to be carried out to determine whether apoA-V directly contributes to NAFLD or is merely a marker for increased TG accumulation in the form of LD.
6. Concluding remarks
The involvement of apoA-V in TG metabolism is slowly evolving but puzzles remain with respect to its primary site of action, intracellular versus extracellular. For a protein whose presence in the plasma is miniscule, it exerts significant effects on TG homeostasis. Whereas previous investigations have focused largely on extracellular events, it is now apparent that the focus is shifting to its intracellular role, since apoA-V may play a role in NAFLD. Such a role is not inconsistent with apoA-V's ability to bind cytoplasmic LD. Studies designed to assess the contribution of apoA-V to lipotoxicity in the liver is a fertile area for future exploration.
Acknowledgements
The authors thank Jennifer Beckstead for assistance with preparation of the Figures.
Footnotes
Work from the authors' laboratory was supported by grants HL-64159 and HL-73061 from the National Institutes of Health.
This article is part of a Special Issue entitled Triglyceride Metabolism and Disease.
References
- 1.O'Brien PJ, Alborn WE, Sloan JH, Ulmer M, Boodhoo A, Knierman MD, Schultze AE, Konrad RJ. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin. Chem. 2005;51:351–359. doi: 10.1373/clinchem.2004.040824. [DOI] [PubMed] [Google Scholar]
- 2.Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 3.Van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J. Biol. Chem. 2001;276:44512–44520. doi: 10.1074/jbc.M106888200. [DOI] [PubMed] [Google Scholar]
- 4.Forte TM, Shu X, Ryan RO. The ins (cell) and outs (plasma) of apolipoprotein A-V. J. Lipid Res. 2009;50 Suppl:S150–S155. doi: 10.1194/jlr.R800050-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen CT, Hegele RA. Genetic bases of hypertriglyceridemic phenotypes. Curr. Opin. Lipidol. 2011 Apr 23; doi: 10.1097/MOL.0b013e3283471972. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Johansen CT, Kathiresan S, Hegele RA. Genetic determinants of plasma triglycerides. J. Lipid Res. 2011;52:189–206. doi: 10.1194/jlr.R009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical, and population relevance of 95 loci mapped for serum lipid concentrations. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen CT, Wang J, Lanktree MB, Cao H, McIntyre AD, Ban MR, Martins RA, Kennedy BA, Hassell RG, Visser ME, Schwartz SM, Voight BF, Elosua R, Salomaa V, O'Donnell CJ, Dallinga-Thie GM, Anand SS, Yusuf S, Huff MW, Kathiresan S, Hegele RA. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat. Genet. 2010;42:684–687. doi: 10.1038/ng.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henneman P, Schaap FG, Havekes LM, Rensen PCN, Frants RR, van Tol A, Hattori H, Smelt AHM, van Dijk KW. Plasma apoAV levels are markedly elevated in severe hypertriglyceridemia and positively correlated with the APOA5 S19W polymorphism. Atherosclerosis. 2007;193:129–134. doi: 10.1016/j.atherosclerosis.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Pennacchio LA, Olivier M, Hubacek JA, Krauss RM, Rubin EM, Cohen JC. Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum. Mol. Genet. 2002;11:3031–3038. doi: 10.1093/hmg/11.24.3031. [DOI] [PubMed] [Google Scholar]
- 11.Palmen J, Smith AJ, Dorfmeister B, Putt W, Humphries SE, Talmud PJ. The functional interaction on in vitro gene expression of APOA5 SNPs, defining haplotype APOA52, and their paradoxical association with plasma triglyceride but not plasma apoAV levels. Biochim. Biophys. Acta. 2008;1782:447–452. doi: 10.1016/j.bbadis.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Talmud PJ, Palmen J, Putt W, Lins L, Humphries SE. Determination of the functionality of common APOA5 polymorphisms. J. Biol. Chem. 2005;280:28215–28220. doi: 10.1074/jbc.M502144200. [DOI] [PubMed] [Google Scholar]
- 13.Kao JT, Wen HC, Chien KL, Hsu HC, Lin SW. A novel genetic variant in the apolipoprotein A5 gene is associated with hypertriglyceridemia. Hum. Mol. Genet. 2003;12:2533–2539. doi: 10.1093/hmg/ddg255. [DOI] [PubMed] [Google Scholar]
- 14.Tang Y, Sun P, Guo D, Ferro A, Ji Y, Chen Q, Fan L. Hum. Mol. Genet. 2003;12:2533–2539. [Google Scholar]
- 15.Pullinger CR, Aouizerat BE, Movsesyan I, Durlach V, Sijbrands EJ, Nakajima K, Poon A, Dallinga-Thie GM, Hattori H, Green LL, Kwok PY, Havel RJ, Frost PH, Malloy MJ, Kane JP. An apolipoprotein A-V gene SNP is associated with marked hypertriglyceridemia among Asian–American patients. J. Lipid Res. 2008;49:1846–1854. doi: 10.1194/jlr.P800011-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lookene, Beckstead JA, Nilsson S, Olivecrona G, Ryan RO. Apolipoprotein A-V-heparin interactions: implications for plasma lipoprotein metabolism. J. Biol. Chem. 2005;280:25383–25387. doi: 10.1074/jbc.M501589200. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson SK, Lookene A, Beckstead JA, Gliemann G, Ryan RO, Olivecrona G. Apolipoprotein A-V interaction with members of the low density lipoprotein receptor gene family. Biochemistry. 2007;46:3896–3904. doi: 10.1021/bi7000533. [DOI] [PubMed] [Google Scholar]
- 18.Beigneux AP, Davies B, Gin P, Weinstein MM, Farber E, Qiao X, Peale F, Bunting S, Walzem RR, Wong JS, Blaner WS, Ding Z, Melford K, Wongsiriroj N, Shu X, de Sauvage F, Ryan RO, Fong LG, Bensadoun A, Young SG. Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gin P, Yin L, Davies BSJ, Weinstein MM, Ryan RO, Bensadoun A, Fong LG, Young SG, Beigneux AP. The acidic domain of GPIHBP1 is important for the binding of lipoprotein lipase and chylomicrons. J. Biol. Chem. 2008;283:29554–29562. doi: 10.1074/jbc.M802579200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu X, Nelbach L, Weinstein MM, Burgess BL, Beckstead JA, Young SG, Ryan RO, Forte TM. Intravenous injection of apolipoprotein A-V reconstituted high density lipoprotein decreases hypertriglyceridemia in apoav−/− mice and requires glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1. Atheroscler. Thromb. Vasc. Biol. 2010;30:2504–2509. doi: 10.1161/ATVBAHA.110.210815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaessen SFC, Dalllinga-Thie GM, Ross CJD, Splint LJ, Castellani LW, Rensen PCN, Hayden MR, Schaap FG, Kuivenhoven JA. Plasma apolipoprotein AV levels in mice are positively associated with plasma triglyceride levels. J. Lipid Res. 2009;50:880–884. doi: 10.1194/jlr.M800551-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaap FG, Rensen PC, Voshol PJ, Vrins C, van der Vliet HN, Chamuleau RA, Havekes LM, Groen AK, van Dijk KW. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J. Biol. Chem. 2004;279:27941–27947. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 23.Shu X, Chan J, Ryan RO, Forte TM. ApoA-V association with intracellular lipid droplets. J. Lipid Res. 2007;48:1445–1450. doi: 10.1194/jlr.C700002-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.van der Vliet HN, Schaap FG, Levels JH, Ottenhoff R, Looije N, Wesseling JG, Groen AK, Chamuleau RA. Adenoviral overexpression of apolipoprotein A-V reduces serum levels of triglycerides and cholesterol in mice. Biochem. Biophys. Res. Commun. 2002;295:1156–1159. doi: 10.1016/s0006-291x(02)00808-2. [DOI] [PubMed] [Google Scholar]
- 25.Shu X, Ryan RO, Forte TM. Intracellular lipid droplet targeting by apolipoprotein A-V requires the carboxyl-terminal segment. J. Lipid Res. 2008;49:1670–1676. doi: 10.1194/jlr.M800111-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tijburg LBM, Nyathi CB, Meijer GW, Geelen MJH. Biosynthesis and secretion of triacylglycerol in rat liver after partial hepatectomy. Biochem. J. 1991;277:723–728. doi: 10.1042/bj2770723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrell GC. Probing Prometheus: fat fueling the fire? J. Hepatol. 2004;40:1252–1255. doi: 10.1002/hep.20522. [DOI] [PubMed] [Google Scholar]
- 28.Shu X, Nelbach L, Ryan RO, Forte TM. Apolipoprotein A-V associates with intrahepatic lipid droplets and influences triglyceride accumulation. Biochim. Biophys. Acta. 2010;1801:605–608. doi: 10.1016/j.bbalip.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pamir N, McMillen TS, Li YI, Lai CM, Wong H, LeBoeuf RC. Overexpression apolipoprotein A5 in mice is not protective against body weight gain and aberrant glucose homeostasis. Metabolism. 2009;58:560–567. doi: 10.1016/j.metabol.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blade AM, Fabritius MA, Hou L, Weinberg RB, Shelness GS. Biogenesis apolipoprotein A-V and its impact on VLDL triglyceride secretion. J. Lipid Res. 2011;52:237–244. doi: 10.1194/jlr.M010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 32.Ploegh HL. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature. 2007;448:435–438. doi: 10.1038/nature06004. [DOI] [PubMed] [Google Scholar]
- 33.Beckstead JA, Wong K, Gupta V, Wan C-P, Cook VR, Weinberg RB, Weers PMM, Ryan RO. The effect of C-terminal truncation on the structural and lipid binding properties of apolipoprotein A-V. J. Biol. Chem. 2007;282:15484–15489. doi: 10.1074/jbc.M611797200. [DOI] [PubMed] [Google Scholar]
- 34.Hickenbottom SJ, Kimmel AR, Londos C, Hurley JH. Structure of a lipid droplet protein; the PAT family member TIP47. Structure. 2004;12:1199–1207. doi: 10.1016/j.str.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Lamant M, Smih F, Harmancey R, Philip-Couderc P, Pathak A, Roncalli J, Galinier M, Collet X, Massabuau P, Senard JM, Rouet P. ApoO, a novel apolipoprotein, is an original glycoprotein up-regulated by diabetes in human heart. J. Biol. Chem. 2006;281:36289–36302. doi: 10.1074/jbc.M510861200. [DOI] [PubMed] [Google Scholar]
- 36.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldberg IJ, Ginsberg HN. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1343–1346. doi: 10.1053/j.gastro.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 38.de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J. Hepatol. 2008;48 Suppl.:104–112. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J. Gastroenterol. 2008;14:2474–2486. doi: 10.3748/wjg.14.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ress C, Moschen AR, Sausgruber N, Tschoner A, Graziadei I, Weiss H, Schgoer W, Ebenbichler CF, Konrad RJ, Patsch JR, Tilg H, Kaser S. The role apolipoprotein A5 in non-alcoholic fatty liver disease. Gut. 2011;60:985–991. doi: 10.1136/gut.2010.222224. [DOI] [PubMed] [Google Scholar]