Abstract
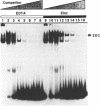
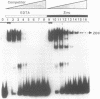
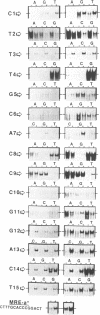
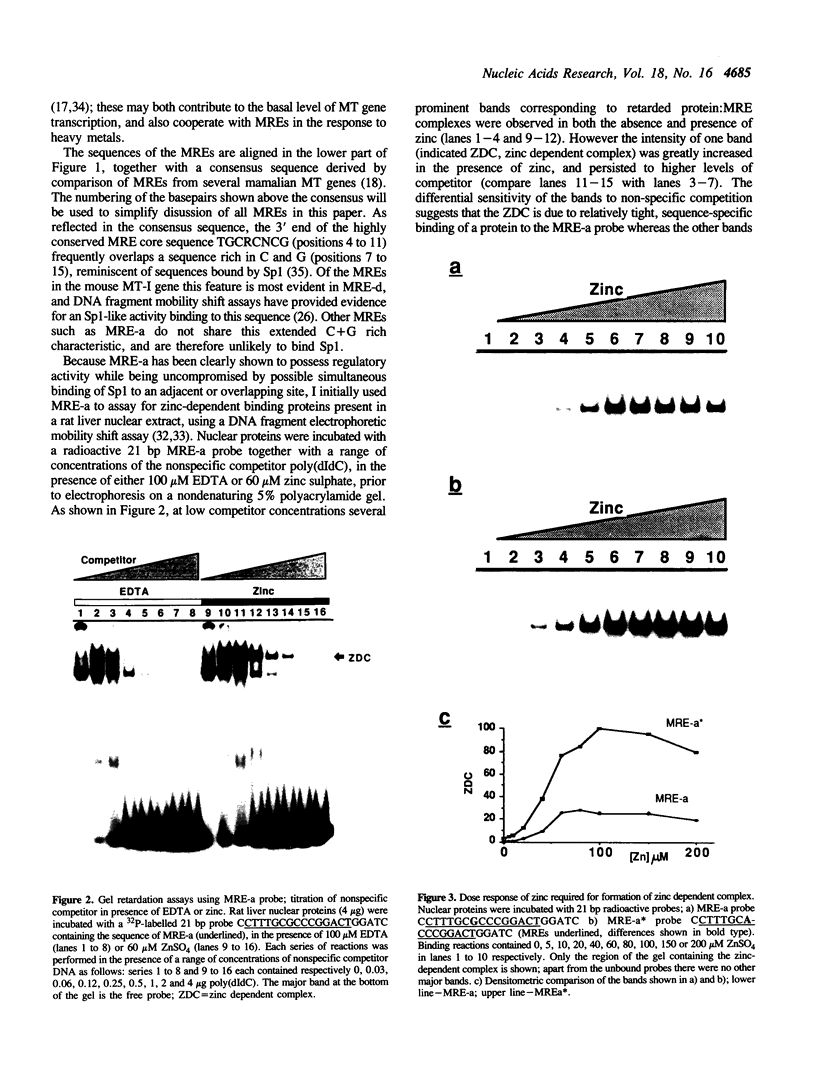
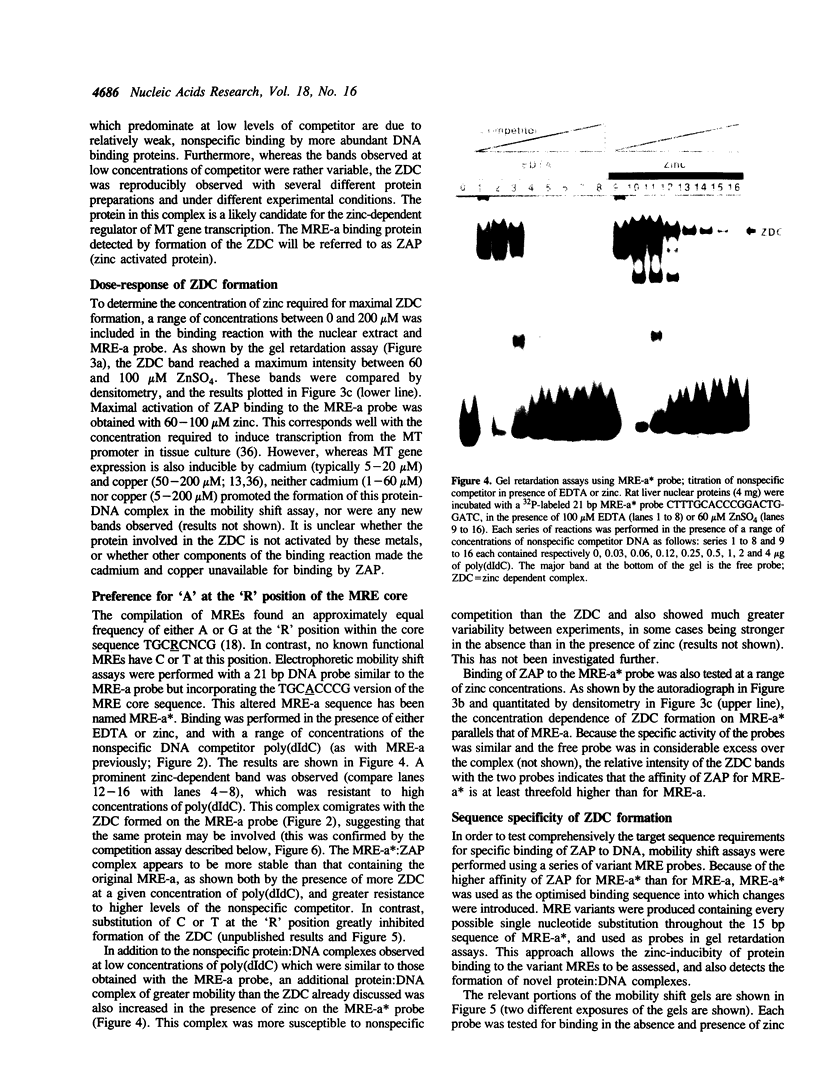
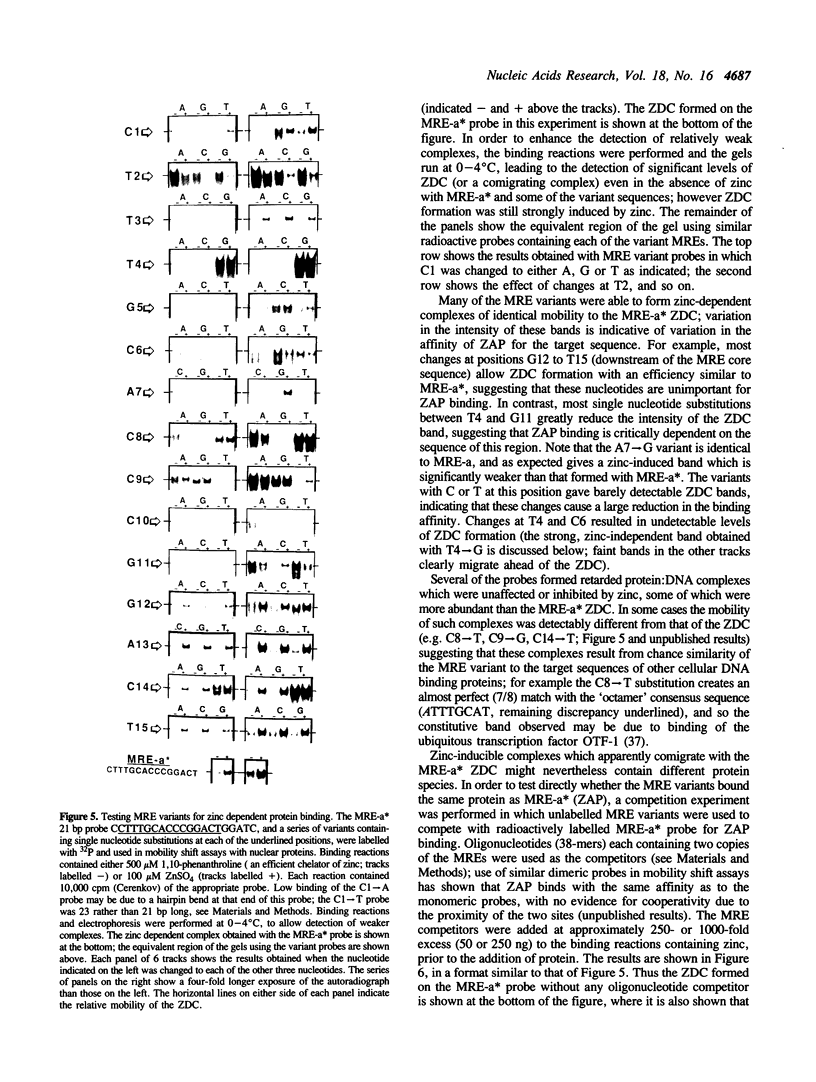
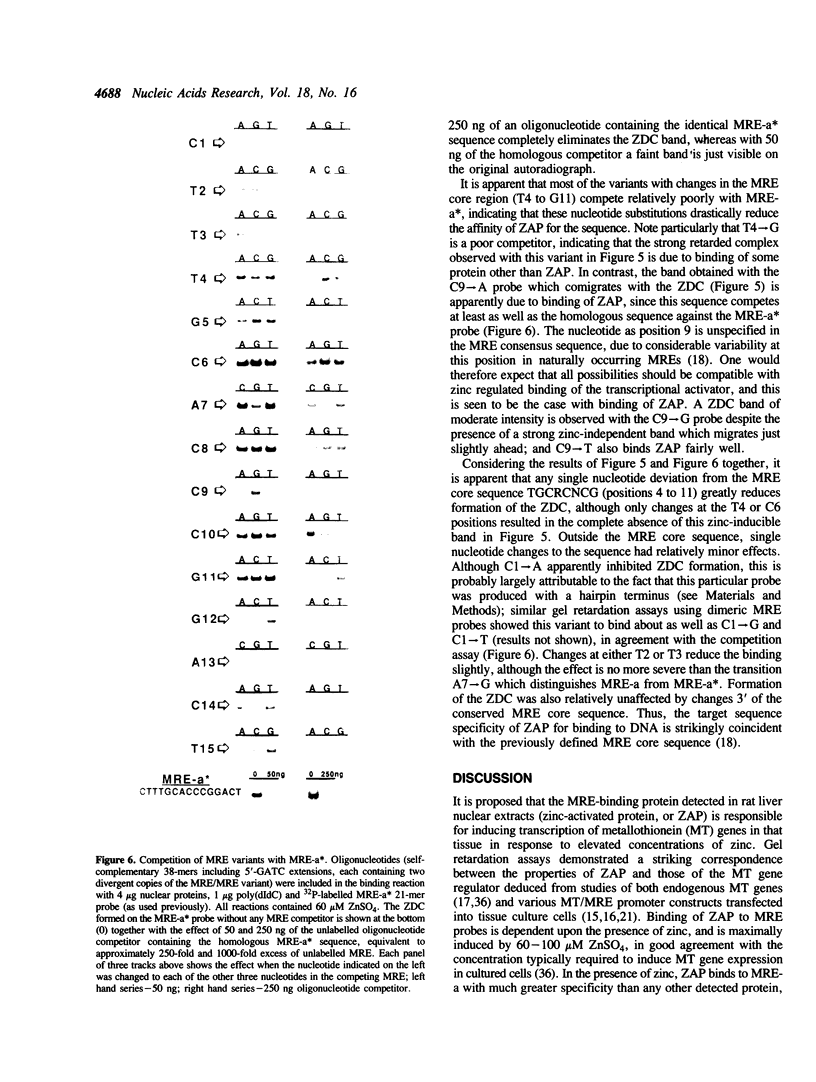
Metallothionein gene transcription is inducible by zinc and other heavy metals, and several metal response elements (MREs) have been mapped within about 200 bp upstream of the site of transcription initiation in several metallothionein genes. Comparison of a number of MREs defined a 15 bp consensus sequence containing a more highly conserved MRE core sequence TGCRCNCG. I have used the proximal MRE of the mouse metallothionein-I gene (MRE-a) in DNA fragment mobility shift assays to detect a protein in rat liver nuclear extracts which binds specifically to the MRE in a zinc-regulated manner. Use of a comprehensive series of variant MRE sequences established that the binding was strongly dependent on the MRE core sequence, whereas changes at the less highly conserved positions had minor effects on binding. This provides strong evidence that the protein detected is responsible for the zinc-responsiveness of the MT genes in liver, and provides a more detailed picture of the regulatory protein:MRE interaction than was previously available.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Taplitz S. J., Wong S., Bristol G., Larkin B., Herschman H. R. Metal-dependent binding of a factor in vivo to the metal-responsive elements of the metallothionein 1 gene promoter. Mol Cell Biol. 1987 Oct;7(10):3574–3581. doi: 10.1128/mcb.7.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A. D., Felber B. K., Walling M. J., Jubier M. F., Schmidt C. J., Hamer D. H. Duplicated heavy metal control sequences of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7392–7396. doi: 10.1073/pnas.81.23.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Cousins R. J. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985 Apr;65(2):238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Hoffman J. S., Quaife C. J., Benditt E. P., Chen H. Y., Brinster R. L., Palmiter R. D. Induction of mouse metallothionein-I mRNA by bacterial endotoxin is independent of metals and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1053–1056. doi: 10.1073/pnas.81.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Induction of metallothionein-I mRNA in cultured cells by heavy metals and iodoacetate: evidence for gratuitous inducers. Mol Cell Biol. 1984 Mar;4(3):484–491. doi: 10.1128/mcb.4.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Fletcher C., Heintz N., Roeder R. G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987 Dec 4;51(5):773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P., Hu S., Hackett R., Hamer D. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell. 1988 Nov 18;55(4):705–717. doi: 10.1016/0092-8674(88)90229-2. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hanas J. S., Hazuda D. J., Bogenhagen D. F., Wu F. Y., Wu C. W. Xenopus transcription factor A requires zinc for binding to the 5 S RNA gene. J Biol Chem. 1983 Dec 10;258(23):14120–14125. [PubMed] [Google Scholar]
- Imbert J., Zafarullah M., Culotta V. C., Gedamu L., Hamer D. Transcription factor MBF-I interacts with metal regulatory elements of higher eucaryotic metallothionein genes. Mol Cell Biol. 1989 Dec;9(12):5315–5323. doi: 10.1128/mcb.9.12.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Heguy A., Dietlin T., Cooke T. Metal-responsive elements act as positive modulators of human metallothionein-IIA enhancer activity. Mol Cell Biol. 1987 Feb;7(2):606–613. doi: 10.1128/mcb.7.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Herschman H. R. Induction of metallothionein in HeLa cells by dexamethasone and zinc. Eur J Biochem. 1981 Jan;113(2):267–272. doi: 10.1111/j.1432-1033.1981.tb05062.x. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. R., Salser S. J., Wold B. Constitutive and metal-inducible protein:DNA interactions at the mouse metallothionein I promoter examined by in vivo and in vitro footprinting. Genes Dev. 1988 Apr;2(4):412–427. doi: 10.1101/gad.2.4.412. [DOI] [PubMed] [Google Scholar]
- Oh S. H., Deagen J. T., Whanger P. D., Weswig P. H. Biological function of metallothionein. V. Its induction in rats by various stresses. Am J Physiol. 1978 Mar;234(3):E282–E285. doi: 10.1152/ajpendo.1978.234.3.E282. [DOI] [PubMed] [Google Scholar]
- Posorske L. H., Cohn M., Yanagisawa N., Auld D. S. Methionyl-tRNA synthetase of Escherichia coli. A zinc metalloprotein. Biochim Biophys Acta. 1979 Jan 25;576(1):128–133. doi: 10.1016/0005-2795(79)90491-4. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Hamer D. H. Cell specificity and an effect of ras on human metallothionein gene expression. Proc Natl Acad Sci U S A. 1986 May;83(10):3346–3350. doi: 10.1073/pnas.83.10.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle P. F., Stuart G. W., Palmiter R. D. Building a metal-responsive promoter with synthetic regulatory elements. Mol Cell Biol. 1985 Jun;5(6):1480–1489. doi: 10.1128/mcb.5.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin C., Hamer D. H. Regulation in vitro of metallothionein gene binding factors. Science. 1987 Mar 13;235(4794):1383–1387. doi: 10.1126/science.3103216. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Chen H. Y., Brinster R. L., Palmiter R. D. A 12-base-pair DNA motif that is repeated several times in metallothionein gene promoters confers metal regulation to a heterologous gene. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7318–7322. doi: 10.1073/pnas.81.23.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Palmiter R. D. Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. 1985 Oct 31-Nov 6Nature. 317(6040):828–831. doi: 10.1038/317828a0. [DOI] [PubMed] [Google Scholar]
- Séguin C., Felber B. K., Carter A. D., Hamer D. H. Competition for cellular factors that activate metallothionein gene transcription. Nature. 1984 Dec 20;312(5996):781–785. doi: 10.1038/312781a0. [DOI] [PubMed] [Google Scholar]
- Séguin C., Prévost J. Detection of a nuclear protein that interacts with a metal regulatory element of the mouse metallothionein 1 gene. Nucleic Acids Res. 1988 Nov 25;16(22):10547–10560. doi: 10.1093/nar/16.22.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin G., Schaffner W. A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J. 1988 Dec 1;7(12):3763–3770. doi: 10.1002/j.1460-2075.1988.tb03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin G., Schaffner W. Heavy metal ions in transcription factors from HeLa cells: Sp1, but not octamer transcription factor requires zinc for DNA binding and for activator function. Nucleic Acids Res. 1988 Jul 11;16(13):5771–5781. doi: 10.1093/nar/16.13.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagle M. K., Palmiter R. D. Coordinate regulation of mouse metallothionein I and II genes by heavy metals and glucocorticoids. Mol Cell Biol. 1985 Feb;5(2):291–294. doi: 10.1128/mcb.5.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]