Abstract
Context:
Iodine is critical for normal thyroid hormone synthesis and brain development during infancy, and preterm infants are particularly vulnerable to the effects of both iodine deficiency and excess. Use of iodine-containing skin antiseptics in intensive care nurseries has declined substantially in recent years, but whether the current dietary iodine intake meets the requirement for hospitalized preterm infants is unknown.
Objective:
The aim of the study was to measure the iodine content of enteral and parenteral nutrition products commonly used for hospitalized preterm infants and estimate the daily iodine intake for a hypothetical 1-kg infant.
Methods:
We used mass spectrometry to measure the iodine concentration of seven preterm infant formulas, 10 samples of pooled donor human milk, two human milk fortifiers (HMF) and other enteral supplements, and a parenteral amino acid solution and soy-based lipid emulsion. We calculated the iodine provided by typical diets based on 150 ml/kg · d of formula, donor human milk with or without HMF, and parenteral nutrition.
Results:
Preterm formula provided 16.4–28.5 μg/d of iodine, whereas unfortified donor human milk provided only 5.0–17.6 μg/d. Adding two servings (six packets) of Similac HMF to human milk increased iodine intake by 11.7 μg/d, whereas adding two servings of Enfamil HMF increased iodine intake by only 0.9 μg/d. The other enteral supplements contained almost no iodine, nor did a parenteral nutrition-based diet.
Conclusions:
Typical enteral diets for hospitalized preterm infants, particularly those based on donor human milk, provide less than the recommended 30 μg/d of iodine, and parenteral nutrition provides almost no iodine. Additional iodine fortification should be considered.
Iodine is a trace element required for the synthesis of T4 and T3, which are critical for normal neurodevelopment in the neonatal period. Worldwide, iodine deficiency resulting in hypothyroidism is the most important preventable cause of cognitive impairment in children (1). However, iodine excess can also be harmful, causing decreased thyroid hormone production and secretion in susceptible individuals (2). Optimal iodine intake provides adequate substrate for thyroid hormone production while avoiding the potential for decreasing thyroid hormone synthesis.
Over 12% of all births in the United States are preterm (<37 completed wk gestation) (3), and preterm infants are a particularly vulnerable population with respect to iodine nutrition. Preterm infants have lower iodine and thyroid hormone stores relative to full-term infants (4) and require relatively more iodine than full-term infants and older children to maintain a positive iodine balance (5, 6), so they are at risk for deficiency without adequate dietary intake. However, hospitalized preterm infants are also susceptible to iodine excess from frequent exposure to iodine-containing skin cleansers such as povidine-iodine, due to immaturity of the preterm skin and resultant high cutaneous absorption. In addition, the ability to escape from the acute thyroid-suppressing effects of iodine (the Wolff-Chaikoff effect) does not mature until 36 wk gestation (7, 8).
After previous studies demonstrated that the use of iodinated skin cleansers in neonatal intensive care unit (NICU) patients was associated with markedly elevated urinary iodine levels (9) and transient hypothyroidism (10, 11), many NICUs in the United States and elsewhere responded by discontinuing the use of povidine-iodine for routine skin cleansing. In these NICUs, dietary iodine is the sole source of iodine for hospitalized infants. Other studies have reported a low iodine content of preterm infant formulas (12) and some samples of human milk (13), but there is little evidence regarding the iodine content of diets used currently for hospitalized preterm infants, particularly diets based on parenteral nutrition and donor human milk, the use of which is on the rise in the United States (14) and is widespread in other countries (15).
The aims of our study were: 1) to measure the iodine content of commercial enteral and parenteral nutrition products and donor human milk typically fed to hospitalized preterm infants; 2) to estimate the total daily dietary iodine intake for a hypothetical 1-kg preterm infant; and 3) to compare our estimates with the recommended daily intake of 30 μg/kg · d.
Materials and Methods
Measurement of iodine content in enteral and parenteral nutrition products
We used ion chromatography-mass spectrometry (16) to measure the iodine content of seven commercially available preterm infant formulas; two commercial human milk fortifiers (HMF); the enteral nutritional supplements medium chain triglyceride (MCT) oil (Mead Johnson Nutrition, Glenview, IL), Beneprotein (Nestle HealthCare Nutrition, Florham Park, NJ), and Polycose (Abbott Nutrition, Columbus, OH); a parenteral amino acid solution (Trophamine; B. Braun Medical Inc., Bethlehem, PA); and soy-based 20% lipid emulsion (Intralipid; Fresenius Kabi, Schaumburg, IL). We also measured the iodine content of 10 samples of donor human milk. Each sample was constituted from three to five different anonymous donors, Holder pasteurized, and frozen according to published guidelines (17).
Calculation of iodine intake for typical hospitalized preterm infant
Using typical enteral (formula and human milk-based) and parenteral diets for preterm infants (Table 1), we calculated the total iodine intake from each component of the diet for a hypothetical 1-kg infant with 150 ml/kg · d of total fluid intake. To estimate the total daily iodine intake, we then summed the daily iodine intake from each component of the diet. For the human milk-based diets, we calculated the total daily iodine intake based on milk with the lowest, median, and highest iodine content.
Table 1.
Typical diets for hospitalized preterm infants
| Enteral |
| Preterm formula (20, 24, or 30 kcal/oz) |
| Transitional formula (22 kcal/oz) |
| Human milk |
| Unfortified (approximately 20 kcal/oz) |
| Fortified |
| 22 kcal/oz (2 packets HMF per 100 ml) |
| 24 kcal/oz (4 packets HMF per 100 ml) |
| 26 kcal/oz (4 packets HMF + 0.87 ml MCT oil per 100 ml) |
| 27 kcal/oz (4 packets HMF + 0.87 ml MCT oil + 1 g Beneprotein per 100 ml) |
| 29 kcal/oz (4 packets HMF + 0.87 ml MCT oil + 1 g Beneprotein + 3 g Polycose per 100 ml) |
| Parenteral |
| Amino acid solution + dextrose + lipid emulsion |
Results
Table 2 shows the iodine content in the seven commercial formulas and parenteral nutrition products and the daily iodine intake based on a 1-kg infant with a total fluid intake of 150 ml/kg · d. The iodine content of preterm and transitional formulas ranged from 109.0 to 190.0 μg/liter, and daily intake for the hypothetical 1-kg infant ranged from 16 to 29 μg/d. Iodine intake from the parenteral amino acid solution and lipid emulsion was negligible.
Table 2.
Iodine content and typical daily iodine intake from commercial infant formula and parenteral nutrition products
| Product | Iodine content (μg/liter) | Typical daily intake (μg/kg · d)a |
|---|---|---|
| Enteral | ||
| Preterm formula | ||
| Premature Enfamil | ||
| 20 kcal/oz | 157 | 24 |
| 24 kcal/oz | 172 | 26 |
| Similac Special Care | ||
| 20 kcal/oz | 115 | 17 |
| 24 kcal/oz | 109 | 16 |
| 30 kcal/oz | 160 | 24 |
| Transitional formula | ||
| Enfacare | 190 | 29 |
| Neosure | 118 | 18 |
| Parenteral | ||
| Trophamine amino acid solution | 2.5 | 0.3 |
| Intralipid lipid emulsion | 15.1 | 0.2 |
Based on a hypothetical 1-kg infant with total fluid intake of 150 ml/kg · d.
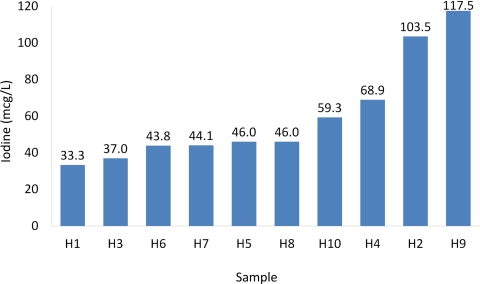
The iodine content in the 10 donor human milk samples ranged from 33.3 to 117.5 μg/liter (Fig.1), with a mean (sd) of 59.5 (28.7) and a median of 46.0 μg/liter. Similac HMF contained 2.2 μg/g of iodine, whereas Enfamil HMF contained almost none (Table 3). The iodine content of other human milk additives is also listed in Table 3; these products also contain very little iodine. Table 4 shows the iodine content in donor human milk-based diets, using the recipes shown in Table 1 and donor human milk with the minimum, median, and maximum iodine content. Without fortification with HMF, donor human milk with the maximum iodine content provided only 17.6 μg/kg · d of iodine. Even with fortification, only human milk with the maximum iodine content (117.5 μg/liter) fortified with Similac HMF (four packets/100 ml) provided close to the recommended 30 μg/kg · d of iodine; all the other human milk-based diets provided less than the recommended amount.
Fig. 1.
Iodine content in 10 samples of donor human milk. Each sample was pooled by the milk bank from three to five different donors, Holder pasteurized, and frozen according to published guidelines (17). To provide the recommended 30 μg/d for preterm infants, human milk would need to contain 200 μg/liter of iodine; all 10 samples had less than this amount.
Table 3.
Iodine content of breast milk additives
| Product | Iodine content |
|---|---|
| HMF | |
| Enfamil HMF | 0.2 μg/g |
| Similac HMF | 2.2 μg/g |
| Other enteral additives | |
| MCT oil | 2.6 μg/liter |
| Beneprotein | 0.1 μg/g |
| Polycose | 0.0 μg/g |
Table 4.
Daily iodine intake from breast milk-based diets
| Diet | Iodine content (μg/liter) | Iodine intake (μg/kg · d)a |
|---|---|---|
| Breast milk with minimum iodine | ||
| Unfortified | 33.3 | 5.0 |
| 22 kcal/oz | ||
| Similac HMF | 72.7 | 10.9 |
| Enfamil HMF | 36.7 | 5.5 |
| ≥24 kcal/oz | ||
| Similac HMF | 111.3 | 16.7 |
| Enfamil HMF | 39.3 | 5.9 |
| Breast milk with median iodine | ||
| Unfortified | 59.3 | 9.0 |
| 22 kcal/oz | ||
| Similac HMF | 85.3 | 12.8 |
| Enfamil HMF | 49.3 | 7.4 |
| ≥24 kcal/oz | ||
| Similac HMF | 124.0 | 18.6 |
| Enfamil HMF | 52.0 | 7.8 |
| Breast milk with maximum iodine | ||
| Unfortified | 117.5 | 17.6 |
| 22 kcal/oz | ||
| Similac HMF | 156.7 | 23.5 |
| Enfamil HMF | 120.7 | 18.1 |
| ≥24 kcal/oz | ||
| Similac HMF | 196.0 | 29.4 |
| Enfamil HMF | 123.3 | 18.5 |
Based on a hypothetical 1-kg infant with total fluid intake of 150 ml/kg · d.
Discussion
We found that typical enteral formula-based diets for preterm neonates provided considerably less iodine than the recommended minimum of 30 μg/kg · d (6, 18). In general, donor human milk provided less iodine than commercial infant formulas, and parenteral nutrition provided almost no iodine. In the past, hospitalized preterm neonates were exposed to large amounts of iodine through povidine-iodine used to clean skin for invasive procedures. Our results suggest that a shift to equally effective noniodinated skin cleansers, while preventing the adverse effects of iodine excess, may have unintended consequences, increasing the risk for negative iodine balance and iodine deficiency, particularly in infants receiving banked donor human milk-based diets or prolonged parenteral nutrition.
None of the donor human milk samples we tested would provide the recommended iodine intake for a growing preterm infant. Our results also demonstrate substantial variability in the iodine content of donor human milk, despite the fact that each of our samples was pooled from three to five different donors. Our results with donor human milk differ from several previous studies that reported a substantially higher iodine content of preterm breast milk; for example, Spanish (12) and Scottish (19) studies of milk provided by lactating mothers of preterm infants of less than 30 wk gestation both reported a mean iodine content of approximately 100 μg/liter, almost twice the mean content of 60 μg/liter in our study. Similarly, a U.S. study (13) of 57 lactating women reported a median breast milk iodine content of 155 μg/liter (range, 2.7 to 1968), also considerably higher than our study and with even greater variability. In another U.S. study, Kirk et al. (20) reported a mean breast milk iodine content of 63.3 μg/liter (range, 4.5 to 184.5), similar to our findings.
The reason for the variability of iodine content of human milk may relate to differences in maternal dietary intake from foods and supplements. Although the United States is a generally iodine-sufficient region, according to the 2005–2008 U.S. National Health and Nutrition Examination Survey, 57% of pregnant women had urinary iodine levels below 150 μg/liter (21). Our results suggest that iodine may be inadequate in the diets of women who donate their milk to milk banks and support recommendations of national and international organizations that all lactating women—including milk donors—take a daily multivitamin containing at least 150 μg of iodine; of note, many prenatal vitamins in the United States contain little iodine (22). It is also possible that Holder pasteurization of the donor milk decreases its iodine content; future studies should examine this possibility.
Our findings suggest that preterm infants receiving prolonged parenteral nutrition do not receive enough iodine. Parenteral nutrition is recommended for all preterm infants born weighing less than 1500 g (23), and many infants—particularly sicker and less mature infants—do not receive full enteral feedings until several weeks after birth. U.S. and European organizations recommend adding only 1 μg/kg · d of iodine to parenteral nutrition, far below the 30 μg/kg · d required to maintain positive iodine balance, but these recommendations assume a substantial degree of skin absorption of topical iodine-containing antiseptics (18). A study (19) of parenterally fed preterm infants of 30 wk gestation or less who were not exposed to iodine-containing skin disinfectants demonstrated evidence of iodine deficiency (low urinary iodine levels) and a negative iodine balance from birth, which reversed by approximately 2 wk of age with the establishment of enteral feeding, suggesting that inadequate iodine in parenteral nutrition has meaningful clinical consequences.
Severe iodine deficiency leads to neurodevelopmental impairment through decreased synthesis of thyroid hormones, which are critical for normal brain development in infancy. The prevalence of postnatal hypothyroxinemia is higher among preterm vs. full-term infants, with estimates ranging from 10% to over 30% in infants of less than 32 wk gestation and/or less than 1500 g (24–27). The phenomenon of lower T4 levels in preterm infants is referred to as the transient hypothyroxinemia of prematurity. Several studies (25, 28, 29) have reported that transient hypothyroxinemia in preterm infants is associated with poorer long-term cognitive and motor outcomes including cerebral palsy, although underlying physiological immaturity and critical illness are also likely to contribute to later neurological impairments (26, 30).
The extent to which iodine status underlies transient hypothyroxinemia of prematurity is unclear. A Spanish study (31) estimated that iodine deficiency accounted for approximately 30% of hypothyroxinemia seen in enterally fed preterm infants of 27–30 wk gestation, in addition to other factors such as immaturity of the hypothalamic-pituitary-thyroid axis, acute prematurity-related illness (e.g. surfactant deficiency, sepsis), and medications such as dopamine. Although a randomized trial (32) of 40–50 vs. 12–16 μg/kg · d iodine supplementation in infant formula in 121 preterm infants of less than 33 wk gestation did not demonstrate a beneficial effect of the higher iodine supplementation on T4 or TSH levels, the extent of participants' exposure to povidine-iodine was not reported. Additionally, only four infants in the trial received predominantly their mothers' own milk, and none received donor human milk. Neurodevelopmental outcomes were not evaluated. In a phase I trial of thyroid hormone supplementation for preterm infants with transient hypothyroxinemia, control infants treated with 30 μg/d of iodine had thyroid hormone levels similar to infants who received the vehicle control (33). Further studies are needed to delineate the impact of iodine nutrition on thyroid function and neurodevelopment in the current era of neonatal intensive care, in populations unexposed to povidine-iodine and fed predominantly human milk.
Our study examined sources of iodine in the preterm diet, but we did not directly measure the iodine status of individual infants fed different diets, nor did we examine the effects of diet on results of newborn screening for congenital hypothyroidism. In the past, rising rates of congenital hypothyroidism detected by newborn screening led to a recognition of iodine overload as an underlying causal factor (34, 35). Of note, the incidence of congenital hypothyroidism has been rising in the United States (27), although the extent to which declining iodine intake relates to this observation is unclear.
In summary, we found that both enteral and parenteral diets for hospitalized preterm infants are low in iodine. Hospitalized preterm infants may be at risk for iodine deficiency, particularly without exposure to iodinated skin cleansers. Ensuring that all lactating women follow recommendations to take a daily multivitamin containing at least 150 μg of iodine is likely to increase the iodine content in human milk. Adding more iodine to parenteral nutrition and commercial HMF may also be warranted.
Acknowledgments
M.B.B. is supported by the National Institutes of Health Grant K23 DK083817.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- HMF
- Human milk fortifier
- MCT
- medium chain triglyceride
- NICU
- neonatal intensive care unit.
References
- 1. Zimmermann MB. 2009. Iodine deficiency. Endocr Rev 30:376–408 [DOI] [PubMed] [Google Scholar]
- 2. Wolff J, Chaikoff IL. 1948. The inhibitory action of iodide upon organic binding of iodine by the normal thyroid gland. J Biol Chem 172:855. [PubMed] [Google Scholar]
- 3. Mathews TJ, Miniño AM, Osterman MJ, Strobino DM, Guyer B. 2011. Annual summary of vital statistics: 2008. Pediatrics 127:146–157 [DOI] [PubMed] [Google Scholar]
- 4. van den Hove MF, Beckers C, Devlieger H, de Zegher F, De Nayer P. 1999. Hormone synthesis and storage in the thyroid of human preterm and term newborns: effect of thyroxine treatment. Biochimie 81:563–570 [DOI] [PubMed] [Google Scholar]
- 5. Ares S, Quero J, de Escobar GM. 2008. Iodine balance, iatrogenic excess, and thyroid dysfunction in premature newborns. Semin Perinatol 32:407–412 [DOI] [PubMed] [Google Scholar]
- 6. Delange F. 2007. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr 10:1571–1580; discussion 1581–1583 [DOI] [PubMed] [Google Scholar]
- 7. Roti E, Gnudi A, Braverman LE. 1983. The placental transport, synthesis and metabolism of hormones and drugs which affect thyroid function. Endocr Rev 4:131–149 [DOI] [PubMed] [Google Scholar]
- 8. Robuschi G, Montermini M, Alboni A, Borciani E, Cersosimo G, Negrotti L, Gnudi A, Safran M, Braverman LE, Roti E. 1987. Cord blood iodothyronine and thyrotropin concentrations in newborns of mothers exposed to povidone iodine in the last trimester. J Endocrinol Invest 10:183–186 [DOI] [PubMed] [Google Scholar]
- 9. Gordon CM, Rowitch DH, Mitchell ML, Kohane IS. 1995. Topical iodine and neonatal hypothyroidism. Arch Pediatr Adolesc Med 149:1336–1339 [DOI] [PubMed] [Google Scholar]
- 10. Smerdely P, Lim A, Boyages SC, Waite K, Wu D, Roberts V, Leslie G, Arnold J, John E, Eastman CJ. 1989. Topical iodine-containing antiseptics and neonatal hypothyroidism in very-low-birthweight infants. Lancet 2:661–664 [DOI] [PubMed] [Google Scholar]
- 11. Chabrolle JP, Rossier A. 1978. Goitre and hypothyroidism in the newborn after cutaneous absorption of iodine. Arch Dis Child 53:495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ares S, Quero J, Durán S, Presas MJ, Herruzo R, Morreale de Escobar G. 1994. Iodine content of infant formulas and iodine intake of premature babies: high risk of iodine deficiency. Arch Dis Child Fetal Neonatal Ed 71:F184–F191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pearce EN, Leung AM, Blount BC, Bazrafshan HR, He X, Pino S, Valentin-Blasini L, Braverman LE. 2007. Breast milk iodine and perchlorate concentrations in lactating Boston-area women. J Clin Endocrinol Metab 92:1673–1677 [DOI] [PubMed] [Google Scholar]
- 14. Cohen RS. 2007. Current issues in human milk banking. NeoReviews 8:e289–e295 [Google Scholar]
- 15. Klingenberg C, Embleton ND, Jacobs SE, O'Connell LA, Kuschel CA. 2012. Enteral feeding practices in very preterm infants: an international survey. Arch Dis Child Fetal Neonatal Ed 97:F56–F61 [DOI] [PubMed] [Google Scholar]
- 16. Valentín-Blasini L, Blount BC, Delinsky A. 2007. Quantification of iodide and sodium-iodide symporter inhibitors in human urine using ion chromatography tandem mass spectrometry. J Chromatogr A 1155:40–46 [DOI] [PubMed] [Google Scholar]
- 17. 2011. Guidelines for the establishment and operation of a donor human milk bank. 3rd ed Fort Worth, TX: Human Milk Banking Association of North America [Google Scholar]
- 18. Zimmermann MB, Crill CM. 2010. Iodine in enteral and parenteral nutrition. Best Pract Res Clin Endocrinol Metab 24:143–158 [DOI] [PubMed] [Google Scholar]
- 19. Ibrahim M, de Escobar GM, Visser TJ, Durán S, van Toor H, Strachan J, Williams FL, Hume R. 2003. Iodine deficiency associated with parenteral nutrition in extreme preterm infants. Arch Dis Child Fetal Neonatal Ed 88:F56–F57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirk AB, Martinelango PK, Tian K, Dutta A, Smith EE, Dasgupta PK. 2005. Perchlorate and iodide in dairy and breast milk. Environ Sci Technol 39:2011–2017 [DOI] [PubMed] [Google Scholar]
- 21. Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. 2011. Iodine status of the U.S. population. National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 21:419–427 [DOI] [PubMed] [Google Scholar]
- 22. Leung AM, Pearce EN, Braverman LE. 2009. Iodine content of prenatal multivitamins in the United States. N Engl J Med 360:939–940 [DOI] [PubMed] [Google Scholar]
- 23. Kleinman RE. 2009. Pediatric nutrition handbook. 6th ed Elk Grove Village, IL: American Academy of Pediatrics [Google Scholar]
- 24. Reuss ML, Leviton A, Paneth N, Susser M. 1997. Thyroxine values from newborn screening of 919 infants born before 29 weeks' gestation. Am J Public Health 87:1693–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Den Ouden AL, Kok JH, Verkerk PH, Brand R, Verloove-Vanhorick SP. 1996. The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very preterm and/or very low birth weight infants. Pediatr Res 39:142–145 [DOI] [PubMed] [Google Scholar]
- 26. Delahunty C, Falconer S, Hume R, Jackson L, Midgley P, Mirfield M, Ogston S, Perra O, Simpson J, Watson J, Willatts P, Williams F. 2010. Levels of neonatal thyroid hormone in preterm infants and neurodevelopmental outcome at 5 1/2 years: millennium cohort study. J Clin Endocrinol Metab 95:4898–4908 [DOI] [PubMed] [Google Scholar]
- 27. Hinton CF, Harris KB, Borgfeld L, Drummond-Borg M, Eaton R, Lorey F, Therrell BL, Wallace J, Pass KA. 2010. Trends in incidence rates of congenital hypothyroidism related to select demographic factors: data from the United States, California, Massachusetts, New York, and Texas. Pediatrics 125(Suppl 2):S37–S47 [DOI] [PubMed] [Google Scholar]
- 28. Reuss ML, Paneth N, Pinto-Martin JA, Lorenz JM, Susser M. 1996. The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. N Engl J Med 334:821–827 [DOI] [PubMed] [Google Scholar]
- 29. Lucas A, Morley R, Fewtrell MS. 1996. Low triiodothyronine concentration in preterm infants and subsequent intelligence quotient (IQ) at 8 year follow up. BMJ 312:1132–1133; discussion 1133–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simpson J, Williams FL, Delahunty C, van Toor H, Wu SY, Ogston SA, Visser TJ, Hume R. 2005. Serum thyroid hormones in preterm infants and relationships to indices of severity of intercurrent illness. J Clin Endocrinol Metab 90:1271–1279 [DOI] [PubMed] [Google Scholar]
- 31. Ares S, Escobar-Morreale HF, Quero J, Durán S, Presas MJ, Herruzo R, Morreale de Escobar G. 1997. Neonatal hypothyroxinemia: effects of iodine intake and premature birth. J Clin Endocrinol Metab 82:1704–1712 [DOI] [PubMed] [Google Scholar]
- 32. Rogahn J, Ryan S, Wells J, Fraser B, Squire C, Wild N, Hughes A, Amegavie L. 2000. Randomised trial of iodine intake and thyroid status in preterm infants. Arch Dis Child Fetal Neonatal Ed 83:F86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. La Gamma EF, van Wassenaer AG, Ares S, Golombek SG, Kok JH, Quero J, Hong T, Rahbar MH, de Escobar GM, Fisher DA, Paneth N. 2009. Phase 1 trial of 4 thyroid hormone regimens for transient hypothyroxinemia in neonates of <28 weeks' gestation. Pediatrics 124:e258–e268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chanoine JP, Boulvain M, Bourdoux P, Pardou A, Van Thi HV, Ermans AM, Delange F. 1988. Increased recall rate at screening for congenital hypothyroidism in breast fed infants born to iodine overloaded mothers. Arch Dis Child 63:1207–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chanoine JP, Pardou A, Bourdoux P, Delange F. 1988. Withdrawal of iodinated disinfectants at delivery decreases the recall rate at neonatal screening for congenital hypothyroidism. Arch Dis Child 63:1297–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]