Abstract
Context:
The relationship of fibroblast growth factor 21 (FGF21) with glucose metabolism and insulin resistance has not been well characterized in community-dwelling adults.
Objective:
The objective of the study was to examine the relationship of FGF21 with glucose metabolism and insulin resistance.
Design:
Serum FGF21, fasting plasma glucose (FPG), glucose tolerance, and insulin resistance were measured in a cross-sectional study, 2002–2007.
Setting:
The study was the Baltimore Longitudinal Study of Aging, a natural history cohort study of aging in community-dwelling men and women.
Participants:
Seven hundred adults, mean age 63.3 yr, participated in the study.
Main Outcome Measures:
FPG, 2-h plasma glucose, homeostasis model of insulin resistance, whole-body insulin sensitivity (Matsuda index), glucose area under the curve (AUC), and insulin AUC were measured.
Results:
Overall, the median (25th and 75th percentiles) FGF21 concentration was 225 (126, 370) pg/ml. The proportion of adults with normal, impaired, and diabetic FPG was 77.0, 21.4, and 1.6%, and those with normal, impaired, and diabetic 2-h plasma glucose was 76.7, 19.1, and 4.1%, respectively. Log serum FGF21 (picograms per milliliter), per 1 sd increase, was associated with an FPG (odds ratio 1.43, 95% confidence interval 1.15, 1.77, P = 0.001) and with 2-h plasma glucose (odds ratio 1.39, 95% confidence interval 1.12, 1.73, P = 0.003), in respective multivariate, ordered logistic regression models, adjusted for potential confounders. Serum FGF21 (picograms per milliliter) was associated with the homeostasis model of insulin resistance, the Matsuda index, glucose AUC, and insulin AUC (all P < 0.0001) in respective multivariable linear regression models adjusted for potential confounders.
Conclusions:
Higher serum FGF21 concentrations were associated with abnormal glucose metabolism and insulin resistance in community-dwelling adults.
Fibroblast growth factor 21 (FGF21), a recently discovered hormone, is emerging as an important endocrine factor involved in glucose and lipid metabolism and energy regulation (1, 2). FGF21 is a member of a family of atypical fibroblast growth factors (FGF) that include FGF19 and FGF23. The lack of a heparin-binding domain, as found on most FGF, allows FGF21 and other atypical FGF to leave tissues of origin and serve as circulating hormones (2). FGF21 is primarily expressed by liver, white adipose tissue, and pancreas. FGF21 mediates its effects on tissues through four classic FGF21 cell surface receptor isotypes 1–4. FGF21 cell surface receptor form a complex with the essential coreceptor β-klotho to mediate FGF21 signal transduction (3). FGF21 stimulates glucose uptake in adipocytes (4) and regulates energy metabolism and enhanced mitochondrial oxidative function through the activation of AMP-activated protein kinase and sirtuin 1 (5). Transgenic mice that overexpress FGF21 had higher glucose clearance and were resistant to diet-induced obesity (4). Administration of recombinant FGF21 to diabetic fatty rats (4) and diabetic rhesus monkeys (6) reduced plasma glucose and triglycerides, lowered low-density lipoprotein (LDL) cholesterol, increased high-density lipoprotein (HDL) cholesterol, and caused a modest weight loss.
In humans, elevated serum FGF21 levels were associated both with obesity and with the metabolic syndrome in a study of more than 200 adults in the Hong Kong Cardiovascular Risk Factor Prevalence Study (7). In a clinic-based study, higher FGF21 concentrations were associated with adverse lipid profiles in patients with coronary heart disease (8). FGF21 had a direct effect on enhancing glucose uptake in cultured skeletal muscle biopsies obtained from patients with and without diabetes (9). Circulating FGF21 levels were higher in patients with diabetes compared with healthy controls (9, 10). In a study of nearly 2000 subjects in Hong Kong, elevated plasma FGF21 levels were an independent predictor of incident type 2 diabetes mellitus over 5.4 yr of follow-up (11).
Although studies suggest that FGF21 is associated with diabetes and glucose metabolism, the relationship between circulating FGF21 and fasting plasma glucose, oral glucose tolerance testing, and insulin resistance have not been well characterized in humans. We postulated that higher fasting plasma glucose, abnormal glucose tolerance, insulin resistance, and other oral glucose tolerance-derived glucose and insulin measurements are associated with elevated FGF21 concentrations. To address this hypothesis, we explored the relationship of FGF21 with fasting plasma glucose, glucose tolerance, insulin resistance, whole-body insulin sensitivity, and other oral glucose tolerance-derived indices in a cohort of community-dwelling men and women.
Materials and Methods
Subjects
The study subjects consisted of participants in the Baltimore Longitudinal Study of Aging (BLSA) who were seen between April 2002 and August 2007. The BLSA is a prospective open cohort study of community-dwelling volunteers, largely from the Baltimore (Maryland)/Washington (DC) area. The study was established in 1958 and is described in detail elsewhere (12). BLSA participants return every 2 yr to the Gerontology Research Center in Baltimore, MD, for 2.5 d of medical, physiological, and psychological examinations. Subjects were excluded from the present study if they had diabetes mellitus and were taking insulin or oral hypoglycemic medications. Blood pressure was measured in the morning, after a light breakfast, with subjects in the seated position, and after a quiet resting period of 5 min. Blood pressure (BP) was measured in both arms with a mercury sphygmomanometer using an appropriately sized cuff. The BP values used in this study are the average of the second and third measurements on both the right and left arms. Height, weight, and waist circumference were measured in all participants. Body mass index was determined as kilograms per square meter and classified as underweight, normal, overweight, and obese, respectively (<18.5, 18.5–24.9, 25.0–29.9, and ≥30 kg/m2) as per the guidelines of the World Health Organization (13). Smoking status was ascertained by a questionnaire that classified each subject as a nonsmoker, former smoker, or current smoker. Use of medications such as statins and β-blockers was ascertained through standardized questionnaires. The BLSA has continuing approval from the Institutional Review Board of the MedStar Research Institute, and the protocol for the present study was also approved by the Institutional Review Board of the Johns Hopkins School of Medicine.
Laboratory methods
Blood samples were drawn from the antecubital vein between 0700 and 0800 h after an overnight fast. Subjects were not allowed to smoke, engage in physical activity, or take medications before the sample was collected. Subjects were sitting in a semireclining chair during glucose tolerance testing (GTT). Fasting plasma and serum were collected at baseline, after which subjects drank 75 g glucose in 300 ml solution (SunDex; Fisherbrand, Pittsburgh, PA), and blood samples were drawn at 5, 10, 15, 20, 40, 60, 80, and 120 min after oral administration for glucose and insulin measurements. Normal fasting glucose, impaired fasting glucose, and diabetic fasting glucose were defined as fasting plasma glucose 99 mg/dl or less, 100–125 mg/dl, and greater than 125 mg/dl, respectively (1). Normal glucose tolerance, impaired glucose tolerance, and diabetic glucose tolerance were defined as 2-h plasma glucose of 139 mg/dl or less, 140–199 mg/dl, and 200 mg/dl or greater, respectively (14). Plasma insulin was measured using an ELISA (Mercodia, Uppsala, Sweden) with inter- and intraassay coefficients of variation less than 4%. Insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR), calculated using fasting glucose and insulin measurements (15). Whole-body insulin sensitivity was estimated using the Matsuda index, calculated as 10,000/square root of the following: [(fasting glucose × fasting insulin) × (mean glucose × mean insulin during GTT)], which provides a good approximation of measurements obtained by the euglycemic insulin clamp technique (16). Integration of the glucose and insulin GTT curves [i.e. area under the curve (AUC)] was calculated by the standard trapezoid method. The insulin and glucose AUC were calculated using all available data for the participant (17).
Concentrations of plasma triglycerides and total cholesterol were determined by an enzymatic method (ABA-200 ATC Biochromatic analyzer; Abbott Laboratories, Irving, TX). The concentration of HDL cholesterol was determined by a dextran sulfate-magnesium precipitation procedure (18). LDL cholesterol concentrations were estimated by using the Friedewald formula (19). Plasma glucose concentration was measured by the glucose oxidase method (Beckman Instruments, Inc., Fullerton, CA). Chronic kidney disease (stage 3) was defined as estimated glomerular filtration rate of less than 60 ml/min per 1.73 m2 using the Modification of Diet in Renal Disease equation of Levey and colleagues (20). Samples were stored continuously at −70 C until the time of the analysis of serum FGF21. Serum FGF21 was measured using ELISA (quantikine human FGF-21 ELISA; R & D Systems, Minneapolis, MN). The samples were analyzed at Johns Hopkins Hospital in the laboratory of one of the investigators (R.D.S.). The intraassay and interassay coefficients of variability were 2.8 and 7.0%, respectively. The assay has a minimum limit of detection of 4.6 pg/ml. No samples were below the lower limit of detection.
Statistical methods
Continuous variables were reported as median (25th and 75th percentiles) for consistency because some of the variables were skewed to higher values. Serum FGF21 was highly skewed and log transformed to obtain a normal distribution. Ordered multivariate logistic regression models were used to examine the relationship of log serum FGF21 (per 1 sd) and demographic, anthropometric, laboratory, and clinical characteristics with fasting plasma glucose and glucose tolerance. Linear regression models were used to examine the relationship of log serum FGF21 and other covariates with HOMA-IR, the Matsuda index, glucose AUC, and insulin AUC. Covariates included in the multivariable models were basic demographic variables (age, gender, and race) and variables that were significantly associated with abnormal fasting glucose, 2-h glucose on oral GTT, or tertiles of HOMA-IR, except for lipids and BP. All multivariable models for abnormal glucose metabolism and insulin resistance were adjusted for the same covariates to maintain consistency in the modeling. Spearman correlations were used to examine correlation between selected variables. All analyses were conducted using SAS (version 9.1.3; SAS Institute, Cary, NC) with type I error of 0.05.
Results
The mean (sd) age of the 700 study subjects was 63.4 (13.7) yr, and the age range was 26.3–92.7 yr. The proportion of adults with normal, impaired, and diabetic fasting plasma glucose was 77.0, 21.4, and 1.6%, respectively, and the proportion with normal, impaired, and diabetic 2-h plasma glucose was 76.7, 19.1, and 4.1%, respectively. Of the 539, 150, and 11 participants with normal, impaired, and diabetic fasting plasma glucose, respectively, 455 (84.4%), 56 (37.3%), and eight (72.7%) also had normal, impaired, and diabetic 2-h plasma glucose (P < 0.0001 by χ2 test). Overall, the median (25th and 75th percentiles) FGF21 concentration was 225 (126, 370) pg/ml. Demographic, disease, and other characteristics of the 700 participants by normal, impaired, and diabetic fasting glucose concentrations are shown in Table 1. There were significant differences in age, gender, body mass index, β-blocker use, systolic and diastolic blood pressure, triglycerides, HDL cholesterol, FGF21, and the proportion of subjects with hypertension, angina, and myocardial infarction between the three different fasting glucose categories. There were no significant differences between the three groups in race, current smoking, statin use, total cholesterol, LDL cholesterol, estimated glomerular filtration rate (eGFR), stroke, heart failure, cancer, osteoarthritis, and chronic kidney disease. The proportion of subjects in this study with stroke and/or heart failure was less than 2%. No participants were taking fibrate medications.
Table 1.
Characteristics of study participants by fasting plasma glucose concentrations in the Baltimore Longitudinal Study of Aging
| Characteristic | Fasting plasma glucose |
Pa | ||
|---|---|---|---|---|
| ≤99 mg/dl (n = 539) | 100–125 mg/dl (n = 150) | >125 mg/dl (n = 11) | ||
| Age (yr) | 61.9 (53.8, 73.1) | 68.1 (58.5, 76.7) | 71.8 (60.9, 75.9) | <0.0001 |
| Male gender (%) | 43.9 | 62.0 | 54.5 | 0.0004 |
| Race, white (%) | 67.7 | 61.3 | 54.6 | 0.24 |
| Body mass index (kg/m2) | 25.7 (23.4, 29.3) | 27.8 (25.4, 31.4) | 31.4 (28.3, 32.7) | <0.0001 |
| Current smoking (%) | 4.4 | 4.0 | 9.1 | 0.73 |
| Statin use (%) | 6.7 | 12.0 | 9.1 | 0.09 |
| β-Blocker use (%) | 10.9 | 22.3 | 36.4 | 0.0004 |
| Systolic blood pressure (mm Hg) | 123 (114, 135) | 130 (122, 140) | 135 (129, 160) | <0.0001 |
| Diastolic blood pressure (mm Hg) | 69 (63, 75) | 72 (66, 78) | 74 (64, 79) | 0.001 |
| Triglycerides (mg/dl) | 86 (64, 114) | 99 (71, 146) | 176 (71, 214) | 0.0002 |
| Total cholesterol (mg/dl) | 194 (172, 218) | 197 (173, 217) | 198 (160, 206) | 0.66 |
| LDL cholesterol (mg/dl) | 116 (95, 139) | 117 (98, 140) | 90 (79, 124) | 0.18 |
| HDL cholesterol (mg/dl) | 58 (47, 71) | 52 (44, 61) | 44 (34, 57) | 0.0002 |
| Serum FGF21 (pg/ml) | 210 (114, 335) | 275 (170, 417) | 405 (221, 499) | <0.0001 |
| eGFR (ml/min per 1.73 m2) | 77.4 (66.7, 89.5) | 77.3 (65.9, 90.1) | 77.5 (65.4, 89.7) | 0.79 |
| Hypertension (%) | 25.4 | 42.0 | 54.5 | <0.0001 |
| Angina (%) | 9.3 | 12.6 | 28.0 | 0.05 |
| Myocardial infarction (%) | 2.0 | 7.3 | 9.1 | 0.003 |
| Stroke (%) | 0.4 | 0.7 | 0.0 | 0.87 |
| Heart failure (%) | 0.4 | 1.3 | 0.0 | 0.37 |
| Cancer (%) | 9.8 | 12.0 | 18.1 | 0.52 |
| Osteoarthritis (%) | 24.8 | 27.3 | 0.0 | 0.13 |
| Chronic kidney disease (%) | 15.2 | 16.7 | 18.2 | 0.87 |
χ2 tests for categorical variables, Kruskal-Wallis tests for continuous variables.
Serum FGF21 was associated with abnormal fasting plasma glucose concentrations in multivariate ordered logistic regression models adjusting for age, race, and gender (model 1) and with addition of body mass index (model 2) and also after further adjusting for eGFR, statin use, β-blocker use, hypertension, angina, and myocardial infarction (Table 2). In the final multivariate model, in addition to FGF21, age, race, gender, and body mass index were also significantly associated with abnormal fasting plasma glucose concentrations.
Table 2.
Multivariate ordered logistic regression models for serum FGF21 and other factors with abnormal fasting plasma glucose concentrations in 700 adults in the Baltimore Longitudinal Study of Aging
| Characteristic | OR | 95% CI | P |
|---|---|---|---|
| Model 1 | |||
| Log serum FGF21 (pg/ml)a | 1.55 | 1.26, 1.91 | <0.0001 |
| Age (yr) | 1.03 | 1.01, 1.04 | 0.001 |
| Male gender | 2.07 | 1.42, 3.02 | 0.0001 |
| White race | 0.51 | 0.35, 0.77 | 0.001 |
| Model 2 | |||
| Log serum FGF21 (pg/ml)a | 1.41 | 1.14, 1.76 | 0.001 |
| Age (yr) | 1.03 | 1.02, 1.05 | 0.0001 |
| Male gender | 2.03 | 1.38, 2.97 | 0.0003 |
| White race | 0.59 | 0.39, 0.89 | 0.01 |
| Body mass index (kg/m2) | 1.11 | 1.07, 1.15 | <0.0001 |
| Model 3 | |||
| Log serum FGF21 (pg/ml)a | 1.43 | 1.15, 1.77 | 0.001 |
| Age (yr) | 1.03 | 1.01, 1.05 | 0.002 |
| Male gender | 1.89 | 1.27, 2.81 | 0.002 |
| White race | 0.65 | 0.42, 1.00 | 0.05 |
| Body mass index (kg/m2) | 1.10 | 1.06, 1.14 | <0.0001 |
| eGFR (ml/min per 1.73 m2) | 1.01 | 0.99, 1.02 | 0.34 |
| Statin use | 1.29 | 0.69, 2.42 | 0.42 |
| β-Blocker use | 1.52 | 0.92, 2.53 | 0.11 |
| Hypertension | 1.27 | 0.83, 1.94 | 0.27 |
| Angina | 0.69 | 0.36, 1.32 | 0.26 |
| Myocardial infarction | 2.20 | 0.86, 5.58 | 0.10 |
Mean (sd) of logFGF21 was 5.36 (0.77) pg/ml.
Similar differences were found in demographic, disease, and other characteristics of 700 participants when classified by normal, impaired, and diabetic 2-h plasma glucose concentrations on oral glucose tolerance testing (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org) as with fasting plasma glucose above, except that there were significant differences in statin use between the three different 2-h plasma glucose categories. Serum FGF21 (per 1 sd increase) was significantly associated with abnormal 2-h plasma glucose concentrations on oral glucose tolerance testing in multivariate ordered logistic regression models adjusting for age, race, and gender (model 1), with addition of body mass index (model 2), and also adjusting further for eGFR, statin use, β-blocker use, hypertension, angina, and myocardial infarction [odds ratio (OR) 1.39, 95% confidence interval (CI) 1.12, 1.73, P = 0.003)] (Supplemental Table 2).
The characteristics of the study participants by tertiles of HOMA-IR are shown in Table 3. Adults with higher HOMA-IR were more likely to be Black, with higher body mass index, higher systolic and diastolic BP, lower use of β-blockers, higher triglycerides, lower HDL cholesterol, and higher FGF21 and were more likely to have hypertension than the participants with lower HOMA-IR. There were no significant differences across tertiles of HOMA-IR by age, gender, current smoking, statin use, total cholesterol, LDL cholesterol, eGFR, angina, myocardial infarction, stroke, heart failure, cancer, osteoarthritis, and chronic kidney disease.
Table 3.
Characteristics of study participants by tertiles of HOMA-IR in the Baltimore Longitudinal Study of Aging
| Characteristic | HOMA-IR |
Pa | ||
|---|---|---|---|---|
| <20.1 (n = 234) | 20.1–35.8 (n = 233) | >35.8 (n = 233) | ||
| Age (yr) | 62.7 (55.2, 73.4) | 64.0 (52.9, 75.1) | 63.7 (56.7, 73.4) | 0.62 |
| Male gender (%) | 47.4 | 48.1 | 48.5 | 0.97 |
| Race, white (%) | 75.2 | 66.5 | 56.6 | <0.0001 |
| Body mass index (kg/m2) | 24.4 (22.6, 27.0) | 25.8 (23.7, 28.7) | 29.5 (25.9, 33.4) | <0.0001 |
| Current smoking (%) | 5.1 | 3.4 | 4.7 | 0.65 |
| Statin use (%) | 7.7 | 9.4 | 6.4 | 0.48 |
| β-Blocker use (%) | 7.2 | 12.0 | 21.4 | <0.0001 |
| Systolic blood pressure (mm Hg) | 122 (112, 131) | 125 (114, 136) | 128 (120, 138) | <0.0001 |
| Diastolic blood pressure (mm Hg) | 68 (63, 74) | 69 (65, 76) | 72 (65, 77) | 0.0007 |
| Triglycerides (mg/dl) | 77 (58, 100) | 86 (66, 115) | 105 (80, 153) | <0.0001 |
| Total cholesterol (mg/dl) | 194 (174, 218) | 191 (171, 218) | 197 (173, 215) | 0.67 |
| LDL cholesterol (mg/dl) | 116 (94, 137) | 112 (93, 134) | 119 (96, 141) | 0.27 |
| HDL cholesterol (mg/dl) | 61 (51, 75) | 58 (48, 70) | 50 (42, 59) | <0.0001 |
| Serum FGF21 (pg/ml) | 195 (104, 309) | 220 (119, 330) | 275 (168, 459) | <0.0001 |
| eGFR (ml/min per 1.73 m2) | 76.3 (66.7, 87.9) | 78.0 (67.4, 90.1) | 77.7 (65.1, 89.8) | 0.49 |
| Hypertension (%) | 20.5 | 30.4 | 37.3 | 0.0003 |
| Angina (%) | 9.8 | 9.8 | 11.1 | 0.88 |
| Myocardial infarction (%) | 3.8 | 3.8 | 2.2 | 0.49 |
| Stroke (%) | 0.4 | 0.8 | 0 | 0.37 |
| Heart failure (%) | 0.4 | 0.8 | 0.8 | 0.78 |
| Cancer (%) | 8.6 | 11.1 | 11.6 | 0.51 |
| Osteoarthritis (%) | 21.3 | 27.0 | 26.6 | 0.29 |
| Chronic kidney disease (%) | 13.8 | 12.6 | 20.2 | 0.05 |
χ2 tests tests for categorical variables, Kruskal-Wallis tests for continuous variables.
The relationship between serum FGF21 and HOMA-IR was examined in multivariable ordered logistic regression models (Table 4). Serum FGF21 was associated with HOMA-IR after adjusting for age, race, and gender (model 1), after addition of body mass index (model 2), and finally after additional adjustment for eGFR, statin use, β-blocker use, hypertension, angina, and myocardial infarction (model 3). In addition to FGF21, race, body mass index, β-blocker use, and myocardial infarction were also significantly associated with FGF21 in the final multivariable model.
Table 4.
Multivariate ordered logistic regression models for serum FGF21 and other risk factors associated with HOMA-IR in 700 adults in the Baltimore Longitudinal Study of Aging
| Characteristic | OR | 95% CI | P |
|---|---|---|---|
| Model 1 | |||
| Serum FGF21 (pg/ml)a | 1.58 | 1.36, 1.84 | <0.0001 |
| Age (yr) | 0.99 | 0.98, 1.01 | 0.82 |
| White race | 0.45 | 0.33, 0.60 | <0.0001 |
| Male gender | 1.10 | 0.83, 1.46 | 0.49 |
| Model 2 | |||
| Serum FGF21 (pg/ml)a | 1.43 | 1.22, 1.67 | <0.0001 |
| Age (yr) | 1.00 | 0.99, 1.01 | 0.48 |
| White race | 0.53 | 0.39, 0.72 | <0.0001 |
| Male gender | 0.95 | 0.71, 1.26 | 0.71 |
| Body mass index (kg/m2) | 1.18 | 1.14, 1.22 | <0.0001 |
| Model 3 | |||
| Serum FGF21 (pg/ml)a | 1.40 | 1.20, 1.64 | <0.0001 |
| Age (yr) | 1.00 | 0.99, 1.01 | 0.79 |
| White race | 0.54 | 0.39, 0.75 | 0.0003 |
| Male gender | 0.92 | 0.68, 1.24 | 0.57 |
| Body mass index (kg/m2) | 1.18 | 1.14, 1.22 | <0.0001 |
| eGFR (ml/min per 1.73 m2) | 1.00 | 0.99, 1.01 | 0.87 |
| Statin use | 0.65 | 0.38, 1.13 | 0.13 |
| β-Blocker use | 2.54 | 1.60, 4.06 | <0.0001 |
| Hypertension | 1.11 | 0.78, 1.57 | 0.57 |
| Angina | 0.99 | 0.58, 1.68 | 0.98 |
| Myocardial infarction | 0.40 | 0.17, 0.97 | 0.04 |
Mean (sd) of logFGF21 was 5.36 (0.77) pg/ml.
Spearman correlations between log serum FGF21 and other GTT-derived insulin and glucose measurements were as follows: log Matsuda index (r = −0.25, P < 0.0001), log glucose AUC (r = 0.28, P < 0.0001), and log insulin AUC (r = 0.20, P < 0.0001). Serum FGF21 (log serum FGF21, per 1 sd) was associated with the Matsuda index (β = −0.15, se = 0.02, P < 0.0001), glucose AUC (β = 0.05, se = 0.01, P < 0.0001), and insulin AUC (β = 0.10, se = 0.02, P < 0.0001), in respective multivariable linear regression models adjusting for age, race, gender, body mass index, eGFR, statin use, β-blocker use, and chronic diseases (hypertension, angina, and myocardial infarction).
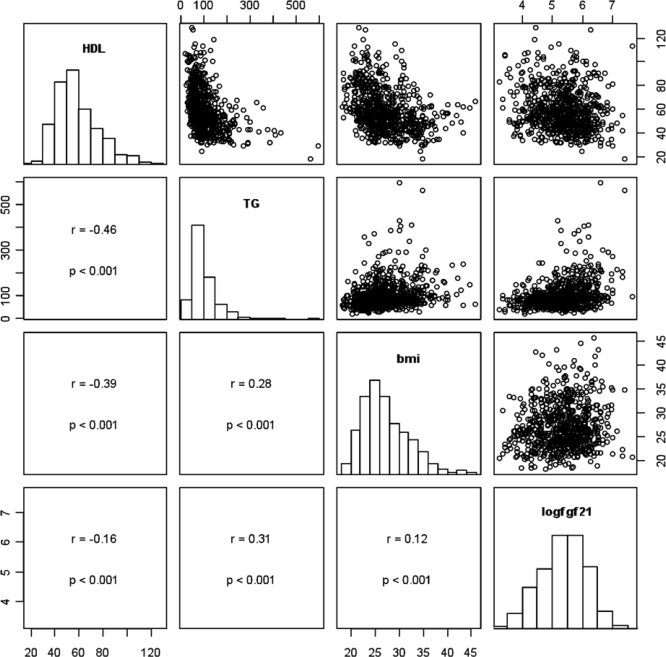
The Spearman correlations and scatterplots of FGF21, HDL cholesterol, triglycerides, and body mass index are shown in Fig. 1. Additional Spearman correlations were examined between FGF21 and total cholesterol (r = −0.0002, P = 0.99) and eGFR (r = −0.19, P < 0.0001), respectively.
Fig. 1.
Paired scatterplots and correlations between HDL cholesterol, triglycerides (TG), body mass index (BMI), and FGF21. On the diagonal, the marginal distributions are shown as histograms. In the lower left of the triangle, the respective correlation coefficients, confidence intervals, and P values are shown. For clarity and because correlations are scale independent, we omitted the axis labels.
Discussion
The present study shows that elevated serum FGF21 concentrations are independently associated with abnormal glucose metabolism and insulin resistance in humans. To our knowledge, this is the first study to demonstrate a relationship between FGF21 levels and abnormal fasting plasma glucose, abnormal glucose tolerance testing, and insulin resistance in a population of community-dwelling adults. The findings of the present study corroborate and extend the relationship of circulating FGF21 and abnormal glucose metabolism described in a clinic-based study of 41 subjects who were selected using the criteria of body mass index, impaired glucose tolerance, and type 2 diabetes mellitus (21). The results of the present study are not consistent with a previous study in which no significant correlation was found between circulating FGF21 levels and fasting plasma glucose; however, the study involved a small sample of 76 healthy controls (22). The association between FGF21 and insulin resistance in the present study also contrasts with a previous study involving a mixed sample of 69 adults selected on the criteria of obesity, type 2 diabetes, or controls (10) and with an investigation in which FGF21 levels were not associated with HOMA-IR in a relatively small sample of 63 adults who underwent hyperglycemic clamp (23). The previous studies of FGF21, glucose metabolism, and insulin resistance (10, 22, 23) had small sample sizes and may have had more limited statistical power to detect associations with FGF21.
In the present study, serum FGF21 was positively correlated with circulating triglycerides, positively associated with body mass index, and negatively correlated with HDL cholesterol. These findings are consistent with a previous study of lipid profiles and FGF21 in mixed sample of patients with coronary heart disease and healthy controls (8) and with clinic-based studies showing an associated between elevated FGF21 and obesity (7). Recent data from the obese ob/ob and diabetic db/db mouse models demonstrate impaired signaling responses in liver and fat after exogenous administration of FGF21, suggesting that obesity is an FGF21-resistant state (24).
The strengths of this study include the population of community-dwelling adults, relatively stronger statistical power associated with the large sample size, the assessment of abnormal glucose metabolism using both fasting plasma glucose and oral glucose tolerance testing, the standardized collection of fasting serum samples at the same 1-h period in the morning from all participants, and oral GTT in which both glucose and insulin were collected over multiple time points over a 2-h period. For studies of FGF21, the timing of sample collection may be important because FGF21 exhibits a circadian rhythm (25). In the present study, all multivariable analyses were carefully controlled for renal function because eGFR was significantly correlated with circulating FGF21 levels, as shown in the present study and in a previous sample of patients on hemodialysis and controls with and without diabetes (26). A limitation of the study is the cross-sectional design because the direction of any causal association of elevated circulating FGF21 and impaired glucose metabolism and insulin resistance cannot be determined with certainty.
FGF21 is induced by prolonged fasting or starvation. In humans, circulating FGF21 levels increased but only after a 7-d fast (22). No increase in FGF21 levels was observed after a 3-d fast in healthy women (25). FGF21 was also induced the liver during the adaptive starvation response in mice (27). These observations raise the question of why circulating FGF21 concentrations should be elevated with subjects with impaired glucose metabolism. Are circulating FGF21 levels increased in adults with impaired glucose metabolism because of FGF21 resistance or do the increased levels represent a compensatory response to facilitate glucose uptake that is blunted by insulin resistance? The present study appears to support the latter mechanism because elevated circulating FGF21 levels were positively associated with HOMA-IR and negatively associated with the Matsuda index, an indicator of whole-body insulin sensitivity.
One prospective epidemiological study suggests that elevated circulating FGF21 is an independent predictor of incident type 2 diabetes mellitus (11). Further longitudinal studies are needed to corroborate these findings and to determine whether elevated FGF21 is a biomarker for individuals who are at risk for developing abnormal glucose metabolism and insulin resistance.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AG027012, R01 AG029148, and R01 HL094507 and the Intramural Research Program, National Institute on Aging, National Institutes of Health.
Disclosure Summary: The authors have no conflicts of interest.
Footnotes
- AUC
- Area under the curve
- BLSA
- Baltimore Longitudinal Study of Aging
- BP
- blood pressure
- CI
- confidence interval
- eGFR
- estimated glomerular filtration rate
- FGF
- fibroblast growth factor
- FGF21
- fibroblast growth factor 21
- GTT
- glucose tolerance testing
- HDL
- high-density lipoprotein
- HOMA-IR
- homeostasis model assessment of insulin resistance
- LDL
- low-density lipoprotein
- OR
- odds ratio.
References
- 1. Kralisch S, Fasshauer M. 2011. Fibroblast growth factor 21: effects on carbohydrate and lipid metabolism in health and disease. Curr Opin Clin Nutr Metab Care 14:354–359 [DOI] [PubMed] [Google Scholar]
- 2. Kliewer SA, Mangelsdorf DJ. 2010. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr 91(Suppl):254S–257S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. 2007. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA 104:7432–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. 2005. FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chau MD, Gao J, Yang Q, Wu Z, Gromada J. 2010. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc Natl Acad Sci USA 107:12553–12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. 2007. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148:774–781 [DOI] [PubMed] [Google Scholar]
- 7. Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A. 2008. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57:1246–1253 [DOI] [PubMed] [Google Scholar]
- 8. Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, Xiao J, Wang X, Feng W, Li X. 2010. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One 5:e15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, Zierath JR, Chibalin AV, Moller DE, Kharitonenkov A, Krook A. 2011. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes mellitus. Diabetes Metab Res Rev 27:286–297 [DOI] [PubMed] [Google Scholar]
- 10. Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, Matoulek M, Dostalova I, Humenanska V, Haluzik M. 2009. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 71:369–375 [DOI] [PubMed] [Google Scholar]
- 11. Chen C, Cheung BM, Tso AW, Wang Y, Law LS, Ong KL, Wat NM, Xu A, Lam KS. 2011. High plasma level of fibroblast growth factor 21 is an independent predictor of type 2 diabetes: a 5.4-year population-based prospective study in Chinese subjects. Diabetes Care 34:2113–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shock NW, Greulich RC, Andres RA, Arenberg D, Casta PT, Lakatta EG, Tobin JP. 1984. Normal human aging: the Baltimore Longitudinal Study of Aging. Washington, DC: U.S. Government Printing Office [Google Scholar]
- 13. World Health Organization 1995. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 854. Geneva: World Health Organization; [PubMed] [Google Scholar]
- 14. Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. 2003. Follow-up report on the diagnosis of diabetes mellitus. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 26:3160–3136 [DOI] [PubMed] [Google Scholar]
- 15. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 16. Matsuda M, DeFronzo RA. 1999. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 17. Kalyani RR, Metter EJ, Ramachandran R, Chia CW, Saudek CD, Ferrucci L. 2012. Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J Gerontol A Biol Sci Med Sci 67:74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warnick G, Benderson J, Albers JJ. 1982. Dextran sulfate-Mg2+ precipitation procedure for quantification of high-density-lipoprotein cholesterol. Clin Chem 28:1379–1388 [PubMed] [Google Scholar]
- 19. Friedewald WT, Levy RI, Frederickson DS. 1972. Estimation of the concentration of low-density-lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- 20. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130:461–470 [DOI] [PubMed] [Google Scholar]
- 21. Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, DeFronzo RA, Tripathy D. 2009. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 32:1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I, Dahlin M, Amark P, Angelin B, Rudling M. 2008. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab 8:169–174 [DOI] [PubMed] [Google Scholar]
- 23. Li H, Bao Y, Xu A, Pan X, Lu J, Wu H, Lu H, Xiang K, Jia W. 2009. Serum fibroblast growth factor 21 is associated with adverse lipid profiles and γ-glutamyltransferase but not insulin sensitivity in Chinese subjects. J Clin Endocrinol Metab 94:2151–2156 [DOI] [PubMed] [Google Scholar]
- 24. Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. 2010. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59:2781–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andersen B, Beck-Nielsen H, Højlund K. 2011. Plasma FGF21 displays a circadian rhythm during a 72 hour fast in healthy female volunteers. Clin Endocrinol (Oxf) 75:514–519 [DOI] [PubMed] [Google Scholar]
- 25. Stein S, Bachmann A, Lössner U, Kratzsch J, Blüher M, Stumvoll M, Fasshauer M. 2009. Serum levels of the adipokine FGF21 depend on renal function. Diabetes Care 32:126–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. 2009. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 106:10853–10858 [DOI] [PMC free article] [PubMed] [Google Scholar]