Abstract
Bilayered detergent-lipid assemblies known as bicelles have been widely used as model membranes in structural biological studies and are being explored for wider applications, including pharmaceutical use. Most studies to date have involved the use of concentrated bicelle mixtures, such that little is known about the capacity of bicellar mixtures to be diluted without unwanted transitions to non-isotropic phases. Here, different detergent/lipid mixtures have been explored, leading to the identification of two different families of bicelles for which it is possible to lower the total amphiphile (detergent+lipid) concentration to <1% (w/v) while retaining isotropic assemblies. These include a novel family of bicelles based on mixtures of 6-cyclohexyl-1-hexylphosphocholine (Cyclofos-6) and the lipid dimyristoylphosphatidylcholine (DMPC). Bicelles formed by these mixtures can be diluted to <0.5% and also have attractive biochemical properties. However, a caveat of our results is that the diffusion coefficients measured for the lipid component of the different bicelles tested were seen to be dependent on sample history, even though all samples were optically transparent. This suggests that the phase behavior of bicelles at low lipid-to-detergent ratios may be more complex than previously appreciated.
Keywords: bicelles, detergents, micelles, mixed micelles, NMR, membranes, membrane proteins, isotropic
Introduction
Bicelles represent a class of model membranes that is increasingly employed in structural biology1–8 and that remains under exploration for wider applications, including pharmaceutical use.9–12 Bicelles are lipid-detergent mixtures that are more lipid-rich than classical mixed micelles, such that the lipid present retains a bilayered state. At the detergent-rich extreme of the bicellar regime assemblies are thought to be discoidal bilayer fragments that are edge-stabilized by an annulus of detergent.4,13–19 At higher lipid-to-detergent q values (lipid-to-detergent mole ratio) bicelles are Swiss cheese-like perforated bilayer sheets in which the holes are edge-stabilized by detergent.6,20–26
Bicelles are appealing for use as model membranes because they provide a bilayered phase in which to solubilize membrane proteins and other hydrophobic molecules while sharing a number of attractive features with detergent micelles such as optical clarity and ease of mixing. Depending on composition and temperature, bicelles either tumble rapidly and isotropically in solution or form phases with high viscosity and long range order.4,6,13–26 Indeed in the viscous q > 2 regime, bicelles can be uniformly aligned using high magnetic field.1,5,27–28 Unlike lipoprotein-phospholipid nanodiscs that have a diameter fixed by the length of the apolipoprotein used to provide bilayer edge-stabilization,29 the size of bicelles can be systematically varied by altering the lipid-to-detergent molar ratio.
One problem sometimes encountered when working with bicelles is that the detergents most commonly used for bicelle formation, CHAPSO and dihexanoylphosphatidylcholine (D6PC), have relatively high critical micelle concentrations (10–15 mM). This significantly limits the degree to which bicelles can be diluted without the lipid phase transforming to vesicles as a result of depleting the edge-stabilizing detergent as the total detergent population (edge-associated + free) shifts towards the soluble monomeric form in order to sustain a CMC concentration of free detergent. Consider the example of bicelles composed of D6PC and dimyristoylphosphatidylcholine (DMPC, a phospholipid). D6PC has a critical micelle concentration of 14 mM.30 When D6PC-DMPC bicelles are diluted, they can be expected to convert into a mixture of free D6PC plus bilayered DMPC vesicles as the total D6PC concentration is lowered to 14 mM or below. Consequently for q= 3 D6PC-DMPC mixtures, bicelles no longer form below a total D6PC+DMPC concentration of approximately 3% w/v (D6PC total = 12 mM), as has previously been experimentally documented.31 For D6PC-rich q = 0.3 D6PC/DMPC bicelles, bicelles do not form below approximately 1% w/v (where the total D6PC concentration is 15 mM), as is shown experimentally in this work. The requirement to work at D6PC+DMPC concentrations of 10 mg/mL or higher creates a formidable expense barrier to using bicelles in applications requiring relatively large volumes of solution, such as in the mobile phase during liquid chromatography.
In this work we explore the development of detergent-lipid mixtures that retain bicelle-like properties even at concentrations below 1% w/v. The focus is on bicelles in the detergent-rich low q regime (q = 0.25–0.5), conditions that are widely used in solution NMR studies of membrane proteins.32–37 Two different systems that retain at least some bicellar properties when diluted below 1% were identified in this work, one of which represents a novel family of bicelles. However, this work also provides evidence that the phase behavior of isotropic lipid-detergent mixtures that are described as “bicelles” may be complex.
Materials and Methods
Preparation of Bicelles
D6PC, 1,2-diheptanoyl-sn-glycero-3-phosphocholine (D7PC),1,2-dioctanoyl-sn-glycero-3-phosphocholine(D8PC), DMPC, 4-cyclohexyl-1-butylphosphocholine (Cyclofos-4), 5-cyclohexyl-1-pentylphosphocholine (Cyclofos-5), 6-cyclohexyl-1-hexylphosphocholine (Cyclofos-6), and 7-cyclohexyl-1-heptylphosphocholine (Cyclofos-7) ware purchased from Avanti Polar Lipids (Alabaster, AL) or Affymetrix-Anatrace (Maumee, OH). Bicelles were prepared by weighing out appropriate amounts of lipid and detergent and co-dissolving both in 25mM sodium phosphate plus 100 mM NaCl and 10% D2O at pH 7.0. Two different methods for mixing samples were employed. In “Method A” samples were mixed by cycles of vortexing, warming in a 318 K water bath, and cooling in an ice bath well beyond the point where samples became optically clear. In “Method B” samples were additionally subjected to 5 cycles of freezing in liquid nitrogen followed by thawing in the presence of bath sonication in warm water.
Use of NMR to Measure Translational Diffusion Coefficients
Translational diffusion coefficients, Dt, were measured using bipolar-gradient based diffusion-ordered spectroscopy (DOSY) experiments38 carried out on a Bruker AMX-600 NMR spectrometer equipped with a cryogenic probe. The diffusion time interval and gradient pulse lengths were fixed for each experiment, with values optimized for each sample. Diffusion time intervals were set to 300 ms for all measurements except D8PC/DMPC bicelles, with corresponding gradient pulse durations being set to 0.75–2.0 ms for bicelle diffusion coefficient measurements. Water diffusion coefficient measurements were carried out using a 30 ms diffusion time and a 0.75 ms gradient pulse duration. The pulsed gradient field strength was incremented linearly from 2% to 95% in 16 steps. Peak intensity versus gradient field strength data was then fit to the following equation:
| (1) |
to determine Dt,39 where I is measured peak intensity, I0 is the reference intensity, γ is the gyromagnetic ratio of the observed nucleus, g is the gradient strength, δ is the length of the gradient pulse, Δ is the diffusion time, and τ is the delay between the bipolar gradient. A 5 sec recycle delay was used for all measurements to allow for effectively complete relaxation between scans. The maximum gradient strength was calibrated to be 65.57 G/cm using a doped water standard sample (Bruker number Z10906; 0.1 mg GdCl3 per mL in D2O with 1% H2O + 0.1% 13CH3OH), in which water has a known diffusion coefficient value of 1.91×10−9m2/s at 298.15 K (Bruker Biospin NMR user manual). The temperature was calibrated to 303.0 ± 0.1 K for each measurement with perdeuterated methanol.40 The translational diffusion coefficients for D6PC/DMPC, D7PC/DMPC and D8PC/DMPC bicelles were measured based on the methyl resonances from the acyl chains of DMPC at 0.81 ppm (Figure 2, top panel). This proton resonance is well separated from any resonances of D6PC, D7PC, and D8PC in the bicelle samples. Data fitting was carried out using Origin 8 (OriginLab, Northampton, MA). Diffusion coefficients measured using DMPC resonances will reflect the Dt of the bicellar (or other isotropic) assemblies because the aqueous solubility of free DMPC is very low (6 nM; http://avantilipids.com). In the absence of a bicelle-forming detergent, DMPC forms higher order lipid aggregates (e.g., vesicles) which are large and yield only broad NMR resonances. Diffusion coefficients for Cyclofos/DMPC based bicelles were measured as above except in the case of very low concentration Cyclofos-6/DMPC bicelles, conditions in which the DMPC methyl peaks overlap severely with peaks from Cyclofos-6. For such samples comparison of the 1D 1H NMR spectra between Cyclofos micelles and Cyclofos/DMPC bicelles shows that peaks in the region from 2.2 ppm to 2.5 ppm arise exclusively from DMPC. Therefore, peaks in this region were used to monitor the translational diffusion for low concentration Cyclofos/DMPC bicelle samples.
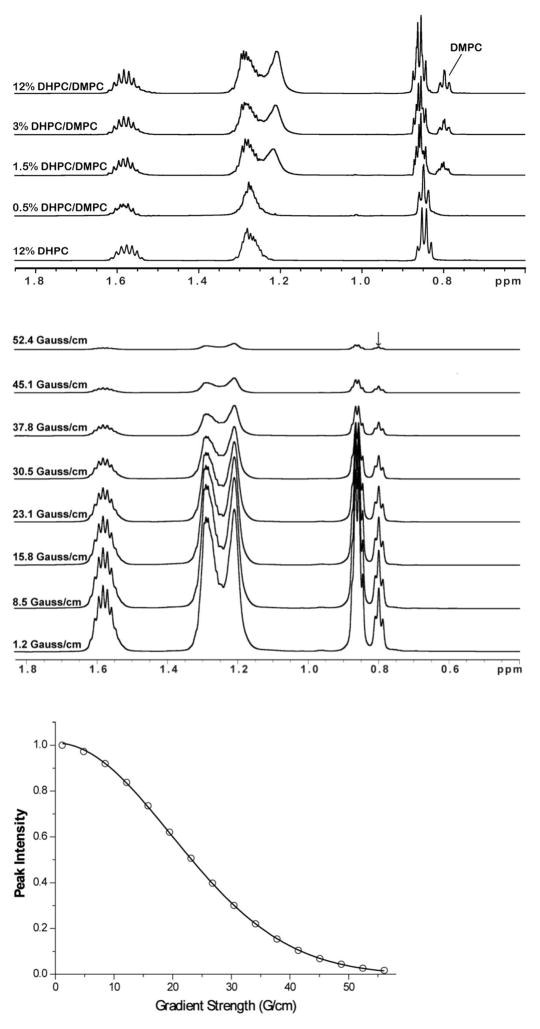
Figure 2.
(Top) 1H NMR spectra of q = 0.3 D6PC-DMPC bicelles at different total amphiphile concentrations and 303 K. The bottom spectrum shows a D6PC-only sample, which provides the basis for distinguishing DMPC peaks from D6PC peaks. These spectra include DMPC peaks at 0.8 and 1.2 ppm that broaden beyond detectability when the isotropic bicellar phase is lost upon dilution to below 1%. The gradient strength-modulated intensity of the DMPC peak at 0.8 ppm was measured as the basis for diffusion coefficient measurements. (Middle) Modulation of 1H NMR DMPC methyl peak intensity as a function of the gradient strength (as shown, in Gauss/cm) when the bipolar-gradient based diffusion-ordered spectroscopy (DOSY) experiments. The sample is the 12% case described for the top panel. (Bottom) Modulation of the DMPC methyl peak intensity as a function of gradient strength and fit of equation 1 to the data determine the translational diffusion coefficient. The sample is the 12% case described for the top panel.
The water diffusion coefficient was also measured in many samples to determine the solution viscosity. The viscosity of the NMR sample (η) can be estimated according to the equation:41
| (2) |
where T is the working temperature and DW,20 and ηW,20 are the diffusion coefficient and viscosity of water at 293 K, respectively. The measured Dt for water was largely independent of the bicelle concentration used in this study, which varied from 0.1% to 12% weight/volume total amphiphile, and clustered around an average Dt value of 2.1×10−9 m2/s at 303 K and 2.5 × 10−9 m2/s at 313 K. Based on the equation above and the approximate constancy of the water translational diffusion coefficients, we estimated sample viscosities.
Measured bicelle diffusion coefficients and solution viscosities were then used to determine the effective hydrodynamic radius of the bicelles. For this the Stokes-Einstein equation was employed:
| (3) |
Dt is the measured diffusion coefficient, Rs is the hydrodynamic radius, η is the measured viscosity, T is the absolute temperature, and kB is the Boltzmann constant. Because this equation assumes a spherical shape and bicelles are not spherical, the determined hydrodynamic radii are effective. They allow varying sizes of bicelles to be qualitatively compared, but do not correspond to true dimensions.
Preparation of the Transmembrane/Cytosolic Domain of the Human Integrin β1 Protein (Integrin β1-TM-CTD) for NMR Spectroscopy
N-terminally 6×His-tagged integrin β1-TM-CTD (residues 719 to 798 of the full length protein) was cloned into a pET16b vector, which was then transformed into BL21(DE3) CodonPlus-RP cells. The transformed cells were plated on a Luria broth/ampicillin/chloramphenicol plate and incubated overnight at 310 K. A single colony was used to inoculate 5 mL of Luria broth medium containing ampicillin and chloramphenicol, which was rotary shaken overnight at room temperature. Approximately 1–2 mL of this culture was then used to inoculate 1 L of 15N-M9 media at room temperature, which was rotary shaken until OD600 reached 0.8, at which point protein expression was induced by adding IPTG to 1 mM, followed by continued rotary shaking for approximately 16 h at room temperature. Cells were harvested by centrifugation and the His6-tagged protein was purified.
Cells were suspended in Lysis buffer (75 mM Tris-HCl, 300 mM NaCl, 0.2 mM EDTA, 0.2 mg/mL PMSF, lysozyme/deoxyribonuclease/ribonuclease at 0.2/0.02/0.02 mg/mL and 5 mM magnesium acetate, pH 7.7) and tumbled at room temperature for ~30 minutes. The cells on ice were probe-sonicated for 5 minutes with cycles of 5 sec on and 5 sec off. The detergent Empigen (30% solution) was added to the cell lysate (1 mL per 10 mL lysate) and mixed at 277 K for 30 min. Solubilized cell lysate was then centrifuged at 20,000 × g for 20 minutes. The supernatant was mixed with Ni(II)-NTA resin (1ml of resin per gram of cells) for 45 min at 277 K. The resin was then collected by centrifugation at 3400 × g for 10 min. The Ni resin was washed with 5 × 1 column volumes of ice cold Emp/A (3% Empigen in 40 mM HEPES, 300 mM NaCl, pH 7.5) and then washed with wash buffer (1.5% Empigen, 40 mM imidazole, 40 mM HEPES, 300 mM NaCl, pH 7.8) until all non-6×His tagged proteins eluted. Next, the resin was flushed using 8 × 1 column volumes of pre-equilibration buffer (20 mM imidazole, 100 mM NaCl, 0.5 mM DTT, and 1% D6PC, pH 6.5) and further with 2 × 1 column volumes of column equilibration buffer (20 mM imidazole,100 mM NaCl, 0.5 mM DTT, 10% D2O, pH 6.5) plus 2% q=0.3 D6PC/DMPC bicelles. The protein was eluted using elution buffer (250 mM imidazole, 0.5 mM DTT, 10% D2O, pH 7.4) plus 2% q=0.3 D6PC/DMPC bicelles. The yield of integrin β1-TM-CTD prepared in this manner from 1 L of culture was ~8 mg pure protein. The pH of the eluted protein solution was adjusted to 6.5 after addition of 1mM EDTA and concentrated using 10 kDa molecular weight cut-off centrifugal ultrafiltration cartridges. For protein purified into q=0.3 Cyclofos-6/DMPC bicelles, the same procedure was used as that described above except that Cyclosfos-6 was substituted for all steps for which use of D6PC is denoted. Purification of the protein using 0.5% Cyclosfos-6/DMPC bicelles yielded a similar amount of integrin as when elution was carried out using a 2% D6PC/DMPC bicelle solution.
2-D 1H-15N TROSY-HSQC spectra were recorded at 600 MHz using a version of the TROSY pulse sequence described in the literature,42 with 1024 × 128 complex data points in the 1H and 15N dimensions and 32 scans per increment. The spectra width was 13 ppm and 30 ppm for 1H and 15N, respectively. The carrier frequency for the 15N dimension was set to 118.5ppm and a recycle delay of 1.5 s was used.
Results
Overview of Bicelle Systems Tested and General Experimental Approach
The exploratory studies of this work focused on q = 0.3 mixtures, reflecting a detergent-to-lipid ratio (3.3) that is at the high end of the composition range believed to remain within the bicellar regime. This is also the q the value most commonly used in solution NMR-based structural studies of membrane proteins that employ bicelles as models membranes.32–37
Four different types of detergents were examined (Figure 1). D6PC was used as a “control” detergent—a detergent that forms bicelles with DMPC but that has a relatively high CMC (14 mM), such that isotropic bicelles do not persist below a high overall detergent+lipid w/v concentration. D6PC/DMPC bicelles also represent the best-characterized bicelle system. We also tested both D7PC and D8PC because they are closely related to D6PC but have much lower critical micelle concentrations (see Table 1). D7PC has previously been shown to form bicelles with DMPC.43,44 We also examined a straight chain alkylphosphocholine (dodecylphosphocholine, DPC). Finally, much attention was devoted to Cyclofos series alkylphosphocholines, for which the chains are terminated by a cyclohexyl moiety. The total number of alkyl chain carbons is 6 plus the number denoted in association with the Cyclofos designation. For example Cyclofos-6 has 12 carbons in the cyclohexyl-terminated chain attached to the phosphocholine head group. CMC values for the detergents are listed in Table 1.
Figure 1.
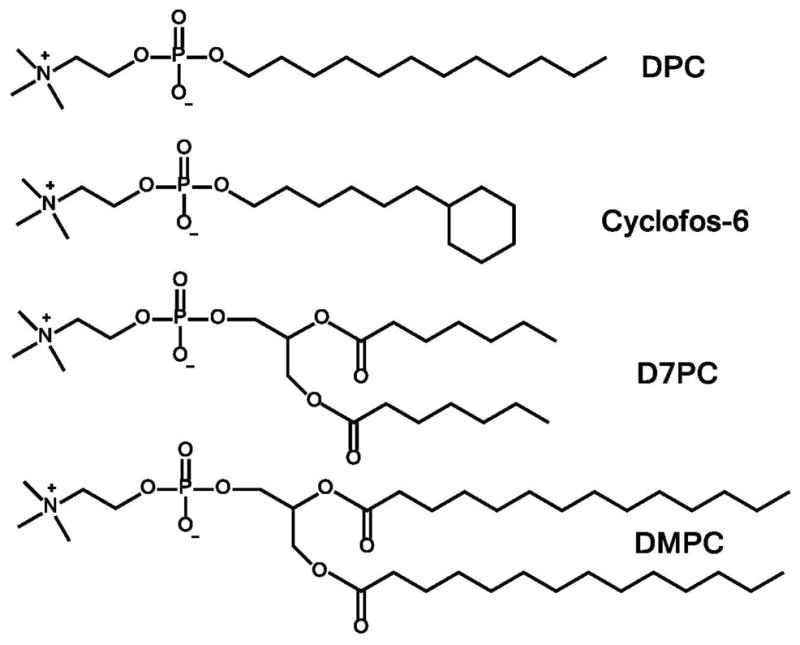
Chemical structures of selected detergents and lipids used in this work.
Table 1.
Translational Diffusion Coefficients (Dt) for DMPC In q = 0.3 Bicelle Mixtures
| Bicelle (Detergent/Lipid) | Weight % (Detergent + Lipid) | Detergent CMC1 (mM) | Temp K | Dt for Bicelles (×10−11m2/s) (Bicelle Preparative Method B) | Effective Hydrodynamic Radius of Bicelles (Å) (Bicelle Preparative Method B) | Dt for Bicelles (×10−11m2/s) (Bicelle Preparative Method A) | Effective Hydrodynamic Radius of Bicelles (Å) (Bicelle Preparative Method A) |
|---|---|---|---|---|---|---|---|
| DPC/DMPC | 12% | 2 | 303 | 8.9±0.2 | 25±1 | 7.2±0.1 | 31±1 |
| D6PC/DMPC | 12% | 14 | 303 | 10.4±0.3 | 22±1 | 9.5±0.1 | 24±1 |
| D6PC/DMPC | 6% | 14 | 303 | 13.9±0.3 | 16±1 | 13.1±0.1 | 17±1 |
| D6PC/DMPC | 3% | 14 | 303 | ND2 | ND | 14.4±0.1 | 16±1 |
| D6PC/DMPC | 1.5% | 14 | 303 | ND | ND | 13.9±0.1 | 16±1 |
| D6PC/DMPC | 1% | 14 | 303 | 18.5±0.3 | 12±1 | 13.4±0.1 | 17±1 |
| D6PC/DMPC | 0.5% | 14 | 303 | ND | ND | No bicelles | No bicelles |
| D6PC/DMPC | 0.25% | 14 | 303 | ND | ND | No bicelles | No bicelles |
| D6PC/DMPC | 0.1% | 14 | 303 | ND | ND | No bicelles | No bicelles |
| D7PC/DMPC | 12% | 1.5 | 303 | 4.0±0.2 | 56±3 | 4.4±0.1 | 51±1. |
| D7PC/DMPC | 6% | 1.5 | 303 | 6.4±0.2 | 35±1 | 6.8±0.1 | 32±1 |
| D7PC/DMPC | 3% | 1.5 | 303 | ND | ND | 8.4±0.1 | 27±1 |
| D7PC/DMPC | 1.5% | 1.5 | 303 | 18.0±0.2 | 12±1 | 9.1±0.1 | 25±1 |
| D7PC/DMPC | 0.5% | 1.5 | 303 | ND | ND | 9.8±0.2 | 23±1 |
| D7PC/DMPC | 0.25% | 1.5 | 303 | 20±0.2 | 11±1 | 9.7±0.3 | 23±1 |
| D7PC/DMPC | 0.1% | 1.5 | 303 | 18.4±0.4 | 12±1 | 10.0±0.3 | 22±1 |
| D8PC/DMPC | 12% | 0.3 | 303 | ND | ND | 0.20±0.01 | 1134±57 |
| D8PC/DMPC | 6% | 0.3 | 303 | ND | ND | 0.19±0.01 | 1194±63 |
| D8PC/DMPC | 3% | 0.3 | 303 | ND | ND | 0.27±0.01 | 840±31 |
| D8PC/DMPC | 1.5% | 0.3 | 303 | ND | ND | 1.1±0.1 | 207±19 |
| D8PC/DMPC | 1% | 0.3 | 303 | ND | ND | 1.1±0.1 | 207±19 |
| D8PC/DMPC | 0.5% | 0.3 | 303 | ND | ND | 2.2±0.1 | 103±5 |
| D8PC/DMPC | 0.25% | 0.3 | 303 | ND | ND | 3.2±0.1 | 71±3 |
| D8PC/DMPC | 0.1% | 0.3 | 303 | ND | ND | 4.7±0.2 | 48±2 |
| Cyclofos-4/DMPC | 12% | 8.5 | 303 | 8.3±0.3 | 27±1 | 6.9±0.1 | 33±1 |
| Cyclofos-5/DMPC | 12% | 4.5 | 303 | 10±0.2 | 22±1 | 7.1±0.1 | 32±1 |
| Cyclofos-7/DMPC | 12% | 0.6 | 303 | 9.5±0.1 | 23±1 | 7.0±0.1 | 32±1 |
| Cyclofos-6/DMPC | 12% | 2.7 | 303 | 10.5±0.2 | 21±1 | 8.4±0.1 | 27±1 |
| Cyclofos-6/DMPC | 12% | 2.7 | 313 | 21.3±0.3 | 12±1 | 16.0±0.1 | 16±1 |
| Cyclofos-6/DMPC | 12% | 2.7 | 293 | 6.6±0.2 | 26±1 | 5.2±0.1 | 33±1 |
| Cyclofos-6/DMPC | 6% | 2.7 | 303 | 14.7±0.3 | 15±1 | 9.4±0.1 | 24±1 |
| Cyclofos-6/DMPC | 3% | 2.7 | 303 | 14.2±0.3 | 16±1 | 11.0±0.1 | 20±1 |
| Cyclofos-6/DMPC | 1.5% | 2.7 | 303 | 16.4±0.2 | 14±1 | 11.0±0.1 | 20±1 |
| Cyclofos-6/DMPC | 0.75% | 2.7 | 303 | ND | ND | 10.5±0.1 | 21±1 |
| Cyclofos-6/DMPC | 0.5% | 2.7 | 303 | 15.0±0.5 | 15±1 | 10.6±0.4 | 21±2 |
| Cyclofos-6/DMPC | 0.3% | 2.7 | 303 | 13.1±0.3 | 17±1 | 6.8±0.1 | 33±1 |
| Cyclofos-6/DMPC | 0.2% | 2.7 | 303 | ND | ND | No bicelles | No bicelles |
| Cyclofos-6/DMPC | 0.1% | 2.7 | 303 | ND | ND | No bicelles | No bicelles |
Bicelle samples were prepared using two different mixing methods, both of which led to optically clear samples that were indistinguishable upon visual inspection. Method A (see methods) was a fairly gentle sample mixing protocol, while method B represents a much more thorough protocol that includes cycles of both mild sonication and freeze/thaw.
1-D 1H NMR spectra were acquired that reveal peaks from both the detergent and DMPC components of the mixtures. The DMPC peaks are only cleanly observed when DMPC is in its isotropic bicellar form (Figure 2, top). Upon transition to much larger assemblies that do not tumble isotropically, DMPC peaks either broaden dramatically or disappear completely as a result of reduced motions and the onset of extensive non-isotropically-averaged 1H-1H dipolar coupling (Figure 2, top). Thus, 1-D 1H NMR can be used to determine the lowest w/v detergent+lipid concentration at which isotropic bicelles persist.
For each detergent+DMPC mixture 1H NMR methods were used to measure the translational diffusion coefficients, Dt, for the bicelles (Figure 2, middle and bottom panels). Dt is a function both of the size and shape of the bicelles and the solution viscosity, the latter of which was observed to vary little in most of the ≤ 12% amphiphile mixtures examined in this work. Rather than invoke a series of assumptions in order to calculate disc dimensions from diffusion coefficients, the more assumption-free approach was taken of calculating hydrodynamic radii for the detergent-DMPC assemblies. While this means the bicelles are treated as spheres, which they are not, this approach does provide a straightforward approach to gaining qualitative insight into size differences between bicelles in solutions of different compositions. Bear in mind that aggregate molecular weight is expected to scale with the radius to the cubed power. The measured bicelle Dt are also useful in assessing the suitability of different bicelle mixtures for NMR spectroscopic use because bicelle rotational correlation time is inversely proportional to the cube of the Dt value45, which will determine NMR linewidths (assuming no contribution from intermediate timescale exchange processes).
D6PC-DMPC Bicelles
For q = 0.3 bicelles the diffusion coefficients measured at 303 K were seen to increase when diluting from 12% bicelles to 1% bicelles, regardless of whether sample mixing method A or B was used. Although part of this increase may be due to a small decrease in viscosity that accompanies dilution, diffusion measurements for water in these samples suggest that a decrease in viscosity cannot account for all of the increases in Dt. A possible explanation is that the size of the aggregates is dependent on the total D6PC+DMPC concentration, with the lipid-detergent assemblies actually getting smaller as the concentration is reduced. Alternatively, there may be other changes in the morphology, self-aggregation, or polydispersity of the lipid-containing isotropic assemblies that account for the reduction in Dt.
Below 1% the shift of D6PC from the bicelles to solution reached the point at which the NMR peaks from DMPC disappear, indicating complete loss of isotropic bicelles and formation of large anisotropic bilayered assemblies in coexistence with free DHPC. This result is expected based on the high CMC of D6PC (see Introduction).
It is surprising that the diffusion coefficient measurements observed for DMPC in D6PC/DMPC bicelles were dependent on whether samples were mixed using method A or method B, with the differences being more pronounced at low % compositions. Inspection of the 1-D 1H NMR spectra from samples prepared with mixing methods A and B were identical and did not reveal differences that shed light on this phenomenon. This dependency of the observed Dt on sample history suggests that the size, shape, and/or morphology of the isotropic lipid-detergent assemblies are dependent on sample history. It is also possible that there is more than one phase present in these samples, with the relative populations of the phases being dependent on sample history.
D7PC-DMPC Bicelles
In the dilute bicelle regime (0.1–3%) the diffusion coefficients for D7PC-DMPC are very similar to those for corresponding D6PC-DMPC mixtures. However, in the more concentrated 6–12% regime, the Dt for D7PC-DMPC is greatly reduced (e.g. larger particle size), suggesting either that a major change in the size the bicelles takes place at the higher concentrations or that some sort of morphological transition has occurred. One possibility is that at the higher concentrations, partitioning of D7PC into the central bilayered domain of the bicelles is increased at the expense of the edge-stabilizing population, which would increase the bicelle size. It has previously been shown that D7PC has a higher affinity for the bilayered domain of bicelles than D6PC.43–44
Notable in light of the goals of this work, Dt indicated that D7PC-DMPC mixtures retain bicelles properties at concentrations even as low as 0.1% total D7PC+DMPC. For D7PC/DMPC mixtures bicelles persist at very low concentrations. However, in the dilute bicelles regime diffusion coefficient measurements were extremely dependent on whether mixing method A or B was employed.
D8PC-DMPC Assemblies
Only mixing method A was employed in characterizing these mixtures. At 303 K in the 3–12% concentration range q = 0.3 D8PC-DMPC assemblies exhibited diffusion coefficients that are more than an order of magnitude lower than the lowest Dt observed for D7PC-DMPC. Only when diluted to 0.1% did D8PC-DMPC bicelles exhibit diffusion coefficients that approached the lowest values observed for D7PC-DMPC. It seems possible that D8PC-DMPC assemblies may be morphologically distinct from isotropic D6PC/DMPC and D7PC/DMPC mixtures, at least in the more concentrated samples. Further studies are required to establish the nature of the phases formed by these mixtures.
Cyclofos/DMPC Bicelles
q = 0.3 bicelles were examined for 12% mixtures of Cyclofos-4, 5, 6, or 7 with DMPC at 303 K. The diffusion coefficient for the Cyclofos-4/DMPC sample was an order of magnitude lower than for the other mixtures, suggesting that the morphology of the assemblies in this mixture is very different from the small isotropic bicelles formed by D6PC/DMPC and D7PC/DMPC mixtures. For DMPC/Cyclofos-5, -6, and -7 mixtures prepared using mixing method A, diffusion coefficients were fairly similar to that observed for the 12% D6PC/DMPC mixture. Of these, Cyclofos-6 yielded the largest Dt, indicative of the smallest aggregate size. For this reason we focused on Cyclofos-6/DMPC mixtures.
As Cyclofos-6/DMPC bicelles were serially diluted, Dt increased to plateau around 1.5% (Table 1). Once again, Dt values were seen to be dependent on sample history (mixing using method A versus method B). Upon further dilution below 0.5%, Dt decreased, suggesting that the CMC is being approached such that the bicelle size is increasing. This is reasonable: the reported CMC of Cyclofos-6 is 2.7 mM (www.anatrace.affymetrix.com), and the total concentration of this detergent in 0.3% q = 0.3 bicelles is 5.4 mM. Below 0.3%, all of the DMPC has shifted out of isotropic assemblies to become spectroscopically invisible, suggesting a transition to vesicles.
Diffusion coefficients were also measured for 3% q = 0.3 Cyclosfos-6/DMPC bicelles at 293 and 313 K. The diffusion coefficients for the bicelles are temperature dependent to an extent that goes well beyond the changes expected from the modest variations in the viscosity of water. Whether the observed changes seen when the temperature is lowered from 303 to 293 K is in any way related to the fact that pure DMPC bilayers exhibit a gel-to-liquid crystalline phase transition temperature at 298 K is unclear, but seems possible because high q bicelles have been shown to undergo a profound morphological change when the temperature is lowered below this temperature.20,31,46
DPC-DMPC Bicelles
The Dt observed for a q = 0.3 12% mixture of dodecylphosphocholine (DPC) and DMPC at 303 K was significantly smaller than for the corresponding Cyclofos-6/DMPC mixture, regardless of whether samples were prepared with mixing method A or B. This suggests there is little benefit to working with DPC-DMPC bicelles relative to Cyclofos-6/DMPC, given that the CMC of DPC is similar to that of Cyclofos-6 (both of which have 12 carbons in their alkyl tails). Accordingly, we did not further examine straight chain alkylphosphocholine/lipid mixtures.
NMR Spectra of an Integral Membrane Protein in Cyclofos-6/DMPC Bicelles
Integrins are the principal receptors that mediate interactions of cells with the extracellular matrix and are composed of transmembrane heterodimers between α and β subunits. The 18 α subunits and 8 β subunits found in mammals are thought to form some 24 different heterodimers. In human and other animals, certain β1-containing integrins are major receptors for extracellular collagen, laminin or fibronectin, and are broadly distributed in many tissues.47–48 For this work we prepared an 80 residue fragment of the human integrin β1 subunit that encompasses its entire transmembrane and cytosolic domains (β1-TM-CTD) and purified it into bicelles. A high total amphiphile concentration (20%) was used in order to maintain a high amphiphile-to-protein ratio, so as to avoid protein aggregation.
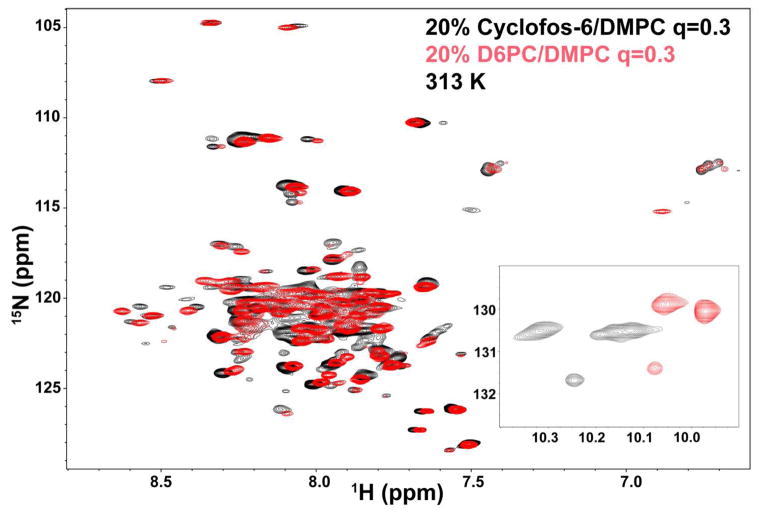
The NMR spectra of β1-TM-CTD in either D6PC/DMPC or Cyclofos-6/DMPC bicelles as q=0.3 are shown in Figure 3. The spectra of are of comparable quality, which are typical of the moderately well-dispersed spectra acquired from helical integral membrane proteins in micelles or bicelles. The spectra from the two bicelle types are similar, suggesting the protein adopts similar conformational states in both types of bicelles. The Cyclofos-6/DMPC bicelles yield similar or perhaps slightly better spectra than D6PC/DMPC (in that several additional peaks can be detected in the spectrum from Cyclofos-6/DMPC—see the 8.5 ppm 1H region). That the tryptophan indole side chain NH resonances (inset to Figure 3) are shifted to higher ppm values and are slightly better dispersed for Cyclofos-6/DMPC suggests that the protein may be slightly better ordered in these bicelles relative to D6PC/DMPC. That three indole peaks are observed in both bicelle mixtures even though the protein contains only two tryptophans suggest that one of the two tryptophan residues populates two different conformations, which are not in rapid exchange.
Figure 3.
Superimposed 600 MHz 2-D 1H,15N-TROSY NMR spectra of integrin β1-TM-CTD in q = 0.3 bicelles composed of either DHPC/DMPC (red) or Cyclofos-6/DMPC (black). The total amphiphile concentration was 20% and the temperature was 313 K. The inset plot shows the tryptophan side chain peaks.
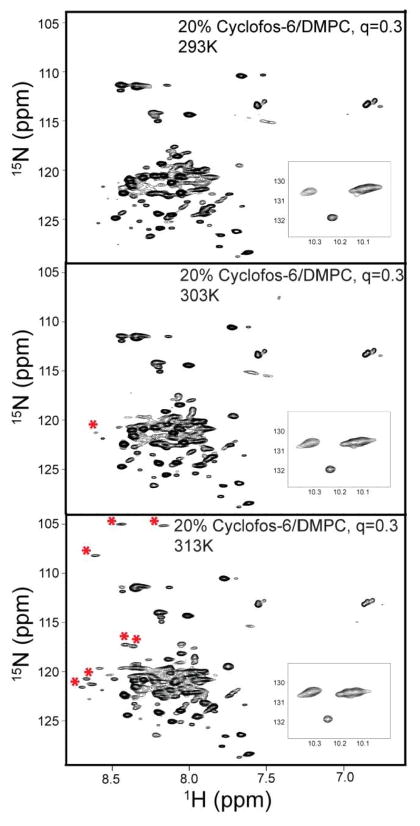
NMR spectra of β1-TM-CTD in q=0.3 Cyclofos-6/DMPC mixtures were also acquired as a function of temperature (Figure 4). As is typical for membrane proteins, the spectral quality increased significantly when the temperature was raised from 293 to 313 K. For example, a number of peaks above 8.5 ppm in the 1H dimension and below 110 ppm in the 15N dimension are seen only at the highest temperature tested (see peaks highlighted with asterisks). These peaks most likely derive from the transmembrane segment, which will have little motion beyond overall bicelle tumbling, the latter of which is known for Cylclosfos-6/DMPC bicelles to be highly temperature dependent (see Table 1).
Figure 4.
Temperature-dependent 600 MHz 2-D 1H,15N-TROSY NMR spectra of integrin β1-TM-CTD in q = 0.3 Cyclofos-6/DMPC bicelles at 20% total amphiphile concentration.
NMR spectra were also acquired for U-15N-β1-TM-CTD prepared in 12% q=0.3 303K D6PC/DMPC and Cylclofos-6/DMPC mixtures that were prepared with bicelles that were mixed using either method A or method B. For each type of bicelle it was observed that the resulting TROSY NMR spectrum of the protein was seen to be identical regardless of mixing method, with the spectral quality from the D6PC/DMPC samples being similar to the spectral quality from the Cylclofos-6/DMPC samples (data not shown).
Discussion
The results of this work suggest that q = 0.3 D7PC/DMPC and Cyclofos-6/DMPC both form small bicelles that are likely to be similar to the relatively well-characterized D6PC/DMPC bicelles, but that have the advantage that they remain isotropic at concentrations lower than the 1% limit observed for q = 0.3 D6PC/DMPC. Isotropic assemblies persist down to 0.3% for Cyclofos-6/DMPC and down to 0.1% for D7PC/DMPC. Use of these bicelles at concentrations of 0.5% or less may lead to considerable cost savings over the use of ≥1% D6PC/DMPC in high volume applications such as liquid chromatography.
Aside from the possibility of working at very low bicelle concentrations, there appears to be little advantage to working with D7PC/DMPC relative to D6PC/DMPC bicelles. The most favorable (highest) observed diffusion coefficients in each of these systems are comparable. On the other hand, when concentrations of D7PC/DMPC bicelles are raised above 3%, as is often required for NMR studies of membrane proteins, the observed Dt for the D7PC/DMPC assemblies falls off steeply, indicating effective aggregate sizes increase significantly at higher % detergent+lipid. This phenomenon would have an adverse effect on NMR linewidths. We suggest that D7PC/DMPC bicelles may best be employed in non-NMR applications where it is desirable to work at as low bicelle concentrations as possible.
Some of the most promising results of this work pertain to a newly introduced system: bicelles composed of the detergent Cyclofos-6 and DMPC. Over the 0.5–12% range, these bicelles yielded diffusion coefficients that are comparable with corresponding D6PC/DMPC mixtures. A possible advantage of Cyclofos-6 is that because of the bulky cyclohexyl group at the end of the alkyl tail of Cylclofos-6, this detergent may be less likely to partition with DMPC in the bilayered domain of bicelles, potentially providing a more native-like bilayered interior relative to D6PC- or D7PC-based bicelles, for which the detergent component is known to significantly partition into the bilayered phase, especially D7PC.43,44 Of course, only future experiments can confirm this hypothesis. It is, however, interesting to note that the bulkiness of the cyclohexyl group of the Cyclofos series has previously been exploited for biochemical reasons. Kay and co-workers found that conventional straight-chain alkyl-based detergents inhibit lipid substrate binding to PagP, a beta-barrel outer membrane enzyme that has a lipid substrate.49 By employing Cyclofos-7 as the detergent used to solubilize PagP for NMR studies, they were able study the complex of PagP with its lipid substrate. This is because the bulky cyclohexyl group of the detergent cannot penetrate the substrate binding site to interfere with substrate binding.
An unusual observation made in this work is that bicelle properties in q = 0.3 bicelles (3.3:1 detergent-to-lipid mole ratio) seem to be dependent on exact sample history, specifically how samples were mixed. This was true for all the bicelle types tested. Dependence of bicelle properties on the method used to prepare them indicates the size, shape, morphology, and/or population distribution of the lipid-detergent assembles depends on sample history. While the coexistence of more than one aggregate type or phase would not be unusual, that the assemblies seem to be kinetically trapped (sample history dependent) is very surprising at such high detergent-to-lipid ratios and also in light of the fact that 1H NMR spectra do not seem to reveal the presence of multiple phases in slow exchange. We do not have a good explanation for the sample history dependence of the diffusion coefficient measurements, which may be worthy of further study. From a practical standpoint, the fact that identical NMR spectra were for the integrin β1-TM-CTD protein in both Cyclofos-6 and D6PC-based bicelles regardless of which mixing method was used suggests that the sample history issues documented herein may not complicate biochemical and biophysical applications of low q bicelles as model membranes.
To conclude, this work has identified two detergent/DMPC systems for which isotropic assemblies persist at total amphiphile concentrations well below 1%: D7PC/DMPC and Cyclofos-6/DMPC mixtures. This work has also introduced Cyclosfos-6/DMPC assemblies as a novel family of bicelles that is both highly dilutable and that appears to have attractive biochemical properties. It is emphasized, however, that the phase behavior of bicelles at low q ratios may be more complex than previously realized.
Abbreviations
- CHAPSO
3-(cholamidopropyl)dimethylammonio-2-hydroxy-1-propanesulfonate
- CMC
critical micelle concentration
- CTD
C-terminal domain
- Cyclofos-4
4-cyclohexyl-1-butylphosphocholine
- Cyclofos-5
5-Cyclohexyl-1-pentylphosphocholine
- Cyclofos-6
6-cyclohexyl-1-hexylphosphocholine
- Cyclofos-7
7-cyclohexyl-1-heptylphosphocholine
- D6PC
1,2-dihexanoyl-sn-glycero-3-phosphocholine(D6PC)
- D7PC
1,2-diheptanoyl-sn-glycero-3-phosphocholine
- D8PC
1,2-dioctanoyl-sn-glycero-3-phosphocholine
- DMPC
1,2-dimyrisotyl-sn-glycero-3-phosphocholine
- Dt
translational diffusion coefficient
- EDTA
ethylenediaminetetraacetic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IPTG
isopropylthiogalactoside
- NMR
nuclear magnetic resonance
- OD
optical density
- NTA
nitriloacetate
- PMSF
phenylmethylsufonylfluoride
- TM
transmembrane
Footnotes
This work was supported by US NIH grants RO1 DK083187, RO1 DC007417, R01DK075594, and U54 GM94608. W. D. V. H. and S. M. are American Heart Association Postdoctoral Fellows (Southeast Affiliate). RZ has a Merit Award from the Department of Veterans affair and is an Established Investigator of the American Heart Association.
References
- 1.De Angelis AA, Opella SJ. Bicelle samples for solid-state NMR of membrane proteins. Nat Protoc. 2007;2:2332–2338. doi: 10.1038/nprot.2007.329. [DOI] [PubMed] [Google Scholar]
- 2.Raschle T, Hiller S, Etzkorn M, Wagner G. Nonmicellar systems for solution NMR spectroscopy of membrane proteins. Curr Opin Struct Biol. 2010;20:471–479. doi: 10.1016/j.sbi.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders CR, Hare BJ, Howard KP, Prestegard JH. Magnetically- Oriented Phospholipid Micelles as a Tool for the Study of Membrane-Associated Molecules. Prog Nucl Mag Res Sp. 1994;26:421–444. [Google Scholar]
- 4.Whiles JA, Deems R, Vold RR, Dennis EA. Bicelles in structure-function studies of membrane-associated proteins. Bioorg Chem. 2002;30:431–442. doi: 10.1016/s0045-2068(02)00527-8. [DOI] [PubMed] [Google Scholar]
- 5.Sanders CR, Prosser RS. Bicelles: a model membrane system for all seasons? Struct Fold Des. 1998;6:1227–1234. doi: 10.1016/s0969-2126(98)00123-3. [DOI] [PubMed] [Google Scholar]
- 6.Prosser RS, Evanics F, Kitevski JL, Al-Abdul-Wahid MS. Current applications of bicelles in NMR studies of membrane-associated amphiphiles and proteins. Biochemistry. 2006;45:8453–8465. doi: 10.1021/bi060615u. [DOI] [PubMed] [Google Scholar]
- 7.Faham S, Bowie JU. Bicelle crystallization: a new method for crystallizing membrane proteins yields a monomeric bacteriorhodopsin structure. J Mol Biol. 2002;316:1–6. doi: 10.1006/jmbi.2001.5295. [DOI] [PubMed] [Google Scholar]
- 8.Vinothkumar KR. Structure of rhomboid protease in a lipid environment. J Mol Biol. 2011;407:232–247. doi: 10.1016/j.jmb.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbosa-Barros L, Barba C, Cocera M, Coderch L, Lopez-Iglesias C, de la Maza A, Lopez O. Effect of bicellar systems on skin properties. Int J Pharm. 2008;352:263–272. doi: 10.1016/j.ijpharm.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Maler L, Graslund A. Artificial membrane models for the study of macromolecular delivery. Methods Mol Biol. 2009;480:129–139. doi: 10.1007/978-1-59745-429-2_9. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez G, Rubio L, Cocera M, Estelrich J, Pons R, de la Maza A, Lopez O. Application of bicellar systems on skin: diffusion and molecular organization effects. Langmuir. 2010;26:10578–10584. doi: 10.1021/la100691m. [DOI] [PubMed] [Google Scholar]
- 12.Rubio L, Alonso C, Rodriguez G, Barbosa-Barros L, Coderch L, De la Maza A, Parra JL, Lopez O. Bicellar systems for in vitro percutaneous absorption of diclofenac. Int J Pharmaceut. 2010;386:108–113. doi: 10.1016/j.ijpharm.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Andersson A, Maler L. Size and shape of fast-tumbling bicelles as determined by translational diffusion. Langmuir. 2006;22:2447–2449. doi: 10.1021/la053177l. [DOI] [PubMed] [Google Scholar]
- 14.Chou JJ, Baber JL, Bax A. Characterization of phospholipid mixed micelles by translational diffusion. J Biomol NMR. 2004;29:299–308. doi: 10.1023/B:JNMR.0000032560.43738.6a. [DOI] [PubMed] [Google Scholar]
- 15.Glover KJ, Whiles JA, Wood MJ, Melacini G, Komives EA, Vold RR. Conformational dimorphism and transmembrane orientation of prion protein residues 110–136 in bicelles. Biochemistry. 2001;40:13137–13142. doi: 10.1021/bi011485m. [DOI] [PubMed] [Google Scholar]
- 16.Lee D, Walter KFA, Bruckner AK, Hilty C, Becker S, Griesinger C. Bilayer in small bicelles revealed by lipid-protein interactions using NMR spectroscopy. J Am Chem Soc. 2008;130:13822–13823. doi: 10.1021/ja803686p. [DOI] [PubMed] [Google Scholar]
- 17.Lind J, Nordin J, Maler L. Lipid dynamics in fast-tumbling bicelles with varying bilayer thickness: effect of model transmembrane peptides. Biochim Biophys Acta. 2008;1778:2526–2534. doi: 10.1016/j.bbamem.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Vold RR, Prosser RS, Deese AJ. Isotropic solutions of phospholipid bicelles: A new membrane mimetic for high-resolution NMR studies of polypeptides. Journal of Biomolecular Nmr. 1997;9:329–335. doi: 10.1023/a:1018643312309. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Su K, Guan X, Sublette ME, Stark RE. Assessing the size, stability, and utility of isotropically tumbling bicelle systems for structural biology. Biochim Biophys Acta. 2010;1798:482–488. doi: 10.1016/j.bbamem.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsaras J, Harroun TA, Pencer J, Nieh MP. “Bicellar” lipid mixtures as used in biochemical and biophysical studies. Naturwissenschaften. 2005;92:355–366. doi: 10.1007/s00114-005-0641-1. [DOI] [PubMed] [Google Scholar]
- 21.Nieh MP, Raghunathan VA, Kline SR, Harroun TA, Huang CY, Pencer J, Katsaras J. Spontaneously formed unilamellar vesicles with path-dependent size distribution. Langmuir. 2005;21:6656–6661. doi: 10.1021/la0508994. [DOI] [PubMed] [Google Scholar]
- 22.Pabst G, Kucerka N, Nieh MP, Rheinstadter MC, Katsaras J. Applications of neutron and X-ray scattering to the study of biologically relevant model membranes. Chem Phys Lipids. 2010;163:460–479. doi: 10.1016/j.chemphyslip.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Van Dam L, Karlsson G, Edwards K. Direct observation and characterization of DMPC/DHPC aggregates under conditions relevant for biological solution NMR. Bba-Biomembranes. 2004;1664:241–256. doi: 10.1016/j.bbamem.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Van Dam L, Karlsson G, Edwards K. Morphology of magnetically aligning DMPC/DHPC aggregates-perforated sheets, not disks. Langmuir. 2006;22:3280–3285. doi: 10.1021/la052988m. [DOI] [PubMed] [Google Scholar]
- 25.Soong R, Macdonald PM. Water diffusion in bicelles and the mixed bicelle model. Langmuir. 2009;25:380–390. doi: 10.1021/la801739a. [DOI] [PubMed] [Google Scholar]
- 26.Soong R, Majonis D, Macdonald PM. Size of bicelle defects probed via diffusion nuclear magnetic resonance of PEG. Biophys J. 2009;97:796–805. doi: 10.1016/j.bpj.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Angelis AA, Jones DH, Grant CV, Park SH, Mesleh MF, Opella SJ. NMR experiments on aligned samples of membrane proteins. Methods Enzymol. 2005;394:350–382. doi: 10.1016/S0076-6879(05)94014-7. [DOI] [PubMed] [Google Scholar]
- 28.Sanders CR, Landis GC. Reconstitution of Membrane-Proteins into Lipid-Rich Bilayered Mixed Micelles for Nmr-Studies. Biochemistry. 1995;34:4030–4040. doi: 10.1021/bi00012a022. [DOI] [PubMed] [Google Scholar]
- 29.Bayburt TH, Sligar SG. Membrane protein assembly into Nanodiscs. Febs Lett. 2010;584:1721–1727. doi: 10.1016/j.febslet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin TL, Chen SH, Gabriel NE, Roberts MF. Use of Small-Angle Neutron-Scattering to Determine the Structure and Interaction of Dihexanoylphosphatidylcholine Micelles. J Am Chem Soc. 1986;108:3499–3507. [Google Scholar]
- 31.Sanders CR, Schwonek JP. Characterization of Magnetically Orientable Bilayers in Mixtures of Dihexanoylphosphatidylcholine and Dimyristoylphosphatidylcholine by Solid-State Nmr. Biochemistry. 1992;31:8898–8905. doi: 10.1021/bi00152a029. [DOI] [PubMed] [Google Scholar]
- 32.Bocharov EV, Mayzel ML, Volynsky PE, Goncharuk MV, Ermolyuk YS, Schulga AA, Artemenko EO, Efremov RG, Arseniev AS. Spatial Structure and pH-dependent Conformational Diversity of Dimeric Transmembrane Domain of the Receptor Tyrosine Kinase EphA1. J Biol Chem. 2008;283:29385–29395. doi: 10.1074/jbc.M803089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bocharov EV, Mayzel ML, Volynsky PE, Mineev KS, Tkach EN, Ermolyuk YS, Schulga AA, Efremov RG, Arseniev AS. Left-Handed Dimer of EphA2 Transmembrane Domain: Helix Packing Diversity among Receptor Tyrosine Kinases. Biophysical Journal. 2010;98:881–889. doi: 10.1016/j.bpj.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau TL, Partridge AW, Ginsberg MH, Ulmer TS. Structure of the integrin beta3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry. 2008;47:4008–4016. doi: 10.1021/bi800107a. [DOI] [PubMed] [Google Scholar]
- 35.Lau TL, Kim C, Ginsberg MH, Ulmer TS. The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling. EMBO J. 2009;28:1351–1361. doi: 10.1038/emboj.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mineev KS, Bocharov EV, Pustovalova YE, Bocharova OV, Chupin VV, Arseniev AS. Spatial structure of the transmembrane domain heterodimer of ErbB1 and ErbB2 receptor tyrosine kinases. J Mol Biol. 2010;400:231–243. doi: 10.1016/j.jmb.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Poget SF, Cahill SM, Girvin ME. Isotropic bicelles stabilize the functional form of a small multidrug-resistance pump for NMR structural studies. J Am Chem Soc. 2007;129:2432–2433. doi: 10.1021/ja0679836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu DH, Chen AD, Johnson CS. An Improved Diffusion-Ordered Spectroscopy Experiment Incorporating Bipolar-Gradient Pulses. J Magn Reson Ser A. 1995;115:260–264. [Google Scholar]
- 39.Stejskal EO, Tanner JE. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J Chem Phys. 1965;42:288–292. [Google Scholar]
- 40.Findeisen M, Brand T, Berger S. A 1H-NMR thermometer suitable for cryoprobes. Magn Reson Chem. 2007;45:175–178. doi: 10.1002/mrc.1941. [DOI] [PubMed] [Google Scholar]
- 41.Cantor CR, Schimmel PR. Techniques for the study of biological structure and function. W. H. Freeman; San Francisco: 1980. [Google Scholar]
- 42.Weigelt J. Single scan, sensitivity- and gradient-enhanced TROSY for multidimensional NMR experiments. J Am Chem Soc. 1998;120:10778–10779. [Google Scholar]
- 43.Triba MN, Warschawski DE, Devaux PF. Reinvestigation by phosphorus NMR of lipid distribution in bicelles. Biophysical Journal. 2005;88:1887–1901. doi: 10.1529/biophysj.104.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Triba MN, Devaux PF, Warschawski DE. Effects of lipid chain length and unsaturation on bicelles stability. A phosphorus NMR study. Biophysical Journal. 2006;91:1357–1367. doi: 10.1529/biophysj.106.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao S, Babon JJ, Norton RS. Protein effective rotational correlation times from translational self-diffusion coefficients measured by PFG-NMR. Biophys Chem. 2008;136:145–151. doi: 10.1016/j.bpc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Sanders CR, 2nd, Prestegard JH. Magnetically orientable phospholipid bilayers containing small amounts of a bile salt analogue, CHAPSO. Biophys J. 1990;58:447–460. doi: 10.1016/S0006-3495(90)82390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 49.Hwang PM, Bishop RE, Kay LE. The integral membrane enzyme PagP alternates between two dynamically distinct states. P Natl Acad Sci USA. 2004;101:9618–9623. doi: 10.1073/pnas.0402324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burns RA, Roberts MF, Dluhy R, Mendelsohn R. Monomer-to-Micelle Transition of Dihexanoylphosphatidylcholine - C-13 Nmr and Raman Studies. J Am Chem Soc. 1982;104:430–438. [Google Scholar]
- 51.Hauser H. Short-chain phospholipids as detergents. Bba-Biomembranes. 2000;1508:164–181. doi: 10.1016/s0304-4157(00)00008-3. [DOI] [PubMed] [Google Scholar]
- 52.Tausk RJ, Karmiggelt J, Oudshoorn C, Overbeek JT. Physical chemical studies of short-chain lecithin homologues. I. Influence of the chain length of the fatty acid ester and of electrolytes on the critical micelle concentration. Biophys Chem. 1974;1:175–183. doi: 10.1016/0301-4622(74)80004-9. [DOI] [PubMed] [Google Scholar]
- 53.Tausk RJ, van Esch J, Karmiggelt J, Voordouw G, Overbeek JT. Physical chemical studies of short-chain lecithin homologues. II. Micellar weights of dihexanoyl- and diheptanoyllecithin. Biophys Chem. 1974;1:184–203. doi: 10.1016/0301-4622(74)80005-0. [DOI] [PubMed] [Google Scholar]