Abstract
Background
The role of intraoperative cholangiography (IOC) in prevention of common bile duct (CBD) injuries and the management of CBD stones is controversial, and current variation in use of IOC has not been well described.
Study Design
Multilevel hierarchical models using data from the Texas Hospital Inpatient Discharge Public Use data files (2001–2008) were used to evaluate the percentage of variance in the use of IOC that was attributable to patient, surgeon, and hospital factors.
Results
A total of 176,981 cholecystectomies were performed in 212 hospitals in Texas. There was wide variation in IOC use, ranging from 2.4% to 98.4% of cases among surgeons and 3.7% to 94.8% of cases among hospitals, even after adjusting for case mix differences. The percentage of variance in IOC use attributable to the surgeon was 20.7% and an additional 25.7% was attributable to the hospital. IOC use was associated with increased age, gallstone pancreatitis or CBD stones, Hispanic race, decreased illness severity, insurance, and later year of cholecystectomy. ERCP (24.0% vs. 14.9%, P<0.0001) and CBD exploration (1.63% vs. 0.42%, P<0.0001) were more commonly performed in patients undergoing IOC.
Conclusions
Uncertainty regarding the benefit of IOC leads to wide variation in use across surgeons and hospitals. The surgeon and hospital are more important determinants of IOC use than patient characteristics. Our study highlights the need for further evaluation of comparative effectiveness of IOC in the prevention of CBD injuries and retained stones taking into account patient risk factors, surgeon skill, cost, and availability of local expertise.
Keywords: acute cholecystitis, gallstone pancreatitis, common bile duct stones, cholecystectomy, intraoperative cholangiography
INTRODUCTION
More than 900,000 cholecystectomies were performed in 2006, making it one of the most frequently performed surgical procedures in the United States.1–3 Intraoperative cholangiography (IOC) performed during cholecystectomy is used for two reasons: 1) to delineate biliary anatomy and 2) to detect common bile duct stones.
Guidelines for laparoscopic biliary tract surgery from the Society for American Gastrointestinal and Endoscopic Surgeons (SAGES) do not include recommendations regarding routine use of IOC, and SAGES indicates that reliable algorithms for selective use have not been developed.4 Because evidence is inconsistent regarding the effectiveness of IOC in the prevention of common bile duct injury and management of common bile duct stones,5–11 routine versus selective IOC use has long been debated among surgeons. Previous studies have documented varying use across surgeons12,13 as well as disagreement regarding clear indications for use.14 However, this variation has not been well described.
Ideally, the use of medical interventions is directed by evidence-based guidelines and determined based on patients’ individual and disease characteristics. Wennberg hypothesized that with increasing uncertainty regarding the optimal treatment course, the use of medical interventions is increasingly determined by physician, and not patient characteristics.15,16 As such, we hypothesized that the uncertainty regarding the indications for IOC use would lead to large variation in use across providers, at both the surgeon and hospital level. To assess the importance of both the surgeon and the hospital in the use of IOC, we used Texas hospital discharge data to conduct a large, population-based, multilevel analysis. Specifically, we evaluated the percentage of variance in the use of IOC that was attributable to patient, surgeon, and hospital factors.
METHODS
The study protocol was approved by the Institutional Review Board at the University of Texas Medical Branch at Galveston, Texas.
Patient Population
Data for this study were obtained from the Texas Hospital Inpatient Discharge Public Use Data Files. The Texas Health Care Information Collect ion Center for Health Statistics of the Texas Department of State Health Services collects quarterly hospital discharge data from all state licensed hospitals to develop administrative reports on use and quality of hospital care in Texas.17 Each record includes patient demographics, hospital information, duration of stay, International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes, ICD-9-CM procedure codes, hospital day of procedure, hospital charges, payer information, and discharge status. Data do not contain any personal identifiers.
The study included all patients discharged from an acute care hospital after undergoing cholecystectomy (ICD-9 procedure codes 51.23, 51.24, 51.21, 51.22) from January 2001 through December 2008. We included patients with diagnosis codes for biliary colic, acute cholecystitis, gallstone pancreatitis, or CBD stones. All other diagnoses were excluded. The diagnostic groups were mutually exclusive. Patients with gallstone pancreatitis were identified first and included any record with any discharge diagnosis code for gallstones (ICD-9 diagnosis codes 574* or 575* with any extension) and a diagnosis of acute pancreatitis (577.0).18,19 The remaining patients were classified as having CBD stones (ICD-9 codes 574.3*, 574.4*, 574.5*, 574.6*, 574.7*, 574.8*, 574.9*), acute cholecystitis (ICD-9 codes 574.0*, 574.1*, 575.0*, 575.1*, 575.2*, 575.3*, 575.4*), or biliary colic (ICD-9 codes 574.2*) in a hierarchical fashion. The overall cohort was restricted to patients 18 years and older.
Patient Characteristics
Independent covariates included age group (continuous age not available in Texas discharge data), gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), indication for cholecystectomy (biliary colic, acute cholecystitis, gallstone pancreatitis, common bile duct stones), type of cholecystectomy (ICD-9 procedure codes: open - 51.21, 51.22; laparoscopic - 51.23, 51.24), insurance coverage (uninsured, Medicare, Medicaid, other insurance), year of cholecystectomy, and severity of illness. The “Severity of Illness” variable, calculated with All Patient Refined-Diagnosis Related Groups (APR-DRG) software, was used to control for patient comorbidity. This variable uses comorbidity, age, and certain procedures to produce a severity of illness score from 0–4, with 4 being the most severe. Categories 0 and 1 were collapsed together in this study because < 150 patients had an illness severity score of 0.
Identification of Hospitals
Texas hospitals that performed fewer than 160 inpatient cholecystectomies during the 8-year period (approximately 20 per year) were excluded from the analysis. With the above patient inclusion/exclusion criteria, this left 176,981 patients undergoing cholecystectomy at 212 Texas hospitals. We did this in order to provide stable estimates of IOC use, eliminating hospitals with too few cases to determine true rates of IOC. Hospital volume was calculated based on the total number of cholecystectomies performed over the study period, and hospitals were stratified into quartiles of hospital cholecystectomy volume. Hospitals belonging to the Council of Teaching Hospitals were classified as teaching hospitals.
Identification of Surgeons
The Texas Inpatient Public Use data file provides a unique physician identifier for the operating surgeon. However, physician identifiers are suppressed when the number of physicians represented in a DRG for a hospital is less than the minimum cell size of five. As such, we were able to identify the individual surgeon in 89% (N=157,618) of the overall cohort. For multilevel modeling, surgeons needed to be clustered within hospitals, but some surgeons operated at more than one hospital. Therefore, we assigned the surgeon to the hospital at which he or she did the most cholecystectomies and did not count procedures performed at other hospitals. This left 3,110 surgeons operating on 82,013 patients for the 3-level models (patients clustered within surgeon and surgeons clustered within hospitals).
Finally, the Texas discharge data physician identifiers are encrypted. We can identify that the same surgeon performed cholecystectomy on different patients, but without surgeon Unique Provider Identification Number (UPIN) or National Provider Identifier (NPI, after 2007) numbers we were unable to obtain any surgeon level characteristics from the American Medical Association Physician Masterfile.
IOC Use
IOC use, identified by ICD-9 procedure code 87.53, was determined at the level of the patient (yes/no), the individual surgeon (% IOC use for all cholecystectomies performed by the individual surgeon, 2001–2008), and the hospital (% IOC use for all cholecystectomies performed at the hospital, 2001–2008).
Statistical Analysis: Variation
Differences in percentage of patients undergoing IOC across strata of patient characteristics were compared using chi-square tests. Logistic regression models were used to estimate the odds of IOC use (dependent variable). Patient characteristics were treated as independent variables. The percentage of variance in IOC use that was attributable to each patient characteristic was calculated with the generalized coefficient of determination (maximum rescaled R2) for unadjusted analyses (each characteristic was evaluated independently without adjustment for other variables) and calculated with the partial generalized coefficient of determination for adjusted analyses (each characteristic analyzed with simultaneous adjustment for other variables).20
Unadjusted variation in IOC use for individual hospitals and individual surgeons was calculated and represented graphically. For unadjusted hospital-level IOC use, all 176,981 patients undergoing cholecystectomy at 212 hospitals were included (cohort already limited to hospitals doing 160 or more cholecystectomies in the time period). For the surgeon-level unadjusted graphs, only surgeons performing 40 or more cholecystectomies from 2001–2008 were included. This left 706 surgeons operating on 144,986 patients within the 212 hospitals for the unadjusted surgeon analysis. This was done to provide stable estimates of variation.
Use of IOC was further evaluated using hierarchical generalized linear models for multilevel analyses. This method is appropriate when there are various levels of data clustered within each other and allows for the estimation and partitioning of variance in IOC use between the patient, surgeon, and hospital levels. Because we were only able to identify the surgeon in 82,013 patients (46.3%) we used both 2-level [patients (level 1) clustered within hospitals (level 2), N=176,981] and 3-level [patients (level 1) clustered within surgeons and surgeons (level 2) clustered within hospitals (level 3)] hierarchical models. For the 3-level hierarchical models, we included all surgeons regardless of their cholecystectomy volume. In this type of model, surgeons doing more cholecystectomies would contribute more heavily to surgeon variance. The final cohort for the 3-level models included 3,110 surgeons performing cholecystectomy on 82,013 patients at 179 Texas hospitals. For surgeons included in the multilevel model doing 5 or more cholecystectomies, the adjusted IOC use was calculated and represented graphically.
As a measure of the importance of the surgeon and the hospital in IOC use, we measured the intraclass correlation coefficient (ICC), which represents the percentage of the total variance in IOC use attributable to the surgeon and hospital. This value was estimated using the threshold technique that is appropriate for dichotomous outcomes.21 Both “null” models, which do not include any patient characteristics, and adjusted models, which include all of these variables, were used. For the adjusted models, results were calculated in two ways. In the first method, a residual ICC was calculated that represents the percentage of variance attributable to surgeon and hospital after adjustment for patient characteristics. In the second, the variance was further partitioned into contributions from the surgeon, hospital, and from important patient characteristics to calculate the percentage of total variance from each component. The 2- and 3-level models were then used to calculate adjusted surgeon and hospital rates of IOC after controlling for patient characteristics.
Statistical significance was accepted at the P<0.05 level. However, given the large sample size, very small differences were highly statistically significant, so we focus on clinically relevant differences in IOC use. SAS 9.2 statistical software (Cary, NC) was used for data management and statistical analysis.
RESULTS
Patient and Tumor Characteristics
Using the Texas Hospital Inpatient Public Use file we identified 176,981 patients aged 18 years and older undergoing inpatient cholecystectomy at 212 hospitals in Texas between 2001 and 2008. Of these, 78,868 (44.6%) had a concurrent IOC. Table 1 shows the percentage of patients who had IOC by stratum of patient characteristics. All P-values are significant due to the large sample size. IOC was more commonly used in older patients, patients with gallstone pancreatitis or common bile duct stones, patients undergoing laparoscopic cholecystectomy, and sicker patients.
Table 1.
Patient Characteristics and Unadjusted IOC use for Patients Undergoing Inpatient Cholecystectomy, Texas Hospital Discharge Data 2001–2008
| n (%) | % with IOC | p Value | |
|---|---|---|---|
| Overall cohort | 176,981 (100) | 44.6 | |
| Age group, y | <0.0001 | ||
| 18–44 | 78,243 (44.2) | 43.2 | |
| 45–54 | 28,884 (16.3) | 43.4 | |
| 55–64 | 25,320 (14.3) | 44.8 | |
| 65–74 | 21,685 (12.2) | 46.8 | |
| 75+ | 22,849 (12.9) | 48.5 | |
| Sex | 0.009 | ||
| Male | 52,744 (30.6) | 44.0 | |
| Female | 119,812 (69.4) | 44.7 | |
| Race/ethnicity | <0.0001 | ||
| White | 85,861 (48.5) | 43.3 | |
| Black | 15,283 (8.6) | 39.0 | |
| Hispanic | 65,576 (37.1) | 47.2 | |
| Other | 10,261 (5.8) | 46.7 | |
| Indication for cholecystectomy | <0.0001 | ||
| Acute cholecystitis | 118,640 (67.0) | 37.0 | |
| Gallstone pancreatitis | 34,126 (19.3) | 62.0 | |
| CBD stones | 20,369 (11.5) | 61.5 | |
| Biliary colic | 3,846 (2.2) | 34.8 | |
| Type of cholecystectomy | <0.0001 | ||
| Laparoscopic | 157,361 (88.9) | 45.1 | |
| Open | 19,620 (11.1) | 40.6 | |
| Insurance | <0.0001 | ||
| Uninsured | 34,879 (19.7) | 40.8 | |
| Medicare | 44,475 (25.1) | 47.2 | |
| Medicaid | 15,295 (8.7) | 48.6 | |
| Other insurance | 82,262 (46.5) | 44.0 | |
| Year of cholecystectomy | <.0001 | ||
| 2001 | 19,691 (11.1) | 42.3 | |
| 2002 | 21,374 (12.1) | 42.6 | |
| 2003 | 21,963 (12.4) | 44.9 | |
| 2004 | 22,834 (12.9) | 44.6 | |
| 2005 | 22,454 (12.7) | 45.5 | |
| 2006 | 23,123 (13.1) | 45.8 | |
| 2007 | 22,695 (12.8) | 45.3 | |
| 2008 | 22,847 (12.9) | 44.9 | |
| Illness severity score | <0.0001 | ||
| 1 | 72,583 (41.0) | 38.0 | |
| 2 | 68,818 (38.9) | 47.8 | |
| 3 | 30,189 (17.1) | 52.8 | |
| 4 | 5,391 (3.0) | 44.5 |
Unadjusted Variation in IOC Use
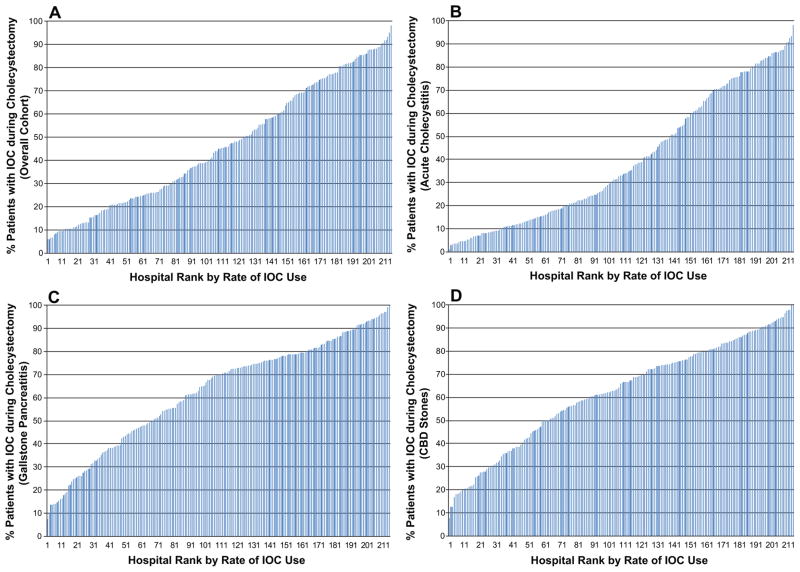
In the 212 Texas hospitals that did more than 160 cholecystectomies, the unadjusted percentage of cholecystectomies performed with IOC at a given hospital ranged from 6.0% to 98.2% (Figure 1A), with IOC use evenly distributed across this range (median use = 45.1%, interquartile range (IQR) 23.6% – 70.5%). Hospital variation in IOC use was evident regardless of indication for cholecystectomy. Unadjusted IOC use ranged from 1.3% to 98.0% of cholecystectomies in patients with acute cholecystitis (median use = 32.9%, IQR 14.6% – 66.6%, N=118,640, Figure 1B). While the range was similar, IOC use was increased in patients with gallstone pancreatitis and CBD stones. 7.6%to 100% of cholecystectomies in patients with gallstone pancreatitis (median use = 70.0%, IQR 45.7% – 79.6%, N=34,126, Figure 1C) and 7.7% to 100% in patients with common bile duct stones (median use = 66.1%, 45.6% – 80.2%, N=20,369, Figure 1D). Similar wide variation was noted in the 3,846 patients undergoing inpatient cholecystectomy for biliary colic (not shown).
Figure 1.
Unadjusted percentage of cholecystectomies performed with IOC in 212 Texas hospitals that did more than 160 cholecystectomies (2001–2008). (A) For all cholecystectomies, regardless of indication, median use = 45.1% (IQR 23.6% – 70.5%). (B) For cholecystectomies done for acute cholecystitis, median use = 32.9% (IQR 14.6% – 66.6%). (C) For cholecystectomies done for gallstone pancreatitis, median use = 70.0% (IQR 45.7% – 79.6%). (D) For cholecystectomies done for CBD stones, median use = 66.1% (IQR 45.6% – 80.2%).
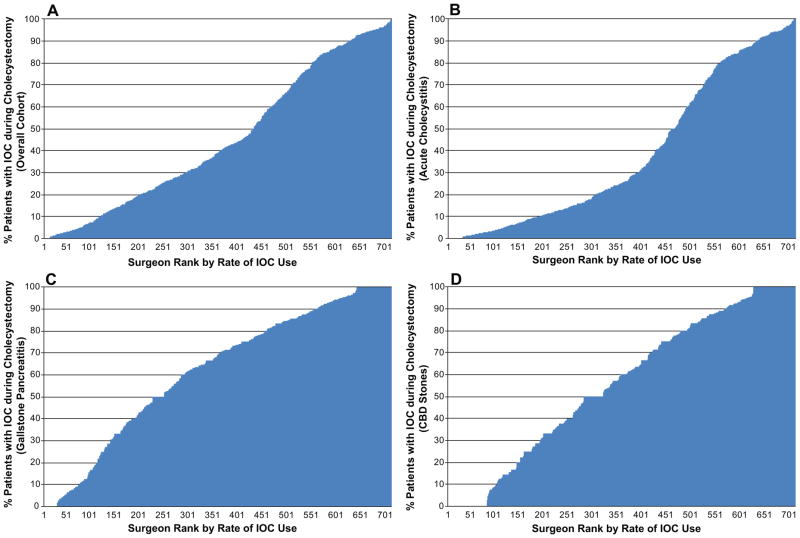
We identified 3,110 surgeons performing cholecystectomy at the 212 Texas hospitals. For the unadjusted analysis we included only surgeons who did 40 or more cholecystectomies during the study period (N=706). Like hospital variation, the variation among surgeons was similarly wide. Surgeon percent IOC use ranged the full spectrum from minimal use to routine use. Figures 2A–D show the unadjusted rates of IOC use across surgeons for the overall cohort, and for patients with acute cholecystitis, gallstone pancreatitis, and common bile duct stones. Based on the shape of the curves, surgeon IOC use for acute cholecystitis seems to be either routinely or very infrequently performed, with the majority of surgeons clustered below 30% of the time or over 80%. For gallstone pancreatitis and CBD stones, surgeon use is more evenly distributed across the spectrum.
Figure 2.
Unadjusted percentage of cholecystectomies performed with IOC by 706 Texas surgeons doing more than 40 cholecystectomies (2001–2008). (A) For all cholecystectomies, regardless of indication, the unadjusted percentage performed with IOC ranged from 0% to 100% (median use = 39.3%, IQR 17.4% – 76.6%). (B) For cholecystectomies done for acute cholecystitis, median use = 25.4% (IQR 9.5% – 73.3%). (C) For cholecystectomies done for gallstone pancreatitis, median use = 69.4% (IQR 39.6% – 87.5%). (D) For cholecystectomies done for CBD stones, median use = 60.0% (IQR 27.6% – 87.0%).
Percentage of Variance in IOC Use Attributable to Patient Characteristics
We investigated the use of IOC by patient characteristics with logistic regression models. Table 2 demonstrates the percentage of the variance in the use of IOC attributable to each patient characteristic in the first column. After adjustment for all the characteristics, the indication for cholecystectomy had the highest percentage of variance, 5.3%. Other patient factors including age, sex, race/ethnicity, type of cholecystectomy, illness severity, insurance status, and year of cholecystectomy contributed to only 1.1% of the overall variance in IOC use.
Table 2.
Percentage of Variance in IOC use Attributable to Patient Characteristics
| Characteristics | Percentage of variance in IOC use* | |
|---|---|---|
| Unadjusted | Adjusted | |
| Indication for cholecystectomy | 6.99 | 5.28 |
| Age | 0.20 | 0.04 |
| Sex | 0.01 | 0.08 |
| Race/ethnicity | 0.35 | 0.34 |
| Type of cholecystectomy | 0.11 | 0.16 |
| Illness severity score | 1.79 | 0.10 |
| Insurance | 0.33 | 0.29 |
| Year of cholecystectomy | 0.08 | 0.07 |
| All patient characteristics | 8.05 | |
The percentage of variance attributable to each characteristic was calculated by the following formula: maximum rescaled R2 × 100%. The R2 values were derived from logistic regression models with IOC use as the dependent variable. For the unadjusted analyses, each patient characteristic was entered separately as an independent variable. For the adjusted analyses, each patient and tumor characteristic was entered as an independent variable, with simultaneous adjustment for the other variables.
Percentage of Variance in IOC Use Attributable to Surgeon and Hospital
The results of the hierarchical linear models are shown in Table 3. We first report the 2-level models (patients clustered within hospitals, N=176,981) given the limitations in surgeon identification. For the subset of patients in whom we can identify the surgeon and assign him/her to a hospital, we report the 3-level model as well (79,343 patients, 1,286 surgeons, 159 hospitals). For each model, we present the residual ICC, which represents the percentage of variance associated with the hospital (2-level) or the surgeon and the hospital (3-level) after adjustment for available patient characteristics. Next, we present the variance further partitioned, showing the percentages of total variance due to surgeon, hospital, and select patient characteristics.
Table 3.
Percentage of Variance in IOC use Attributable to Surgeon and Hospital, Multilevel model
| Characteristics | Percentage of variance in IOC use* |
|---|---|
| adjusted with patient characteristics | |
| 2–level model (Patient, Hospital) | |
|
| |
| MODEL 1 | |
| No. of patients | 176,981 |
| No. of hospitals | 212 |
| Residual ICC (% variance) – Hospital*† | 29.7% |
|
| |
| MODEL 2 (Partitioned variance)* ‡ | |
| Indication for cholecystectomy | 6.8% |
| All other patient characteristics | 0.3% |
| Hospital | 27.6% |
|
| |
| 3–level model (Patient, Surgeon, Hospital) | |
|
| |
| MODEL 3 | |
| No. of patients | 82,013 |
| No. of surgeons | 3,110 |
| No. of hospitals | 179 |
| Residual ICC (% variance) – Surgeon*† | 20.0% |
| Residual ICC (% variance) – Hospital*† | 25.3% |
|
| |
| MODEL 4 (Partitioned variance)* ‡ | |
| Indication for cholecystectomy | 7.2% |
| All other patient characteristics | 0.4% |
| Surgeon | 19.1% |
| Hospital | 23.8% |
Hierarchical generalized linear models: 2–level model has patient characteristics entered as “level 1” variables and hospital characteristics entered as “level 2” variables. 3–level model has patient characteristics entered as “level 1” variables, surgeon identifiers entered as “level 2” variables, and hospital identifiers entered as “level 3” variables. ICC = intraclass correlation coefficient.
The percentage of variance attributable to the surgeon and hospital are calculated with a threshold model, after simultaneous adjustment of all available patient characteristics. The denominator for the calculation of the percentage was composed of the variance attributable to the hospital (2–level model) or surgeon and hospital (3–level model), after adjustment for available patient characteristics, and the variance attributable to unexplained patient variables plus error.
The variance was further partitioned using a threshold model so that the percentages of total variance contributed by the surgeon, hospital, and specific patient characteristics are presented. Results are presented as the percentage of total variance attributable to the indicated characteristic. The denominator is total variance, which is composed of the variance attributable to the hospital (2–level model) or surgeon and hospital (3–level model) after adjustment for available patient characteristics, the variance attributable to available patient characteristics, and the variance attributable to unexplained patient characteristics plus error.
2-Level Hierarchical Model (Patient and Hospital)
The 2-level model represents the percentage of variance attributable to the hospital, with patients (N=176,981) clustered within hospitals (N=212, Table 3). The percentage of variance in IOC use attributable to the hospital (residual ICC) was 29.7%, after controlling for patient characteristics (Table 3, Model 1). This estimate did not change after controlling for hospital volume and hospital teaching status in addition to patient characteristics (Model 1, column 2). When the variance was further partitioned to include the percentage of total variance due to patient characteristics, the total variance attributable hospital was still 27.6%, which was higher than the variance due to indication for cholecystectomy (6.8%) and all other measured patient characteristics (0.3%, Table 3, Model 2).
3-Level Hierarchical Model (Patient, Surgeon, and Hospital)
In the 3-level model (82,013 patients, 3,110 surgeons, 179 hospitals), the surgeon contribution to variance in IOC was significant, but did not diminish the amount of variance attributable to the hospital. In other words, surgeon variation in IOC use did not explain the observed hospital variation. The percentage of variance in IOC use attributable to the surgeon was 20.0% and an additional 25.3% was attributable to the hospital, independent of the surgeon (Table 3, Model 3). Again, there was no change in percentage of variance attributed to the surgeon or to the hospital after adjusting for hospital characteristics. The contribution of patient characteristics to variance in IOC use was the same in the model 4, which was further partitioned to include the indication for cholecystectomy and other patient factors.
Rates of IOC Use Attributable to the Surgeon and Hospital
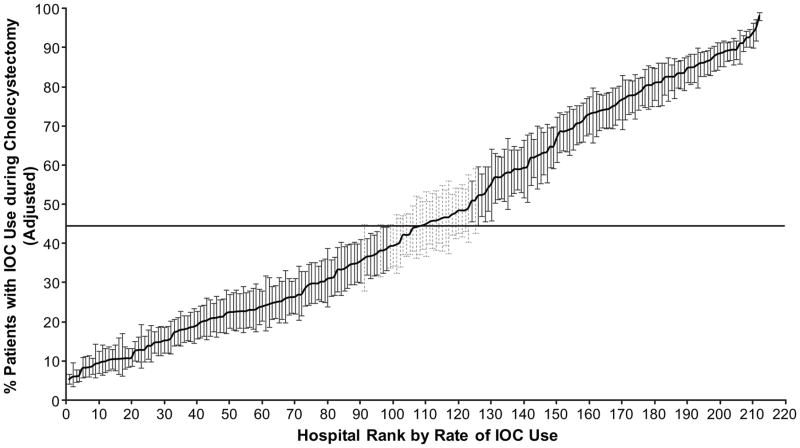
As another measure of hospital variation in IOC use, hospital-specific rates of IOC use during cholecystectomy were calculated using the 2-level hierarchical generalized linear model (Figure 3), adjusted for patient characteristics (including indication for cholecystectomy)and plotted by hospital rank in IOC use, from lowest to highest. This model accounts for differences in reliability of individual rates resulting from variations in the size of the panel of patients for each hospital, with each hospital-specific rate adjusted toward the mean of the overall rate as a factor of panel size. Hospital rates based on a large numbers of patients will result in very little adjustment toward the mean rate and vice versa for hospital rates based on smaller numbers of patients. Variability was evident even after controlling for patient factors. Hospital IOC use in the 2-level model ranged from 5.2% to 96.2%. Eighty-seven percent of hospitals performed IOC significantly above or below the mean rate of use (44.5%), with 96 hospitals (45.3%) performing IOC at rates significantly lower than the mean rate and 88 hospitals (41.5%) at rates above the mean (P<0.05, Figure 3).
Figure 3.
Rates of IOC based on the 2-level hierarchical model. Rates of IOC use for 212 hospitals (>160 cholecystectomies) by rank, from lowest to highest IOC use, from 2001 through 2008. The rates were calculated by use of hierarchical generalized linear modeling (2-level), adjusted for patient characteristics. This model also accounts for differences in reliability of individual rates resulting from variations in the size of the panel of patients for each hospital. Each hospital-specific rate was adjusted toward the mean of the overall rate as a factor of panel size (i.e., a hospital with a large number of patients will result in very little adjustment toward the mean rate, whereas a hospital with a small number of patients will have more adjustment). The horizontal line represents the overall mean rate of IOC use. Error bars represent 95% confidence intervals for the IOC rates of individual hospitals. Black error bars represent hospitals that have rates statistically significantly (p<0.05) above or below the mean rate (44.5%) and light gray barsrepresent rates that are not different from the mean rate.
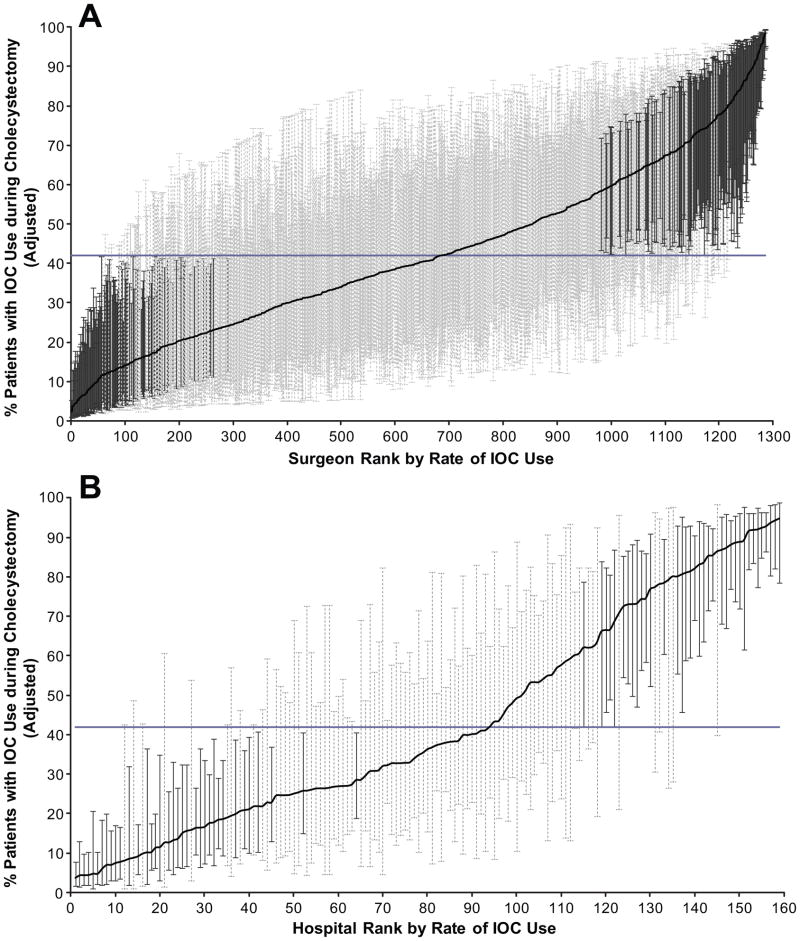
Surgeon- and hospital- specific rates of IOC use were calculated using the adjusted 3-level model (Figure 4A and B). When calculating adjusted rates, we did so only for surgeons doing >5 cases (1,286 surgeons at 159 hospitals). IOC use based on the 3-level model ranged from 2.4% to 98.3% among surgeons and 3.7% to 94.8% among hospitals, after adjusting for case mix differences. Three hundred eleven (24.1%) of surgeons (11.2% below and 12.9% above, Figure 4A) and 45.3% of hospitals (22.6% below and 22.6% above, Figure 4B) had rates of IOC use significantly different (P<0.05) from the mean IOC rate of 41.9%. In comparing Figure 4B and Figure 3 you can see that hospital variability decreased after controlling for surgeon level factors, demonstrating that some of the variability at the hospital-level was attributable to the surgeon, consistent with the residual ICCs reported in Table 3.
Figure 4.
(A) Adjusted rates of IOC use for 1,286 surgeons, from lowest to highest IOC use, from 2001 through 2008. (B) Adjusted rates of IOC use for 159 hospitals by rank, from lowest to highest IOC use, from 2001 through 2008. Rates of IOC based on the 3-level hierarchical model. Rates were only calculated for surgeons doing 5 or more cholecystectomies (79,343 patients, 1,286 surgeons, at 159 hospitals). This model also accounts for differences in reliability of individual rates resulting from variations in the size of the panel of patients for each surgeon and hospital. Each surgeon- and hospital-specific rate was adjusted toward the mean of the overall rate as a factor of panel size (ie, surgeons or hospitals with a large number of patients will result in very little adjustment toward the mean rate, whereas surgeons or hospitals with a small number of patients will have more adjustment). The horizontal line represents the overall mean rate of IOC use (41.9%). Error bars represent 95% confidence intervals for the IOC rates of individual hospitals. Black error bars represent (A) surgeons or (B) hospitals that have rates statistically significantly (p<0.05) above or below the mean rate and light gray bars represent rates that are not different from the mean rate.
Factors Independently Associated with IOC Use in the Multilevel Model (Table 4)
Table 4.
Three–Level Hierarchical Generalized Linear Models, Factors Associated with IOC Use
| Variable | OR (95% CI), n=82,013 |
|---|---|
| Age group, y | |
| 18–44 | 0.91 (0.83, 1.01) |
| 45–54 | 0.97 (0.88, 1.07) |
| 55–64 | 0.96 (0.87, 1.06) |
| 65–74 | 0.96 (0.88, 1.04) |
| 75+ | REF |
| Female (vs. male) | 1.09 (1.04, 1.14) |
| Race/ethnicity | |
| White | 0.92 (0.87, 0.97) |
| Hispanic | REF |
| Black | 0.91 (0.85, 0.99) |
| Other | 0.99 (0.91, 1.10) |
| Indication for Cholecystectomy | |
| Biliary colic (reference) | REF |
| Acute cholecystitis | 0.84 (0.74, 0.96) |
| Gallstone pancreatitis | 4.35 (3.79, 5.00) |
| CBD stones | 2.89 (2.51, 3.32) |
| Year of surgery | |
| 2001 | REF |
| 2002 | 1.17 (1.08, 1.27) |
| 2003 | 1.36 (1.25, 1.48) |
| 2004 | 1.44 (1.32, 1.56) |
| 2005 | 1.54 (1.42, 1.68) |
| 2006 | 1.59 (1.46, 1.73) |
| 2007 | 1.55 (1.42, 1.69) |
| 2008 | 1.44 (1.27, 1.64) |
| Insurance | |
| Uninsured | 0.94 (0.89, 0.99) |
| Medicare | 1.02 (0.94, 1.10) |
| Medicaid | 1.09 (1.01, 1.18) |
| Other insurance | REF |
| Laparoscopic cholecystectomy (vs. open) | 1.11 (1.00, 1.24) |
| Illness severity | |
| 1 | 1.29 (1.12, 1.47) |
| 2 | 1.33 (1.17, 1.52) |
| 3 | 1.24 (1.09, 1.42) |
| 4 | REF |
In the 2-level hierarchical model controlling for patient and hospital factors, IOC use was most strongly associated with the indication for cholecystectomy. IOC use was also positively associated with increased age, Hispanic race, decreased illness severity, insurance (other than Medicare/Medicaid vs. none) and year of cholecystectomy, with IOC being more common in later years of the study (Table 4). Factors predicting IOC were identical in the 3-level model with the exception of age, which reached statistical significance.
IOC Use and ERCP/CBD Exploration
Patients undergoing IOC were more likely to undergo ERCP during the same admission (21.2% vs. 14.7%, P<0.0001). The timing of ERCP varied with IOC use. In the IOC group, 36.7% of ERCPs were performed preoperatively with the remainder being done on the day of operation or after. In the no IOC group 71.2% of ERCPs were performed prior to the day of operation (P<0.0001). In addition to increased overall ERCP use, CBD exploration, while uncommon, was performed almost four times more often (1.63% vs. 0.42%, P<0.0001) in the IOC group. The same patterns persisted across hospital quartiles of IOC use and CBD exploration.
DISCUSSION
To our knowledge, this is the first study to comprehensively describe variation in IOC use during cholecystectomy using multilevel models to examine the independent contributions of patient, surgeon, and hospital factors. There was wide variation in IOC use, ranging from 2% to 98% of the time among surgeons and 4% to 95% of the time among hospitals, even after adjusting for case mix differences in multilevel models. Nearly half of the variance in IOC use was attributable to the surgeon (20%) and hospital (25%). These results provide support for the supposition that IOC use is strongly influenced by surgeon preference.22,23 More of the variance was attributable to the surgeon and the hospital than to available patient characteristics such as indication for cholecystectomy, which only accounted for 7% of the variance in IOC use. Thus, the surgeon and/or hospital to which a patient presents may be more important determinants of whether that patient will undergo IOC than the patient’s diagnosis and other characteristics.
The significant surgeon and hospital variation in IOC use observed in our study is likely multifactorial. Uncertainty regarding the effectiveness of IOC in preventing bile duct injury and retained stones is likely a major contributor. Little to no level 1 evidence exists, and previous studies have significant weaknesses. Given the low incidence of bile duct injury,7 single-institution studies may be underpowered to demonstrate a difference.5,8–10,24 However, large population-based studies are subject to selection bias.6,7 In addition, previous studies have shown conflicting results regarding the benefit of IOC. As a result, the routine use of IOC during cholecystectomy remains debated and controversial among surgeons.5–11,24,25
In addition to uncertainty regarding the effectiveness of IOC, surgeon factors such as skill and preference may influence use of IOC. Surgeon decisions may be influenced by training bias, risk-taking behavior,13 ability to perform laparoscopic IOC and CBD exploration, and familiarity and knowledge of biliary anatomy. In addition, surgeons may make decisions about IOC use based on the local availability of alternative methods of bile duct clearance (ERCP) and time to perform IOC at their institution. For example, a surgeon with strong familiarity with the biliary anatomy, excellent ERCP capability at his or her institution, but limited ability to perform laparoscopic CBD exploration may choose a different method of bile duct clearance for a patient with suspected stones, and may not do an IOC if anatomy is clear at laparoscopy. However, another surgeon at an institution with limited ERCP availability and laparoscopic CBD exploration experience may choose to do an IOC.
In our 3-level models, 25% of the variance in IOC use was at the hospital level, indicating that hospital factors influence IOC use independent of surgeon factors. These hospital factors, not measured in our study, may include availability of ERCP, actual time to get an IOC at the individual institution, method for obtaining IOC (fluoroscopy vs. plain x-ray), radiology availability, and culture (surgeons influencing each other’s behavior). Two prospective studies reported that it takes about 15 minutes to perform an IOC26,27. However it may take significantly longer at hospitals where fluoroscopy is not readily available, surgeons are not skilled, and it is not routinely done.12
Variation in IOC use could have significant cost implications. Cost estimates for performing IOC range from $100–700.28–30 However, additional costs may be incurred for stone removal procedures, many of which may be unnecessary since it is estimated that nearly half of patients with common bile duct stones pass the stones spontaneously within weeks of cholecystectomy.31 Given the frequency of cholecystectomy in the U.S., increased costs per patient equate to considerable costs at the population level. Evidence regarding the cost effectiveness of IOC in the prevention of CBD injury is inconsistent.30,32,33 A cost-effectiveness analysis evaluating the management CBD stones found laparoscopic cholecystectomy with IOC to be the most cost-effective treatment strategy if the probability of CBD stones was >4%. However, this was only true if the patient was referred for ERCP if stones are detected. CBD exploration in this setting rendered an IOC strategy no longer cost-effective.33
Previous studies have evaluated the role of physician characteristics in practice variation.34–38 Residual ICCs, or the percent of variation attributable to physicians after adjusting for patient characteristics, are lowest when clear guidelines exist, such as in resource utilization in diabetics (ICC 1–4%)36 and use of prescription beta-blockers (ICC 7–9%),34 and highest when indications are unclear, such as the use of androgen deprivation in prostate cancer (ICC 21%).37 The amount of practice variation attributable to the surgeon and the hospital in our study is substantially higher than previous studies of provider variation. As such, efforts directed at changing physician behavior would likely have a significant impact on outcomes.34,36,37 However, in order to change physician behavior regarding IOC use, we must first define appropriate use.
There are potential benefits and harms of IOC use during cholecystectomy. Use of IOC may serve as a system-level intervention to prevent major injury to the common bile duct, minimize extent of injury, and protect against medical malpractice claims.22 IOC use can also detect suspected or unsuspected common bile duct stones,39 allowing for preemptive removal (endoscopic retrograde cholangiopancreatography (ERCP), common bile duct exploration) before they become symptomatic or lead to complications such as biliary obstruction, cholangitis, and pancreatitis.40 However, IOC increases operative time,22 involves radiation exposure for patients and staff, and carries a small risk of complications.22,41 In addition, recent case series evaluating routine IOC demonstrate relatively high rates of false positive cholangiograms, high rates of detection of asymptomatic stones leading to unnecessary procedures for stone removal, and no decrease in gallstone-related readmissions.24,42–44 While not the focus of this study, these findings are consistent with the increased ERCP and CBD exploration rates seen at the patient and hospital level of IOC use in our study.
The variation in IOC use observed in this study highlights the need for further evaluation of the comparative effectiveness of IOC in the prevention of bile duct injuries and retained stones. Future studies should take into account patient risk factors, surgeon skill, cost, and availability of local resources such as ERCP. We propose using the observed surgeon and hospital variation in IOC use as a tool to study the comparative effectiveness of IOC in various settings. Case mix differences are unlikely to explain the degree of variation observed and we suspect patients presenting for inpatient cholecystectomy to be relatively similar across geographic regions. As such, the wide variation will provide a natural experiment, with patients essentially “randomized” among high and low IOC users (both surgeon and hospital), thereby controlling for unmeasured confounders. The goal should be to identify specific subgroups of patients in whom IOC would be effective, thereby aiding physician decision-making and allowing us to focus efforts on changing physician behavior where it will be most beneficial.
Our study has several limitations. First, the Texas hospital discharge data lack specific information about patient presentation that may influence the use of IOC, including liver function tests, bilirubin levels, and radiology reports to document findings such as CBD dilation or suspected CBD stones. These factors may contribute to some of the unexplained patient variance or may account for a portion of the observed surgeon and hospital variance. However, while disease characteristics might vary somewhat between panels of patients for a given surgeon or hospital, we do not suspect they would vary enough to account for a significant portion of the observed surgeon- and hospital-level variation.
In order to evaluate surgeon-level factors, our cohort was restricted to the cases in which we could identify the surgeon. In addition, for surgeons operating at more than one hospital, we only included the cases from the highest volume hospital, decreasing our sample size to 82,013 patients. For this reason, we also performed and reported the 2-level models with patients clustered within hospitals. In the 2-level model, the surgeon variance was likely partly accounted for within the hospital and patient levels of variation. The factors predicting IOC use were essentially unchanged in the 2- and 3-level models, and hospital level variance was very similar between the two samples.
Our study evaluated inpatient cholecystectomies only. Variation in IOC use during cholecystectomy may differ in outpatient compared to inpatient settings. In addition, generalizability may also be limited as all patients are from Texas, which has higher rates of Hispanic and uninsured patients than other areas of the country.
In conclusion, the substantial surgeon and hospital variation observed in this study raises questions regarding optimal use of IOC. Based on the wide variation, it is unlikely that routine IOC is necessary or feasible in all patients across various settings. Future research should evaluate the comparative effectiveness of IOC in the prevention of CBD injuries and retained stones and identify subgroups of patients in whom IOC is most beneficial. Guidelines regarding optimal use of IOC would aid physician decision-making, decreasing practice variation across surgeons and hospitals.
Acknowledgments
This study is supported by following grants: K05CA134923, K07CA130983.
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 123rd Annual Meeting, Hot Springs, VA, December 2011.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Center for Health Statistics. Health, United States, 2009: With Special Feature on Medical Technology. Washington, DC: U.S. Government Printing Office; 2010. [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. National Health Statistics Reports: 2006 National Hospital Discharge Survey. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 3.National Center for Health Statistics. National Health Statistics Reports: Ambulatory Surgery in the United States, 2006. Washington, DC: U.S. Department of Health and Human Services; 2009. [Google Scholar]
- 4.Overby DW, Apelgren KN, Richardson W, Fanelli R Society of American G Endoscopic S. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc. 2010 Oct;24(10):2368–2386. doi: 10.1007/s00464-010-1268-7. [DOI] [PubMed] [Google Scholar]
- 5.Akolekar D, Nixon SJ, Parks RW. Intraoperative cholangiography in modern surgical practice. Digestive surgery. 2009;26(2):130–134. doi: 10.1159/000206150. [DOI] [PubMed] [Google Scholar]
- 6.Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003 Apr 2;289(13):1639–1644. doi: 10.1001/jama.289.13.1639. [DOI] [PubMed] [Google Scholar]
- 7.Flum DR, Koepsell T, Heagerty P, Sinanan M, Dellinger EP. Common bile duct injury during laparoscopic cholecystectomy and the use of intraoperative cholangiography: adverse outcome or preventable error? Arch Surg. 2001 Nov;136(11):1287–1292. doi: 10.1001/archsurg.136.11.1287. [DOI] [PubMed] [Google Scholar]
- 8.Hamad MA, Nada AA, Abdel-Atty MY, Kawashti AS. Major biliary complications in 2,714 cases of laparoscopic cholecystectomy without intraoperative cholangiography: a multicenter retrospective study. Surg Endosc. 2011 Dec;25(12):3747–3751. doi: 10.1007/s00464-011-1780-4. [DOI] [PubMed] [Google Scholar]
- 9.Horwood J, Akbar F, Davis K, Morgan R. Prospective evaluation of a selective approach to cholangiography for suspected common bile duct stones. Ann R Coll Surg Engl. 2010 Apr;92(3):206–210. doi: 10.1308/003588410X12628812458293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2006 Jun;20(6):868–874. doi: 10.1007/s00464-005-0425-x. [DOI] [PubMed] [Google Scholar]
- 11.Traverso LW. Intraoperative cholangiography lowers the risk of bile duct injury during cholecystectomy. Surg Endosc. 2006 Nov;20(11):1659–1661. doi: 10.1007/s00464-006-0122-4. [DOI] [PubMed] [Google Scholar]
- 12.Massarweh NN, Devlin A, Elrod JA, Symons RG, Flum DR. Surgeon knowledge, behavior, and opinions regarding intraoperative cholangiography. Journal of the American College of Surgeons. 2008 Dec;207(6):821–830. doi: 10.1016/j.jamcollsurg.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Massarweh NN, Devlin A, Symons RG, Broeckel Elrod JA, Flum DR. Risk tolerance and bile duct injury: surgeon characteristics, risk-taking preference, and common bile duct injuries. Journal of the American College of Surgeons. 2009 Jul;209(1):17–24. doi: 10.1016/j.jamcollsurg.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 14.Parra-Membrives P, Diaz-Gomez D, Vilegas-Portero R, Molina-Linde M, Gomez-Bujedo L, Lacalle-Remigio JR. Appropriate management of common bile duct stones: a RAND Corporation/UCLA Appropriateness Method statistical analysis. Surg Endosc. 2010 May;24(5):1187–1194. doi: 10.1007/s00464-009-0748-0. [DOI] [PubMed] [Google Scholar]
- 15.Wennberg J, Gittelsohn A. Variations in medical care among small areas. Scientific American. 1982 Apr;246(4):120–134. doi: 10.1038/scientificamerican0482-120. [DOI] [PubMed] [Google Scholar]
- 16.Wennberg JE, Barnes BA, Zubkoff M. Professional uncertainty and the problem of supplier-induced demand. Soc Sci Med. 1982;16(7):811–824. doi: 10.1016/0277-9536(82)90234-9. [DOI] [PubMed] [Google Scholar]
- 17.Texas Department of State Health Services. [Accessed June 1, 2011.];Texas Health Care Information Collection Center. http://www.dshs.state.tx.us/thcic/Hospitals/HospitalData.shtm.
- 18.Nguyen GC, Tuskey A, Jagannath SB. Racial disparities in cholecystectomy rates during hospitalizations for acute gallstone pancreatitis: a national survey. Am J Gastroenterol. 2008 Sep;103(9):2301–2307. doi: 10.1111/j.1572-0241.2008.01949.x. [DOI] [PubMed] [Google Scholar]
- 19.Trust MD, Sheffield KM, Boyd CA, et al. Gallstone pancreatitis in older patients: Are we operating enough? Surgery. 2011 Sep;150(3):515–525. doi: 10.1016/j.surg.2011.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagelkerke NJD. A note on general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
- 21.Snijders TA, Bosker RJ. Multilevel analysis: an introduction to basic and advanced multilevel modeling. Thousand Oaks: SAGE Publications; 1999. [Google Scholar]
- 22.Massarweh NN, Flum DR. Role of intraoperative cholangiography in avoiding bile duct injury. Journal of the American College of Surgeons. 2007 Apr;204(4):656–664. doi: 10.1016/j.jamcollsurg.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 23.Nuzzo G, Giuliante F, Giovannini I, et al. Re: Role of intraoperative cholangiography in avoiding bile duct injury. Journal of the American College of Surgeons. 2007 Oct;205(4):e5–6. doi: 10.1016/j.jamcollsurg.2007.07.014. author reply e6. [DOI] [PubMed] [Google Scholar]
- 24.Buddingh KT, Weersma RK, Savenije RA, van Dam GM, Nieuwenhuijs VB. Lower rate of major bile duct injury and increased intraoperative management of common bile duct stones after implementation of routine intraoperative cholangiography. Journal of the American College of Surgeons. 2011 Aug;213(2):267–274. doi: 10.1016/j.jamcollsurg.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Kharbutli B, Velanovich V. Management of preoperatively suspected choledocholithiasis: a decision analysis. J Gastrointest Surg. 2008 Nov;12(11):1973–1980. doi: 10.1007/s11605-008-0624-6. [DOI] [PubMed] [Google Scholar]
- 26.Catheline JM, Turner R, Paries J. Laparoscopic ultrasonography is a complement to cholangiography for the detection of choledocholithiasis at laparoscopic cholecystectomy. Br J Surg. 2002 Oct;89(10):1235–1239. doi: 10.1046/j.1365-2168.2002.02198.x. [DOI] [PubMed] [Google Scholar]
- 27.Halpin VJ, Dunnegan D, Soper NJ. Laparoscopic intracorporeal ultrasound versus fluoroscopic intraoperative cholangiography: after the learning curve. Surg Endosc. 2002 Feb;16(2):336–341. doi: 10.1007/s00464-001-8325-1. [DOI] [PubMed] [Google Scholar]
- 28.Traverso LW, Roush TS, Koo K. CBD stones--outcomes and costs. Laparoscopic transcystic techniques other than choledochoscopy. Surg Endosc. 1995 Nov;9(11):1242–1244. [PubMed] [Google Scholar]
- 29.Podnos YD, Gelfand DV, Dulkanchainun TS, et al. Is intraoperative cholangiography during laparoscopic cholecystectomy cost effective? Am J Surg. 2001 Dec;182(6):663–669. doi: 10.1016/s0002-9610(01)00808-x. [DOI] [PubMed] [Google Scholar]
- 30.Flum DR, Flowers C, Veenstra DL. A cost-effectiveness analysis of intraoperative cholangiography in the prevention of bile duct injury during laparoscopic cholecystectomy. Journal of the American College of Surgeons. 2003 Mar;196(3):385–393. doi: 10.1016/S1072-7515(02)01806-9. [DOI] [PubMed] [Google Scholar]
- 31.Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg. 2004 Jan;239(1):28–33. doi: 10.1097/01.sla.0000103069.00170.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livingston EH, Miller JA, Coan B, Rege RV. Costs and utilization of intraoperative cholangiography. J Gastrointest Surg. 2007 Sep;11(9):1162–1167. doi: 10.1007/s11605-007-0209-9. [DOI] [PubMed] [Google Scholar]
- 33.Brown LM, Rogers SJ, Cello JP, Brasel KJ, Inadomi JM. Cost-effective treatment of patients with symptomatic cholelithiasis and possible common bile duct stones. Journal of the American College of Surgeons. 2011 Jun;212(6):1049–1060. e1041–1047. doi: 10.1016/j.jamcollsurg.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaulieu MD, Blais R, Jacques A, Battista RN, Lebeau R, Brophy J. Are patients suffering from stable angina receiving optimal medical treatment? QJM: monthly journal of the Association of Physicians. 2001 Jun;94(6):301–308. doi: 10.1093/qjmed/94.6.301. [DOI] [PubMed] [Google Scholar]
- 35.Cowen ME, Strawderman RL. Quantifying the physician contribution to managed care pharmacy expenses: a random effects approach. Med Care. 2002 Aug;40(8):650–661. doi: 10.1097/00005650-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Hofer TP, Hayward RA, Greenfield S, Wagner EH, Kaplan SH, Manning WG. The unreliability of individual physician “report cards” for assessing the costs and quality of care of a chronic disease. JAMA. 1999 Jun 9;281(22):2098–2105. doi: 10.1001/jama.281.22.2098. [DOI] [PubMed] [Google Scholar]
- 37.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst. 2006 Jun 21;98(12):839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sixma HJ, Spreeuwenberg PM, van der Pasch MA. Patient satisfaction with the general practitioner: a two-level analysis. Med Care. 1998 Feb;36(2):212–229. doi: 10.1097/00005650-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Amott D, Webb A, Tulloh B. Prospective comparison of routine and selective operative cholangiography. ANZ J Surg. 2005 Jun;75(6):378–382. doi: 10.1111/j.1445-2197.2005.03393.x. [DOI] [PubMed] [Google Scholar]
- 40.Metcalfe MS, Ong T, Bruening MH, Iswariah H, Wemyss-Holden SA, Maddern GJ. Is laparoscopic intraoperative cholangiogram a matter of routine? Am J Surg. 2004 Apr;187(4):475–481. doi: 10.1016/j.amjsurg.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 41.Zacharakis E, Angelopoulos S, Kanellos D, et al. Laparoscopic cholecystectomy without intraoperative cholangiography. J Laparoendosc Adv Surg Tech A. 2007 Oct;17(5):620–625. doi: 10.1089/lap.2006.0220. [DOI] [PubMed] [Google Scholar]
- 42.Byrne MF, McLoughlin MT, Mitchell RM, et al. For patients with predicted low risk for choledocholithiasis undergoing laparoscopic cholecystectomy, selective intraoperative cholangiography and postoperative endoscopic retrograde cholangiopancreatography is an effective strategy to limit unnecessary procedures. Surg Endosc. 2009 Sep;23(9):1933–1937. doi: 10.1007/s00464-008-0250-0. [DOI] [PubMed] [Google Scholar]
- 43.Nugent N, Doyle M, Mealy K. Low incidence of retained common bile duct stones using a selective policy of biliary imaging. Surg. 2005 Oct;3(5):352–356. doi: 10.1016/s1479-666x(05)80115-5. [DOI] [PubMed] [Google Scholar]
- 44.Khan OA, Balaji S, Branagan G, Bennett DH, Davies N. Randomized clinical trial of routine on-table cholangiography during laparoscopic cholecystectomy. Br J Surg. 2011 Mar;98(3):362–367. doi: 10.1002/bjs.7356. [DOI] [PubMed] [Google Scholar]