Abstract
Antibody responses against infectious agents are an important component in the prevention of disease. The avidity of antibodies for their antigens relates to their functional efficiency, and is a fundamental aspect in the investigation of humoral responses. Modified ELISAs are used to estimate avidity through the use of chaotropic agents and the measurement of the degree to which they disrupt the interaction between antibody and antigen. The theory behind the assay is the higher the avidity of an interaction the less susceptible it is to the effects of the chaotropic agent. The goal of this study was to generate a modified ELISA where a complex, multimeric coating-antigen, human papillomavirus (HPV) virus-like particles (VLP), was used to measure the avidity of anti-HPV antibodies generated following vaccination with HPV VLPs. A series of chaotropic agents were evaluated in the assay for their effectiveness in measuring avidity. Guanidine hydrochloride (GuHCl) was selected as a chaotropic reagent with the ability to disrupt antibody and antigen interactions, while not affecting the integrity of the plate-bound VLP. Two methods of determining the avidity index were assessed and shown to be comparable. This assay was then successfully applied to measure the avidity of anti-HPV VLP serum antibodies in samples from a HPV L1 VLP vaccine clinical trial. Overall, the assay was highly reproducible and captured a wide range of antibody avidities. Therefore, a GuHCl-modified ELISA is an acceptable method that can be used to determine HPV-specific antibody avidity indices within a clinical trial setting.
Keywords: HPV vaccine, antibody, avidity
Introduction
The human immune system is able to recognize and respond to most foreign material that is introduced within the body. Antibodies exert their functions through general and unique properties. An example of a general property is the isotype of the antibody and the functions that go with it. A unique property of an antibody, such as its avidity, relates to the antibody’s antigen-binding site and is different for each individual antibody clone. Avidity has been investigated less routinely, especially in the context of HPV L1 VLP vaccination.
Avidity of antibody interactions has been measured mainly through the use of two methods. Biospecific interaction analysis (i.e. Biacore) is the most accurate measurement, but is expensive and not readily accessible. A modified ELISA has been traditionally used to measure the overall avidity of a serum sample. The method, sometimes referred to as a “bind and break” ELISA [1–5], uses chaotropic reagents to disrupt the interaction between antibodies and their specific antigens. The rationale of using the chaotropic agent is to disrupt the antigen binding of low avidity antibodies, effectively “eluting” them from the well before quantifying the antibodies bound to the antigen. The remaining more avid antibodies in the well are then measured and compared to the amount of antibodies detected in a control well that was not exposed to the chaotropic reagent. The chaotropic agent is introduced to a standard ELISA in a variety methods, such as exposure to a single [6–13] or a range of concentrations [10, 14–17], or repeated exposure by the incorporation into washing buffers [10]. The avidity can be either expressed as the molar concentration that reduces the control OD signal by half, a percentage of the control OD signal that remains after treatment with the chaotropic agent or a more complex calculation involving the ratio between treated and untreated wells. The avidity ELISA has been reported to correlate with the results from more accurate and technically advanced measurements of avidity, including Biospecific interaction analysis [12, 18] and equilibrium dialysis [19].
Antibody avidity against a plethora of antigen types has been studied. Antigens used to coat wells have included cell lysates [9, 14, 20–21], polysaccharide complexes [6, 10, 15–17], hapten-protein conjugates [18], peptide antigens [12] and purified, monomeric proteins [11, 13]. We were interested in measuring the avidity of antibodies generated in response to a human papillomavirus (HPV) virus-like particle (VLP) vaccine, which is a more complex antigen than has been used to date. The VLPs are formed by the protein-protein interactions of the HPV L1 proteins [22–23]. Ideally the chaotropic agent would only disrupt antibody/antigen interactions, but it is possible that the chaotrope would disrupt the protein-protein interactions essential to the integrity of the VLP. This could denature the VLPs, rendering them unable to be recognized by a VLP-specific antibody. Most published reports have not consider this issue, but it may be due to the less complex antigens that were used. One report mentions evaluating this potential effect, and no effect was observed on a polysaccharide based antigen [17].
Here we evaluated multiple chaotropic agents in a modified ELISA designed to measure antibody avidity in serum from HPV L1 VLP vaccine recipients. We identified guanidine hydrochloride (GuHCl) as an ideal chaotropic reagent that over a wide range of concentrations does not affect the plate-bound antigen, yet is still able to disrupt antibody/antigen interactions. We performed the modified ELISA following two different common assay approaches and showed that the results from the two approaches correlated strongly. We further showed that the assay is highly reproducible and is able to measure a range of avidities in serum samples from HPV-16/18 L1 VLP vaccine recipients. Thus, GuHCl is a reliable reagent for assessment of antibody avidity indices (AI) in serum from HPV L1 VLP vaccine recipients.
Materials and Methods
Participants and Serum Samples
7466 women participated in a double blind, controlled, randomized, phase III study of the efficacy of an HPV16/18 VLP vaccine in Costa Rica. Details of recruitment and study design have been published [24]. Briefly, participants were randomized to receive either an HPV16/18 AS04-adjuvanted vaccine (GSK Bio, Rixensart, Belgium) or a control hepatitis A vaccine (GSK Bio) at 0- (enrollment), 1- and 6-month. All participants provided informed consent, and the trial was approved by human subjects review committees at the National Cancer Institute (NCI) and Costa Rica. For the present study, a subgroup of 50 women, who were scheduled to receive three doses of the HPV16/18 AS04-adjuvanted vaccine, was randomly selected and the month 12 sample (6 months after the completion of the vaccine regiment) was analyzed. Seven individuals were excluded from the analysis because they had evidence of pre-existing HPV neutralizing antibodies. The month 12 samples from the 43 seronegative participants were chosen testing because all of the samples had a sufficient antibody titer required for the avidity ELISA as described in following sections. A subset of 16 serum samples, also collected at month 12 after enrollment from a previous Phase I/II clinical trial of a monovalent HPV16 L1 VLP vaccine [25] were used to determine ideal assay conditions for sample dilution and chaotrope concentration.
ELISA
Anti-Human papillomavirus IgG antibodies were detected by an enzyme-linked immunosorbent assay (ELISA), as previously described [26–27]. Polystyrene flat-bottom microtiter plates (MaxiSorp, high binding; Nunc, Thermo Fisher Scientific, USA) were coated with 100 μl of HPV16 L1 VLP at a concentration of 2.7 μg of protein per ml. The plates were incubated at 4°C and washed with a saline-based buffer containing Tween 20. After blocking the plates with blocking buffer containing 4% Skim Milk and 0.2% Tween 20 (volume/volume) in phosphate-buffered saline, the plates were again washed. Then 100 μl of two-fold serially diluted serum (diluted in blocking buffer) was added to each well. All samples were tested in duplicate. The plates were incubated for 1 hr at room temperature. After washing four times, a solution of peroxidase-labeled goat anti-human IgG (KPL, Inc., Gaithersburg, MD) was added for 1 hr. Plates were then developed with a tetramethylbenzidine substrate solution (KPL, Inc.). After 25 min of incubation in the dark at room temperature, the reaction was stopped by the addition of 100 μl of 0.36N H2SO4 to each well. The absorbance at 450 nm and 620 nm were measured with a microtiter plate reader (Spectramax M5; Molecular Device, Sunnyvale, CA). To control for the background reaction, four wells were filled with blocking buffer only. To calculate the titers of the samples, eight wells were filled with 100 μl of 1:100 diluted pooled sera from healthy, unimmunized individuals, presumably negative for HPV antibodies. The mean optical density (OD) of the pooled normal serum plus 3 standard deviations was used as cutoff to determine the titer of the samples. The data were fitted to a graph representing on the y-axis the absorbance and on the x-axis the dilutions of the samples. The reported value was the dilution interpolated from the curve for each sample that would give an OD value equal to the healthy, unimmunized control-based cut-off value.
Selecting a Chaotropic Reagent
To rapidly screen chaotropes and their effects on the structure HPV16 VLP, VLP-coated wells were treated with increasing concentrations of different chaotropes. The four chaoptropes tested were: urea (1–8 M; Sigma-Aldrich, St. Louis, MO) dissolved in PBS containing 0.2% Tween-20 (PBST); diethylamine (DEA; 1.6–50 mM; Sigma-Aldrich) diluted in PBST, with resulting pH values of 7–11; guanidine hydrochloride (GuHCl; 0.5–3.5 M; Sigma-Aldrich) dissolved in PBST containing 10 mg/ml bovine serum albumin; ammonium thiocyanate (NH4SCN, 0.25–1.75 M, Sigma-Aldrich) dissolved in PBST. 100 μl per well of chaotropes were added to VLP-coated wells in duplicates. After a washing step, diluted serum was added and allowed to incubate for 1 hour. The remainder of the assay was the same as the ELISA assay. The percentage of the untreated, control well OD remaining after exposure to the different concentrations of the chaotropes was determined. GuHCl was selected for further investigation because it best preserved the antibody binding over a wide range of concentrations. The effects of GuHCl treatment on plate-bound VLP was further scrutinized by using the V5 antibody. V5 is a mouse monoclonal antibody that recognizes conformational dependent epitopes [28] of HPV16 VLP and is a neutralizing antibody [29], allowing for the evaluation of the fitness of biologically relevant VLP structures. Briefly, HPV16 VLP-coated wells were exposed to concentrations of GuHCl ranging from 0.5 to 3.5 M for 15 minutes. After a washing step, V5 antibody was incubated with the plate-bound HPV16 VLP for one hour. The amount of V5 bound in the wells was detected using a HPR-conjugated anti-mouse IgG antibody (Bethyl Laboratories, Inc., Montgomery, TX). The remainder of the assay is the same as the ELISA assay described above. The amount of V5 bound to GuHCl-treated HPV16 VLP was compared to the amount of V5 bound to untreated HPV16 VLP and reported.
Two Assay Approaches for the Modified ELISA Using Chaotropic Elution
Two modified assay set-ups were evaluated and compared in this study. The first (Method A) is based on a method reported by Polanec et al [30]. Briefly, plates were initially coated as described in the ELISA assay described above. Serum samples were diluted and used in the assay such that they yielded an optical density of 1.0±0.5 in the detailed ELISA assay. 100 μl of the diluted serum was added to each well for 1 hour with shaking. All incubations were done at room temperature. After a washing step, 100 μl of 0.5 to 3.5 M GuHCl was added for 15 minutes. Buffers without GuHCl were added to control wells. The plates were washed and the remainder of the assay is the same as the above detailed ELISA assay. The second approach (Method B) was based on the report of Vermont et al [31]. Briefly, plates were initially coated as described in the ELISA assay described above. Serum samples were serially diluted (2-fold, 5 dilutions) such that dilutions tested fell on the linear range of the described ELISA assay. 100 μl of the diluted serum was added to each well for 1 hour with shaking. All incubations were done at room temperature. After a washing step, all wells were treated with a single concentration of GuHCl for 15 minutes. Buffers without GuHCl were added to control wells. The plates were washed and the remainder of the assay is the same as the above detailed ELISA assay. A total of four concentrations of GuHCl (0.5, 1, 2, and 3 M) were evaluated.
Methods to Calculate Avidity Index Values
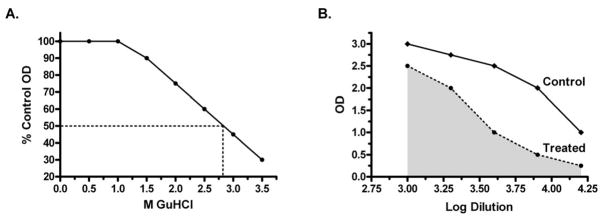
Avidity Index (AI) values were differentially calculated based on the assay approach utilized. Assays performed as described by Polanec (Method A) were analyzed using the method provided in the same report [30]. Here, the absorbance readings for each serum sample treated with a different GuHCl concentrations were converted to the percentage of the untreated, control OD remaining (% = (treated OD/untreated OD)*100). The data was fitted to a graph representing on the y-axis the percentage of the control OD and on the x-axis the molar concentration of GuHCl (Fig. 1A) The AI value determined in Method A was the extrapolated molar concentration of GuHCl required to reduce the absorbance of the untreated, control well by 50%. Alternatively, assays performed as described by Vermont were analyzed using the method reported by Perciani et al. [8] based on the area under the curves of treated and untreated wells (Method B). Curves were generated by plotting the OD value on the y-axis versus the log of the serum dilution factors on the x-axis. For each serum sample a curve depicting the data from GuHCl treated and untreated wells was generated (Fig. 1B). The area under the curve was determined for both scenarios using Prism 4 (GraphPad Software Inc., USA). The AI value calculated as described by Perciani et al. was reported as the ratio between two areas (AI = treatedarea/untreatedarea).
FIG. 1.
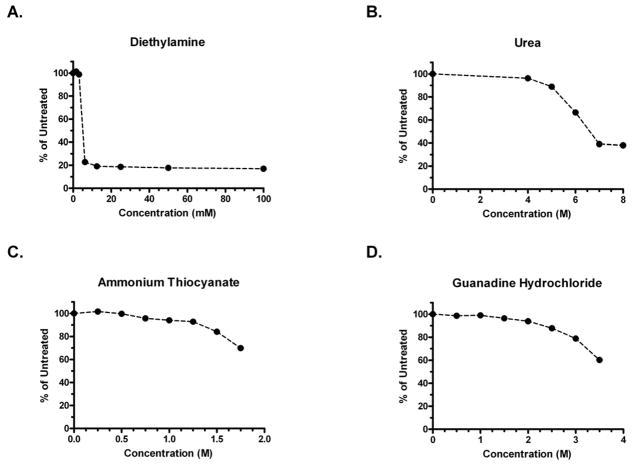
Effects of chaotropic agents on the plate-bound HPV16 L1 VLPs. The effects of chaotropic agents (A-Diethylamine, B- Urea, C- Ammonium Thiocyanate, D- Guanidine Hydrochloride) were compared by treating VLP-coated wells before the addition of serum samples from HPV16 VLP vaccinated individuals. The results are presented as the percentage of the OD signal observed in untreated wells in a representative experiment.
Statistical analysis
The AI of samples was analyzed on the natural logarithmic scale. A nested ANOVA was used to evaluate the samples. Estimates of the variability associated with each subject (σ2s) with day for each subject (σ2d), and with duplicates on the same day for a given subject (σ2d) were obtained. Letting zijk denote the AI measurement for sample i (i=1,2,3,…,43) on day j(i) (j=1,2) and using duplicate k(ij) (k=1,2) the statistical model is written: Log (zijk) = μ + ai + bj(i) + ε(ij) Here, μ is the average level of the AI, and ai, bj(i), and ε(ij) are normal independent variates with means zero and variances σ2s, σ2d, σ2e, respectively. Restricted maximum likelihood estimates of the variance components were obtained using the SAS procedure PROC VARCOMP. From the estimates of variance components two measures of assay reproducibility (coefficient of variation and intraclass correlation coefficient) were estimated. The variance of the natural logarithm of an AI was calculated by the “delta method” (Lehmann) and was approximately the square of the CV. The ICC is the proportion of the total variability explained by AI differences among subject (ICC = (σ2s ÷ (σ2s + σ2d + σ2e) × 100)). Spearman coefficients of correlation were calculated using Prism 4 (GraphPad Software Inc., USA).
RESULTS
Selection of the chaotrope guanidine hydrochloride (GuHCl) for use in the modified ELISA
Four chaotropic agents were initially identified and evaluated based on their description in the literature. The four were urea [7, 11–13, 20, 30], ammonium thiocyanate [7–8, 10, 13–17, 21, 30], GuHCl [7, 13, 30], and diethylamine [13, 30]. It was paramount in this study to determine the factors that were leading to the reduction of OD signals in the assay because of concerns over the chaotropic agents potential to alter the structure of the plate-bound VLP. To assess this, plate-bound VLP were exposed to a range of concentrations of the specified chaotropes, followed by incubation with serum from HPV16 VLP immunized patients. The antibody bound in wells treated with chaotropes were then compared to the amount in an untreated reference well. The OD values were used as the basis of the calculations. The criteria to be considered a viable option for future use was that the chaotrope did not reduce the antibody binding at any point by more than 20% within the working concentration range selected. With these limits, it was assumed that the integrity of the plate-bound VLP was preserved. Diethylamine treatment clearly decreased the potential of the VLP to be recognized by antibodies (FIG. 1A) and urea had a substantial effect on the antibody’s ability to bind at concentrations of 6 M and above (FIG. 1.B). However, both ammonium thiocyanate and GuHCl appeared to be viable candidates (FIG. 1C, D) as no significant drop in antibody binding was observed over the majority of concentrations tested. GuHCl was chosen for further testing for two reasons. The first was that GuHCl appeared to have a larger dynamic range than ammonium thiocyanate in terms of the range of applicable molar concentrations (usable molar concentrations of 3.5 and 1.5, respectively). Secondly, GuHCl treatment consisted of a 15 minute incubation as opposed to 30 minutes for ammonium thiocyanate, allowing for a shortened assay protocol.
The choice of GuHCl as the chaotropic reagent was further scrutinized by analyzing the ability of a monoclonal antibody, V5, to bind plate-bound VLP after being exposed to GuHCl. V5 was used in chosen because it is a neutralizing HPV16-specific antibody [29] that recognizes an epitope that is conformationally dependent [28]. V5 is a representative example of the antibodies we hope to be able to measure the avidity with this modified ELISA. When compared to untreated VLP, greater than 90% preservation of V5 binding occurred with concentrations of GuHCl ranging for 0.5 to 3.0 M (Table 1). These experiments confirm that GuHCl is chaotropic reagent amendable to the evaluation of the avidity in patient samples because it has minimal effect on the integrity of the VLP and has a relatively broad dynamic range.
Table 1.
Preservation of V5 binding following VLP exposure to GuHCl
| M GuHCl | V5 Dilution Factor | ||
|---|---|---|---|
| 4 × 104 | 8 × 104 | 16 × 104 | |
| 0.5 | 99.9 | 99.2 | 98.2 |
| 1.0 | 93.2 | 91.3 | 89.1 |
| 1.5 | 96.5 | 94.5 | 93.0 |
| 2.0 | 97.8 | 96.1 | 94.9 |
| 2.5 | 98.9 | 97.7 | 96.5 |
| 3.0 | 99.2 | 98.1 | 97.1 |
| 3.5 | 73.2 | 68.0 | 65.1 |
Results presented as the % of the binding in untreated, control wells
Comparison of two methods used to calculate AI values based on the GuHCl modified ELISA
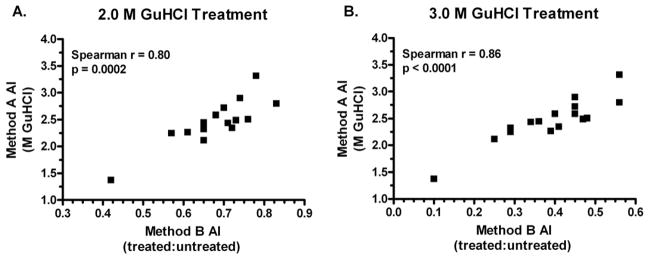
A variety of modifed ELISAs and calculations of AI values have been reported. Two primary methods of calculating the avidity index were compared in this study, the one described by Polanec et al.[30] and another used by Perciani et al. [8]. There are two variables that are treated differently between the two approahces, sample dilution or GuHCl concentration. The approach by Polanec et al. varies the concentrations of GuHCl used to ensure that all avidities within a population of samples are measured. A single dilution of serum that yields an OD which falls on the linear portion of the ELISA assay (target OD of approximately 1.000) is used in the Polanec approach. The Polanec AI value is derived from a graph plotting the percentage of the control OD against the concentration of GuHCl. The AI value is the molar concentration of GuHCl that results in a 50% reduction of the control OD (FIG 2A). Alternatively, the calculation by Perciani et al. relies on the assay set-up as described by Vermont [31]. Here the serum sample is serially diluted and subsequently treated with a single, optimized concentration of GuHCl. Four concentrations of GuHCl (0.5, 1.0, 2.0 or 3.0 M) were tested here because the optimal concentration to mediate an effect in all serum samples was not known. The AI value determined according Perciani et al. is derived from a graph plotting OD values against serum dilution factors. Two lines are plotted, one representing untreated wells and one representing wells treated with GuHCl. This AI value reflects the ratio between the area under the curve calculated for either conditions (FIG 2B). Both methods were applied to a set of 16 serum samples from patients enrolled in a phase II clinical trial of a unadjuvanted HPV16 L1 VLP vaccine. The results are shown in Table 2. Treatments with 0.5 or 1.0 M GuHCl were not effective in disrupting the antigen/antibody binding as small ranges of values were observed (0.86 – 1.00 and 0.73 – 0.94, respectively). A concentration between 2.0 and 3.0 M appear to most effective in determining AI values because they were better able to differentiate between individual samples as evidenced by the increased ranges of values (results of 0.10 to 0.56). The AI values calculated using the approach by Polanec et al. from 16 individuals were then compared to the results of the analysis done according to Perciani et al. using these two concentrations of GuHCl. A strong correlation was observed between the methods when treatment with optimal chaotrope cncentrations of either 2.0 or 3.0 M GuHCl were used in the Perciani et al. calculation method (Spearman ρ = 0.80–0.86, p ≤ 0.0002, FIG. 3A and 3B). This result shows that the two methods can be used interchangeably when the correct conditions for a given sample population are used.
FIG. 2.
Graphs depicting the AI value determination using two different methods (A- Method described by Polanec et al. [30] and B - by Perciani et al [8]).
Table 2.
Avidity Index calculations using two different assay set-ups and conditions
| Method A (Polanec et al.)a | Method B (Perciani et al.)b | ||||
|---|---|---|---|---|---|
| 0.5M | 1.0 M | 2.0 M | 3.0 M | ||
| Number | 16 | 16 | 16 | 16 | 16 |
| Mean | 2.47 | 0.94 | 0.85 | 0.68 | 0.39 |
| Median | 2.47 | 0.94 | 0.85 | 0.69 | 0.41 |
| Minimum | 1.38 | 0.86 | 0.73 | 0.42 | 0.10 |
| Maximum | 3.32 | 1.00 | 0.94 | 0.83 | 0.56 |
Presented as the concentration of GuHCl (M).
Presented as the fraction of untreated, control wells under the four different GuHCl concentration treatments
FIG. 3.
Correlations observed between two different methods and conditions for determing AI values (Method A described by Polanec et al. and Method B by Perciani et al.). 2M GuHCl is shown in panel A and 3 M GuHCl treatment is shown in panel B. Month 12 serum samples from 16 individuals vaccinated with an unadjuvanted HPV16 L1 VLP were tested for AI values as described in the Materials and Methods. The Spearman correlation between the two values is reported with the corresponding p-value.
For our purposes we chose the Polanec method for our further analyses. One potential issue with this method is that variation in the target OD value between samples or assays could impact the final AI calculation. To evaluate this possibility, samples were serially-diluted five times using 2-fold dilutions. The reference OD values covered by this strategy ranged from 0.4 to 3.0. When the AI values for 16 serum samples were evaluated using the 5 different reference OD, an average variation of 11.0% was observed indicating that the dilution of the serum sample had little effect on the final avidity index value when using the Polanec method.
Evaluation of the performance of the avidity ELISA using GuHCl elution
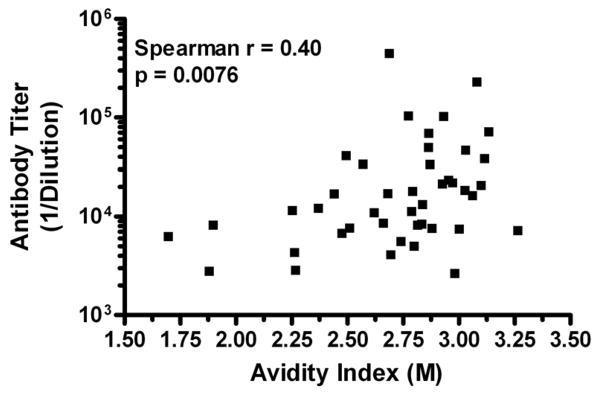
The reproducibility of AI values using the approach by Polanec et al. was evaluated by determining how consistent measurements were in different plates over different days. Samples from 43 clinical trial participants immunized with an adjuvanted, bi-valent HPV16/18 L1 VLP vaccine were each tested over 2 days and in duplicate plates per day. Within each plate samples were plated in duplicate. An excellent overall reproducibility was observed for the assay. A coefficient of variation (CV) of 3.6% and an intraclass correlation coefficient (ICC) of 97.6% was calculated. The CV between plates and days were 2.9% and 2.2%, respectively. As shown in Table 2, the range of AI of the samples was wide with a median AI of 2.72 M displaying the robustness of the assay. The range of AI values for this sample set indicates that GuHCl concentrations of 1.5 to 3.0 M were optimal. The concern over GuHCl effects on the integrity of plate-bound VLP was once again evaluated using these 43 samples. For each sample, four wells were screened: two VLP-coated wells were treated with GuHCl before serum sample addition (pre-treatment) and two were treated after serum sample addition (post-treatment) The pre-treatment effect of GuHCl over the concentration range of 1.5 to 3.0 M was confirmed to be minimal (median reduction in OD = 13.75%; Inter-Quartile Range = 8.2% – 22.6%). Lastly, we looked at the correlation between titers and the avidity of HPV16 L1-specific IgG antibodies within the serum samples. A moderate correlation (Spearman ρ = 0.40, p = 0.0076) was observed between these parameters (FIG. 4). Based on HPV16 L1-specific IgG titer tertiles, individuals with high antibody titers had a high AI, while individuals with low antibody titers could have either a low or high AI (Table 4).
FIG. 4.
Low correlation observed between HPV16-specific antibody titers and avidity in vaccinated individuals. Month 12 serum samples from 43 individuals vaccinated with an adjuvanted HPV16/18 L1 VLP were screened for antibody titers and avidities as described in the Materials and Methods. The Spearman correlation between the two values is reported with the corresponding p-value.
Table 4.
Avidity values analyzed by tertile distribution of HPV16-specific antibody titers
| Antibody Titer Grouping | Titer Tertile | no. | Mean* | SD | Median* | Minimum* | Maximum* | r value** |
|---|---|---|---|---|---|---|---|---|
| Low | ≤8215 | 14 | 2.52 | 0.47 | 2.60 | 1.70 | 3.26 | 0.12 |
| Medium | 8215 – 21395 | 14 | 2.73 | 0.25 | 2.79 | 2.25 | 3.10 | 0.34 |
| High | ≥21395 | 15 | 2.88 | 0.19 | 2.93 | 2.49 | 3.13 | −0.06 |
Reported as the AI (Molar Concentration of GuHCl)
Spearman correlation between the anti-HPV antibody titer and AI
Discussion
The avidity and quantity of a serum antibody are key factors in how effective the antibody will be in preventing infection and disease [15, 32–34]. Avidity has been assessed in clinical trials in the pediatric settings and correlated with the effectiveness of vaccines against bacterial infections [35–39]. However, to our knowledge, avidity of anti-HPV L1 antibodies developed in the context of vaccination or infection has not been formally addressed in clinical trials. Here for the first time, an avidity ELISA assay has been established and validated that approximates the binding strength of an IgG antibodies specific for an HPV VLP in serum from vaccinated adults in the setting of a clinical trial.
The modified ELISA assay depends on the use of chaotropic agents that disrupt the interaction between proteins. The effects of the chaotropic agents are non-specific, and while their effects are not well understood, the results from a modified ELISA have been correlated with more accurate measurements of avidity using Biacore [12, 18–19]. Therefore, a modified ELISA represents an acceptable alternative for avidity measurement. However, the integrity of plate-bound antigens is a important consideration, especially when analyzing responses raised against a complex, multimeric protein structure. We evaluated four chemicals typically used in modified avidity ELISAs and GuHCl was selected because it had the largest range of concentrations that minimized alterations to the plate-bound VLP, while presumably capable of disrupting the interaction antigen/antibody interactions. We showed this both using patient serum samples and a monoclonal antibody against HPV16. GuHCl has been previously used in evaluations of antibody avidity after infection with Rubella virus [7, 30] and autoantigens in the setting of diabetes [13]. The optimal range of GuHCl concentrations identified here (1.5 to 3.0 M) caused less than a 20% reduction in OD signals.
Multiple methods for calculating AI values have been described [8, 30–31]. Here we show that the results between AI values calculated using standard approaches adopted by Perciani et al. or Polanec et al. are highly comparable at the working concentrations of GuHCl. We chose to move forward with the method adopted by Polanec et al. for a number of reasons. First, the range of antibody titers observed in HPV VLP vaccine recipients can vary 100-fold within a single time point and 1000-fold between samples. This variability in titers between individuals would make identifying a set of serum dilution factors to use in calculating AI values according to the method used by Perciani et al. problematic. At best different sample dilutions would have to be used between time points and at worst a set of dilutions would have to be found for each individual. In contrast, we can identify a single, optimal dilution for each individual based on the results from the ELISA assay that can then be used in the Polanec et al. method of AI calculation. Secondly, not every concentration of GuHCl used in the Perciani et al. method was comparably to the Polanec et al. method. Using samples from after the completion of the vaccine regiment required a treatment with 2.0 to 3.0 M GuHCl while lower concentrations had minor effects. However, at collection time points before completion of the vaccine regiment the lower concentrations may be more ideal to achieve the required levels of reduction in OD. This is based on an assumption that avidity maturation is occurring in the context of the vaccination. If so, this means that each time point would require the identification of the optimal concentration of the GuHCl to be used, which would not be practical in clinical studies with large sample size. The approach by Polanec et al. alleviates this problem because the full range of GuHCl concentrations is applied to every sample guaranteeing that we can generate data for each individual from which an AI value can be calculated. Based on these practical considerations, we feel that when dealing with populations with a large range of antibody titers and unknown AI values, it is more effective to use the method adopted by Polanec et al.
The modified ELISA assay described here was highly reproducible as evidenced by the very low coefficients of variation (CV) obtained in measurements done in different plates and in between days. The assay was applied to serum from 43 HPV VLP vaccine recipients and a range of avidities were reliably measured. The high ICC reflect the fact that most variability observed is between the avidity measurements among different individuals rather than assay driven. The results of this analysis can be compared to and correlated with other parameters of immunity including the absolute titers of HPV-specific antibodies. Preliminary evidence presented here indicates that there was only a modest correlation between avidity and antibody titers. Thus, future studies are planned to further address the role of avidity in protection against natural infection with HPV and in context of vaccination. This assay will be useful in studying how avidity changes over time and in response to different HPV vaccine formulations.
In conclusion, we developed and validated a simple modified ELISA that can be reliably applied to measure serum HPV antibody avidity in clinical samples from large epidemiological studies. In doing so, it was important to take in account interactions that the chaotropic agent affected. We showed that the four chemicals we evaluated had measurable and at times significant effects on the plate-bound antigens. We took careful steps to measure this effect and understand under which conditions it was minimized. The results of this study showed that GuHCl is a viable reagent to use in avidity assays that rely on detecting responses against HPV L1 VLP and potentially other complex plate-bound antigens and that avidity can be measured in a reproducible fashion using our assay.
Table 3.
HPV16-specific Serum Antibody Response*
| no. | Mean | SD | Median | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Antibody Titers | 43 | 37338 | 75503 | 13316 | 2650 | 447870 |
| Avidity Indices | 43 | 2.72 | 0.35 | 2.80 | 1.70 | 3.26 |
Serum samples collected from participants in a phase III clinical trial of an HPV16/18 L1 VLP vaccine
Acknowledgments
All participants provided informed consent, and the trial was approved by human subjects review committees at the National Cancer Institute (NCI) and Costa Rica.
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the NCI. The trial is sponsored by the NCI, and conducted with support from the Ministry of Health of Costa Rica. This project has been funded in part by the NCI Intramural Research Program, the National Institutes of Health Office for Research on Women’s Health (ORWH). Vaccine was provided for our trial by GlaxoSmithKline Biologicals (GSK Bio, Rixensart, Belgium), under a Clinical Trials Agreement with the NCI. GSK Bio also provided support for aspects of the trial associated with regulatory submission needs of the company under grant FDA BB-IND 7920. Laboratory testing was performed at the NCI-sponsored SAIC-Frederick, Inc. HPV Immunology Laboratory in Frederick, MD. The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. The NCI and Costa Rica investigators make final editorial decisions on this and subsequent publications; GSK Bio has the right to review and comment.
We also would like to acknowledge Dr. Clayton Harro at the Johns Hopkins Bloomberg School of Public Health for the specimens from the Phase I/Phase II NCI/JHU clinical trial of a monovalent HPV-16 L1 VLP vaccine.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Names and Affiliations of the Costa Rica Vaccine Trial (CVT) group members are as follow
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica
Mario Alfaro (Cytologist)
Manuel Barrantes (Field Supervisor)
M. Concepcion Bratti (co-Investigator)
Fernando Cárdenas (General Field Supervisor)
Bernal Cortés (Specimen and Repository Manager)
Albert Espinoza (Head, Coding and Data Entry)
Yenory Estrada (Pharmacist)
Paula Gonzalez (co-Investigator)
Diego Guillén (Pathologist)
Rolando Herrero (co-Principal Investigator)
Silvia E. Jimenez (Trial Coordinator)
Jorge Morales (Colposcopist)
Lidia Ana Morera (Head Study Nurse)
Elmer Pérez (Field Supervisor)
Carolina Porras (co-Investigator)
Ana Cecilia Rodriguez (co-Investigator)
Maricela Villegas (Clinic M.D.)
University of Costa Rica, San José, Costa Rica
Enrique Freer (Director, HPV Diagnostics Laboratory)
Jose Bonilla (Head, HPV Immunology Laboratory)
Sandra Silva (Head Microbiologist, HPV Diagnostics Laboratory)
Ivannia Atmella (Microbiologist, Immunology Laboratory)
Margarita Ramírez (Microbiologist, Immunology Laboratory)
United States National Cancer Institute, Bethesda, MD, USA
Allan Hildesheim (co-Principal Investigator & NCI co-Project Officer)
Douglas R. Lowy (HPV Virologist)
Nora Macklin (Trial Coordinator)
Mark Schiffman (Medical Monitor & NCI co-Project Officer)
John T. Schiller (HPV Virologist)
Mark Sherman (QC Pathologist)
Diane Solomon (Medical Monitor & QC Pathologist)
Sholom Wacholder (Statistician)
SAIC-Frederick, Inc., NCI-Frederick, Frederick, MD, USA
Ligia Pinto (Head, HPV Immunology Laboratory)
Alfonso Garcia-Pineres (Scientist, HPV Immunology Laboratory)
Womens and Infants’ Hospital, Providence, RI, USA
Claire Eklund (QC Cytology)
Martha Hutchinson (QC Cytology)
DDL Diagnostic Laboratory, The Netherlands
Wim Quint (HPV DNA Testing)
Leen-Jan van Doorn (HPV DNA Testing)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dziemian E, Zarnowska H, Kolodziej-Sobocinska M, Machnicka B. Determination of the relative avidity of the specific IgG antibodies in human toxocariasis. Parasite Immunol. 2008;30:187–90. doi: 10.1111/j.1365-3024.2007.01010.x. [DOI] [PubMed] [Google Scholar]
- 2.Motran CC, Cerban FM, Rivarola HW, Vottero de Cima E. Characterization of autoantibodies generated in mice by immunization with the C-terminal region of Trypanosoma cruzi ribosomal P1 and P2 proteins. Clin Immunol. 1999;91:17–24. doi: 10.1006/clim.1998.4678. [DOI] [PubMed] [Google Scholar]
- 3.Narita M, Matsuzono Y, Takekoshi Y, et al. Analysis of mumps vaccine failure by means of avidity testing for mumps virus-specific immunoglobulin G. Clin Diagn Lab Immunol. 1998;5:799–803. doi: 10.1128/cdli.5.6.799-803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raviprakash K, Porter KR, Kochel TJ, et al. Dengue virus type 1 DNA vaccine induces protective immune responses in rhesus macaques. J Gen Virol. 2000;81:1659–67. doi: 10.1099/0022-1317-81-7-1659. [DOI] [PubMed] [Google Scholar]
- 5.Yasodhara P, Ramalakshmi BA, Sarma MK. A new approach to differentiate recent vs chronic toxoplasma infection: Avidity elisa in toxoplasma serology. Indian J Med Microbiol. 2001;19:145–8. [PubMed] [Google Scholar]
- 6.Anttila M, Eskola J, Ahman H, Kayhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998;177:1614–21. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- 7.Hedman K, Seppala I. Recent rubella virus infection indicated by a low avidity of specific IgG. J Clin Immunol. 1988;8:214–21. doi: 10.1007/BF00917569. [DOI] [PubMed] [Google Scholar]
- 8.Perciani CT, Peixoto PS, Dias WO, Kubrusly FS, Tanizaki MM. Improved method to calculate the antibody avidity index. J Clin Lab Anal. 2007;21:201–6. doi: 10.1002/jcla.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med. 2003;9:1209–13. doi: 10.1038/nm918. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Steiner S, Holder PF, Gomez de Leon P, Spear W, Hennessy TW, Carlone GM. Avidity determinations for Haemophilus influenzae Type b anti-polyribosylribitol phosphate antibodies. Clin Diagn Lab Immunol. 2005;12:1029–35. doi: 10.1128/CDLI.12.9.1029-1035.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rychert J, Amedee AM. The antibody response to SIV in lactating rhesus macaques. J Acquir Immune Defic Syndr. 2005;38:135–41. doi: 10.1097/01.qai.0000148947.03416.b5. [DOI] [PubMed] [Google Scholar]
- 12.Ward KN, Dhaliwal W, Ashworth KL, Clutterbuck EJ, Teo CG. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J Med Virol. 1994;43:367–72. doi: 10.1002/jmv.1890430409. [DOI] [PubMed] [Google Scholar]
- 13.Westerlund A, Ankelo M, Ilonen J, Knip M, Simell O, Hinkkanen AE. Absence of avidity maturation of autoantibodies to the protein tyrosine phosphatase-like IA-2 molecule and glutamic acid decarboxylase (GAD65) during progression to type 1 diabetes. J Autoimmun. 2005;24:153–67. doi: 10.1016/j.jaut.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Arias-Bouda LM, Kuijper S, Van der Werf A, Nguyen LN, Jansen HM, Kolk AH. Changes in avidity and level of immunoglobulin G antibodies to Mycobacterium tuberculosis in sera of patients undergoing treatment for pulmonary tuberculosis. Clin Diagn Lab Immunol. 2003;10:702–9. doi: 10.1128/CDLI.10.4.702-709.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breukels MA, Jol-van der Zijde E, van Tol MJ, Rijkers GT. Concentration and avidity of anti-Haemophilus influenzae type b (Hib) antibodies in serum samples obtained from patients for whom Hib vaccination failed. Clin Infect Dis. 2002;34:191–7. doi: 10.1086/338259. [DOI] [PubMed] [Google Scholar]
- 16.Harris SL, Tsao H, Ashton L, Goldblatt D, Fernsten P. Avidity of the immunoglobulin G response to a Neisseria meningitidis group C polysaccharide conjugate vaccine as measured by inhibition and chaotropic enzyme-linked immunosorbent assays. Clin Vaccine Immunol. 2007;14:397–403. doi: 10.1128/CVI.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero-Steiner S, Musher DM, Cetron MS, et al. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999;29:281–8. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 18.McCloskey N, Turner MW, Goldblatt TD. Correlation between the avidity of mouse-human chimeric IgG subclass monoclonal antibodies measured by solid-phase elution ELISA and biospecific interaction analysis (BIA) J Immunol Methods. 1997;205:67–72. doi: 10.1016/s0022-1759(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald RA, Hosking CS, Jones CL. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J Immunol Methods. 1988;106:191–4. doi: 10.1016/0022-1759(88)90196-2. [DOI] [PubMed] [Google Scholar]
- 20.de Souza VA, Pannuti CS, Sumita LM, de Andrade Junior HF. Enzyme-linked immunosorbent assay-IgG antibody avidity test for single sample serologic evaluation of measles vaccines. J Med Virol. 1997;52:275–9. doi: 10.1002/(sici)1096-9071(199707)52:3<275::aid-jmv7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;86:83–7. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 22.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89:12180–4. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirnbauer R, Taub J, Greenstone H, et al. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993;67:6929–36. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. Jama. 2007;298:743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 25.Pinto LA, Edwards J, Castle PE, et al. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188:327–38. doi: 10.1086/376505. [DOI] [PubMed] [Google Scholar]
- 26.Dessy FJ, Giannini SL, Bougelet CA, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4:425–34. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 27.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 28.Christensen ND, Dillner J, Eklund C, et al. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology. 1996;223:174–84. doi: 10.1006/viro.1996.0466. [DOI] [PubMed] [Google Scholar]
- 29.Roden RB, Armstrong A, Haderer P, et al. Characterization of a human papillomavirus type 16 variant-dependent neutralizing epitope. J Virol. 1997;71:6247–52. doi: 10.1128/jvi.71.8.6247-6252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polanec J, Seppala I, Rousseau S, Hedman K. Evaluation of protein-denaturing immunoassays for avidity of immunoglobulin G to rubella virus. J Clin Lab Anal. 1994;8:16–21. doi: 10.1002/jcla.1860080105. [DOI] [PubMed] [Google Scholar]
- 31.Vermont CL, van Dijken HH, van Limpt CJ, de Groot R, van Alphen L, van Den Dobbelsteen GP. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infect Immun. 2002;70:584–90. doi: 10.1128/IAI.70.2.584-590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chargelegue D, Stanley CM, O’Toole CM, Colvin BT, Steward MW. The affinity of IgG antibodies to gag p24 and p17 in HIV-1-infected patients correlates with disease progression. Clin Exp Immunol. 1995;99:175–81. doi: 10.1111/j.1365-2249.1995.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ek T, Mellander L, Hahn-Zoric M, Abrahamsson J. Avidity of tetanus and Hib antibodies after childhood acute lymphoblastic leukaemia - implications for vaccination strategies. Acta Paediatr. 2006;95:701–6. doi: 10.1080/08035250500459717. [DOI] [PubMed] [Google Scholar]
- 34.Schlesinger Y, Granoff DM. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. Jama. 1992;267:1489–94. [PubMed] [Google Scholar]
- 35.Denoel PA, Goldblatt D, de Vleeschauwer I, Jacquet JM, Pichichero ME, Poolman JT. Quality of the Haemophilus influenzae type b (Hib) antibody response induced by diphtheria-tetanus-acellular pertussis/Hib combination vaccines. Clin Vaccine Immunol. 2007;14:1362–9. doi: 10.1128/CVI.00154-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longworth E, Borrow R, Goldblatt D, et al. Avidity maturation following vaccination with a meningococcal recombinant hexavalent PorA OMV vaccine in UK infants. Vaccine. 2002;20:2592–6. doi: 10.1016/s0264-410x(02)00151-2. [DOI] [PubMed] [Google Scholar]
- 37.Nair N, Gans H, Lew-Yasukawa L, Long-Wagar AC, Arvin A, Griffin DE. Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J Infect Dis. 2007;196:1339–45. doi: 10.1086/522519. [DOI] [PubMed] [Google Scholar]
- 38.Poolman J, Kaufhold A, De Grave D, Goldblatt D. Clinical relevance of lower Hib response in DTPa-based combination vaccines. Vaccine. 2001;19:2280–5. doi: 10.1016/s0264-410x(00)00517-x. [DOI] [PubMed] [Google Scholar]
- 39.Wuorimaa T, Dagan R, Vakevainen M, et al. Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J Infect Dis. 2001;184:1211–5. doi: 10.1086/323648. [DOI] [PubMed] [Google Scholar]