Abstract
The human Organic Cation/Carnitine Transporter (hOCTN2), is a high affinity cation/carnitine transporter expressed widely in human tissues and is physiologically important for the homeostasis of L-carnitine. The objective of this study was to elucidate the substrate requirements of this transporter via computational modelling based on published in vitro data. Nine published substrates of hOCTN2 were used to create a common features pharmacophore that was validated by mapping other known OCTN2 substrates. The pharmacophore was used to search a drug database and retrieved molecules that were then used as search queries in PubMed for instances of a side effect (rhabdomyolysis) associated with interference with L-carnitine transport. The substrate pharmacophore was comprised of two hydrogen bond acceptors, a positive ionizable feature and ten excluded volumes. The substrate pharmacophore also mapped 6 out of 7 known substrate molecules used as a test set. After searching a database of ~800 known drugs, thirty drugs were predicted to map to the substrate pharmacophore with L-carnitine shape restriction. At least 16 of these molecules had case reports documenting an association with rhabdomyolysis and represent a set for prioritizing for future testing as OCTN2 substrates or inhibitors. This computational OCTN2 substrate pharmacophore derived from published data partially overlaps a previous OCTN2 inhibitor pharmacophore and is also able to select compounds that demonstrate rhabdomyolysis, further confirming the possible linkage between this side effect and hOCTN2.
Keywords: human Organic Cation/Carnitine Transporter (hOCTN2), carnitine, pharmacophore, transporters
INTRODUCTION
Drug transporter in vitro data generation, computational modeling and knowledge of the substrate requirements or structure activity relationships (SAR) is at least a decade behind that of comparable efforts in characterizing drug metabolizing enzymes. Very few transporters other than P-glycoprotein and BCRP 1–3 have been characterized extensively in vitro and in silico. We therefore need to accelerate in silico modeling for other transporters in order to predict drug-transporter interactions, drug-drug interactions and the potential for toxicity. Generating drug transporter models could also enable design and optimization of drugs that may improve specificity and uptake. While such models may also enable repurposing of drugs 4, 5 that are either found to be substrates or inhibitors of transporters, such that they could find new therapeutic indications.
One approach we have taken recently with several human drug transporters is to use a combination of computational and in vitro approaches which follow iterative cycles, to increase the number of molecules with transporter inhibition or substrate data 6–11. For example, there is no crystal structure or three dimensional (3D) protein model of the human Organic Cation/Carnitine Transporter (hOCTN2), which is a high affinity cation/carnitine transporter expressed widely in human tissues 12. hOCTN2 is physiologically important for the homeostasis of the endogenous compound L-carnitine, transporting it in a sodium dependent manner 13. L-carnitine is involved in intermediary metabolism 13 and holds a primary role in facilitating the transport of long-chain fatty acids into mitochondria, allowing β-oxidation for energy production 14, 15. This transporter can also be targeted to increase uptake to the CNS and has been used in a prodrug strategy with drugs conjugated to L-carnitine 14. An approach to study the substrate requirements of hOCTN2 could assist in these targeting and prodrug efforts and also predict molecules that cause drug-induced secondary carnitine deficiency.
In two previous studies, we generated and validated computational models for inhibitors of hOCTN2 6, 9. Besides these studies on inhibitor pharmacophores, which resulted in models with a positive ionizable feature, two hydrophobes and a hydrogen bond acceptor (or third hydrophobic feature), we are aware of only one other report investigating the structural requirements of hOCTN2 inhibition 15. This study used L-carnitine and cephaloridine to build a pharmacophore with a constantly positively charged nitrogen atom and a carboxyl, nitrile or ester group connected by a 2–4-atom linker 15. To our knowledge to this point there have been no computational studies to define the pharmacophore or structure activity relationships of OCTN2 substrates. The goal of our current study was to use substrate data from our laboratory 14 and others, to build and test the first substrate pharmacophore for hOCTN2, which could be useful for selecting or avoiding novel molecules that target this transporter.
EXPERIMENTAL SECTION
Pharmacophore development
Computational molecular modeling studies were carried out using Discovery Studio 2.5.5 (Accelrys, San Diego, CA). Compounds listed in Table 1 represent known substrates predominantly from our laboratory or the literature and were used for common feature pharmacophore generation. The CAESAR algorithm 16 was used to generate upto 255 conformers per molecule with an energy threshold of 20kcal/mol. Excluded volumes were also added during pharmacophore generation. Common feature pharmacophore models attempt to describe the arrangement of key features that are important for biological activity and their generation has been widely described 17, 18.
Table 1.
Molecules used for hOCTN2 common features substrate pharmacophore generation. MaxOmitFeat (set at zero for all molecules) and Principal are required by DiscoveryStudio software. Principal = 2 represents the most active, 0 = less active or inactive.
| Substrate | Affinity for hOCTN2 Km (μM) | Principal | References |
|---|---|---|---|
| Acetyl-L-Carnitine | 8.5 | 2 | 13 |
| Ipratropium | 53 | 1 | 22 |
| Ketoprofen-Glycine-L-Carnitine | 58.5 | 1 | 14 |
| Ketoprofen-L-Carnitine | 77 | 1 | 14 |
| L-Carnitine | 5.3 | 2 | 9 |
| Mildronate | 26 | 1 | 24 |
| Naproxen-L-Carnitine | 257 | 0 | 14 |
| Valproyl-Glycolic Acid-L-Carnitine | 161 | 0 | 14 |
| Valproyl L-Carnitine | 132 | 0 | 14 |
A common features pharmacophore was initially developed using L-carnitine and acetyl-L-carnitine, (which had the best affinity for OCTN2). Ipratropium, ketoprofen-glycine-L-carnitine, mildronate and ketoprofen-L-carnitine were mapped to this. Hence, the pharmacophore was based on the five of the most active compounds (using Km data) that were screened. The remaining compounds were used as less active compounds whose features were excluded from the pharmacophore, in order to highlight features of the five most active compounds. This pharmacophore was compared with that generated previously for a set of inhibitors 6 using the ‘pharmacophore comparison protocol’. An additional pharmacophore with shape restriction was developed. This shape restriction used the van der Waals shape of L-carnitine and represents a method to limit the search space to that of the shape for this known substrate.
Test set evaluation
Following development of the pharmacophore using the substrates listed in Table 1, we performed an extensive literature search to find additional substrates for OCTN2. The structures of the eight compounds (listed in Table 2) as putative substrates were either found in ChemSpider (http://www.chemspider.com/) or drawn manually and saved as mol or sdf files. This test set was used in Discovery Studio to create a 3D database. Oxaliplatin could not be used due to it being a coordination complex. The remaining seven compounds were searched with the OCTN2 substrate pharmacophore with and without shape feature.
Table 2.
Molecules used as a test set for the hOCTN2 common features substrate pharmacophore. NA = 3D conformers not generated for database, NM = did not map to the pharmacophore. Fit Value = higher values are desirable.
| Purported Substrate/Inhibitor of OCTN2 | Details | Pharmacophore without shape Fit value | Pharmacophore with shape Fit Value | References |
|---|---|---|---|---|
| Oxaliplatin | Oxaliplatin transport in OCTN2 over-expressing HEK293 cells. | NA | NA | 25 |
| Verapamil | hOCTN2 expressed in MDCKII and LLC-PK1 cells (EC50 = 25μM). | 0.58 | NM | 20 |
| Spironolactone | hOCTN2 expressed in MDCKII and LLC-PK1 cells (EC50 = 26μM). | Only mapped when feature miss-enabled | NM | 20 |
| Pyrilamine | Mouse kidney slice uptake (Km = 236μM). | 0.72 | NM | 21 |
| Nipecotic acid-L-carnitine | Uptake in mouse brain. | 1.64 | NM | 26 |
| Betaine | hOCTN2 in Xenopus oocytes (uptake rate = 36.7 pmol/30 min). | 0.73 | 0.72 | 27 |
| Propionyl-L-carnitine | No published Km but is a substrate | 2.70 | 2.48 | 28 |
| Palmitoyl-L-carnitine | No published Km but is a substrate | 2.20 | NM | 39 |
Database searching
The pharmacophore with the shape restriction using the van der Waals shape of L-carnitine was used to search the SCUT database of 796 compounds (656 drugs in the SCUT 2008 database plus additional drug metabolites and drugs of abuse). This database was previously created using structures in the MDL SDF format prior to conversion to a 3D Catalyst database after generating up to 100 molecule conformations with the FAST conformer generation method, with the maximum energy threshold of 20 kcal/mol 19. The quality of the molecule mapping to the substrate pharmacophore was determined by the FitValue, which is dependent on the proximity of a compound to the pharmacophore feature centroids and the weights assigned to each centroid, where a higher FitValue represents a better fit. Compounds returned as mapping to the pharmacophore (Table 3) were used to search PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) using the compound name and ‘rhabdomyolysis’ as search terms.
Table 3.
Results of SCUT FDA drug database search using the hOCTN2 shape feature pharmacophore model. Fit Value = higher values are desirable.
| Molecule | Pharmacological action | hOCTN2 Substrate Pharmacophore with shape Fit value | Examples of literature related to rhabdomyolysis |
|---|---|---|---|
| Metaproterenol | Sympathomimetic bronchodilator | 2.65 | |
| Isoproterenol | β1 and β2 stimulant | 2.65 | 29 |
| Phenylephrine | α-adrenergic agonist | 2.64 | 41 |
| Epinephrine | β-adrenergic agonist | 2.64 | 30 |
| Pamidronic Acid | Inhibition of normal and abnormal bone resorption | 2.56 | 42 |
| Cocaine_Metabolite_Ecgonine | Serotonin– norepinephrine– dopamine reuptake inhibitor | 2.46 | 31 |
| Miglitol | α-glucosidase inhibitor, delays ingestion of ingested carbohydrates | 2.15 | |
| Alendronate | Bone resorprtion osteoporosis | 2.13 | 44 |
| Terbutaline | Sympathomimetic | 2.09 | 32 |
| Triethanolamine | Ceruminolytic | 2.04 | |
| Acyclovir | Interferes with viral DNA synthesis | 1.96 | 33 |
| Norepinephrine | Peripheral vasoconstrictor acting on both arterial and venous beds | 1.86 | |
| Amifostine | Anticancer | 1.85 | |
| Cocaine_Metabolite_Ecgonine_Methyl_Ester | Serotonin– norepinephrine– dopamine reuptake inhibitor | 1.84 | 31 |
| Methyldopa | Centrally acting antihypertensive | 1.58 | |
| Dopamine | Positive inotrope | 1.58 | |
| Bethanechol Chloride | Stimulates smooth muscle cholinergic receptors in bladder and GI tract | 1.53 | |
| Decitabine | Inhibits DNA methyltransferase | 1.48 | |
| Cidofovir | Viral DNA inhibitor | 1.21 | |
| Aminocaproic Acid | Inhibits fibrinolysis via inhibition of TPA substances | 1.16 | 34 |
| Levodopa | Precursor for dopamine, norepinephrine, and epinephrine | 1.05 | 35 |
| Tizanidine | α-2 adrenergic agonist | 0.79 | |
| Emtricitabine | Nucleoside reverse transcriptase inhibitors | 0.75 | |
| Lamivudine | Inhibits HIV reverse transcriptase | 0.73 | 36 |
| Amphetamine_Analog_MDEA | Serotonin, norepinephrine, and dopamine releasing agent | 0.51 | 37 |
| Amphetamine_Analog_MDMA | Serotonin, norepinephrine, and dopamine releasing agent | 0.50 | 37 |
| Cytarabine | Interferes with DNA synthesis | 0.29 | 38 |
| Gemcitabine | Inhibits ribonucleotide reductase, produces false nucleotide bas-inhibiting DNA synthesis | 0.14 | 39 |
| Pregabalin | Binds to the α2δ subunit of the voltage-dependent calcium channel | 0.02 | |
| Ethambutol | Inhibits cellular metabolism | 0.003 |
RESULTS
OCTN2 substrate pharmacophore
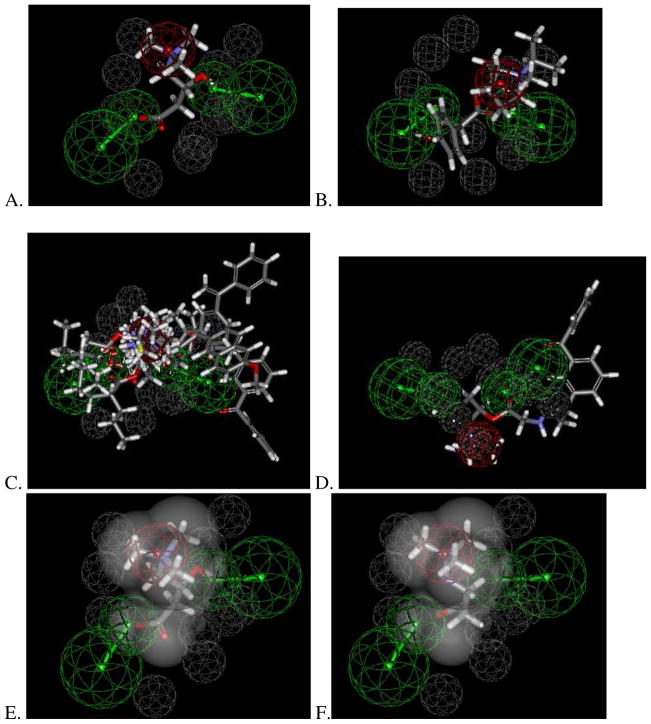
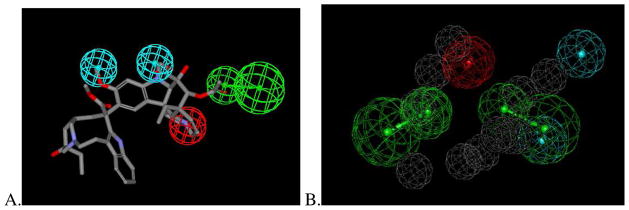
The common features pharmacophore derived from nine molecules (that have been published by us or others as OCTN2 substrates, Table 1) consisted of two hydrogen bond acceptor features, a positive ionizable feature and ten excluded volumes (Figure 1A). These molecules and their interactions with the pharmacophore can be visualized to understand how feature mapping relates to Km values. Larger training set compounds such as ipratropium (Figure 1B) and drugs conjugated to L-carnitine (ketoprofen-L-carnitine, ketoprofen-glycine-L-carnitine, naproxen-L-carnitine, valprolyl-glycolic acid-L-carnitine) extend outside of the pharmacophore features (Figure 1C and 1D) and had Km values 53–257μM 16, 22. With the pharmacophore using the van der Waals surface around L-carnitine (Figure 1E), mildronate (Km 26μM 20) fit within this volume but did not map the hydrogen bond acceptor features when compared with the highest affinity substrate, L-carnitine (Km 5.3 μM 9), which was used to create the shape (Figure 1F). This suggests mapping the hydrogen bond acceptors is important for higher affinity interactions with OCTN2 as shown with the training set compounds. This substrate pharmacophore (Figure 1A) partially overlapped a previously generated inhibitor pharmacophore (Figure 2A) with the positive ionizable feature and hydrogen bond acceptor feature common to both having an RMSD 0.27 Å (Figure 1B). These two features are therefore likely important to suggest molecules that can interact as either substrates, inhibitors or both. The features unique to each pharmacophore are therefore likely to determine whether a compound is a substrate or inhibitor.
Figure 1.
Common features hOCTN2 substrate pharmacophore that consists of a positive ionizable feature (red) and two hydrogen bond acceptors (green) with 10 excluded volumes (grey). The pharmacophore was developed using L-carnitine and acetyl-L-carnitine, (which had the best affinity for OCTN2). Ipratropium, ketoprofen-glycine-L-carnitine, mildronate and ketoprofen-L-carnitine were mapped to this. Hence, the pharmacophore was based on the five of the most active compounds (using Km data) that were screened. Naproxen-L-carnitine, valproyl-glycolic acid-L-carnitine and valpoyl L-carnitine were used as less active compounds whose features were excluded from the pharmacophore. A. L- carnitine; B. ipratropium; C. All conjugated compounds; D Ketoprofen glycine-L-carnitine; E. van der Waals surface around L-carnitine, F. Mildronate. For ketoprofen glycine-L-carnitine in panel D, there was poorer mapping to the hydrogen bond acceptors.
Figure 2.
Comparison of the hOCTN2 inhibitor 6 and substrate pharmacophores (Figure 1), with two features in common including a positive ionizable (red) and one hydrogen bond acceptor (green). A. hOCTN2 inhibitor pharmacophore 6, hydrophobic features in cyan B. overlap of hOCTN2 inhibitor and substrate pharmacophores showing how left most hydrogen bond acceptor and positive ionizable features overlap in both models.
OCTN2 substrate pharmacophore testing
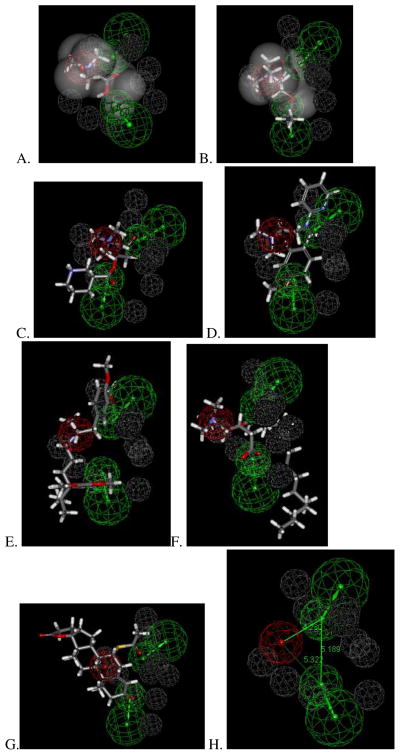
This new OCTN2 substrate pharmacophore also mapped six out of seven known substrate molecules to differing extents that could be converted to a 3D database for use as a test set (Table 2). Betaine (Figure 3A) and propionyl-L-carnitine (Figure 3B) mapped to the shape plus feature pharmacophore and may be indicative of higher affinity substrates, while nipecotic acid-L-carnitine, pyrilamine, verapamil and palmitoyl-L-carnitine (Figure 3C–F) only map these features to varying extents when the L-carnitine shape is omitted (Table 2), with large portions of the molecule outside the key three pharmacophore features. These latter compounds were however still selected by the pharmacophore and this may suggest they have lower affinity (e.g. pyrilamine is reported to have a Km of 236μM in mouse kidney slices 21, Table 2). Spironolactone only mapped two out of three features, missing the positive ionizable feature (Figure 3G) and projects most of the structure into an area not covered in the original pharmacophore (Figure 1C). This would also be indicative that this compound is likely to be a low affinity substrate compared with L-carnitine. The OCTN2 substrate inter-feature distances (Figure 1H) of 4.2–5.3Å suggests that high affinity substrates will be relatively small like L-carnitine.
Figure 3.
Test set mapping to the hOCTN2 substrate pharmacophore that consists of positive ionizable feature (red) and two hydrogen bond acceptors (green) with 10 excluded volumes (grey). A. betaine mapped to shape feature hypothesis, B. Propionyl-L-carnitine mapped to shape feature hypothesis, C. Nipecotic acid-L-carnitine, D. Pyrilamine, E. Verapamil, F. Palmitoyl-L-carnitine, G. Spironolactone, (allowing one feature missing) mapped two out of three features, missing the positive ionizable feature and H. Inter-feature distances.
Database searching with OCTN2 pharmacophore
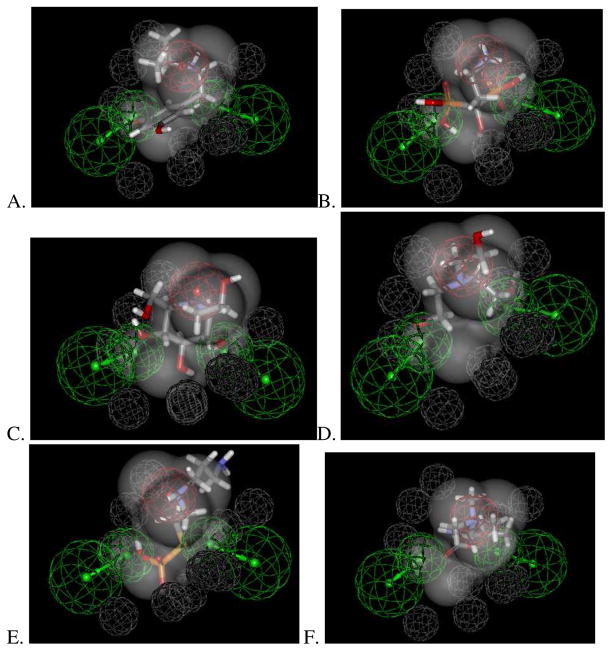
After searching a database of ~800 known drugs with the OCTN2 substrate pharmacophore and the van der Waals shape of L-carnitine, thirty drugs or metabolites with a diverse array of pharmacological mechanisms were predicted to map (Table 3). Several of these drugs or metabolites were of a very similar size and shape to L-carnitine (Figure 4) as required by this pharmacophore and had high fit values (>2.0) which would provide some confidence of likely affinity. After searching PubMed, 16 of these predicted OCTN2 substrate molecules had case reports or papers which documented an association with rhabdomyolysis (Table 3), a side effect that can result from inhibition of L-carnitine transport 6, 9. To our knowledge none of these compounds have been tested in vitro as inhibitors of OCTN2.
Figure 4.
Examples of compounds mapping to hOCTN2 substrate shape-feature pharmacophore. A. Metaproteronel, B. Pamidromic acid, C. Miglitol, D. Triethanolamine, E. Amifostine, F Bethanechol.
DISCUSSION
Computational models for transporters
While there has been a recent discussion 22 of the transporters that are clinically important in drug absorption and disposition, and the in vitro systems for assessing drug interactions, there is still a deficit in the knowledge of substrate and inhibitor requirements for most transporters. If we are to characterize as many of the human drug transporters as possible then in vitro data generation has to be combined with computational efforts 6–11, as we have suggested previously, if we are to catch up with drug metabolizing enzyme characterization. The current study represents one such approach for hOCTN2 substrates by using previously published data from us and other laboratories to produce the first OCTN2 substrate pharmacophore. We have also illustrated how additional literature data can be used to validate this pharmacophore and a prospective database search can be coupled with literature searching to further suggest an important relationship with a toxicological side-effect. At the same time it prioritizes additional compounds that could be tested in vitro in future.
In a previous study, we generated an in silico common features pharmacophore model for inhibitors of hOCTN2 using initial in vitro cell culture data from our laboratory 9. The hOCTN2 inhibitor pharmacophore was used to identify additional more potent inhibitors of OCTN2 that were then obtained and tested in vitro. The validated inhibitor OCTN2 pharmacophore was shown to accurately prioritize compounds for testing and also identified an array of novel low micromolar inhibitors from diverse therapeutic classes of drugs. This initial work, also lead us to identify an association between compounds that were more likely to cause rhabdomyolysis (a relatively rare condition resulting in a form of muscle weakness). For example, HMG-CoA reductase inhibitors alone or in combination with other medications, can cause rhabdomyolysis 9. One possible mechanism for this side effect is due to the inhibition of L-carnitine uptake into muscle and/or inhibition of L-carnitine re-absorption in the kidney, which are well known to be both mediated by hOCTN2 9. We found for OCTN2 inhibitors if their Cmax/Ki ratio was higher than 0.0025, they were more likely to cause rhabdomyolysis 9. Follow up work resulted in quantitative structure–activity relationship (QSAR) of OCTN2 inhibitors and a Bayesian model, both of which were subjected to validation with external molecules 6. Treating the testing data as binary, the Bayesian method correctly identified over 80% of the compounds whereas the pharmacophore could correctly classify over 70% of the compounds. The additional results from this second study also confirmed the earlier observation of increased likelihood of rhabdomyolysis and the Cmax/Ki ratio higher than 0.0025 6. We hypothesized that OCTN2 substrates may also be associated with rhabdomyolyis.
OCTN2 substrate pharmacophore
There has been no previous development of an OCTN2 substrate pharmacophore. The current study therefore presents the first substrate pharmacophore for hOCTN2. We found that it partially overlaps the inhibitor pharmacophore features with a positive ionizable feature and hydrogen bond acceptor in common (Figure 2B). This is surprising because both pharmacophore models were generated with different sets of molecules and yet the distance between a positive ionizable and hydrogen bond acceptor is almost identical. The features that differ suggests hOCTN2 inhibitors have hydrophobic features while substrates require an additional hydrogen bonding feature. It could also be quite complex to dissociate these two activities, because recognition by OCTN2 requires the same two minimal features, namely the positive ionizable feature and hydrogen bond acceptor. This OCTN2 substrate model also mapped six out of seven of the additional suggested literature substrates (and or inhibitors) that were used as a test set (Figure 3, Table 2). This provides validation that the model can identify substrates that are also not in the training set. We have previously used a set of FDA approved drugs for pharmacophore searching to identify new compounds for testing against other transporters 8, 10. In this study we searched the FDA drugs with the OCTN2 substrate pharmacophore with the L-carnitine van der Waals surface and retrieved 30 drugs that mapped to the key features. These molecules belonged to many different therapeutic classes with diverse pharmacological actions (Table 3), an observation in line with our previous work on searching the same database with the OCTN2 inhibitor pharmacophore 6, 9. As we have previously shown the association of OCTN2 and rhabdomyolysis 6, 9, we used this side effect as a surrogate for likely interaction with this transporter. It is possible that we may be selecting for a mixture of potential OCTN2 substrates and inhibitors in this case, which remains to be verified. Following literature searching with each compound that mapped to the pharmacophore, we identified associations with rhabdomyolysis for sixteen of these thirty molecules (Figure 4, Table 3). We are not aware that any of these compounds have been tested as substrates (or inhibitors) of OCTN2 in vitro and this may represent future work. This pharmacophore and literature mining approach could be taken with other transporters to accelerate our understanding of their pharmacophores.
In the current study, the retrieval of compounds associated with rhabdomyolysis could suggest that such compounds are either substrates or solely inhibitors of hOCTN2. Further experimental validation of these predictions is warranted to enable the update and clarification of the model. However, from the various analyses in this study, the OCTN2 substrate model appears valid since other known substrates (Table 2, Figure 3) map to differing extents, lending confidence in the predictions. The combination of in vitro and computational methods in the same laboratory is certainly a compelling approach to take. However, our results here and elsewhere 6–11, 25–28 suggest that considerably more research can be achieved by leveraging the already available literature transporter data using even a small number of substrates, and linking findings such as potential clinical effects associated with a transporter, e.g. rhabdomyolysis derived from our prior studies 6, 9. The small number and limited diversity of substrates used in this study is a limitation although we have made use of all of the available OCTN2 substrate data published to date that we are aware of.
This pharmacophore and data mining approach if it is to be used more widely requires a well curated structure activity database for each transporter, ideally one that is publically accessible and perhaps contains other clinically relevant data 23. Computational models 24 could result in a more efficient use of in vitro resources for transporter studies, focusing effort on compounds most likely to be substrates or inhibitors for a particular transporter of interest.
However one question is still likely to remain: What do the pharmacophore models represent? In many cases, the pharmacophore models may be a synthesis or consensus of multiple binding sites (whether substrate or inhibitor), or perhaps an average of the key features if the transporter is promiscuous 31–33. The drug-L-carnitine conjugates are much larger than the L-carnitine pharmacophore itself and these molecules extend far beyond the pharmacophore features in this study. The pharmacophore has also only explored substitution of these conjugates at one position which may be a limitation and this could be evaluated by future experimental testing of conjugates substituted elsewhere.
In summary, we have defined the first substrate pharmacophore for hOCTN2, which is different to the previously published hOCTN2 inhibitor pharmacophores. Using a small set of additional substrates we validated the substrate pharmacophore, showing that they map to differing degrees. Our work reveals some features in common and also some differentiating features between OCTN2 substrate and inhibitor pharmacophores that can be exploited in modifying molecules to enable uptake via this transporter.
Acknowledgments
S.E. gratefully acknowledges Dr. Matthew D. Krasowski for his assistance in creating the SCUT 2008 database supplemented with metabolites and drugs of abuse. S.E. also thanks Accelrys (San Diego, CA) for making Discovery Studio available. This work was supported in part by National Institutes of Health Grant DK67530.
ABBREVIATIONS
- LLC-PK1
Pig kidney epithelial cells
- MDCK
Madin-Darby canine kidney
- QSAR
quantitative structure-activity relationship
References
- 1.Matsson P, Pedersen JM, Norinder U, Bergstrom CA, Artursson P. Identification of novel specific and general inhibitors of the three major human ATP-binding cassette transporters P-gp, BCRP and MRP2 among registered drugs. Pharm Res. 2009;26(8):1816–31. doi: 10.1007/s11095-009-9896-0. [DOI] [PubMed] [Google Scholar]
- 2.Matsson P, Englund G, Ahlin G, Bergstrom CA, Norinder U, Artursson P. A global drug inhibition pattern for the human ATP-binding cassette transporter breast cancer resistance protein (ABCG2) J Pharmacol Exp Ther. 2007;323(1):19–30. doi: 10.1124/jpet.107.124768. [DOI] [PubMed] [Google Scholar]
- 3.Ekins S. Drug transporter pharmacophores. In: Ecker G, Chiba P, editors. Transporters as drug carriers: structure, function, substrates. Vol. 44. Wiley-VCH Verlag GmbH & Co KGaA; Weinheim: 2009. pp. 215–227. [Google Scholar]
- 4.Ekins S, Williams AJ, Krasowski MD, Freundlich JS. In silico repositioning of approved drugs for rare and neglected diseases. Drug Disc Today. 2011;16:298–310. doi: 10.1016/j.drudis.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Ekins S, Williams AJ. Finding promiscuous old drugs for new uses. Pharm Res. 2011;28:1786–1791. doi: 10.1007/s11095-011-0486-6. [DOI] [PubMed] [Google Scholar]
- 6.Diao L, Ekins S, Polli JE. Quantitative Structure Activity Relationship for Inhibition of Human Organic Cation/Carnitine Transporter. Mol Pharm. 2010;7:2120–2130. doi: 10.1021/mp100226q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahadduri PM, Polli JE, Swaan PW, Ekins S. Targeting drug transporters - combining in silico and in vitro approaches to predict in vivo. Methods Mol Biol. 2010;637:65–103. doi: 10.1007/978-1-60761-700-6_4. [DOI] [PubMed] [Google Scholar]
- 8.Zheng X, Ekins S, Rauffman JP, Polli JE. Computational models for drug inhibition of the Human Apical Sodium-dependent Bile Acid Transporter. Mol Pharm. 2009;6:1591–1603. doi: 10.1021/mp900163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diao L, Ekins S, Polli JE. Novel Inhibitors of Human Organic Cation/Carnitine Transporter (hOCTN2) via Computational Modeling and In Vitro Testing. Pharm Res. 2009;26:1890–1900. doi: 10.1007/s11095-009-9905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C, Bahadduri PM, Polli JE, Swaan PW, Ekins S. Rapid Identification of P-glycoprotein Substrates and Inhibitors. Drug Metab Dispos. 2006;34:1976–1984. doi: 10.1124/dmd.106.012351. [DOI] [PubMed] [Google Scholar]
- 11.Ekins S, Johnston JS, Bahadduri P, D’Souzza VM, Ray A, Chang C, Swaan PW. In Vitro And Pharmacophore Based Discovery Of Novel hPEPT1 Inhibitors. Pharm Res. 2005;22:512–517. doi: 10.1007/s11095-005-2505-y. [DOI] [PubMed] [Google Scholar]
- 12.Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem. 1998;273(32):20378–82. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 13.Rebouche CJ, Paulson DJ. Carnitine metabolism and function in humans. Annu Rev Nutr. 1986;6:41–66. doi: 10.1146/annurev.nu.06.070186.000353. [DOI] [PubMed] [Google Scholar]
- 14.Diao L, Polli JE. Synthesis and in vitro characterization of drug conjugates of l-carnitine as potential prodrugs that target human Octn2. J Pharm Sci. 2011;100:3802–3816. doi: 10.1002/jps.22557. [DOI] [PubMed] [Google Scholar]
- 15.Todesco L, Bur D, Brooks H, Torok M, Landmann L, Stieger B, Krahenbuhl Pharmacological manipulation of L-carnitine transport into L6 cells with stable overexpression of human OCTN2. Cell Mol Life Sci. 2008;65:1596–1608. doi: 10.1007/s00018-008-8065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Ehlers T, Sutter J, Varma-O’brien S, Kirchmair J. CAESAR: a new conformer generation algorithm based on recursive buildup and local rotational symmetry consideration. J Chem Inf Model. 2007;47(5):1923–32. doi: 10.1021/ci700136x. [DOI] [PubMed] [Google Scholar]
- 17.Ekins S, Chang C, Mani S, Krasowski MD, Reschly EJ, Iyer M, Kholodovych V, Ai N, Welsh WJ, Sinz M, Swaan PW, Patel R, Bachmann K. Human pregnane X receptor antagonists and agonists define molecular requirements for different binding sites. Mol Pharmacol. 2007;72:592–603. doi: 10.1124/mol.107.038398. [DOI] [PubMed] [Google Scholar]
- 18.Clement OO, Mehl AT. HipHop: Pharmacophore based on multiple common-feature alignments. In: Guner OF, editor. Pharmacophore perception, development, and use in drug design. IUL; San Diego: 2000. pp. 69–84. [Google Scholar]
- 19.Krasowski MD, Siam MG, Iyer M, Pizon AF, Giannoutsos S, Ekins S. Chemoinformatic methods for predicting interference in drug of abuse/toxicology immunoassays. Clin Chem. 2009;55(6):1203–13. doi: 10.1373/clinchem.2008.118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grube M, Meyer zu Schwabedissen HE, Prager D, Haney J, Moritz KU, Meissner K, Rosskopf D, Eckel L, Bohm M, Jedlitschky G, Kroemer HK. Uptake of cardiovascular drugs into the human heart: expression, regulation, and function of the carnitine transporter OCTN2 (SLC22A5) Circulation. 2006;113(8):1114–22. doi: 10.1161/CIRCULATIONAHA.105.586107. [DOI] [PubMed] [Google Scholar]
- 21.Kato S, Kato Y, Nakamura T, Sugiura T, Kubo Y, Deguchi Y, Tsuji A. Genetic deficiency of carnitine/organic cation transporter 2 (slc22a5) is associated with altered tissue distribution of its substrate pyrilamine in mice. Biopharm Drug Dispos. 2009;30(9):495–507. doi: 10.1002/bdd.681. [DOI] [PubMed] [Google Scholar]
- 22.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3):215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekins S, Williams AJ. Precompetitive Preclinical ADME/Tox Data: Set It Free on The Web to Facilitate Computational Model Building to Assist Drug Development. Lab on a Chip. 2010;10:13–22. doi: 10.1039/b917760b. [DOI] [PubMed] [Google Scholar]
- 24.Ekins S, Ecker GF, Chiba P, Swaan PW. Future directions for drug transporter modelling. Xenobiotica. 2007;37(10):1152–70. doi: 10.1080/00498250701646341. [DOI] [PubMed] [Google Scholar]
- 25.Jong N, Nakanishi T, Liu J, Tamai I, McKeage M. Oxaliplatin transport mediated by organic cation/carnitine transporters OCTN1 and OCTN2 in over-expressing HEK293 cells and rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.111.181297. [DOI] [PubMed] [Google Scholar]
- 26.Napolitano C, Scaglianti M, Scalambra E, Manfredini S, Ferraro L, Beggiato S, Vertuani S. Carnitine conjugate of nipecotic acid: a new example of dual prodrug. Molecules. 2009;14(9):3268–74. doi: 10.3390/molecules14093268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner CA, Lukewille U, Kaltenbach S, Moschen I, Broer A, Risler T, Broer S, Lang F. Functional and pharmacological characterization of human Na(+)-carnitine cotransporter hOCTN2. Am J Physiol Renal Physiol. 2000;279(3):F584–91. doi: 10.1152/ajprenal.2000.279.3.F584. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, Chen J, Conway SJ, Ganapathy V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J Pharmacol Exp Ther. 1999;290(3):1482–92. [PubMed] [Google Scholar]
- 29.Schofield PM, Beath SV, Mant TG, Bhamra R. Recovery after severe oxprenolol overdose complicated by rhabdomyolysis. Hum Toxicol. 1985;4(1):57–60. doi: 10.1177/096032718500400106. [DOI] [PubMed] [Google Scholar]
- 30.Eisenburger P, Laggner AN, Lenz K, Druml W. Acute renal failure and rhabdomyolysis after inadvertent intra-arterial infusion of excessive doses of epinephrine during cardiopulmonary resuscitation. Wien Klin Wochenschr. 2000;112(4):174–6. [PubMed] [Google Scholar]
- 31.Byard RW, Summersides G, Thompson A. Confluent muscle pallor: a macroscopic marker of cocaine-induced rhabdomyolysis. Forensic Sci Med Pathol. 2011 doi: 10.1007/s12024-011-9229-6. [DOI] [PubMed] [Google Scholar]
- 32.Blake PG, Ryan F. Rhabdomyolysis and acute renal failure after terbutaline overdose. Nephron. 1989;53(1):76–7. doi: 10.1159/000185707. [DOI] [PubMed] [Google Scholar]
- 33.Pirounaki M, Liatsos G, Elefsiniotis I, Skounakis M, Moulakakis A. Unusual onset of varicella zoster reactivation with meningoencephalitis, followed by rhabdomyolysis and renal failure in a young, immunocompetent patient. Scand J Infect Dis. 2007;39(1):90–3. doi: 10.1080/00365540600798809. [DOI] [PubMed] [Google Scholar]
- 34.Seymour BD, Rubinger M. Rhabdomyolysis induced by epsilon-aminocaproic acid. Ann Pharmacother. 1997;31(1):56–8. doi: 10.1177/106002809703100109. [DOI] [PubMed] [Google Scholar]
- 35.Lyoo CH, Lee MS. Rhabdomyolysis induced by severe levodopa induced dyskinesia in a patient with Parkinson disease. J Neurol. 2011 doi: 10.1007/s00415-011-6041-x. [DOI] [PubMed] [Google Scholar]
- 36.Adani GL, Baccarani U, Risaliti A, Bresadola F, Della Rocca G, Viale P. Rhabdomyolysis due to Lamivudine administration in a liver transplant recipient. Am J Transplant. 2005;5(3):634. doi: 10.1111/j.1600-6143.2005.00691.x. [DOI] [PubMed] [Google Scholar]
- 37.Skrinska VA, Gock SB. Measurement of 3,4-MDMA and related amines in diagnostic and forensic laboratories. Clin Lab Sci. 2005;18(2):119–23. [PubMed] [Google Scholar]
- 38.Truica CI, Frankel SR. Acute rhabdomyolysis as a complication of cytarabine chemotherapy for acute myeloid leukemia: case report and review of literature. Am J Hematol. 2002;70(4):320–3. doi: 10.1002/ajh.10152. [DOI] [PubMed] [Google Scholar]
- 39.Vicente E, Zafra M, Garcia-Martinez E, de la Pena FA. Acute rhabdomyolysis as a complication of paclitaxel-gemcitabine chemotherapy for ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2009;145(2):226. doi: 10.1016/j.ejogrb.2009.04.029. [DOI] [PubMed] [Google Scholar]