Abstract
The mosquito midgut plays a central role in the sporogonic development of malaria parasites. We have found that polyclonal sera, produced against mosquito midguts, blocked the passage of Plasmodium falciparum ookinetes across the midgut, leading to a significant reduction of infections in mosquitoes. Anti-midgut mAbs were produced that display broad-spectrum activity, blocking parasite development of both P. falciparum and Plasmodium vivax parasites in five different species of mosquitoes. In addition to their parasite transmission-blocking activity, these mAbs also reduced mosquito survivorship and fecundity. These results reveal that mosquito midgut-based antibodies have the potential to reduce malaria transmission in a synergistic manner by lowering both vector competence, through transmission-blocking effects on parasite development, and vector abundance, by decreasing mosquito survivorship and egg laying capacity. Because the intervention can block transmission of different malaria parasite species in various species of mosquitoes, vaccines against such midgut receptors may block malaria transmission worldwide.
Of the several hundred species of Anopheles mosquitoes worldwide, only about 20 are major vectors of human malaria. Several components contribute to this fact, including the propensity of a given species to feed on humans, the average longevity of a mosquito species, and the innate ability of a species to permit development of the parasite (i.e., vector competence). Different intervention strategies attempt to reduce malaria transmission by reducing these various components. Reducing vector competence is the primary objective behind two rapidly evolving areas of research in malaria and other vector-borne diseases, namely the replacement of natural vector populations with populations genetically engineered for refractoriness and transmission-blocking immunity (TBI).
Transmission-blocking vaccines in malaria are envisioned to inhibit the developmental stages of the malaria parasites in the mosquito midgut. The first 48 h after ingestion of an infective blood meal is the most critical time period for malaria parasite development within the mosquito. It is during this time that the parasite fertilization takes place and the resulting zygotes transform into motile ookinetes. Ookinetes traverse the midgut and lodge between the basal lamina and midgut epithelia, where they encyst and develop into sporozoite-producing oocysts.
Several studies with rodent and human malaria parasites have revealed that the ookinete-to-oocyst transition is the most vulnerable link in sporogonic development of malaria parasites (1–3). Thus, the developing parasite stages in the mosquito midgut provide an ideal target for transmission-blocking vaccines. Efforts in transmission-blocking vaccines in malaria have focused primarily on the use of specific parasite antigens as targets of immune intervention in the mosquito midgut. Antibodies against parasite antigens block fertilization and/or prevent the movement of ookinetes across the mosquito midgut, when ingested by the mosquito (4–6).
We are investigating an alternate approach, using mosquito midgut antigens as targets of immune intervention in TBI. This approach uses the same underlying principle of rendering competent vectors incompetent, but targets mosquito antigens that parasites encounter during sporogonic development. An advantage of mosquito-based TBI is that antibodies directed against mosquito midgut antigens also could reduce mosquito survival and/or fecundity and have a cumulative effect on parasite transmission.
Previous studies by us and others using polyclonal anti-midgut antibodies have shown inhibition of sporogonic development in the rodent malaria parasite, Plasmodium berghei, and the human malaria parasite, Plasmodium vivax (7–9). The aim of this study was to: (i) investigate whether mAbs against midgut antigens can block the development of two major human malaria parasites, P. falciparum and P. vivax, in several mosquito species; and (ii) determine whether anti-midgut antibodies reduce mosquito fecundity and survivorship. The results of the study reveal that antibodies against specific midgut antigens can block the development of P. falciparum and P. vivax in different species of mosquitoes, as well as significantly reduce egg-laying capacity and survival. These findings provide additional avenues for the development of transmission-blocking vaccines for malaria, as well as, other vector-borne human infectious diseases.
Methods
Antibody Production.
Anopheles gambiae mosquito midguts were dissected and snap-frozen in sterile PBS plus protease inhibitors, iodoacetamide (5.0 μM), pepstatin A (1.0 μM), leupeptin (1.0 μM), EDTA (0.5 μM), pefabloc (1.0 mM), and aprotinin (1%). BALB/c mice were immunized with 100 μg of midgut proteins in Freund's complete adjuvant and boosted twice with 50 μg of midgut proteins in Freund's incomplete adjuvant. For mAb production spleen cells from hyperimmune mice were fused with Sp2/0 myeloma cells in the presence of polyethylene glycol 1500, as described by Kohler and Milenstein (10). After 2 weeks, culture supernatants were screened for the presence of anti-midgut antibodies by ELISA. Positive cultures were expanded and subcloned by limiting dilution.
Western Blot Analysis.
An. gambiae mosquito midguts were dissected and snap-frozen in sterile PBS plus protease inhibitors. After heating in sample buffer at 95°C for 5 min, ca. 10 μg of midgut protein (10 midguts) was electrophoretically separated on a 4–20% SDS-polyacrylamide gradient mini gel (Bio-Rad), under reducing conditions. Proteins were transferred to a nitrocellulose membrane, and membrane strips were incubated overnight at 4°C with 5 ml of mAb supernatant. To determine whether mAbs were specific for carbohydrate or noncarbohydrate antigenic determinants, alternate nitrocellulose strips were treated with 20 mM periodate (Bio-Rad), as described by Woodward et al. (11). Membrane strips were washed with PBS containing 0.3% Tween 20 (PBS-TW) and incubated 1 h at room temperature with peroxidase-conjugated goat anti-mouse IgG antibodies, diluted 1:3,000 in PBS-TW. After washing with PBS-TW, bound antibodies were detected by using 3,3′ diaminobenzidine and 30% hydrogen peroxide. Relative molecular weights were estimated by using broad-range molecular weight markers.
Transmission-Blocking Assay.
For P. falciparum assays, polyclonal sera were mixed 1:1 with naive sera (human or mouse depending on experiment) and administered together with infectious P. falciparum gametocyte cultures to mosquitoes via membrane feeders. mAb MG25E was assayed by mixing 0.67 mg mAb per ml of human blood containing infectious P. falciparum gametocytes. Gametocyte cultures containing control serum (human or mouse), and separately, a biologically irrelevant mAb (NYLS3, directed against Plasmodium yoelii liver-stage antigen), served as negative controls. For the P. vivax assays, mAbs MG25E, MG24C, or MG4B were assayed by mixing 1.0 mg mAb per ml of chimpanzee blood containing P. vivax gametocytes. Normal chimpanzee sera and an irrelevant antibody, either IB11, directed against P. vivax bloodstage parasites, or a commercial polyclonal IgG mouse antibody (Sigma) served as separate negative controls. For all experiments, unfed mosquitoes were removed and engorged mosquitoes were incubated at 24°C. For each experimental group, mosquitoes were dissected and blood meals were examined for ookinetes (27–30 h postinfection for P. falciparum and 24–28 h for P. vivax). Eight to 11 days later, mosquitoes from each experimental group were dissected and midguts were examined for oocysts. On days 18–21, the remaining mosquitoes were dissected and salivary glands were examined for sporozoites.
Survival and Fecundity Assays.
Chimpanzee blood containing P. vivax gametocytes was mixed with either 1.0 mg of mAb MG25E, 24C, or 4B and fed to Anopheles stephensi mosquitoes by individual water jacketed membrane feeders. Normal chimpanzee sera served as a negative control. Mosquitoes were held at 25°C and mortality was monitored daily for 7 days. Ten individual females from each group were kept in oviposition vials after ingestion. Vials were monitored and eggs were counted daily for 10 days. The experiments reported herein were conducted according to the principles set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Statistical Analysis.
Appropriate transformations were performed for statistical comparison of means by t test and ANOVAs (Tukey's studentized and Duncan's multiple range) and computation of confidence intervals. Nonparametric analysis also was performed with similar results. Infection prevalence rates were compared with χ2 analyses.
Results
We first tested the potential of midgut-based TBI against the most important human parasite species, P. falciparum, by using anti-midgut hyperimmune mouse sera with known transmission-blocking activity against Plasmodium berghei (7). Immune sera were pooled and fed to An. stephensi mosquitoes in conjunction with infective P. falciparum gametocytes. Mosquitoes were examined at the appropriate times thereafter for ookinetes, oocysts, and sporozoites. The prevalence of oocysts in the mosquitoes that were fed anti-mosquito midgut immune sera was significantly less compared with control groups (Table 1). Similarly, the sporozoite prevalence rate in immune-fed mosquitoes was also significantly less than either control group (P < 0.05).
Table 1.
Prevalance of P. falciparum development in An. stephensi mosquitoes
| Treatment | Ookinete | Oocyst | Sporozoite |
|---|---|---|---|
| Normal human serum | 83% | 90% | 53% |
| (6) | (50) | (15) | |
| Naive mouse serum | 62% | 59%* | 44% |
| (8) | (51) | (32) | |
| Anti-mosquito midgut serum | 62% | 2%** | 4%** |
| (8) | (50) | (23) |
Infection prevalence is expressed as percentages of infected mosquitoes per total examined (number of mosquitoes examined). Statistical comparisons were made within each lifestage among the three treatments. Treatments that have a single asterisk are significantly different from the normal human serum control at the 0.05 level of significance. Treatments with double asterisks are significantly different from both the normal human serum control and the naive mouse serum groups at the 0.05 level of significance.
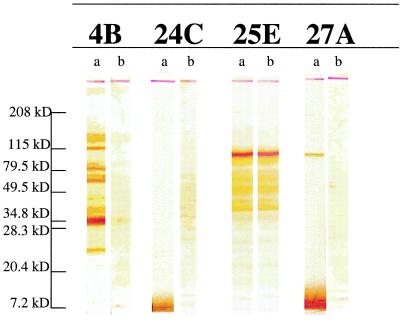
To identify specific midgut proteins that interact with parasites and are potentially responsible for TBI activity, mAbs were produced by immunizing mice with An. gambiae midgut lysate. Seventeen mAbs of various isotypes (IgG1, IgG2a, IgG3) reacted with An. gambiae midgut lysates by ELISA. Western blot analysis revealed four different profiles of antigenic reactivity. Antibody MG25E reacted against a 110-kDa antigen; MG1A, 10F, 12B, 17A, 18E, 19E, 21A, and 24C reacted with a 7-kDa antigen; MG27A reacted with both a 110-kDa and 7-kDa antigen; and MG4B, 5B, 11C, 15B, and 23A reacted with multiple bands ranging from 150 to 7 kDa. We initially selected one mAb from each Western blot profile (Fig. 1) (MG27A, MG25E, MG24C, and MG4B) for assessing their TBI activity. However, MG27A could not be produced in sufficient amounts and was not tested for TBI activity. Before the TBI assay, we determined whether mAbs MG25E, MG24C, and MG4B were directed against protein or carbohydrate moeities of the antigen. MG25E antibody recognizes protein component(s) in the 110-kDa antigen, and the other two antibodies, MG24C and MG4B, were directed against the carbohydrate domains of the proteins (Fig. 1).
Figure 1.
Western blot analysis of mAbs against An. gambiae midgut lysates. Seventeen mAbs were analyzed. Shown are representative mAbs of the four different patterns observed: MG4B (IgG2a), MG24C (IgG1), MG25E (IgG2a), and MG27A (IgG3). Lanes a, midgut antigens were treated under standard SDS/PAGE reducing conditions. Lanes b, in addition to standard SDS/PAGE reducing conditions midgut antigens were treated with 20 mM periodate, which cleaves carbohydrate groups from polypeptide chains.
We further investigated the binding pattern of MG25E, MG24C, and MG4B to the midgut tissue by confocal microscopy (data not shown). Analysis of everted whole-mount and crysectioned midguts revealed several different staining patterns. Only MG25E antibody displayed immunofluorescent staining that was consistent with surface localization of the antigen. Cryosections of midguts demonstrated staining of the surface of midgut epithelial cells, while staining of everted whole mounts showed a patchy fluorescence that seemed to define membrane borders.
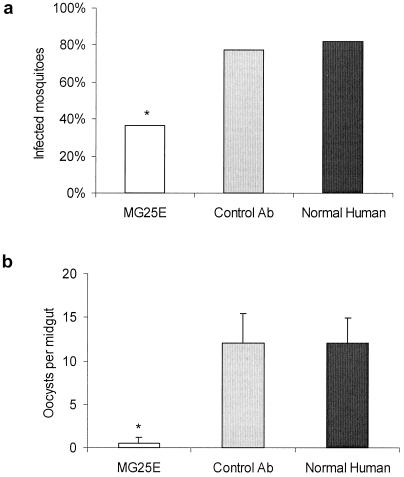
We first conducted experiments to test the spectrum of inhibitory activity for mAb MG25E. In the first experiment, we fed An. stephensi mosquitoes the MG25E mAb with cultured gametocytes of P. falciparum. Normal human serum and an irrelevant mAb (NYLS3) served as negative controls. The mAb MG25E had no effect on ookinete production compared with control groups (data not shown). However, prevalence and intensity of oocyst infection was significantly affected (Fig. 2). There was >50% oocyst prevalence reduction in the mosquitoes that received MG25E antibody (P < 0.0001); further, for those mosquitoes that developed oocyst infection, there was a significant reduction in the intensity of infection (P < 0.0001). In a second experiment, An. stephensi mosquitoes were fed two concentrations (1.0 mg and 0.5 mg per ml) of mAb MG25E with gametocytes of P. vivax harvested from an infected chimpanzee. Normal chimpanzee serum and a P. vivax bloodstage antigen-specific mAb (IB11) were used as negative controls. We found an antibody dose-dependent reduction in the prevalence and intensity of oocysts found in the MG25E-fed mosquitoes (data not shown).
Figure 2.
Transmission-blocking effect of anti-mosquito midgut mAb MG25E on P. falciparum development in An. stephensi mosquitoes. (a) Infection prevalence is expressed as geometric means. * denotes statistical significance at the 0.05 level of MG25E (n = 76) as compared with the normal human (n = 55) and control Ab (n = 66) groups. (b) Parasite oocyst infection intensities are expressed as geometric mean ± SEM of infected mosquitoes. * denotes statistical significance at the 0.05 level of MG25E as compared with the normal human and control Ab groups.
We next assessed the transmission-blocking effects of MG25E antibody with gametocytes of P. vivax in An. stephensi, Anopheles freeborni, Anopheles albimanus, and Anopheles farauti mosquitoes (Table 2). We did not find significant affect on the prevalence of oocyst infection. However, significantly lower intensity of oocyst infection was noted as compared with chimpanzee serum control in An. albimanus and An. farauti (P < 0.008 and P < 0.0002, respectively). The intensity of infection in An. stephensi receiving MG25E with an infective blood meal was significantly less than those fed IB11. However, in An. freeborni, the reduction of oocyst intensity did not reach the level of significance when compared with chimpanzee serum control (P < 0.08). The P. vivax blood stage-specific antibody, mAB IB11, caused reduction in the intensity of oocyst infection in An. stephensi, An. farauti, and An. albimanus. This reduction may be caused by a shared and/or cross-reactive epitope between bloodstage antigen and mosquito-specific antigen. Two important conclusions can be drawn from the results of these studies. First, mAb MG25E reduced oocyst prevalence by blocking the conversion of ookinetes to oocysts. Second, this blocking effect had a broad spectrum of activity against different parasite species developing in different vector species.
Table 2.
Decreased intensity of P. vivax oocysts in four species of Anopheles mosquitoes
| Treatment | An. stephensi | An. freeborni | An. albimanus | An. farauti |
|---|---|---|---|---|
| Chimpanzee serum | 149 ± 7.8 | 20.4 ± 38.3 | 89.9 ± 36.5 | 62.8 ± 9.5 |
| (11) | (11) | (9) | (28) | |
| mAb IB11 | 30.5 ± 3.3* | 12.9 ± 4.6 | 11 ± 5 | 4.2 ± 1.6* |
| (11) | (12) | (11) | (24) | |
| MG25E | 5.2 ± 1.2** | 1.3 ± 0.3 | 6.5 ± 3.5* | 2.1 ± 1* |
| (19) | (18) | (9) | (11) |
Oocyst intensity is expressed as geometric mean ± SEM (number of mosquitoes examined). Statistical comparisons were made among all treatments. Treatments that have a single asterisk are significantly different from the chimpanzee serum control at the 0.05 level of significance. Treatments with double asterisks are significantly different from both the chimpanzee serum control and the mAb IB11 groups at the 0.05 level of significance.
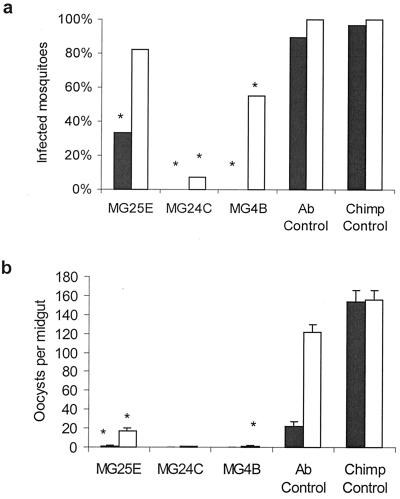
In a subsequent experiment, we compared the transmission-blocking activities of two additional anti-midgut mAbs with MG25E against P. vivax. Three antibodies, MG25E, MG24C, and MG4B, were tested in An. stephensi and An. gambiae mosquitoes. Normal chimpanzee serum and a commercial polyclonal IgG mouse antibody were used as controls. As in previous experiments with P. falciparum, we did not find any significant effect on the ookinete production in the two mosquito species (data not shown). Control mouse polyclonal IgG antibody did not affect oocyst prevalence, but it reduced oocyst intensity. We don't know the reason for the inhibitory effect of this commercial polyclonal antibody. It is likely that the observed effect was due to nonspecific cross-reactivity or some inhibitory contaminants in the commercial preparation. However, the transmission-blocking activity of midgut-specific antibodies was highly significant (Fig. 3). There was a 20% reduction in oocyst prevalence of An. stephensi given MG25E with the blood meal compared with those given normal chimpanzee serum. However, this difference did not reach a level of statistical significance (P > 0.2). The reduction in prevalence of oocysts in mosquitoes fed MG24C was 93% (P < 0.001), and for those fed MG4B, the reduction was 45% (P < 0.05) (Fig. 3a). Of the anti-midgut antibody-fed mosquitoes that were positive for oocysts, the intensity of oocyst infection was also significantly lower. The percent reduction in intensity of infection by antibodies MG25E, MG24C, or MG4B was 83%, 99%, and 98.6%, respectively (P < 0.0001) (Fig. 3b).
Figure 3.
Transmission-blocking effect of anti-mosquito midgut mAb MG25E, MG24C, or MG4B, on P. vivax development in An. gambiae (filled bars) and An. stephensi (open bars) mosquitoes. (a) Oocyst infection prevalence in mosquitoes allowed to feed on MG25E (n = 18 and 22 for An. gambiae and An. stephensi, respectively), MG24C (n = 3 and 15), MG4B (n = 9 and 11), Ab control (n = 28 and 37), or chimpanzee serum control (n = 28 and 39) is expressed as geometric means. * denotes statistical significance at the 0.05 level as compared with the chimpanzee and Ab control groups. (b) Oocyst infection intensity is expressed as geometric mean ± SEM of infected mosquitoes fed MG25E, MG24C, MG4B, Ab control, or chimpanzee control. * denotes statistical significance at the 0.05 level as compared with the chimpanzee and Ab control groups.
The effects of anti-midgut mAbs on oocyst prevalence and intensity were more pronounced in An. gambiae mosquitoes, against which the mAbs were originally raised (Fig. 3). Oocyst prevalence was reduced by 66%, 100%, and 100% in An. gambiae given MG25E (P < 0.001), MG24C (P < 0.0.1), or MG4B (P < 0.001) with a blood meal (Fig. 3a). There was a significant decrease in the number of oocysts in the mosquitoes fed antibody MG25E (>98% reduction, P < 0.0001), with no oocysts seen in mosquitoes fed MG24C or MG4B (Fig. 3b). The normal mouse polyclonal antibodies had a slight, albeit not statistically significant, inhibitory effect on the sporogonic events in An. gambiae. These results indicate that there are probably several epitopes on the mosquito midgut that can be used in the development of midgut-based TBI vaccines.
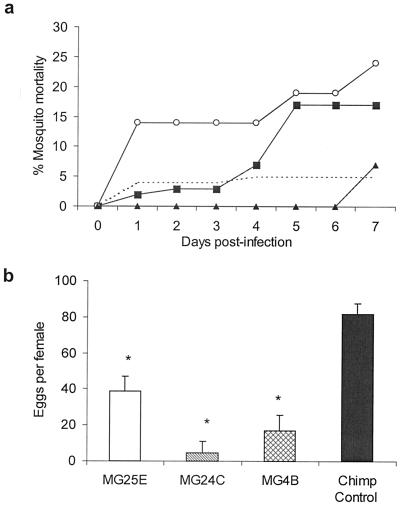
We next assessed the affect of anti-midgut antibodies on mosquito survival and fecundity in An. stephensi. The addition of MG25E, MG24C, or MG4B to the blood meal increased mortality to 17%, 24%, and 7%, as compared with 5% in the chimpanzee serum-fed mosquitoes (Fig. 4a). As compared with the chimpanzee control, the mortality at day 7 in the MG25E and MG24C groups was statistically significant. In a follow-up experiment designed to determine the effects of anti-midgut antibodies on fecundity, An. stephensi mosquitoes were allowed to feed on blood supplemented with normal chimpanzee serum or midgut antibodies MG25E, MG24C, or MG4B. Compared with the number of eggs per female in the mosquitoes fed normal chimpanzee sera, addition of the anti-midgut antibodies resulted in a significant reduction in the number of eggs (Fig. 4b). These results suggest that anti-mosquito antibodies that exhibit transmission-blocking activity also reduce mosquito fecundity.
Figure 4.
Effects of anti-mosquito midgut mAb MG25E, MG24C, or MG4B on mortality and fecundity of An. stephensi. (a) Mosquito mortality was monitored daily after feeding mosquitoes a P. vivax-infected blood meal along with MG25E (n = 40, ■), MG24C (n = 21, ○), MG4B (n = 14, ▴), or chimpanzee serum (n = 101, dashed line). Results from a representative experiment are shown. (b) Individual An. stephensi female mosquitoes were kept in oviposition vials after ingestion of a P. vivax-infected blood meal along with MG25E (n = 10), MG24C (n = 8), MG4B (n = 9), or chimpanzee serum (n = 10). Eggs were monitored and counted daily. Results are presented as geometric mean ± SEM. * denotes statistical significance at the 0.05 level as compared with the chimpanzee control group.
Discussion
This study was undertaken to investigate the potential of mosquito midgut antigens as targets of transmission-blocking vaccines against P. falciparum and P. vivax, which are responsible for almost all malaria-attributable morbidity and mortality. mAbs were developed against midgut antigens of An. gambiae, which is a major vector in sub-Saharan Africa. The transmission-blocking effects of An. gambiae midgut-based mAbs, which react with specific midgut proteins, were tested in several anopheline mosquito species using gametocytes of P. falciparum and P. vivax. The rationale for using several anopheline species in transmission-blocking assays was to determine whether An. gambiae-specific antibodies can elicit inhibitory effects within a broad range of vector species.
The results of transmission-blocking experiments have revealed that mAbs against mosquito midgut antigens specifically target the ookinete-oocyst transition and are effective in blocking the development of both P. falciparum and P. vivax parasites in several species of anopheline mosquitoes. The finding of midgut-based TBI in both human and rodent malaria models is noteworthy because different Plasmodium species display different developmental kinetics in the mosquito (12–16).
For instance, rodent plasmodial species, such as P. berghei, develop quickly and exit the midgut well before peak secretion of mosquito digestive enzymes. Conversely, P. falciparum develops more slowly and ookinetes do not begin midgut attachment and penetration until digestive enzymes are at their peak (24–30 h). Because most of the host IgG in anopheline bloodmeals is digested within 48 h (17–19), we were concerned that anti-midgut antibodies might be degraded before having a chance to exert their effect. The results of experiments have shown that the effect of anti-midgut antibodies are of sufficient duration to block the passage of a “slow developer” such as P. falciparum.
There are several advantages of a transmission-blocking vaccine based on mosquito antigens. First, the intervention can work against different species of malaria parasites transmitted by different species of mosquitoes. Second, mosquito midgut-based TBI would have the added benefit of potentially decreasing mosquito survivorship and/or fecundity (20–25). Third, anti-midgut antibodies also may disrupt mosquito digestion/absorption enough to retard normal oocyst development in previously infected mosquitoes. The indirect effects on mosquito survivorship, fecundity, and parasite development may be cumulative and could substantially impact malaria transmission.
The results of the studies reported here provide data in support of the first two hypotheses. We have found that mAbs against midgut antigens inhibit parasite development in An. stephensi mosquitoes and that the same antibodies inhibit the development of P. vivax in An. stephensi, An. freeborni, An. albimanus, and An. farauti. It is important to note that the antibodies against An. gambiae midguts elicited TBI in several mosquito species, which suggest that during their passage through the midgut, ookinetes may use receptors common to many anopheline vectors.
Mosquito transmission of malaria is comprised of several components whose relative contributions have been described mathematically in the Ross–MacDonald equation of vectorial capacity (28). For example, mosquito longevity has a powerful influence on transmission as it is intimately tied with the extrinsic incubation period of the parasite and thus influences transmission in an exponential fashion. Vector competence (i.e., proportion of mosquitoes that become infectious after feeding on a gametocytemic host) influences transmission in a linear fashion. To understand better the cumulative effect that antibody-mediated mortality and TBI activity might have on malaria transmission, we substituted our experimentally derived values into the vectorial capacity equation. Daily mosquito survival rates (calculated from Fig. 4a) were 0.992, 0.973, 0.990, and 0.964 for chimp control sera, MG25E, MG4B, and MG24C, respectively. Using our TBI efficacy data for P. vivax in An. stephensi (Fig. 3a) and assuming an extrinsic incubation period of 13 days, we asked the hypothetical question if 100 mosquitoes fed on each treatment, how many infectious mosquitoes would be remaining at the end of the extrinsic incubation period to transmit malaria? For example, in the chimp control, there would be (100 mosquitoes) × (0.99213 surviving) × (100% infectivity), or 90 infectious mosquitoes remaining at the end of 13 days. When calculations were performed for each treatment group, the estimated yield of infectious mosquitoes were 90, 57, 47, and 4 mosquitoes for chimp control sera, MG25E, MG4B, and MG24C, respectively. These calculations illustrate the degree to which a mosquito midgut-based vaccine can, in theory, reduce transmission.
Our experiments also indicate that anti-mosquito midgut antibodies may reduce mosquito fecundity. Although the effect of reducing fecundity by itself may not be great, it is the simultaneous accumulation of all three effects (i.e., TBI, mosquito mortality, and reduced fecundity) that provide the appeal of this approach. We can think of no other single intervention strategy that attacks malaria transmission by attacking three critical components all at once.
The mosquito antigen-based TBI approach offers another advantage over some parasite antigen-based TBI approaches. Like the successful anti-tick vaccine, Bm86 (26, 27), midgut-based transmission-blocking antigens are immunologically concealed, which would suggest that there is no basis for selection and nothing to drive antigenic variation, as there is with circulating parasites. Some may suggest, however, that the application of immune pressure on natural populations of mosquitoes may lead to the emergence and selection of variant mosquitoes. It is important to keep in mind that selective pressures of a midgut-based TBI vaccine would be directed only against those mosquito phenotypes that are susceptible to an antibody-mediated loss in fitness (i.e., mosquito mortality and reduced fecundity). There would be no selective pressures against the parasites or against the vector parasite interaction. Even if a midgut-based TBI vaccine were, over time, to select for mosquito populations immune to the insecticidal properties of the vaccine, it is unlikely that the transmission blocking efficacy of the vaccine would diminish. This supposition is supported by our observations that mosquitoes that survived the lethal effects of ingested mAbs contained few or no parasites.
We don't know whether the genes encoding the midgut antigens of wild mosquitoes are monomorphic, but the data suggests that variability, even if it exists, may not pose a problem in the use of midgut antigens as targets of transmission-blocking vaccines. As we proceed in the development of this concept, it would be important to determine the variability of gene encoding the TBI antigens from natural populations of mosquitoes. We also would like to know whether comparable inhibitory results are obtained with gametocytes from naturally infected individuals.
It is also important to note that the inhibitory effects of mosquito midgut antigen-based antibodies are observed for P. falciparum and P. vivax in several species of mosquitoes. This observation implies that the midgut antigenic epitopes targeted by these antibodies (as will be the case for protein reactive antibody MG25E) are conserved. In support of our observations, a recent study has proposed a universal mechanism of mosquito midgut penetration by the malaria parasite (29). In this in vitro study, identical events during invasion for P. gallinaceum and P. berghei were observed in Aedes aegypti and An. stephensi, respectively.
In conclusion, we have found that mosquito midgut-based TBI is effective and has broad-spectrum activity in blocking transmission of malaria. The precise mechanisms by which anti-midgut antibodies disrupt the ookinete-oocyst transition remain to be determined. However, the fact that mAbs can significantly impede parasite development in different parasite-mosquito systems suggests the presence of a universal family of midgut proteins that plasmodial ookinetes use to traverse the midgut. Although the antigens identified in this study provide a starting point for the development of midgut antigen-based malaria transmission-blocking vaccine, additional studies may lead to the identification of other midgut antigens that also could be used as targets of immune intervention. Such midgut-based antigens could serve as effective TBI immunogens against multiple species of malaria parasites transmitted by different species of vectors worldwide. The use of midgut antigens in vaccine formulations would not have the safety concerns as may be the case with salivary gland antigens, because the human host does not come in contact with these antigens during mosquito bites. We propose that a combination of mosquito antigens involved in host-parasite interactions together with parasite antigens could form a highly effective multivalent malaria vaccine. Such a multivalent malaria vaccine may control and/or prevent malaria in immunized hosts, due to antiparasitic components in the vaccine, as well as reduce transmission by different anopheline vectors, due to anti-midgut components in the vaccine. Furthermore, midgut-based TBI also could find uses against other arthropod-transmitted human diseases (e.g., dengue and filariasis), in which case one midgut antigen-based vaccine may block transmission of many vector-borne diseases.
Acknowledgments
We thank Dr. John Barnwell (Centers for Disease Control and Prevention) for kindly providing mAb IB11. Dr. Yupin Charoenvit (Naval Medical Research Center) kindly provided the mAb NYLS3. We also thank OraLee Branch for assistance with statistical analysis. This work was funded in-part by National Institutes of Health Grants AI 17828 and AI 43006.
Abbreviation
- TBI
transmission-blocking immunity
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Vaughan J A, Noden B H, Beier J C. J Parasitol. 1992;78:716–724. [PubMed] [Google Scholar]
- 2.Vaughan J A, Noden B H, Beier J C. Am J Trop Med Hyg. 1994;51:233–243. doi: 10.4269/ajtmh.1994.51.233. [DOI] [PubMed] [Google Scholar]
- 3.Gouagna L C, Mulder B, Noubissi E, Tchuinkam T, Verhave J P, Boudin C. Trop Med Int Health. 1998;3:21–28. doi: 10.1046/j.1365-3156.1998.00156.x. [DOI] [PubMed] [Google Scholar]
- 4.Carter R, Chen D H. Nature (London) 1976;263:57–60. doi: 10.1038/263057a0. [DOI] [PubMed] [Google Scholar]
- 5.Gwadz R W. Science. 1976;193:1150–1151. doi: 10.1126/science.959832. [DOI] [PubMed] [Google Scholar]
- 6.Kaslow D C. Curr Opin Immunol. 1993;5:557–565. doi: 10.1016/0952-7915(93)90037-s. [DOI] [PubMed] [Google Scholar]
- 7.Lal A A, Schriefer M, Qari S H, Goldman I F, Azad A F, Collins W E. Infect Immun. 1994;62:316–318. doi: 10.1128/iai.62.1.316-318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramasamy M S, Ramasamy R. Med Vet Entomol. 1990;4:161–166. doi: 10.1111/j.1365-2915.1990.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 9.Srikrishnaraj K A, Ramasamy R, Ramasamy M S. Med Vet Entomol. 1995;9:353–357. doi: 10.1111/j.1365-2915.1995.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 10.Kohler G, Milenstein C. Nature (London) 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 11.Woodward M P, Young W W, Jr, Bloodgood R A. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan J A, Narum D, Azad A F. J Parasitol. 1991;77:758–761. [PubMed] [Google Scholar]
- 13.Vaughan J A, Hensley L, Beier J C. J Parasitol. 1994;80:674–681. [PubMed] [Google Scholar]
- 14.Ramasamy M S, Kulasekera R, Wanniarachchi I C, Srikrishnaraj K A, Ramasamy R. Med Vet Entomol. 1997;11:290–296. doi: 10.1111/j.1365-2915.1997.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 15.Robert V, le Goff G, Gouagna L C, Sinden M, Kieboom J, Kroneman R, Verhave J P. Ann Trop Med Parasitol. 1998;92:115–118. doi: 10.1080/00034989860247. [DOI] [PubMed] [Google Scholar]
- 16.Lackie A M, Gavin S J. Med Vet Entomol. 1989;3:225–230. doi: 10.1111/j.1365-2915.1989.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 17.Vaughan J A, Wirtz R A, do Rosario V E, Azad A F. Am J Trop Med Hyg. 1990;42:10–16. doi: 10.4269/ajtmh.1990.42.10. [DOI] [PubMed] [Google Scholar]
- 18.Billingsley P F, Hecker H. J Med Entomol. 1991;28:865–871. doi: 10.1093/jmedent/28.6.865. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland G B, Ewen A B. J Insect Physiol. 1974;20:655–660. doi: 10.1016/0022-1910(74)90187-5. [DOI] [PubMed] [Google Scholar]
- 20.Alger N E, Cabrera E J. J Econ Entomol. 1972;65:165–168. doi: 10.1093/jee/65.1.165. [DOI] [PubMed] [Google Scholar]
- 21.Hatfield P R. Med Vet Entomol. 1988;2:331–338. doi: 10.1111/j.1365-2915.1988.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramasamy M S, Ramasamy R, Kay B H, Kidson C. Med Vet Entomol. 1988;2:87–93. doi: 10.1111/j.1365-2915.1988.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 23.Srikrishnaraj K A, Ramasamy R, Ramasamy M S. Med Vet Entomol. 1993;7:66–68. doi: 10.1111/j.1365-2915.1993.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramasamy M S, Raschid L K, Srikrishnaraj A, Ramasamy R. J Med Entomol. 1996;33:162–164. doi: 10.1093/jmedent/33.1.162. [DOI] [PubMed] [Google Scholar]
- 25.Opdebeeck J P, Wong J Y M, Jackson L A, Dobson C. Parasite Immunol. 1988;10:405–410. doi: 10.1111/j.1365-3024.1988.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 26.Willadsen P, McKEnna R V. Parasite Immunol. 1991;13:605–616. doi: 10.1111/j.1365-3024.1991.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 27.Fragoso H, Rad P H, Ortiz M, Rodriguez M, Redondo M, Herrera L, de la Fuente J. Vaccine. 1998;16:1990–1992. doi: 10.1016/s0264-410x(98)00116-9. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald G. The Epidemiology and Control of Malaria. London: Oxford Univ. Press; 1957. [Google Scholar]
- 29.Zieler H, Dvorak J A. Proc Natl Acad Sci USA. 2000;97:11516–11521. doi: 10.1073/pnas.97.21.11516. [DOI] [PMC free article] [PubMed] [Google Scholar]