Abstract
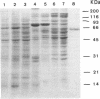
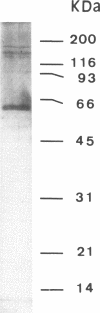
The preparation of quantities of poly(ADP-ribose) glycohydrolase sufficient for detailed structural and enzymatic characterizations has been difficult due to the very low tissue content of the enzyme and its lability in late stages of purification. To date, the only purification of this enzyme to apparent homogeneity has involved a procedure requiring 6 column chromatographic steps. Described here is the preparation of an affinity matrix which consists of ADP-ribose polymers bound to dihydroxyboronyl sepharose. An application is described for the purification of poly(ADP-ribose) glycohydrolase from calf thymus in which a single rapid affinity step was used to replace 3 column chromatographic steps yielding enzyme of greater than 90% purity with a 3 fold increase in yield. This matrix should also prove useful for other studies of ADP-ribose polymer metabolism and related clinical conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-Ela N., Jacobson E. L., Jacobson M. K. Labeling methods for the study of poly- and mono(ADP-ribose) metabolism in cultured cells. Anal Biochem. 1988 Oct;174(1):239–250. doi: 10.1016/0003-2697(88)90541-6. [DOI] [PubMed] [Google Scholar]
- Althaus F. R., Richter C. ADP-ribosylation of proteins. Enzymology and biological significance. Mol Biol Biochem Biophys. 1987;37:1–237. [PubMed] [Google Scholar]
- Alvarez-Gonzalez R., Jacobson M. K. Characterization of polymers of adenosine diphosphate ribose generated in vitro and in vivo. Biochemistry. 1987 Jun 2;26(11):3218–3224. doi: 10.1021/bi00385a042. [DOI] [PubMed] [Google Scholar]
- Alvarez-Gonzalez R., Juarez-Salinas H., Jacobson E. L., Jacobson M. K. Evaluation of immobilized boronates for studies of adenine and pyridine nucleotide metabolism. Anal Biochem. 1983 Nov;135(1):69–77. doi: 10.1016/0003-2697(83)90732-7. [DOI] [PubMed] [Google Scholar]
- Burzio L. O., Riquelme P. T., Ohtsuka E., Koide S. S. Evidence for two variants of poly(adenosine diphosphate ribose) glycohydrolase in rat testis. Arch Biochem Biophys. 1976 Mar;173(1):306–319. doi: 10.1016/0003-9861(76)90264-2. [DOI] [PubMed] [Google Scholar]
- Hatakeyama K., Nemoto Y., Ueda K., Hayaishi O. Purification and characterization of poly(ADP-ribose) glycohydrolase. Different modes of action on large and small poly(ADP-ribose). J Biol Chem. 1986 Nov 15;261(32):14902–14911. [PubMed] [Google Scholar]
- Jacobson M. K., Payne D. M., Alvarez-Gonzalez R., Juarez-Salinas H., Sims J. L., Jacobson E. L. Determination of in vivo levels of polymeric and monomeric ADP-ribose by fluorescence methods. Methods Enzymol. 1984;106:483–494. doi: 10.1016/0076-6879(84)06052-3. [DOI] [PubMed] [Google Scholar]
- Jonsson G. G., Menard L., Jacobson E. L., Poirier G. G., Jacobson M. K. Effect of hyperthermia on poly(adenosine diphosphate-ribose) glycohydrolase. Cancer Res. 1988 Aug 1;48(15):4240–4243. [PubMed] [Google Scholar]
- Juarez-Salinas H., Sims J. L., Jacobson M. K. Poly(ADP-ribose) levels in carcinogen-treated cells. Nature. 1979 Dec 13;282(5740):740–741. doi: 10.1038/282740a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miwa M., Ishihara M., Takishima S., Takasuka N., Maeda M., Yamaizumi Z., Sugimura T., Yokoyama S., Miyazawa T. The branching and linear portions of poly(adenosine diphosphate ribose) have the same alpha(1 leads to 2) ribose-ribose linkage. J Biol Chem. 1981 Mar 25;256(6):2916–2921. [PubMed] [Google Scholar]
- Miwa M., Nakatsugawa K., Hara K., Matsushima T., Sugimura T. Degradation of poly(adenosine diphosphate ribose) by homogenates of various normal tissues and tumors of rats. Arch Biochem Biophys. 1975 Mar;167(1):54–60. doi: 10.1016/0003-9861(75)90440-3. [DOI] [PubMed] [Google Scholar]
- Miwa M., Sugimura T. Splitting of the ribose-ribose linkage of poly(adenosine diphosphate-robose) by a calf thymus extract. J Biol Chem. 1971 Oct 25;246(20):6362–6364. [PubMed] [Google Scholar]
- Miwa M., Tanaka M., Matsushima T., Sugimura T. Purification and properties of glycohydrolase from calf thymus splitting ribose-ribose linkages of poly(adenosine diphosphate ribose). J Biol Chem. 1974 Jun 10;249(11):3475–3482. [PubMed] [Google Scholar]
- Ménard L., Poirier G. G. Rapid assay of poly(ADP-ribose) glycohydrolase. Biochem Cell Biol. 1987 Jul;65(7):668–673. doi: 10.1139/o87-088. [DOI] [PubMed] [Google Scholar]
- Oka J., Ueda K., Hayaishi O., Komura H., Nakanishi K. ADP-ribosyl protein lyase. Purification, properties, and identification of the product. J Biol Chem. 1984 Jan 25;259(2):986–995. [PubMed] [Google Scholar]
- Oka J., Ueda K., Hayaishi O. Snake venom phosphodiesterase: simple purification with Blue Sepharose and its application to poly(ADP-ribose) study. Biochem Biophys Res Commun. 1978 Feb 28;80(4):841–848. doi: 10.1016/0006-291x(78)91321-9. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Tanuma S., Kawashima K., Endo H. Purification and properties of an (ADP-ribose)n glycohydrolase from guinea pig liver nuclei. J Biol Chem. 1986 Jan 15;261(2):965–969. [PubMed] [Google Scholar]
- Tavassoli M., Tavassoli M. H., Shall S. Effect of DNA intercalators on poly(ADP-ribose) glycohydrolase activity. Biochim Biophys Acta. 1985 Mar 1;827(3):228–234. doi: 10.1016/0167-4838(85)90207-9. [DOI] [PubMed] [Google Scholar]
- Tavassoli M., Tavassoli M. H., Shall S. Isolation and purification of poly(ADP-ribose) glycohydrolase from pig thymus. Eur J Biochem. 1983 Oct 3;135(3):449–455. doi: 10.1111/j.1432-1033.1983.tb07672.x. [DOI] [PubMed] [Google Scholar]
- Wielckens K., Bredehorst R., Hilz H. Quantification of protein-bound ADP-ribosyl and (ADP-ribosyl)n residues. Methods Enzymol. 1984;106:472–482. doi: 10.1016/0076-6879(84)06051-1. [DOI] [PubMed] [Google Scholar]
- Wielckens K., George E., Pless T., Hilz H. Stimulation of poly(ADP-ribosyl)ation during Ehrlich ascites tumor cell "starvation" and suppression of concomitant DNA fragmentation by benzamide. J Biol Chem. 1983 Apr 10;258(7):4098–4104. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zahradka P., Ebisuzaki K. Poly(ADP-ribose) polymerase is a zinc metalloenzyme. Eur J Biochem. 1984 Aug 1;142(3):503–509. doi: 10.1111/j.1432-1033.1984.tb08314.x. [DOI] [PubMed] [Google Scholar]