Abstract
Methylmercury (MeHg) is an environmental neurotoxicant associated with aberrant central nervous system (CNS) functions. In this study, we examined the protective effect of a novel anti-inflammatory and cytoprotective nonapeptide, termed IIIM1, against MeHg-induced toxicity in cultured rat neonatal rat primary astrocytes. Astrocytes were pretreated for 66 hours with 5µg/ml IIIM1 (4.95 µM) followed by 6 hour exposure to MeHg (5 µM). MeHg significantly increased F2-isoprostane generation, a lipid peroxidation biomarker of oxidative injury and this effect was significantly reduced upon pre-treatment with IIIM1. The MeHg-induced increase in levels of prostaglandin E2 (PGE2), biomarkers of inflammatory responses, was also decreased in the peptide-treated cells. Mass spectrometry analysis revealed no chemical or binding interaction between MeHg and IIIM1, indicating that intracellular cytoprotective mechanism of action accounts for the neuroprotection rather than direct intracellular neutralization of the neurotoxicant with the peptide. These findings point to therapeutic potential for IIIM1 in a plethora of conditions associated with reactive oxygen species (ROS) generation. The implication of these findings may prove beneficial in designing new treatment modalities that efficiently suppress neurotoxicity, triggered not only by MeHg, but also by other metals and environmental agents, as well as chronic disease conditions that inherently increase reactive radical production and inflammatory signaling.
INTRODUCTION
Methylmercury (MeHg) is a time-honored established human neurotoxicant formed through the methylation of inorganic mercury in the aquatic environment. MeHg is one of the most poisonous environmental chemicals associated with aberrant central nervous system (CNS) functions (Grandjean et al., 2003, Steuerwald et al., 2000). Several studies have associated maternal exposure to MeHg with neurological as well as neurodevelopmental deficits and pointed to the selective detrimental effects of MeHg on neurogenesis (Grandjean et al., 1999, Grandjean et al., 2003, Kjellstrom et al., 1989). In addition to direct damaging neurons induced by MeHg, previous studies have established a prominent role of astrocytes in mediating MeHg neurotoxicity (Allen et al., 2001a, Allen et al., 2001b, Aschner et al., 2000, Shanker et al., 2005).
MeHg induces an excessive amount of synaptic glutamate by inhibiting astrocytic glutamate uptake and stimulating neuronal presynaptic glutamate efflux (Aschner et al., 1993; Aschner, 1996; Allen et al., 2001a, Allen et al., 2001b, Aschner et al., 2000), thus leading to neuronal excitotoxicity and cell death. Damage associated with MeHg correlates with brain areas with dense glutamatergic innervation and the ionotropic glutamate receptor N-methyl-D-aspartate (NMDA) antagonist, dizocilpine (MK801) protects against neuronal damage induced by in vivo MeHg (Lafon-Cazal, et al., 1993). In addition, MeHg disrupts astrocytic and microglial cellular redox homeostasis through excessive generation of reactive oxygen species (ROS) (Farina et al., 2011b, Farina et al., 2011a, Ni et al., 2010, Shanker et al., 2005, Yin et al., 2011). MeHg selectively inhibits astrocytic (uptake systems for cystine and cysteine transport (Allen et al., 2001a; Shanker et al., 2001a; 2001b), compromising glutathione (GSH) synthesis and the CNS redox potential (Aschner et al., 1994). Compared with astrocytes, neurons have lower levels of GSH as well as of a second putative antioxidant, metallothionein (MT) (Ni et al., 2010; Maret, 1994) making them more susceptible to the effects of increased ROS. Since GSH synthesis is dependent upon precursors derived from astrocytes, MeHg-induced inhibition of cystine transport and astrocytic GSH production would ultimately lead to decreased neuronal GSH levels and increased glutamate toxicity. MeHg-induced ROS formation in astrocytes can be attenuated by antioxidants (Shanker et al., 2002), reversing its functional effects on glutamate uptake inhibition (Allen et al., 2001b). Finally, additional data invoke astrocytic cPLA2 as a target for MeHg toxicity, supporting the notion that cPLA2-stimulated hydrolysis and release of arachidonic acid (AA) play a role in MeHg-induced neurotoxicity (Shanker et al., 2002).
MeHg-induced ROS is associated with failure of energy metabolism, depletion of intracellular ATP and ADP, and the disruption of calcium homeostasis, lipid peroxidation and the dissipation of the mitochondrial membrane potential (Aschner et al. 2007; Yin et al. 2007; 2011; Ni et al., 2010; Ni et al., 2011). Moreover, MeHg causes the activation of cytosolic phospholipase A2 (cPLA2) release of arachidonic acid (AA) and inflammatory response (Shanker et al., 2004, Yin et al., 2011), leading to shortcomings in the glutamine/glutamate cycle (Yin et al., 2007). These damaging changes gradually lead to the failure of astrocytes to control the extracellular milieu, eventually triggering neuronal damage.
We have recently discovered a novel nonapeptide, histone H2A fragment 36–44 (KGHYAERVG) termed IIIM1 that demonstrated anti-inflammatory and anti-oxidant activities. The peptide ameliorated skin irritation in guinea pigs topically exposed to sulfur mustard (SM), a powerful skin irritant and vesicant (Brodsky et al., 2008). In addition, administration of IIIM1 reduced SM-induced ear swelling in mice in a dose-dependent manner (Brodsky et al., 2008). Furthermore, peptide-transfected human keratinocytes showed higher resistance against SM cytotoxicity as compared to control cells (Brodsky et al., 2008). The peptide reduced carrageenan-induced hind paw swelling in mice and rats (Schussheim et al., 2011). IIIM1 was also active in experimental systems of chronic inflammation. For instance, in experimental autoimmune encephalitis, an animal model of multiple sclerosis, a significant reduction in neurological symptoms was observed in mice and rats treated with the peptide (Shapira et al., 2010). In addition, marked decrease in pathological symptoms was observed in IIIM1-treated MRL/lpr mice, an animal model of systemic lupus erythematosus (Shapira et al., 2011). These three recent reports (Schussheim et al., 2011, Shapira et al., 2010, Shapira et al., 2011) clearly highlight the beneficial effects of IIIM1 in in vitro experimental models of pathology, suggesting excellent bioavailability of the peptide in cells and tissues, including the central nervous system (Shapira et al., 2010). The mechanism of anti-inflammatory action of IIIM1 might involve antioxidant activity expressed by the concentration-dependent suppressing effect of IIIM1 on oxidative burst of activated neutrophils (Brodsky et al., 2008, Schussheim et al., 2011). This was corroborated by the reduction in skin edema caused by the intradermal injection of glucose oxidase (Brodsky et al., 2008), an enzyme that catalyzes the oxidation of glucose to glucoronic acid and hydrogen peroxide.
Notably, several earlier studies have indicated a potential role for mediators of inflammation in MeHg toxicity (Chang, 2011, Gardner et al., 2010, Ilback et al., 1996), which could synergistically increase the deleterious pro-oxidant effects of MeHg. Consequently, the present study was carried out examine the effects of IIIM1 on astrocytic cytotoxicity, oxidative injury and inflammation induced by MeHg. We found that IIIM1 pretreatment effectively attenuated toxicity of this hazardous metal and suppressed MeHg-induced alteration in markers of cytotoxicity (MTT and LDH), lipid peroxidation (F2-isoprostanes) and inflammation (prostaglandin E2).
MATERIALS AND METHODS
Primary cortical astrocyte cultures
Astrocyte cultures were prepared according to previously established protocols (Aschner et al., 1992). Astrocytes were isolated from cerebral cortices of newborn (1-day-old) Sprague-Dawley rats. Pups were decapitated under halothane-anesthesia and the cerebral cortices were dissected out. The meninges were removed and the cortices were digested with bacterial neutral protease (Dispase, Invitrogen). Astrocytes were then recovered by the repeated removal of dissociated cells and plated in 6- or 12-well plates at a density of 1 × 105/ml. Twenty-four hours after the initial plating, the media were changed to preserve the adhering astrocytes and to remove neurons and oligodendrocytes. The cultures were maintained at 37°C in a 95% air/5% CO2 incubator for 3 weeks in Minimum Essential Medium (MEM) with Earle's salts supplemented with 10% fetal bovine serum, 100 U/ml of penicillin and 100 µg/ml of streptomycin. The media were changed twice per week. Our experience dictates that the purity of these cultures is > 95%-positive for the astrocyte-specific marker, glial fibrillary acidic protein (GFAP). All experiments were performed 3 weeks post isolation when the astrocytes reached confluency.
Astrocytic viability and cytotoxicity assays
Once confluent, astrocytes (~ 3 weeks in culture) were pretreated for 66 hours with IIIM1 (5 µg/ml; equivalent to 4.95 µM; this was the optimal concentration for the protective activity as determined in preliminary experiments) followed by 6-hour exposure to MeHg (5 µM) or vehicle. This peptide concentration is physiologically relevant since a similar concentration (7.4µM) was detected in the blood of mice administered with an active dose of the peptide (Shapira et al., 2010). Cell viability was assessed by measuring formazan production after the addition of 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma Chemical Co., St. Louis, MO). The number of surviving cells was determined by measuring the optical density (OD) of the dissolved formazan product at A570 nm after the addition of MTT for 1 hour according to the manufacturer's instructions. Untreated negative controls were run together with the treated cells, and plates with reagent only served as background controls. Each experiment was performed in a minimum 3 wells and independently repeated in triplicates.
Cytotoxicity, reflecting cell membrane integrity, was assessed by measuring the activity of lactate dehydrogenase (LDH) in the culture media by colorimetric method (Promega, Madison, WI, USA). Cultured astrocytes were pretreated for 66 hours with IIIM1 (5 µg/ml; equivalent to 4.95 µM) followed by 6 hour exposure to MeHg (5 µM) or vehicle. Next, the supernatant was transferred to a 96-well plate and incubated with the reaction mixture for 30 minutes at room temperature allowing for the development of color changes. The optical density was measured at A492 nm with a spectrophotometer (Molecular Devices, VMax Kinetic Microplate Reader). Each experiment with different groups of astrocytes cultures was performed in at least 3 wells and independently repeated in triplicates.
Quantification of F2-isoprostanes (F2-IsoPs)
Upon completion of the experiments, primary astrocytes cultures were rapidly harvested, flash frozen in liquid nitrogen, and stored at −80 °C until analysis. Total F2-IsoPs were determined with a stable isotope dilution method with detection by gas chromatography/mass spectrometry and selective ion monitoring as previously described (Montine et al., 2002). Cultured astrocytes were pretreated for 66 hours with IIIM1 (5 µg/ml; equivalent to 4.95 µM) followed by 6 hour exposure to MeHg (5 µM) or vehicle. Next, astrocytes were resuspended in 0.5 ml of methanol containing 0.005% butylated hydroxytoluene, sonicated and then subjected to chemical saponification using 15% KOH to hydrolyze bound F2-IsoPs. The cell lysates were adjusted to a pH of 3, followed by the addition of 0.1 ng of 4H2-labeled 15-F2α-IsoP internal standard. F2-IsoPs were subsequently purified by C18 and silica Sep-Pak extraction and by thin layer chromatography. They were then analyzed by pentafluorobenzyl ester, a trimethylsilyl ether derivative, via gas chromatography, negative ion chemical ionization mass spectrometry (Milatovic and Aschner, 2009).
Quantification of prostaglandin E2 (PGE2)
PGE2 was measured with a stable isotope dilution gas chromatographic/negative ion chemical ionization-mass spectrometric assay (Awas et al., 1996). Briefly, following addition of [4H2]-PGE2 (1.28 ng) to astrocytes homogenates, the samples were acidified to pH 3 with 1 N HCl and extracted on a C18 Sep-Pak. PGE2 was eluted with ethyl acetate:heptane and evaporated under a stream of N2. PGE2 in methoxylamine solution was extracted with ethyl acetate and evaporated with N2. The pentafluorobenzyl esters were purified by thin layer chromatography (PGE2 and PGD2 methyl esters are used as TLC standards), converted to O-methyloxime pentafluorobenzyl ester trimethylsilyl derivatives, and PGE2 dissolved in undecane that was dried over a bed of calcium hydride. Gas chromatographic/negative ion chemical ionization-mass spectrometric analysis was performed as described previously with the M-181 ions for PGE2 (m/z 526) and the [4H2]-PGE2 as internal standard (m/z 528) (Milatovic and Aschner, 2009).
Mass spectrometry analysis
In order to determine whether IIIM1 neutralizes MeHg by binding the peptide (50µg/ml) was incubated with MeHg (50µM50 µM) for 72 hours and subjected to mass spectrometry (MS) analysis. The ratio of MeHg to IIIM1 under these conditions is about 1:1, thus recapitulating the ratio that was used in the cytotoxic assays described above. The samples were nanosprayed into the Orbi-trap MS system in 50% CH3CN 1% CHOOH solution. MS was carried out with Orbi-trap XL (Thermo Finnigen) using nanospray attachment (Wilm and Mann, 1996).
Statistical analysis
Measurements of MTT, LDH, F2-IsoPs, and PGE2 were conducted in duplicate or triplicate wells/experiment, and the mean from a minimum of three independent experiments was used for statistical analysis. The means of each independent experiment was used as the statistical unit, n (not the total number of wells). The data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni’s correction for multiple comparisons with statistical significance of p<0.05. All analyses were carried out with GraphPad Prism 4.02 for Windows (GraphPad Software, San Diego, CA).
RESULTS
IIIM1 reverses the MeHg-induced astrocytic cell death
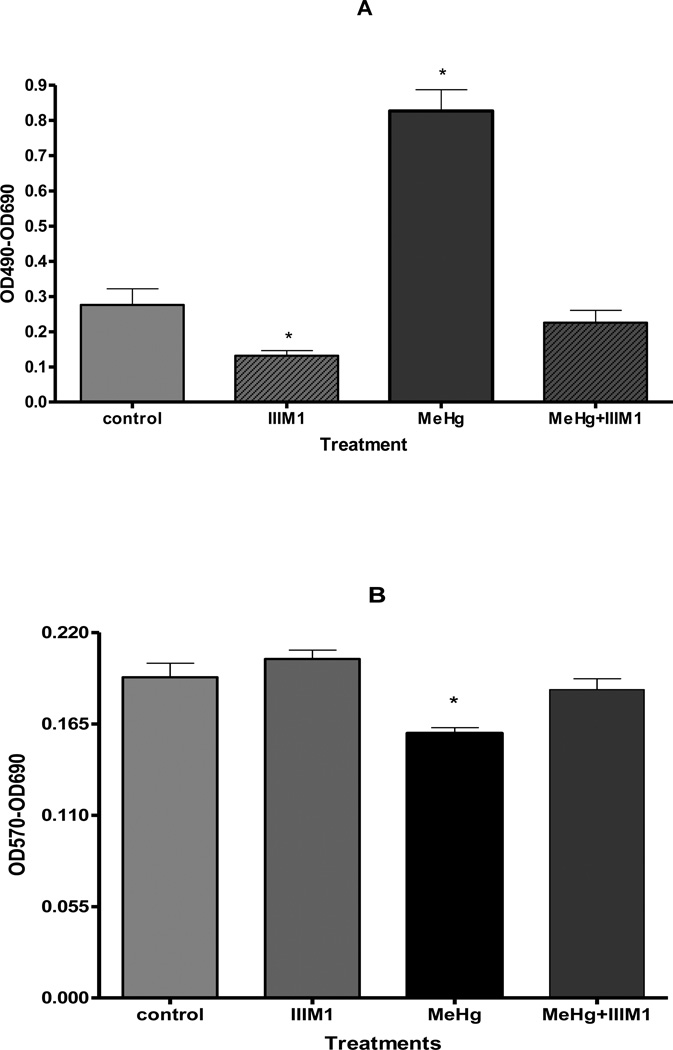
We tested ability of IIIM1 to modulate the overall cytotoxicity of MeHg in cultured astrocytes with the lactate dehydrogenase (LDH) assay. LDH activity in the culture media is reversely correlated with cell viability. As shown in Figure 1A, the activity of LDH significantly increased after 6 hour treatment with 5 µM MeHg (p < 0.001), reflecting decreased cell viability. However, when astrocytes were pretreated for 66 hours with IIIM1 (5 µg/ml) prior to exposure to MeHg (5 µM for 6 h) LDH activity in the media was unchanged and indistinguishable from the controls. Interestingly, astrocytes treated with IIIM1 showed significantly (p < 0.005) lower level of LDH release compared to control/non MeHg-treated cells indicating that IIIM1 may stabilize the cells and possibly protects against spontaneous damage inherent to culturing the cells in 21% atmospheric oxygen concentration.
Figure 1.
Effect of IIIM1 on viability of primary rat astrocytes in the presence and absence of MeHg treatment. Viability/cytotoxicity was measured by LDH assay (A) and MTT assay (B). Activity of lactate dehydrogenase (LDH) released into the media was measured spectrophotometrically according to the manufacturer’s protocol. The quantity of purple formazan product in MTT assay was measured at 570 nm. Cultured astrocytes were pretreated for 66 hours with IIIM1 (5 µg/ml; 4.95 µM) followed by 6 hour exposure to MeHg (5 µM) or vehicle. Data represent mean ± SEM (bars) values from 3 independent experiments. *p<0.05 versus control by one-way ANOVA followed by Bonferroni’s multiple comparison test.
Cell viability was also measured by the MTT assay. The absorbance of the MTT reduction to its formazan salt (wavelength of 570 nm), a product of MTT reduction by dehydrogenases, is positively correlated with cell viability. As shown in Figure 1B, after MeHg treatment for 6 hours, the absorbance was significantly reduced compared to control/non MeHg-treated astrocytes. However, when cultured astrocytes were pretreated for 66 hours with IIIM1 (5 µg/ml) prior to exposure to MeHg (5 µM for 6 h) MTT activity was fully recovered to levels indistinguishable from controls. While statistically insignificant, results from the MTT experiments also show that the absorbance following IIIM1 treatment may be increased compared to control/non MeHg-treated astrocytes, attesting to an inherent neuroprotective effect of IIIM1 on mitochondrial function.
Effects of IIIM1 on MeHg-induced astrocytic F2 – IsoPs and PGE2 formation
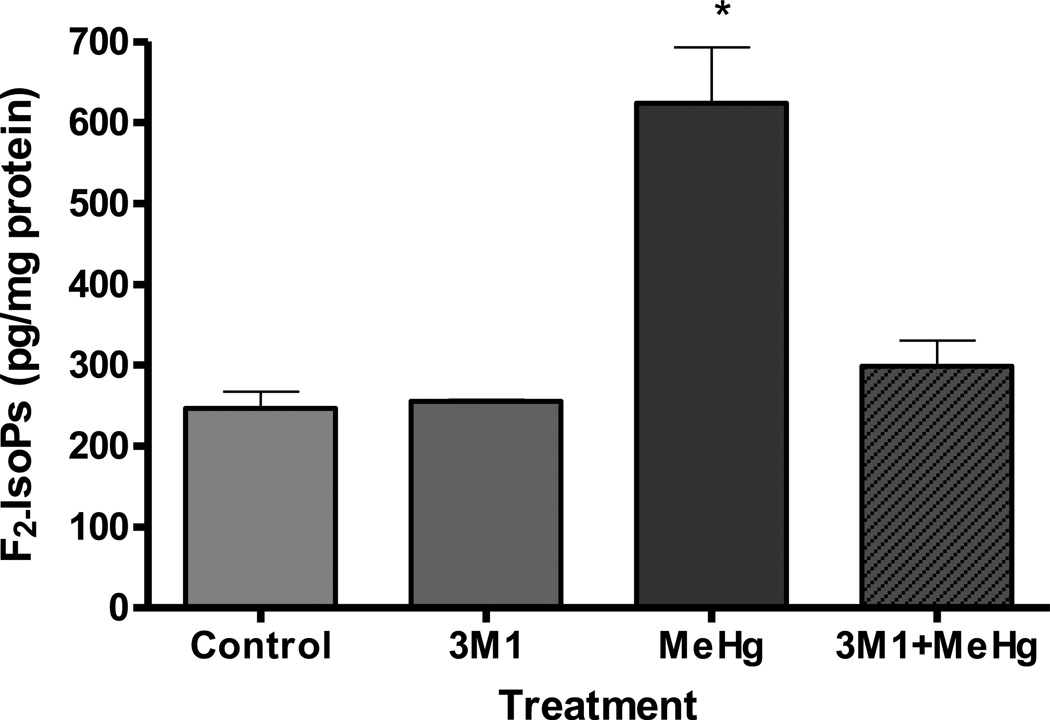
We tested the ability of IIIM1 to suppress oxidative stress in astrocytes exposed to MeHg by measuring the levels of F2-IsoPs, a lipid peroxidation biomarker of oxidative injury. Six-hour exposure to 5 µM MeHg was associated with a significant increase in astrocytic F2-isoPs levels (p<0.05) (Figure 2). Pretreatment for 66 hours with IIIM1 (5 µg/ml; 4.95 µM) prior to exposure to MeHg (5 µM for 6 h) fully restored F2-IsoPs to levels indistinguishable from controls. These results indicate that IIIM1 pretreatment fully suppress MeHg-induced oxidative stress.
Figure 2.
Effects of IIIM1 on the F2 – IsoPs formation in cultured astrocytes in the presence and absence of MeHg treatment. Rat primary astrocytes cultures were incubated for 66 hours at 37°C with IIIM1 (5 µg/ml; 4.95 µM) followed by 6 hour exposure to MeHg (5 µM) or vehicle. Total F2-IsoPs were determined with a stable isotope dilution method with detection by gas chromatography/mass spectrometry and selective ion monitoring. Data represent the mean ± S.E.M. from three independent experiments. * p<0.05 versus control by one-way ANOVA followed by Bonferroni’s multiple comparison tests.
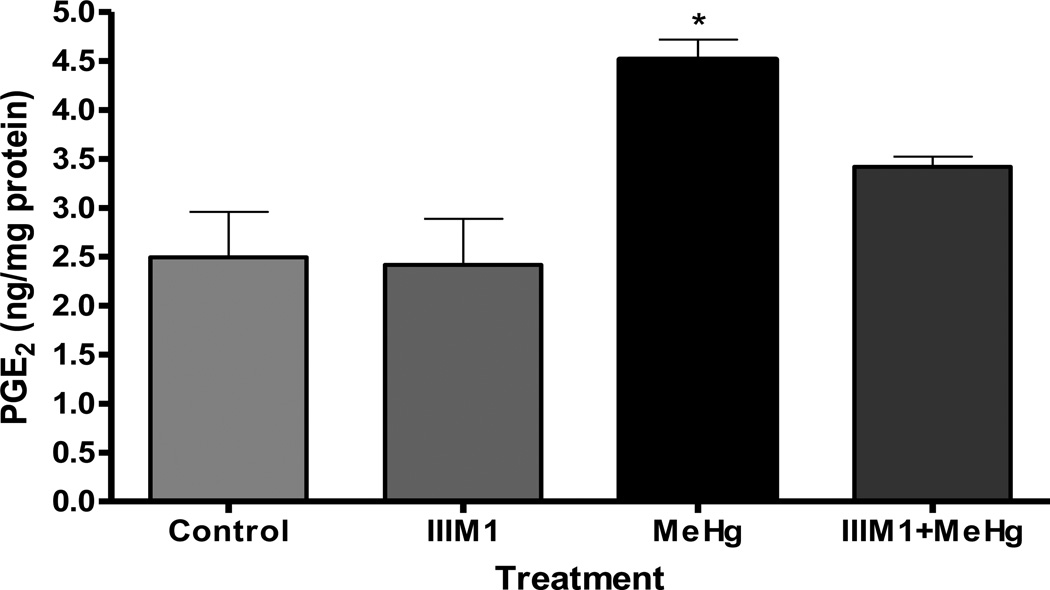
PGE2, a biomarker of proinflammatory responses, was also evaluated. Astrocytes treated for 6 hours with 5 µM of MeHg showed significant increase (p<0.05) in PGE2 levels, indicative of inflammatory responses (Figure 3). Sixty-six-hour pretreatment with IIIM1 prior to exposure to 5 µM MeHg fully restored PGE2 to levels indistinguishable from the controls. As shown in Figure 3, PGE2 levels were indistinguishable between cultured astrocytes treated with only IIIM1 and control.
Figure 3.
Effects of IIIM1 on on PGE2 formation in cultured astrocytes in the presence and absence of MeHg treatment. Rat primary astrocytes cultures were incubated for 66 hours at 37°C with IIIM1 (5 µg/ml; 4.95 µM) followed 6 hour exposure to MeHg (5 µM) or vehicle. PGE2 as a biomarker of inflammation was also measured by using a stable isotope dilution gas chromatographic/negative ion chemical ionization-mass spectrometric assay. Data represent the mean ± S.E.M. from three independent experiments. * p<0.05 versus control by one-way ANOVA followed by Bonferroni’s multiple comparison tests.
Mass spectrometry analysis
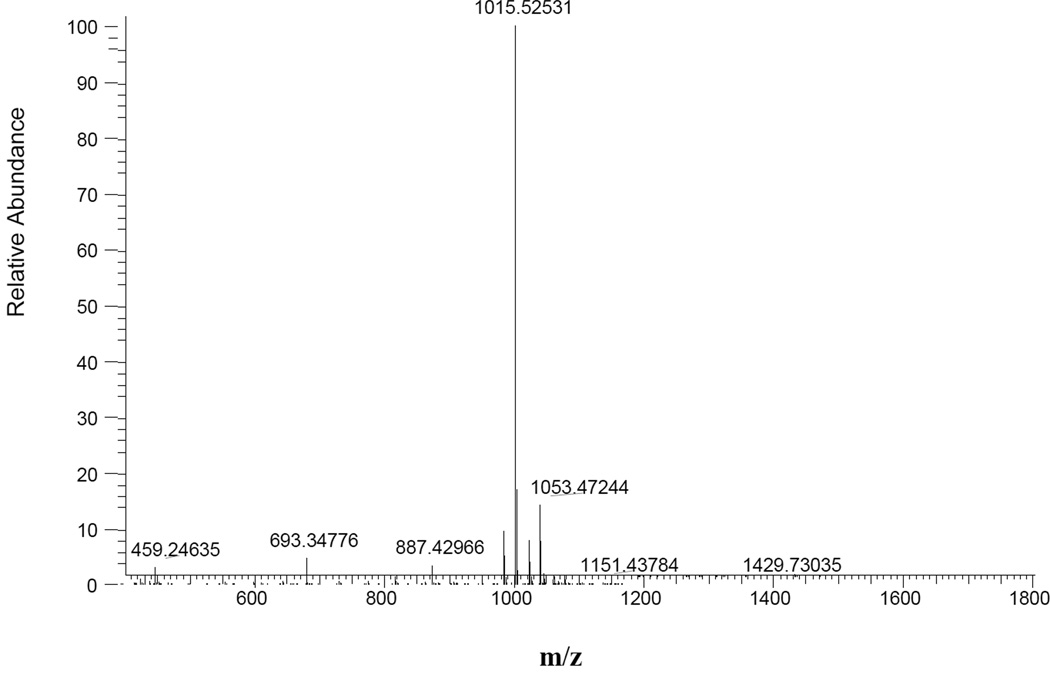
Mass spectrometry analysis revealed no chemical or binding interaction between MeHg and IIIM1 (Figure 4), the relevant peaks were the peptide (1015.5) and an addition of potassium ion (1053.4). This indicates intracellular cytoprotective mechanism of action for IIIM1 rather than direct neutralization of MeHg.
Figure 4.
Mass spectrometry of IIIM1 incubated with MeHg. The peptide was incubated with MeHg at equivalent molar concentrations (50 µg/ml IIIM1 incubated with 50 µM MeHg). Peak 1015.5 is IIIM1 and the minor peak of 1053.4 is an addition of potassium ion to the peptide.
DISCUSSION
To our knowledge, this is the first study to investigate the efficacy of IIIM1 in reducing MeHg-induced cytotoxicity in primary rat neonatal astrocyte cultures. The implication of these findings may prove beneficial in designing new treatment modalities that efficiently suppress neurotoxicity, triggered not only by MeHg, but also by other metals and environmental agents, as well as chronic disease conditions that inherently increase ROS production and inflammatory signaling.
Our results corroborate previous findings on the ability of MeHg to decrease viability and induce free-radical mediated damage to astrocytes. For example, utilizing a selective probe for mitochondrial reactive oxygen intermediates, Yin et al (2011) demonstrated a significant increase in intracellular astrocytic superoxide anion, hydrogen peroxide and hydroxyl radicals in response to MeHg treatment. These studies indicated that the mitochondrial electron transport chain is an early, primary site for ROS formation (Farina et al., 2011a, Franco et al., 2007, Franco et al., 2010, Roos et al., 2011, Yin et al., 2011). Lipid peroxidation secondary to increased mitochondrial and cellular permeability alterations, involving glutathione (GSH) depletion and an increase in intramitochondrial calcium concentrations (Hood et al., 2010) feed a vicious cycle whereby these disturbances ROS formation lead to cellular dysfunction and demise.
Results from our study also showed that MeHg exposure induced increase in biomarkers of proinflammatory response, PGE2. MeHg has also been reported to stimulate interleukin-6 (IL-6) release from the rat C6 glioma cells, the human U251HF glioma cells and the human retina pigment epithelial (ARPE-19) cells (Chang, 2007). In accordance with our results, exposure of glial cells to MeHg can induce the release of IL-6, a pro-inflammatory cytokine (Chang, 2011), corroborating that the neurotoxicity of MeHg has an inflammatory component. Similarly, inflammatory mediator release is also reported in mast cells following mercury (Hg) exposure (Kempuraj et al., 2010). Murine mast cell line and mouse bone marrow-derived cultured mast cells showed increase in histamine and cytokines, such as IL-4, IL-6 and tumor necrosis factor-alpha (TNF-α) along with vascular endothelial growth factor (VEGF). These observations are consistent with disruption of the blood-brain-barrier and consequent brain inflammation, as suggested by Walczak-Drzewiecka et al. (2005), most likely via the activation of c-Jun N-terminal kinase (JNK).
We have demonstrated that pretreatment with IIIM1 effectively suppressed MeHg-induced cytotoxicity, oxidative injury and inflammation, restoring MTT and LDH, F2-IsoPs and PGE2 to levels inherent to control, non MeHg-treated astrocytes. These findings were supported by the MTT assay showing significant decrease in cell toxicity upon treatment with the peptide. The beneficial effect of IIIM1 in MeHg-exposed cells was further corroborated by its pronounced effect on F2-IsoPs and PGE2 levels which are parameters of oxidative stress and inflammation (Milatovic et al., 2011). The marked effect on these two factors is in line with the cytoprotective activity of IIIM1 in astrocytes exposed to MeHg. This type of activity is well correlated with the known cytoprotective/anti-inflammatory/counter-irritating properties of IIIM1. For instance, peptide-transfected keratinocytes constitutively producing IIIM1 showed pronounced resistance against the cytotoxic effect of the alkylating agent sulfur mustard (Brodsky et al., 2008) which belong to a group of compounds that induce reactive oxygen species (ROS) formation and oxidative damage (Gray et al., 2010, Sharma et al., 2008). This cytoprotective/anti-inflammatory effect of IIIM1 is also reflected by its counter-irritating activity against skin vesicants, suppression of oxidative burst of activated neutrophils, reduction of skin edema caused by the intradermal injection of glucose oxidase (Brodsky et al., 2008) and decreased carrageenan-induced hind paw swelling in mice and rats (Schussheim et al., 2011). IIM1 was more efficacious in reversing the MeHg-induced effect on MTT vs. LDH. These observations do not exclude the possibility that the primary site of MeHg damage is within the mitochondria, with secondary dissipation of ion gradients and massive efflux of LDH. While the studies are not sufficiently refined to address the primary site of damage, they do support a role for the mitochondria in mediating MeHg-induced neurotoxicity. Furthermore, future studies will be necessary to address whether the protective mechanism of IIIM1 is mediated by membrane receptors and how efficiently it crosses the cellular membrane (see below).
The ability of IIIM1 to reduce IsoPs and PGE2 may also be associated with its beneficial effects in chronic inflammatory diseases. For instance, in experimental autoimmune encephalitis, an animal model of multiple sclerosis, a significant reduction in neurological symptoms was observed in mice and rats treated with the peptide (Shapira et al., 2010). In addition, marked decrease in pathological symptoms was observed in IIIM1-treated MRL/lpr mice, an animal model of systemic lupus erythematosus (Shapira et al., 2011). Both multiple sclerosis and lupus animal models showed reduction in inflammatory mediators such as IL17 and IL12 upon treatment with IIIM1 (Shapira et al., 2010, Shapira et al., 2011). Taking into account (i) previous studies on the beneficial effects of IIIM1 in experimental models of pathological conditions related to inflammation and oxidative stress (Schussheim et al., 2011, Shapira et al., 2010, Shapira et al., 2011), as well as the (ii) proinflammatory (Chang, 2007) and pro-oxidative properties of MeHg (Aschner et al., 2007, Farina et al., 2011b, Farina et al., 2011a), these data are in line with the cytoprotective/anti-inflammatory activity of the peptide against the toxic effects of MeHg in cultured astrocytes.
Recent studies demonstrated that IIIM1 induces proliferation of T-regulatory lymphocytes (Tregs) both in vivo and in vitro (Shapira et al., 2010). Incubation of isolated human and mouse Tregs with the peptide caused hyperproliferation and induction of production of a specific peptide (Shapira et al., 2010). Further studies using FACS analysis have shown the presence of the peptide inside the cells (unpublished results), which presumably affects intracellular mechanism of action leading to its immunological and pharmacological effects. It might be that the same mode of action applies for the cultured astrocytes; namely, the peptide penetrates the cells and elicits intracellular mechanisms that suppress the MeHg-induced oxidative stress leading to increased cell viability. These cellular modifications may occur not only in a prophylactic treatment but also in a post exposure mode where the cells are under oxidative attack, which can be counteracted by the peptide. Whether peptide penetration is receptor-mediated or by a non-specific mechanism and, what kind of alterations in gene expressions are involved in IIIM1-induced protection remain open questions to be addressed in future studies.
Acknowledgments
MA was supported in part by funds from NIH ES R01 07331.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Allen JW, Mutkus LA, Aschner M. Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res. 2001a;902:92–100. doi: 10.1016/s0006-8993(01)02375-7. [DOI] [PubMed] [Google Scholar]
- 2.Allen JW, Shanker G, Aschner M. Methylmercury inhibits the in vitro uptake of the glutathione precursor, cystine, in astrocytes, but not in neurons. Brain Res. 2001b;894:131–140. doi: 10.1016/s0006-8993(01)01988-6. [DOI] [PubMed] [Google Scholar]
- 3.Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem. 1992;58:730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- 4.Aschner M, Du YL, Gannon M, Kimelberg HK. Methylmercury-induced alterations in excitatory amino acid transport in rat primary astrocytic cultures. Brain Res. 1993;602:181–186. doi: 10.1016/0006-8993(93)90680-l. [DOI] [PubMed] [Google Scholar]
- 5.Aschner M, Mullaney KJ, Wagoner D, Lash LH, Kimelberg HK. Intracellular glutathione (GSH) levels modulate mercuric chloride (MC)- and methylmercuric chloride (MeHgCl)-induced amino acid release from neonatal rat primary astrocytes cultures. Brain Res. 1994;664:133–140. doi: 10.1016/0006-8993(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 6.Aschner M. Methylmercury in astrocytes- what possible significance? Neurotoxicology. 1996;17:93–106. [PubMed] [Google Scholar]
- 7.Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res. 2007;40:285–291. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- 8.Aschner M, Yao CP, Allen JW, Tan KH. Methylmercury alters glutamate transport in astrocytes. Neurochem Int. 2000;37:199–206. doi: 10.1016/s0197-0186(00)00023-1. [DOI] [PubMed] [Google Scholar]
- 9.Awas JA, Soteriou MC, Drougas JG, Stokes KA, Roberts LJ, 2nd, Pinson CW. Plasma prostaglandin E1 concentrations and hemodynamics during intravenous infusions of prostaglandin E1 in humans and swine. Transplantation. 1996;61:1624–1629. doi: 10.1097/00007890-199606150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky B, Erlanger-Rosengarten A, Proscura E, Shapira E, Wormser U. From topical antidote against skin irritants to a novel counter-irritating and anti-inflammatory peptide. Toxicol Appl Pharmacol. 2008;229:342–350. doi: 10.1016/j.taap.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Chang JY. Methylmercury causes glial IL-6 release. Neurosci Lett. 2007;416:217–220. doi: 10.1016/j.neulet.2007.01.076. [DOI] [PubMed] [Google Scholar]
- 12.Chang JY. Methylmercury-induced IL-6 release requires phospholipase C activities. Neurosci Lett. 2011;496:152–156. doi: 10.1016/j.neulet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farina M, Aschner M, Rocha JB. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol. 2011b doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farina M, Rocha JB, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 2011a;89:555–563. doi: 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco JL, Braga HC, Stringari J, Missau FC, Posser T, Mendes BG, et al. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem Res Toxicol. 2007;20:1919–1926. doi: 10.1021/tx7002323. [DOI] [PubMed] [Google Scholar]
- 16.Franco JL, Posser T, Missau F, Pizzolatti MG, Dos Santos AR, Souza DO, et al. Structure-activity relationship of flavonoids derived from medicinal plants in preventing methylmercury-induced mitochondrial dysfunction. Environ Toxicol Pharmacol. 2010;30:272–278. doi: 10.1016/j.etap.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner RM, Nyland JF, Silbergeld EK. Differential immunotoxic effects of inorganic and organic mercury species in vitro. Toxicol Lett. 2010;198:182–190. doi: 10.1016/j.toxlet.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandjean P, Budtz-Jorgensen E, White RF, Jorgensen PJ, Weihe P, Debes F, et al. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am J Epidemiol. 1999;150:301–305. doi: 10.1093/oxfordjournals.aje.a010002. [DOI] [PubMed] [Google Scholar]
- 19.Grandjean P, White RF, Weihe P, Jorgensen PJ. Neurotoxic risk caused by stable and variable exposure to methylmercury from seafood. Ambul Pediatr. 2003;3:18–23. doi: 10.1367/1539-4409(2003)003<0018:nrcbsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Gray JP, Mishin V, Heck DE, Laskin DL, Laskin JD. Inhibition of NADPH cytochrome P450 reductase by the model sulfur mustard vesicant 2-chloroethyl ethyl sulfide is associated with increased production of reactive oxygen species. Toxicol Appl Pharmacol. 2010;247:76–82. doi: 10.1016/j.taap.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hood JE, Jenkins JW, Milatovic D, Rongzhu L, Aschner M. Mefloquine induces oxidative stress and neurodegeneration in primary rat cortical neurons. Neurotoxicology. 2010;31:518–523. doi: 10.1016/j.neuro.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Ilback NG, Wesslen L, Fohlman J, Friman G. Effects of methyl mercury on cytokines, inflammation and virus clearance in a common infection (coxsackie B3 myocarditis) Toxicol Lett. 1996;89:19–28. doi: 10.1016/s0378-4274(96)03777-0. [DOI] [PubMed] [Google Scholar]
- 23.Kempuraj D, Asadi S, Zhang B, Manola A, Hogan J, Peterson E, et al. Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation. 2010;7:20. doi: 10.1186/1742-2094-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjellstrom T, Kennedy P, Wallis S. Physical and mental development of children with prenatal exposure to mercury from fish. Stage 2. Interviews and psychological tests at age 6, Solna, Sweden. National Swedish Environmental Board Report. 1989:3642. [Google Scholar]
- 25.Lafon-Cazal M, Pietri S, Culcasi M, Boeckaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 26.Maret M. Oxidative metal release from metallothionein via zincthiol/disulfide interchange. Proc. Natl. Acad. Sci. USA. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milatovic D, Aschner M. Measurement of Isoprostanes as Markers of Oxidative Stress in Neuronal Tissue. Curr Protoc toxicol. 2009;2009(12):41–44. doi: 10.1002/0471140856.tx1214s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milatovic D, Montine TJ, Aschner M. Prostanoid signaling: dual role for prostaglandin E2 in neurotoxicity. Neurotoxicology. 2011;32:312–319. doi: 10.1016/j.neuro.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montine TJ, Milatovic D, Gupta RC, Valyi-Nagy T, Morrow JD, Breyer RM. Neuronal oxidative damage from activated innate immunity is EP2 receptor-dependent. J Neurochem. 2002;83:463–470. doi: 10.1046/j.1471-4159.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 30.Ni M, Li X, Yin Z, Jiang H, Sidoryk-Wegrzynowicz M, Milatovic D, et al. Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol Sci. 2010;116:590–603. doi: 10.1093/toxsci/kfq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni M, Li X, Yin Z, Sidoryk-Węgrzynowicz M, Jiang H, Farina M, Rocha JB, Syversen T, Aschner M. Comparative study on the response of rat primary astrocytes and microglia to methylmercury toxicity. Glia. 2011;59:810–820. doi: 10.1002/glia.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roos DH, Puntel RL, Farina M, Aschner M, Bohrer D, Rocha JB, et al. Modulation of methylmercury uptake by methionine: prevention of mitochondrial dysfunction in rat liver slices by a mimicry mechanism. Toxicol Appl Pharmacol. 2011;252:28–35. doi: 10.1016/j.taap.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schussheim Y, Aschner M, Brodsky B, Proscura E, Erlanger-Rosengarten A, Feldman R, et al. Anti-inflammatory effects of peptide fragments of H2A histone and Oryza Sativa Japonica protein. Peptides. 2011;32:125–130. doi: 10.1016/j.peptides.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Shanker G, Allen JW, Mutkus LA, Aschner M. Methylmercury inhibits cysteine uptake in cultured primary astrocytes, but not in neurons. Brain Res. 2001a;914:159–165. doi: 10.1016/s0006-8993(01)02791-3. [DOI] [PubMed] [Google Scholar]
- 35.Shanker G, Aschner M. Identification and characterization of uptake systems for cystine and cysteine in cultured astrocytes and neurons: evidence for methylmercury-targeted disruption of astrocytic transport. J Neurosci Res. 2001b;66:998–1002. doi: 10.1002/jnr.10066. [DOI] [PubMed] [Google Scholar]
- 36.Shanker G, Mutkus LA, Walker S, Aschner M. Methylmercury enhances arachidonic acid release and cytosolic phospholipase A2 expression in primary cultures of neonatal astrocytes. Mol. Brain Res. 2002;106:1–11. doi: 10.1016/s0169-328x(02)00403-5. [DOI] [PubMed] [Google Scholar]
- 37.Shanker G, Hampson RE, Aschner M. Methylmercury stimulates arachidonic acid release and cytosolic phospholipase A2 expression in primary neuronal cultures. Neurotoxicology. 2004;25:399–406. doi: 10.1016/j.neuro.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Shanker G, Syversen T, Aschner JL, Aschner M. Modulatory effect of glutathione status and antioxidants on methylmercury-induced free radical formation in primary cultures of cerebral astrocytes. Brain Res Mol Brain Res. 2005;137:11–22. doi: 10.1016/j.molbrainres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Shapira E, Brodsky B, Proscura E, Nyska A, Erlanger-Rosengarten A, Wormser U. Amelioration of experimental autoimmune encephalitis by novel peptides: involvement of T regulatory cells. J Autoimmun. 2010;35:98–106. doi: 10.1016/j.jaut.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Shapira E, Proscura E, Brodsky B, Wormser U. Novel peptides as potential treatment of systemic lupus erythematosus. Lupus. 2011;20:463–472. doi: 10.1177/0961203310389484. [DOI] [PubMed] [Google Scholar]
- 41.Sharma M, Vijayaraghavan R, Ganesan K. Comparison of toxicity of selected mustard agents by percutaneous and subcutaneous routes. Indian J Exp Biol. 2008;46:822–830. [PubMed] [Google Scholar]
- 42.Steuerwald U, Weihe P, Jorgensen PJ, Bjerve K, Brock J, Heinzow B, et al. Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. J Pediatr. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- 43.Walczak-Drzewiecka A, Wyczolkowska J, Dastych J. c-Jun N-terminal kinase is involved in mercuric ions-mediated interleukin-4 secretion in mast cells. Int Arch Allergy Immunol. 2005;136:181–190. doi: 10.1159/000083892. [DOI] [PubMed] [Google Scholar]
- 44.Wilm M, Mann M. Analytical properties of the nanoelectrospray ion source. Anal Chem. 1996;68:1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- 45.Yin Z, Lee E, Ni M, Jiang H, Milatovic D, Rongzhu L, et al. Methylmercury-induced alterations in astrocyte functions are attenuated by ebselen. Neurotoxicology. 2011;32:291–299. doi: 10.1016/j.neuro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO, et al. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131:1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]