Abstract
Context:
Clinically overt thyroid dysfunction is associated with alterations in triglyceride (TG) metabolism. The effect of subclinical thyroid disease on very-low-density lipoprotein (VLDL) kinetics is not known.
Objective:
Our objective was to investigate whether subclinical thyroid disease is associated with alterations in hepatic VLDL metabolism.
Design and Outcomes:
We measured VLDL-TG and VLDL-apolipoprotein B-100 (apoB-100) kinetics by infusing stable isotopically labeled tracers, in conjunction with mathematical modeling.
Setting and Participants:
Ten women with subclinical hypothyroidism, 10 women with subclinical hyperthyroidism, and 25 euthyroid women, matched on age, body mass index, and percent body fat, were studied in the Clinical Research Unit at Washington University School of Medicine.
Results:
Plasma VLDL-TG concentrations were 0.75 ± 0.13, 0.51 ± 0.06, and 0.37 ± 0.07 mmol/liter (P = 0.029), and hepatic VLDL-TG secretion rates were 6.5 ± 0.7, 5.0 ± 0.4, and 4.1 ± 0.6 μmol/liter·min (P = 0.026) in hypothyroid, euthyroid, and hyperthyroid women, respectively. The differences in VLDL-TG secretion rates were due to differences in the incorporation of systemic plasma free fatty acids into VLDL-TG (4.3 ± 0.3, 3.1 ± 0.3, and 2.5 ± 0.3 μmol/liter·min in hypothyroid, euthyroid, and hyperthyroid women, respectively; P = 0.005). Plasma VLDL-apoB-100 concentration and hepatic secretion rate did not differ among groups (P > 0.400), so the molar ratios of VLDL-TG to VLDL-apoB-100 secretion rates were 21,469 ± 3,477, 16,025 ± 1,273, and 11,889 ± 1,319 in hypothyroid, euthyroid, and hyperthyroid women, respectively (P = 0.019).
Conclusions:
Subclinical thyroid disease affects hepatic VLDL-TG but not VLDL-apoB-100 metabolism: subclinical hypothyroidism increases, whereas subclinical hyperthyroidism decreases, hepatic VLDL-TG secretion rate compared with the euthyroid state. Plasma VLDL-TG concentration is greater in subclinical hypothyroid than euthyroid and hyperthyroid subjects, due to greater secretion of large, TG-rich VLDL particles from the liver.
Subclinical thyroid disease, defined as increased (hypothyroidism) or decreased (hyperthyroidism) plasma TSH concentration in conjunction with normal free T4 and total T3 concentrations (1), is common and occurs in approximately 10% of adults in the United States (2). Subclinical thyroid disease has important clinical implications because alterations in thyroid function are associated with alterations in plasma lipids (3). Subclinical hyperthyroidism (S-Hyper) is associated with a decrease, whereas subclinical hypothyroidism (S-Hypo) is associated with an increase, in plasma triglyceride (TG) concentrations (2, 4, 5). The mechanism responsible for the observed relationship between subclinical thyroid dysfunction and plasma TG concentration is not known but likely involves an alteration in very-low-density lipoprotein (VLDL)-TG metabolism because VLDL-TG accounts for at least 65% of circulating TG (6). Therefore, a better understanding of VLDL kinetics in people with subclinical thyroid dysfunction would provide new insights into the relationship between thyroid function and hepatic lipoprotein metabolism.
The purpose of the present study was to evaluate the interrelationships among subclinical thyroid dysfunction, plasma TG concentration, and VLDL-TG and VLDL-apolipoprotein B-100 (apoB-100) metabolism. Accordingly, we measured VLDL-TG and VLDL-apoB-100 kinetics in vivo in women with S-Hypo and S-Hyper and in euthyroid women, matched on age, body mass index (BMI), and percent body fat, by using stable isotopically labeled tracers and compartmental modeling.
Subjects and Methods
Subjects
Ten women with S-Hypo, 10 women with S-Hyper, and 25 euthyroid women, matched on age, BMI, and percent body fat, participated in this study (Table 1). S-Hypo and S-Hyper were diagnosed at screening on the basis of high (>4.5 mU/liter) and low (<0.45 mU/liter) plasma TSH concentration, respectively, in conjunction with normal plasma free T4 and total T3 concentrations (1). Fat mass and fat-free mass were assessed by using dual-energy x-ray absorptiometry (Delphi-W densitometer; Hologic, Waltham, MA). Written informed consent was obtained from all subjects before participation in the study, which was approved by the Human Studies Committee of Washington University School of Medicine.
Table 1.
Subject characteristics
| S-Hypo | Euthyroid | S-Hyper | P value | |
|---|---|---|---|---|
| Age (yr) | 37 ± 3 | 36 ± 2 | 36 ± 3 | 0.975 |
| Weight (kg) | 92 ± 7 | 88 ± 4 | 90 ± 7 | 0.849 |
| BMI (kg/m2) | 33 ± 2 | 32 ± 1 | 32 ± 3 | 0.894 |
| Body fat (% body weight) | 41 ± 3 | 41 ± 2 | 40 ± 3 | 0.936 |
| Fat mass (kg) | 40 ± 5 | 37 ± 3 | 37 ± 5 | 0.921 |
| Fat-free mass (kg) | 53 ± 3 | 51 ± 1 | 53 ± 2 | 0.671 |
| TSH (mU/liter) | 11.2 ± 2.9a,b | 1.7 ± 0.2 | 0.3 ± 0.1 | <0.001 |
| Free T4 (ng/liter) | 8.8 ± 0.5a | 9.3 ± 0.4a | 11.3 ± 0.8 | 0.021 |
| Total T3 (ng/liter) | 976 ± 94 | 866 ± 68 | 1057 ± 196 | 0.435 |
| Insulin (pmol/liter) | 62 ± 12 | 61 ± 7 | 77 ± 20 | 0.571 |
| Glucose (mmol/liter) | 5.0 ± 0.1 | 5.1 ± 0.1 | 5.2 ± 0.1 | 0.559 |
| FFA (μmol/liter) | 445 (350–567) | 422 (370–480) | 482 (370–627) | 0.565 |
| VLDL-apoB-100 (nmol/liter) | 63 (42–95) | 51 (42–61) | 46 (30–71) | 0.400 |
| VLDL-TG (mmol/liter) | 0.75 ± 0.13a,b | 0.51 ± 0.06 | 0.37 ± 0.07 | 0.029 |
Values are means ± sem, except for FFA and VLDL-apoB-100 concentrations, which are means (95% confidence intervals).
Value is significantly different from S-Hyper group at P ≤ 0.05.
Value is significantly different from euthyroid group at P ≤ 0.05.
Experimental protocol
Each subject completed a 12-h isotope tracer infusion study to assess plasma free fatty acid (FFA), VLDL-TG, and VLDL-apoB-100 kinetics. Subjects were admitted to the Clinical Research Unit at Washington University School of Medicine in the evening before the study and consumed a standard meal at 1900 h. The following morning, after subjects fasted overnight, a catheter was inserted into a forearm vein to administer stable isotopically labeled tracers. A second catheter was inserted into a contralateral hand vein, which was heated to 55 C with a thermostatically controlled box, to obtain arterialized blood samples. At 0600 h, a bolus of [1,1,2,3,3-2H5]glycerol was injected, and a primed-constant infusion of [5,5,5-2H3]leucine and a constant infusion of [2,2-2H2]palmitate were initiated. Blood samples were obtained before starting the tracer infusions and at 5, 15, 30, 60, 90, and 120 min and then every hour until 12 h to determine VLDL-TG, VLDL-apoB-100, and plasma FFA kinetics (7).
Sample analyses
Plasma VLDL was prepared by ultracentrifugation (7). VLDL-TG concentrations were determined by using an enzymatic spectrophotometric kit (Sigma Chemical Co., St. Louis, MO), and VLDL-apoB-100 concentrations were measured by using a turbidimetric immunoassay (Wako Pure Chemical Industries, Osaka, Japan). Plasma glycerol, palmitate, and leucine tracer-to-tracee ratios in plasma and in VLDL were determined by using gas chromatography-mass spectrometry (7). Plasma TSH, free T4, and total T3 concentrations were measured by using ELISA kits (IBL-America, Minneapolis, MN). Plasma insulin concentration was measured by using a RIA (Linco Research, St. Louis, MO). Plasma FFA concentrations were quantified by gas chromatography (HP 5890 Series II GC; Hewlett-Packard, Palo Alto, CA).
Calculations
The fractional turnover rates of VLDL-TG and of VLDL-apoB-100 were determined by fitting the tracer-to-tracee ratio time courses of free glycerol and leucine in plasma and in VLDL to a compartmental model, and VLDL-TG and VLDL-apoB-100 secretion and clearance rates were calculated as previously described (7). The proportion of fatty acids in VLDL-TG derived from systemic plasma FFA (generated by lipolysis of sc adipose tissue TG) and nonsystemic fatty acids (generated by lipolysis of intrahepatic TG, hepatic lipolysis of circulating TG, and hepatic de novo lipogenesis) was calculated by using a multicompartmental model (7). The molar ratio of VLDL-TG and VLDL-apoB-100 secretion rates was calculated to provide an index of the average TG content of newly secreted VLDL particles (7). Palmitate and total FFA rates of appearance (Ra) in plasma were calculated as previously described (7).
Statistical analysis
All data were tested for normality, and nonnormally distributed variables were log-transformed for analysis and back-transformed for presentation. Differences among groups were assessed by using one-way ANOVA and Fisher's least significant difference post hoc tests. Trend analysis was performed for selected variables of interest to describe a linear or quadratic component of the trend from the S-Hyper to euthyroid to S-Hypo groups. Results are presented as means ± sem or as means and 95% confidence intervals. The relationship between variables was examined by using Pearson's correlation analysis.
Results
No differences were detected among groups in plasma FFA, VLDL-apoB-100, insulin, and glucose concentrations and in body composition (Table 1). Plasma VLDL-TG concentration increased with progressive increases in plasma TSH concentration from S-Hyper to euthyroid to S-Hypo subjects (P value for linear trend = 0.029) and was greater in S-Hypo than in euthyroid (P = 0.050) and S-Hyper women (P = 0.009) (Table 1). No significant differences were detected between groups in total FFA Ra (348 ± 26, 329 ± 24, and 327 ± 36 μmol/min, respectively, P = 0.890) or FFA Ra normalized for fat-free mass (P = 0.880) (Fig. 1A).
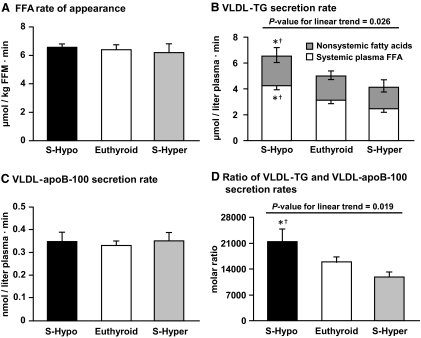
Fig. 1.
A, FFA Ra in plasma in S-Hypo, euthyroid, and S-Hyper women. No significant differences were detected in FFA Ra among groups (P = 0.880). B, Hepatic VLDL-TG secretion rate in S-Hypo, euthyroid, and S-Hyper women. Statistical significance symbols located above the bars refer to total rate of VLDL-TG secretion, and those located inside the bars refer to the secretion rate of VLDL-TG derived from systemic plasma FFA. The total rate of VLDL-TG secretion (P = 0.026) and the absolute secretion rate of VLDL-TG derived from systemic plasma FFA (P = 0.005) were significantly different among groups. C, Hepatic VLDL-apoB-100 secretion rate in S-Hypo, euthyroid, and S-Hyper women. No significant differences were detected in VLDL-apoB-100 secretion rates among groups (P = 0.831). D, Molar ratio of VLDL-TG and VLDL-apoB-100 secretion rates, an index of the average TG content of newly secreted VLDL particles by the liver, in S-Hypo, euthyroid, and S-Hyper women. Molar ratio values were significantly different among groups (P = 0.019). Data are presented as means ± sem. *, Value is significantly different from S-Hyper group at P ≤ 0.05; †, value is significantly different from euthyroid group at P ≤ 0.05.
Total VLDL-TG secretion rate increased from S-Hyper to euthyroid to S-Hypo subjects (P value for linear trend = 0.026) and was greater in S-Hypo than in euthyroid (P = 0.041) and S-Hyper women (P = 0.008) (Fig. 1B). The relative contribution of systemic and nonsystemic fatty acids to total VLDL-TG production did not differ among groups (P = 0.723). Systemic plasma FFA accounted for 68 ± 5, 65 ± 3, and 63 ± 4%, and nonsystemic fatty acids accounted for 32 ± 5, 35 ± 3, and 37 ± 4% of all fatty acids in VLDL-TG in S-Hypo, euthyroid, and S-Hyper women, respectively (Fig. 1B). The absolute secretion rate of VLDL-TG from systemic plasma FFA was greater in S-Hypo than in S-Hyper (P = 0.002) and euthyroid (P = 0.015) women, whereas the absolute secretion rate of VLDL-TG from nonsystemic fatty acids did not differ among groups (P = 0.608) (Fig. 1B). VLDL-TG secretion rate was positively correlated with plasma VLDL-TG concentration (r = 0.507, P < 0.001), whereas VLDL-TG plasma clearance rate was not different among groups (33 ± 6, 32 ± 3, and 39 ± 7 ml/min for S-Hypo, euthyroid, and S-Hyper women, respectively, P = 0.505).
Hepatic VLDL-apoB-100 secretion rate was not different among groups (P = 0.831) (Fig. 1C). Therefore, the molar ratio of VLDL-TG and VLDL-apoB-100 secretion rates decreased from S-Hypo to euthyroid to S-Hyper women (P value for linear trend = 0.019) and was significantly greater in S-Hypo than in euthyroid (P = 0.052) and S-Hyper women (P = 0.005) (Fig. 1D).
Discussion
In this study, we evaluated VLDL kinetics in women with normal thyroid function and subclinical thyroid disease to gain a better understanding of the metabolic mechanisms responsible for the alterations in plasma TG concentrations associated with subclinical thyroid dysfunction. Our data demonstrate that hepatic secretion of VLDL-TG increases progressively from S-Hyper to the euthyroid state to S-Hypo, so that VLDL-TG secretion rate is greater in S-Hypo than in euthyroid and S-Hyper women.
The differences in hepatic VLDL-TG secretion rates among our subject groups are likely an important contributor to the differences observed in plasma VLDL-TG concentrations, because we found a direct correlation between VLDL-TG secretion and plasma concentration and no differences among groups in VLDL-TG plasma clearance rates. The absence of an effect of subclinical thyroid dysfunction on VLDL-TG clearance is consistent with data from a recent study that found S-Hypo is not associated with alterations in total plasma TG fractional catabolic rate (8).
The secretion rate of VLDL-apoB-100 is greater in people who have overt hypothyroidism than those who are euthyroid, because of increased secretion of small VLDL2 particles (9). In contrast, we found subclinical thyroid disease was not associated with alterations in hepatic VLDL-apoB-100 secretion (i.e. number of VLDL particles secreted by the liver). Therefore, the molar ratio of VLDL-TG to VLDL-apoB-100 secretion rates was greater in S-Hypo women than in euthyroid and S-Hyper women, indicating the secretion of large, TG-rich nascent VLDL particles in our S-Hypo subjects.
The effects of thyroid function on in vivo VLDL-TG metabolism in patients with clinically overt thyroid disease have not been clear because of contradictory results among studies, demonstrating that hepatic secretion rate of VLDL-TG is lower (10), greater (11), or not different (12) in hyperthyroid than in euthyroid subjects, and greater or not different in hypothyroid than in euthyroid subjects (11, 12). The reasons for this inconsistency could be related to differences between groups in 1) subject characteristics, such as BMI and sex distribution because obesity and sex have independent effects on VLDL-TG metabolism (13); 2) the method used to measure VLDL-TG kinetics (14); 3) the clinical severity and duration of thyroid disease (15); and 4) the potential confounding influence of therapy for hypo- or hyperthyroidism on VLDL metabolism. To minimize these potential confounding factors, we studied only women who were carefully matched on age and BMI and who were either euthyroid or had subclinical thyroid disease.
We did not detect an effect of subclinical alterations in thyroid function on adipose tissue lipolytic activity (FFA Ra). In contrast, overt hyperthyroidism is associated with increased FFA Ra (16) and plasma FFA concentration (10, 11, 17), whereas overt hypothyroidism is associated with lower (11) or the same (18) lipolytic rates and plasma FFA concentrations compared with euthyroid subjects. Therefore, our data suggest that subclinical thyroid disease is not associated with alterations in adipose tissue lipolytic activity.
In conclusion, hepatic VLDL-TG secretion rate and the average TG content of newly secreted VLDL particles are greater in middle-aged obese women with S-Hypo than in those with S-Hyper. These data help explain the alterations in plasma TG concentrations associated with subclinical thyroid dysfunction.
Acknowledgments
We thank Freida Custodio, Jennifer Shew, and Dr. Adewole Okunade for technical assistance and the study subjects for their participation.
This study was supported by the American Heart Association Grant 0365436Z and National Institutes of Health Grants HD057796, DK 37948, DK 56341 (Nutrition Obesity Research Center), RR 00954 (Biomedical Mass Spectrometry Resource), and UL1 RR024992 (Clinical and Translational Science Award).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- apoB-100
- Apolipoprotein B-100
- BMI
- body mass index
- FFA
- free fatty acid
- Ra
- rate of appearance
- S-Hyper
- subclinical hyperthyroidism
- S-Hypo
- subclinical hypothyroidism
- TG
- triglyceride
- VLDL
- very-low-density lipoprotein.
References
- 1. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ. 2004. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 291:228–238 [DOI] [PubMed] [Google Scholar]
- 2. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. 2000. The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- 3. Duntas LH. 2002. Thyroid disease and lipids. Thyroid 12:287–293 [DOI] [PubMed] [Google Scholar]
- 4. Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, Michelangeli V. 2005. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med 165:2467–2472 [DOI] [PubMed] [Google Scholar]
- 5. Tanaci N, Ertugrul DT, Sahin M, Yucel M, Olcay I, Demirag NG, Gursoy A. 2006. Postprandial lipemia as a risk factor for cardiovascular disease in patients with hypothyroidism. Endocrine 29:451–456 [DOI] [PubMed] [Google Scholar]
- 6. Wahl PW, Warnick GR, Albers JJ, Hoover JJ, Walden CE, Bergelin RO, Ogilvie JT, Hazzard WR, Knopp RH. 1981. Distribution of lipoproteins triglyceride and lipoprotein cholesterol in an adult population by age, sex, and hormone use- The Pacific Northwest Bell Telephone Company health survey. Atherosclerosis 39:111–124 [DOI] [PubMed] [Google Scholar]
- 7. Magkos F, Patterson BW, Mittendorfer B. 2007. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. J Lipid Res 48:1204–1211 [DOI] [PubMed] [Google Scholar]
- 8. Sigal GA, Medeiros-Neto G, Vinagre JC, Diament J, Maranhão RC. 2011. Lipid metabolism in subclinical hypothyroidism: plasma kinetics of triglyceride-rich lipoproteins and lipid transfers to high-density lipoprotein before and after levothyroxine treatment. Thyroid 21:347–353 [DOI] [PubMed] [Google Scholar]
- 9. Packard CJ, Shepherd J, Lindsay GM, Gaw A, Taskinen MR. 1993. Thyroid replacement therapy and its influence on postheparin plasma lipases and apolipoprotein-B metabolism in hypothyroidism. J Clin Endocrinol Metab 76:1209–1216 [DOI] [PubMed] [Google Scholar]
- 10. Sandhofer F, Sailer S, Braunsteiner H. 1966. [Fatty acid and triglyceride metabolism in thyroid gland hyperfunction]. Klin Wochenschr 44:1389–1393 (German) [DOI] [PubMed] [Google Scholar]
- 11. Nikkilä EA, Kekki M. 1972. Plasma triglyceride metabolism in thyroid disease. J Clin Invest 51:2103–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abrams JJ, Grundy SM, Ginsberg H. 1981. Metabolism of plasma triglycerides in hypothyroidism and hyperthyroidism in man. J Lipid Res 22:307–322 [PubMed] [Google Scholar]
- 13. Mittendorfer B, Patterson BW, Klein S. 2003. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am J Clin Nutr 77:573–579 [DOI] [PubMed] [Google Scholar]
- 14. Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. 2002. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res 43:223–233 [PubMed] [Google Scholar]
- 15. Keyes WG, Heimberg M. 1979. Influence of thyroid status on lipid metabolism in the perfused rat liver. J Clin Invest 64:182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elks ML, Manganiello VC. 1985. Effects of thyroid hormone on regulation of lipolysis and adenosine 3′,5′-monophosphate metabolism in 3T3-L1 adipocytes. Endocrinology 117:947–953 [DOI] [PubMed] [Google Scholar]
- 17. Beylot M, Martin C, Laville M, Riou JP, Cohen R, Mornex R. 1991. Lipolytic and ketogenic fluxes in human hyperthyroidism. J Clin Endocrinol Metab 73:42–49 [DOI] [PubMed] [Google Scholar]
- 18. Haluzik M, Nedvidkova J, Bartak V, Dostalova I, Vlcek P, Racek P, Taus M, Svacina S, Alesci S, Pacak K. 2003. Effects of hypo- and hyperthyroidism on noradrenergic activity and glycerol concentrations in human subcutaneous abdominal adipose tissue assessed with microdialysis. J Clin Endocrinol Metab 88:5605–5608 [DOI] [PubMed] [Google Scholar]