Abstract
Context:
Mild abnormalities of thyroid function have been associated with both beneficial and detrimental effects on mortality.
Objective:
Our objective was to determine the association between continuous TSH as well as categories of thyroid function with total and cause-specific mortality in a cohort of older men.
Design, Setting, and Participants:
Data were analyzed from the Osteoporotic Fractures in Men (MrOS) study, a cohort of community-dwelling U.S. men aged 65 yr and older. A total of 1587 participants randomly selected for thyroid function testing were included in this analysis. TSH and free T4 were measured at baseline, and four categories of thyroid function were defined. (subclinical hyperthyroid; euthyroid; subclinical hypothyroid TSH <10 mIU/liter; and subclinical hypothyroid, TSH ≥10 mIU/liter.)
Main Outcome Measure:
Total mortality, cardiovascular (CV) and cancer deaths were confirmed by review of death certificates.
Results:
There were 432 deaths over a mean follow-up of 8.3 yr. In fully adjusted models, there was no association between baseline TSH and any death [relative hazard (RH) = 1.01 per mIU/liter, 95% confidence interval (CI) = 0.95–1.06], CV death (RH = 1.05 per mIU/liter, 95% CI 0.96–1.15), or cancer death (RH = 0.96 per mIU/liter, 95% CI = 0.85–1.07). There was also no statistically significant association between thyroid function category and total or cause-specific mortality, but few men (n = 8) had subclinical hypothyroidism with TSH levels of 10 mIU/liter or higher.
Conclusions:
A single measurement of thyroid function did not predict total or cause-specific mortality in this cohort. These data support neither a beneficial nor a detrimental effect of subclinical thyroid dysfunction in older men.
Summary:
Subclinical thyroid dysfunction is not associated with an increased risk of all-cause or CV mortality in older men.
Although there is little controversy regarding the treatment of overt thyroid failure, the appropriate management of subclinical thyroid dysfunction remains unknown. The association between subclinical thyroid disease and adverse outcomes, including mortality, remains controversial. Subclinical hyperthyroidism [suppressed TSH and normal free T4 (FT4)] has been associated with atrial fibrillation and all-cause and cardiovascular (CV) mortality (1, 2). Several prospective cohort studies have also examined the association between subclinical hypothyroidism (elevated TSH and normal FT4) and mortality with conflicting results (1, 3–5).
The inconsistent results from prospective studies may be due to differences in the age, sex, and other participant characteristics. Studies of older cohorts have found either no association with or decreased risk of mortality with higher TSH, suggesting there may be an age-dependent difference in the relationship between thyroid function and adverse outcomes (1, 5, 6). A recent meta-analysis of over 55,000 participants using subject-level data showed an increased risk of coronary heart disease mortality in individuals with subclinical hypothyroidism and found no interaction with age; however, many of the included studies did not focus on older adults (7).
The relationship between subclinical thyroid dysfunction and mortality may also differ by sex. Some (4), but not all (1, 6), studies have found an increased risk of mortality in men but not women with subclinical hypothyroidism. Despite significant differences in the prevalence of thyroid dysfunction between men and women, the relationship between subclinical thyroid disease and mortality has not been examined in an exclusively male cohort. Although more prevalent in women, subclinical hypothyroidism occurs not infrequently in men, with up to 15% of men over age 60 affected (8, 9).
Using data from the Osteoporotic Fractures in Men (MrOS) study, a large cohort of community-dwelling older men, we sought to determine the relationship between baseline thyroid function and mortality in this population.
Subjects and Methods
Study population
The MrOS study is a prospective cohort of 5994 community-dwelling ambulatory men designed to study healthy aging and fracture risk. Eligible men were at least 65 yr old and able to walk without assistance. Participants were recruited by mailings to the Department of Motor Vehicles and voter registration databases, community and senior newspaper advertisements, and presentations targeted at seniors in the communities surrounding the clinical sites (10). Participants were enrolled from March 2000 to April 2002 at one of six U.S. clinical centers. Details of the MrOS study design and cohort have been previously reported (10, 11). The Institutional Review Board at each clinical center approved the study protocol, and written informed consent was obtained from all participants.
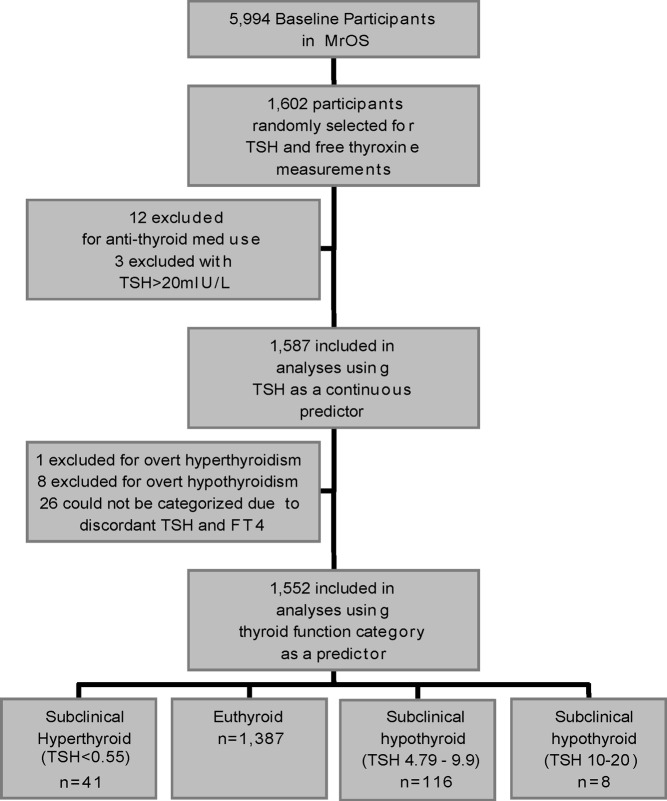
At baseline, serum was collected and archived at −120 C (11). TSH and FT4 were measured in a randomly selected sample of the baseline cohort (n = 1602). This analysis was restricted to MrOS participants with thyroid hormone measurements. Among participants with thyroid function measurements, those taking antithyroid medications (n = 12) were excluded due to the variable effect of these medications on thyroid function tests. Because this analysis was designed to examine the effects of subclinical thyroid dysfunction, three participants with outlying TSH values (>20 mIU/liter) were also excluded (Fig. 1).
Fig. 1.
Participant flow diagram.
Thyroid hormone measurements
TSH was measured using a third-generation assay (Siemens Diagnostics, Deerfield, IL). The reference range for this assay is 0.55–4.78 mIU/liter, and the coefficient of variation at 2.08 mIU/liter is 2.4%. FT4 was measured with a competitive immunoassay (Siemens Diagnostics) in the same participants. The reference range for FT4 by this assay is 0.70–1.85 ng/dl, and the coefficient of variation at 1.09 ng/dl is 4.1%. All 1587 participants with measured TSH values were included in analyses using TSH as a continuous predictor of mortality.
Thyroid function categories
Thyroid function categories were created based on previous literature on adult thyroid function and degrees of subclinical hypothyroidism (12). Four categories were defined: subclinical hyperthyroid (TSH <0.55 mIU/liter, FT4 0.7–1.85 ng/dl), euthyroid (TSH 0.55–4.78 mIU/liter, FT4 0.7–1.85 ng/dl), subclinial hypothyroid with mildly elevated TSH (TSH 4.79–9.9 mIU/liter, FT4 0.7–1.85 ng/dl), and subclinical hypothyroid with markedly elevated TSH (TSH 10–20 mIU/liter, FT4 0.7–1.85 ng/dl). Twenty-six participants were not categorized by thyroid function due to discordance between TSH and FT4 values that suggested possible nonthyroid illness. Most (n = 25) of these participants had normal TSH values and low FT4; one had low TSH and low FT4. These 26 participants were not included in analyses using thyroid function category as a predictor. Because our aim was to study mild thyroid dysfunction rather than overt disease, we also excluded overt hyperthyroidism (TSH <0.55 mIU/liter, FT4 >1.85 ng/dl, n = 1) or overt hypothyroidism (TSH >4.78mIU/liter, FT4 <0.7 ng/dl, n = 8) from our analysis of thyroid function categories. The final cohort for our analyses using thyroid function categories as a predictor of mortality included 1552 participants.
Assessment of outcomes
Total mortality was the primary outcome for this analysis. Participant vital status was adjudicated by a physician at the coordinating center using state death certificates with International Classification of Diseases, Ninth Revision (ICD-9) codes for cause of death, plus additional medical records, if available (8). Secondary outcomes included CV death and cancer death. Deaths were determined through January 2011.
Assessment of covariates
Medical history, race, and smoking status were obtained using a self-administered questionnaire. Participants were asked to report diagnoses previously given by a physician or other healthcare provider, such as hypertension, thyroid disease, heart attack, coronary, or myocardial infarction, cancer, or stroke. During an interview-administered questionnaire, participants were asked to rate their overall health, and this response was subsequently dichotomized to fair or poor health vs. good or excellent health (13). Prescription medication use at baseline was ascertained by a participant-completed log of all medications taken for at least 1 month and confirmed during the clinical interview (11). Medications were classified using a hierarchical drug dictionary based upon the Iowa Drug Information System codes (College of Pharmacy, University of Iowa, Iowa City, IA) (14).
Height was measured using a wall-mounted stadiometer and weight by a balance beam scale. Body mass index (BMI) was calculated as kilograms per meter (2). Systolic blood pressure was measured using an 8-MHz Doppler probe to determine the ankle-arm index (11).
Total, high-density lipoprotein, and low-density lipoprotein cholesterol and triglycerides assays were performed on fasting serum from the baseline clinic visit at the Oregon Veterans Affairs Clinical Lab (Portland, OR) using a Roche Integra 800 automated analyzer (Roche Diagnostics Corp., Indianapolis, IN).
Statistical analysis
Baseline characteristics of the cohort were compared by survival status and by thyroid function category using χ2 tests for categorical variables and t tests for continuous variables. For continuous variables that significantly deviated from a normal distribution, the appropriate nonparametric test was employed. In comparing characteristics between thyroid function categories, the Fisher's exact test was used for categorical variables due to small numbers of participants in some categories. If an overall difference was detected, baseline characteristics were compared between each thyroid function category and euthyroid participants, using an unpaired t test or the Wilcoxon rank sum test for nonparametric data.
Covariates were chosen based on previous literature and biological plausibility. These covariates included several potential confounders: age, race (White vs. non-White), smoking status (current, past, or never), thyroid hormone use, and clinic site. Additional covariates were chosen for their association with the outcome of total mortality: history of non-skin cancer and self-reported health status. Risk factors for death that could be mediated by thyroid dysfunction [hypertension, lipid levels, BMI, coronary heart disease (CHD), stroke, diabetes, and congestive heart failure (CHF)] were excluded from the model to avoid overadjustment.
We used Cox proportional hazards regression models to determine the relative hazard (RH) and 95% confidence interval (CI) of death for every 1 mlU/L increase in TSH. Models were initially adjusted for age, and subsequent multivariate (MV) models were adjusted for age, race, smoking status, thyroid hormone use, history of non-skin cancer, self-reported health status, and clinic site.
We then performed identical Cox proportional hazards models to determine the RH and 95% CI of death for each category of thyroid function (subclinical hyperthyroid, euthyroid, subclinical hypothyroid with TSH <10 mIU/liter, and subclinical hypothyroid with TSH ≥10 mIU/liter) using euthyroid participants as the reference category.
Analyses were performed with FT4 as a continuous predictor using the same models as for TSH and thyroid function categories. All analyses were repeated using CV death and cancer death as secondary outcomes.
Finally, we tested for interaction with age (age as a continuous variable as well as age above and below the median of 73 yr), thyroid hormone use, and prevalent CHD status using a cutoff of P < 0.1 as suggestive of interaction.
To address the possibility of a nonlinear association between TSH and mortality, we plotted the RH of any death, CV death, and cancer death per decile of TSH for visual inspection. These plots did not suggest a U-shaped or J-shaped association, therefore justifying the use of linear models. We tested the proportional hazards assumption using both graphical methods and the Schoenfeld test and found no statistical evidence of violation (P > 0.05 for all).
Results
Baseline characteristics
The mean age of the cohort was 74 yr, and nearly 90% of participants were White. Baseline characteristics, described in Table 1, were generally similar between thyroid function categories, although as expected, thyroid-specific characteristics were significantly different between groups. Participants with subclinical hyperthyroidism had the highest FT4 levels. FT4 levels were significantly higher in those with subclinical hyperthyroidism compared with their euthyroid counterparts, and participants with subclinical hypothyroidism (both those with TSH <10 and ≥10 mIU/liter) had lower FT4 than euthyroid individuals (P < 0.01 for all comparisons). Thyroid hormone use and reported history of hyper- or hypothyroidism also differed significantly between thyroid function categories.
Table 1.
Baseline characteristics by thyroid function category
| Subclinical hyperthyroid (n = 41) | Euthyroid (n = 1387) | Subclinical hypothyroid, TSH <10 (n = 116) | Subclinical hypothyroid, TSH ≥ 10 (n = 8) | P valuea | |
|---|---|---|---|---|---|
| Age (yr) | 74.1 (5.9) | 73.6 (5.8) | 76.4 (6.4)b | 78.1 (7.5) | <0.01 |
| White race | 36 (87.8) | 1244 (89.7) | 106 (91.4) | 8 (100) | 0.8 |
| Smoking status | 0.5 | ||||
| Never | 15 (36.6) | 499 (36) | 48 (41.4) | 5 (62.5) | |
| Past | 26 (63.4) | 835 (60) | 66 (56.9) | 3 (37.5) | |
| Current | 0 | 53 (3.8) | 2 (1.7) | 0 | |
| BMI (kg/m2) | 27.9 (4.4) | 27.4 (3.7) | 27.3 (3.5) | 24.5 (4.0) | 0.12 |
| Systolic BP (mm Hg) | 136.7 (18.7) | 141.8 (19.3) | 143.6 (19.7) | 145.3 (17.9) | 0.25 |
| TSH (mIU/liter) | 0.32 (0.16)b | 2.14 (0.97) | 6.12 (1.25)b | 13.19 (2.46)b | <0.01 |
| FT4 (ng/dl) | 1.16 (0.21)b | 1.00 (0.14) | 0.92 (0.14)b | 0.87 (0.10)b | <0.01 |
| Total cholesterol (mg/dl) | 195.2 (42.7) | 192.9 (32.9) | 190.5 (31.0) | 205.1 (37.4) | 0.61 |
| LDL cholesterol (mg/dl) | 112.9 (36.7) | 114.0 (29.8) | 111.0 (29.1) | 109.1 (35.5) | 0.8 |
| HDL cholesterol (mg/dl) | 55.8 (18.5)b | 49.2 (14.5) | 48.8 (15.4) | 60.5 (19.4) | 0.01 |
| Triglycerides (mg/dl) | 132.2 (66.8) | 148.7 (91.2) | 153.5 (96.2) | 177.5 (232.0) | 0.4 |
| Baseline history of | |||||
| Diabetes | 4 (9.8) | 153 (11.0) | 17 (14.7) | 1 (12.5) | 0.6 |
| CHD | 7 (17.1) | 320 (23.1) | 37 (31.9) | 2 (25.0) | 0.1 |
| Non-skin cancer | 5 (12.2) | 240 (17.3) | 21 (18.1) | 2 (25.0) | 0.7 |
| Stroke | 2 (4.9) | 83 (6.0) | 7 (6.0) | 1 (12.5) | 0.7 |
| CHF | 3 (7.3) | 61 (4.4) | 8 (6.9) | 1 (12.5) | 0.2 |
| Hypertension | 16 (39.0) | 617 (44.5) | 52 (44.8) | 1 (12.5) | 0.3 |
| High thyroid or Graves' disease | 3 (7.3) | 19 (1.4) | 4 (3.5) | 1 (12.5) | <0.01 |
| Low thyroid or hypothyroid | 15 (36.6) | 83 (6.0) | 20 (17.2) | 2 (25.0) | <0.01 |
| Self-reported health (good/excellent) | 36 (87.8) | 1189 (85.8) | 98 (84.5) | 5 (62.5) | 0.3 |
| Thyroid hormone use | 15 (36.6) | 80 (6.0) | 22 (19.8) | 2 (25.0) | <0.01 |
| Statin use | 11 (26.8) | 376 (28.3) | 29 (26.1) | 1 (12.5) | 0.7 |
Results are mean (sd) or n (%). BP, Blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P value for overall χ2, one-way ANOVA, or Kruskall-Wallis test.
P < 0.05 vs. euthyroid using t test or Wilcoxon rank sum test.
During a mean follow-up of 8.3 yr, 432 men died. Deaths were further classified as cancer (n = 134), CV (n = 150), or other (n = 148). Factors associated with death included age, smoking status, and history of diabetes, CHF, CHD, stroke, and non-skin cancer. Participants who died were more likely to have reported thyroid hormone use (n = 49, 11.8 vs. 6.0%, P < 0.01) than those who survived. There was no significant difference in mean TSH or FT4 levels between groups based on vital status (P = 0.2 and P = 0.3, respectively).
When compared with the overall MrOS cohort, participants included in this study were comparable with regard to age, race, smoking status, and self-reported health status (overall MrOS cohort mean age 74 yr, 89% Caucasian/non-Hispanic, 86% reporting good or excellent health) (11).
Thyroid function and total mortality
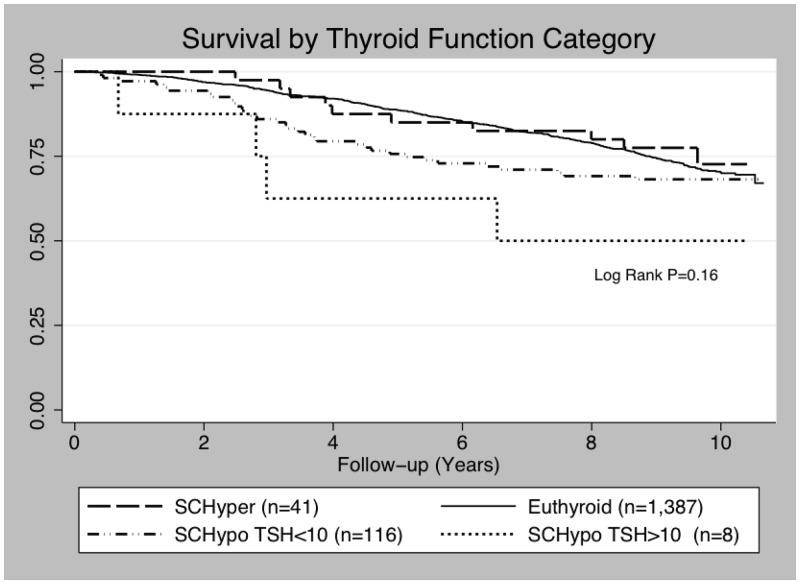
There was no significant association between TSH level analyzed as a continuous variable and risk of death in age-adjusted (RH = 1.00 per mIU/liter increase in TSH, 95% CI 0.95–1.06, P = 0.87) or MV-adjusted models (RH = 1.01 per mIU/liter, 95% CI = 0.95–1.06, P = 0.85) (Table 2). Results were similar after excluding thyroid hormone users (n = 112) (MV-adjusted RH = 0.97 per mIU/liter, 95% CI = 0.91–1.04, P = 0.33). There was also no statistically significant difference in risk of death between thyroid function groups; the RH were suggestive of higher mortality in participants with subclinical hypothyroidism and TSH ≤10 mIU/liter; however, results were based on a small number or participants (n = 8, or n = 6 excluding thyroid hormone users) and CI were wide (MV-adjusted RH = 1.95, 95% CI = 0.72–5.26) (Table 2 and Fig. 2). Further adjustment for self-reported history of high thyroid or Graves' disease did not change results (data not shown).
Table 2.
RH of any death by thyroid function category
| Aged-adjusted RH (95% CI) | MV-adjusted RHa (95% CI) | |
|---|---|---|
| Entire cohort (n = 1587) | ||
| Per 1 mlU/L increase in TSH | 1.00 (0.95–1.06) | 1.01 (0.95–1.06) |
| By thyroid function category | ||
| Subclinical hyperthyroid (n = 41) | 0.87 (0.46–1.62) | 0.78 (0.41–1.48) |
| Euthyroid (n = 1,387) | Referent | Referent |
| SCHypo, TSH <10 (n = 116) | 1.01 (0.71–1.44) | 0.94 (0.65–1.36) |
| SCHypo, TSH ≥10 (n = 8) | 1.78 (0.66–4.78) | 1.95 (0.72–5.26) |
| Excluding thyroid hormone users (n = 1467) | ||
| Per 1 mlU/L increase in TSH | 0.97 (0.91–1.03) | 0.97 (0.91–1.04) |
| By thyroid function category | ||
| Subclinical hyperthyroid (n = 26) | 0.64 (0.26–1.54) | 0.65 (0.27–1.59) |
| Euthyroid (n = 1,248) | Referent | Referent |
| SCHypo, TSH <10 (n = 89) | 0.87 (0.58–1.32) | 0.87 (0.58–1.31) |
| SCHypo, TSH ≥10 (n = 6) | 1.36 (0.34–5.48) | 1.59 (0.40–6.40) |
SCHypo, Subclinical hypothyroid.
Adjusted for age, race, smoking status, clinic site, history of non-skin cancer, and self-reported health status. Models including thyroid hormone users were also adjusted for thyroid hormone use.
Fig. 2.
Kaplan-Meier survival by thyroid function category.
When our analyses were restricted to the 1387 participants with TSH values within the euthyroid range (0.55–4.78 mIU/liter), results still showed no association between TSH level and mortality (MV-adjusted RH = 0.95 per mIU/liter, 95% CI = 0.85–1.06).
Thyroid function and CV death
There was no association between continuous TSH and risk of CV death (MV-adjusted RH = 1.05 per mIU/liter, 95% CI = 0.96–1.15; excluding thyroid hormone users, RH = 1.04 per mIU/liter, 95% CI = 0.95–1.15). Results were suggestive of greater CV death in participants with subclinical hypothyroidism and TSH ≤10 mIU/liter; however, as previously noted, there were very few individuals in this category, and the result approached but did not meet statistical significance (MV-adjusted RH = 3.95, 95% CI = 0.97–16.12) (Table 3).
Table 3.
RH of CV death by thyroid function category
| Aged-adjusted RH (95% CI) | MV-adjusted RHa (95% CI) | |
|---|---|---|
| Entire cohort (n = 1587) | ||
| Per 1 mlU/L increase in TSH | 1.06 (0.97–1.15) | 1.05 (0.96–1.15) |
| By thyroid function category | ||
| Subclinical hyperthyroid (n = 41) | 1.21 (0.49–2.96) | 1.12 (0.45–2.77) |
| Euthyroid (n = 1387) | Referent | Referent |
| SCHypo, TSH <10 (n = 116) | 1.28 (0.77–2.14) | 1.05 (0.60–1.85) |
| SCHypo, TSH ≥10 (n = 8) | 3.32 (0.82–13.45) | 3.95 (0.97–16.12) |
| Excluding thyroid hormone users (n = 1467) | ||
| Per 1 mlU/L increase in TSH | 1.03 (0.94–1.14) | 1.04 (0.95–1.15) |
| By thyroid function category | ||
| Subclinical hyperthyroid (n = 26) | 1.30 (0.48–3.52) | 1.23 (0.45–3.35) |
| Euthyroid (n = 1248) | Referent | Referent |
| SCHypo, TSH <10 (n = 89) | 1.05 (0.58–1.91) | 1.03 (0.57–1.88) |
| SCHypo, TSH ≥10 (n = 6) | 3.32 (0.82–13.49) | 3.79 (0.93–15.49) |
SCHypo, Subclinical hypothyroid.
Adjusted for age, race, smoking status, clinic site, history of non-skin cancer, and self-reported health status. Models including thyroid hormone users were also adjusted for thyroid hormone use.
Thyroid function and cancer death
There was no association between continuous TSH and cancer death (MV-adjusted RH = 0.96 per mIU/liter, 95% CI = 0.85–1.07) or thyroid function category and cancer death [MV-adjusted RH per mIU/liter = 0.80 (0.29–2.23) for subclinical hyperthyroid vs. euthyroid, 0.88 (0.44–1.74) for subclinical hypothyroid TSH <10 mIU/liter vs. euthyroid]. There were no incident cancer deaths in individuals with subclinical hypothyroidism and TSH ≤10 mIU/liter and therefore no RH reported for this category.
Subgroup analyses/testing for interaction
Although testing for interaction suggested an age-dependent effect of TSH level on total mortality (P = 0.04 for interaction using age as a continuous variable, P = 0.1 using age above or below the median of 73 yr), MV-adjusted results stratified by age greater than or less than the median were minimally different [for participants ≥73 yr (n = 866), RH = 1.04 per mIU/liter, 95% CI = 0.98–1.10; for participants <73 yr (n = 721), RH = 0.93 per mIU/liter, 95% CI - 0.80–1.08]. Even among the oldest participants, the RH for any death was only slightly different from that for younger age groups [for highest quartile of age (mean age 82 yr, range 79–99), RH = per mIU/liter 1.07, 95% CI = 1.01–1.15 vs. lowest quartile of age, RH per mIU/liter = 0.94, 95% CI = 0.77–1.15).
There was also evidence to suggest that thyroid hormone use (P = 0.02 for interaction) might modify the effect of TSH on mortality. Although this interaction term was statistically significant, when results were stratified by thyroid hormone use, the MV-adjusted RH were only slightly different [RH = 1.07 per mIU/liter (0.99–1.18) in thyroid hormone users; RH = 0.97 per mIU/liter (0.90–1.04) in nonusers]. There was no evidence of an interaction with prevalent CHD status for total mortality (P = 0.15 for interaction).
We found no evidence of interaction with age, thyroid hormone use, or prevalent CHD status for CV death (Table 4).
Table 4.
Subgroup analyses and testing for interaction
| MV-adjusted RHa (95% CI) per unit increase in TSH |
||||
|---|---|---|---|---|
| All death | P for interaction | CV death | P for interaction | |
| Age | ||||
| <73 yr | 0.93 (0.80–1.08) | 0.04 | 1.15 (0.94–1.41) | 0.59 |
| ≥73 yr | 1.04 (0.98–1.10) | 1.05 (0.95–1.17) | ||
| Prevalent CHD | ||||
| No (n = 1215) | 0.96 (0.90–1.04) | 0.15 | 0.98 (0.85–1.12) | 0.29 |
| Yes (n = 372) | 1.09 (0.99–1.20) | 1.15 (1.00–1.32) | ||
| Thyroid hormone use | ||||
| No (n = 1467) | 0.97 (0.90–1.04) | 0.02 | 1.03 (0.93–1.14) | 0.16 |
| Yes(n = 120) | 1.07 (0.99–1.18) | 1.28 (0.93–1.77) | ||
Adjusted for age, race, smoking status, clinic site, history of non-skin cancer, and self-reported health status. Models including thyroid hormone users also adjusted for thyroid hormone use.
Additional analyses
FT4 and mortality
Analyses using FT4 as a continuous predictor revealed no association with any death, CV death, or cancer death. For example, for any death, the MV-adjusted RH per unit increase in FT4 was 1.09 (95% CI = 0.59–2.00) and for CV death the RH was 1.71 (95% CI = 0.61–4.84). Results were unchanged after exclusion of thyroid hormone users.
Thyroid hormone use and mortality
In univariate analyses, thyroid hormone use was associated with an increased risk of death. After adjustment for TSH level and other potential confounders, thyroid hormone use remained an independent predictor of any death (RH = 1.4, 95% CI = 1.04–1.90) and cancer death (RH = 1.94, 95% CI = 1.17–3.21) but not CV death (RH = 1.01, 95% CI = 0.56–1.81).
Discussion
In this cohort of community-dwelling older men, we found no evidence of an association between TSH or FT4 level and all-cause or cause-specific mortality. There was also no statistically significant difference in risk of all-cause or cause-specific death between categories of thyroid function. Among the four categories of thyroid function, there was a suggestion of higher CV mortality in participants with subclinical hypothyroidism and TSH levels ≤10 mIU/liter; however, there were few participants in this group (n = 8), and the CI was very wide, limiting interpretation of this result. Of note, this is the same category of individuals (subclinical hypothyroid with TSH ≥10 mIU/liter) who, in the recent meta-analysis by Rodondi et al. (7) using subject level data of over 55,000 participants, were found to have an increase in CHD events and CHD mortality. This may be due to a dose-dependent relationship between thyroid hypofunction and adverse CV outcomes.
Our findings are consistent with several other studies of subclinical thyroid dysfunction in the elderly. In particular, the Cardiovascular Health Study and the Health, Aging, and Body Composition Study similarly failed to find increased mortality in participants with subclinical thyroid dysfunction (1, 6). The Leiden 85+ study, in which individuals were enrolled at age 85 and followed for a mean 3.7 yr, found increased survival with higher TSH levels, suggesting a protective effect of subclinical hypothyroidism in the elderly (5). These findings have not been replicated, however, possibly due to the lack of other cohorts including sufficient numbers of participants with advanced age. We did not find evidence of a protective effect of higher TSH levels, although the MrOS cohort is younger than the Leiden study participants (mean age 74 vs. 85 yr). However, even among the oldest quartile of participants in the MrOS cohort, whose mean age (82 yr) was comparable to that of the Leiden study, higher TSH level was not associated with decreased mortality. We also did not observe an increased risk of all-cause or CV mortality in participants with subclinical hyperthyroidism, although this has been reported in other cohorts (2).
There are several proposed mechanisms by which both subclinical hyperthyroidism and subclinical hypothyroidism might cause increased mortality or CV disease. Subclinical hyperthyroidism and increasing FT4 concentration even within the euthyroid range has been associated with an increased incidence of atrial fibrillation (1, 15), which might increase myocardial oxygen demand and precipitate CHD events. Subclinical hypothyroidism has been associated with atherogenic dyslipidemia, hypertension, and impaired endothelial function, all of which may confer increased risk of mortality, and specifically CHD mortality, in the general population (16). In older adults, however, the high prevalence of conventional risk factors may supersede the contribution of any one factor, and therefore, subclinical hypothyroidism may not independently increase risk. Furthermore, lower levels of active thyroid hormone, as indicated by a higher TSH, may be protective in old age by decreasing the metabolic rate (17, 18).
Recently, there has been controversy over what constitutes normal thyroid function, particularly in older adults, and some researchers have advocated for an increase in the upper limit of the normal range for TSH (19). Our findings do not support a significant association between minor abnormalities in thyroid function and mortality and therefore may provide support for a more generous upper limit of normal TSH for older individuals.
In addition to the results of our primary analysis, we also observed a differential effect of TSH by thyroid hormone use status for any death (P = 0.02 for interaction) but not for CV death. In MV-adjusted models, thyroid hormone use was unexpectedly associated with an increased risk of any death and cancer death, even after adjustment for TSH level. One possible explanation for these associations is that thyroid hormone use is a marker of overall poor health, and although we adjusted for self-reported health status, residual confounding may persist. Additionally, this analysis was limited by assessment of thyroid hormone use at baseline only and would not account for medication initiation or cessation during follow-up. These unexpected findings are in contrast with previous studies that reported no difference in mortality between thyroid hormone users and nonusers (20, 21). Importantly, analyses stratified by thyroid hormone use did not reveal a marked difference in RH of death (Table 4), and exclusion of thyroid hormone users did not substantially change our results. Therefore, we adjusted for thyroid hormone use in our MV models.
Our study has several strengths and limitations. One notable strength is that all participants had measured TSH and FT4, which allows for appropriate classification into thyroid function categories. In addition, the MrOS cohort allows us to examine the relationship between thyroid dysfunction and mortality in the unique and important population of community-dwelling older men.
One weakness of our study is that thyroid function was measured only once, despite evidence that abnormal TSH will spontaneously normalize in a substantial proportion (up to 25%) of individuals (22, 23). This would most likely result in nondifferential misclassification and may have biased our results toward the null, because participants categorized as having abnormal thyroid function ultimately became euthyroid. Furthermore, although we were able to define thyroid function categories using both TSH and FT4, there may still be individuals with nonthyroid illness who were mistakenly classified as subclinical hyperthyroid in the setting of a low TSH but normal FT4 level. We addressed this by comparing FT4 values in individuals classified as subclinical hyperthyroid with euthyroid participants and found FT4 to be significantly higher in the subclinical hyperthyroid group. This argues against potential bias due to inclusion of individuals with chronic nonthyroidal illness in the subclinical hyperthyroid category, because these participants would be more likely to have lower (although possibly normal range) FT4 levels. In addition, T3 levels were not measured in this cohort, and therefore, hyperthyroid individuals with isolated T3 toxicosis may not have been identified. However, based on the previously reported low prevalence of T3 toxicosis (<3% of individuals with low TSH and normal FT4) (24), it is unlikely that we misclassified many hyperthyroid individuals as having subclinical hyperthyroidism.
In addition, we were underpowered to examine an association between thyroid function category and death due to the small number of participants with abnormal TSH values in this cohort of community-dwelling men. Finally, our secondary outcomes of cause-specific mortality were based primarily on death certificates. The primary cause of death is sometimes difficult to ascertain in older adults, and documentation may therefore be inaccurate.
In summary, we found no evidence of increased mortality in individuals with subclinical hyper- or hypothyroidism in this cohort of older men. Additional studies should be performed to determine whether the effect of thyroid function on mortality is age dependent and whether treatment of minor thyroid function abnormalities is warranted.
Acknowledgments
Disclosure Summary: A.C.W., S.H., M.S., E.S.L., E.O., and D.C.B. have nothing to disclose. K.E.E., A.R.H., and H.A.F. receive research grants from the NIA and NIAMS. E.B.-C. receives a research grant from the National Institutes of Health (NIH).
The Osteoporotic Fractures in Men (MrOS) Study is supported by NIH funding. Support was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging, the National Center for Research Resources, and the NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Footnotes
- CHD
- Coronary heart disease
- CHF
- congestive heart failure
- CI
- confidence interval
- CV
- cardiovascular
- FT4
- free T4
- MrOS
- Osteoporotic Fractures in Men
- MV
- multivariate
- RH
- relative hazard.
References
- 1. Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. 2006. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. 2001. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 358:861–865 [DOI] [PubMed] [Google Scholar]
- 3. Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, Michelangeli V. 2005. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med 165:2467–2472 [DOI] [PubMed] [Google Scholar]
- 4. Imaizumi M, Akahoshi M, Ichimaru S, Nakashima E, Hida A, Soda M, Usa T, Ashizawa K, Yokoyama N, Maeda R, Nagataki S, Eguchi K. 2004. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab 89:3365–3370 [DOI] [PubMed] [Google Scholar]
- 5. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. 2004. Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]
- 6. Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. 2005. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 165:2460–2466 [DOI] [PubMed] [Google Scholar]
- 7. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J. 2010. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JS, Buzková P, Fink HA, Vu J, Carbone L, Chen Z, Cauley J, Bauer DC, Cappola AR, Robbins J. 2010. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 170:1876–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biondi B, Cooper DS. 2008. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 29:76–131 [DOI] [PubMed] [Google Scholar]
- 10. Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. 2005. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26:557–568 [DOI] [PubMed] [Google Scholar]
- 11. Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. 2005. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study: a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585 [DOI] [PubMed] [Google Scholar]
- 12. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ. 2004. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 291:228–238 [DOI] [PubMed] [Google Scholar]
- 13. Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR. 2007. High-trauma fractures and low bone mineral density in older women and men. JAMA 298:2381–2388 [DOI] [PubMed] [Google Scholar]
- 14. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. 1994. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10:405–411 [DOI] [PubMed] [Google Scholar]
- 15. Gammage MD, Parle JV, Holder RL, Roberts LM, Hobbs FD, Wilson S, Sheppard MC, Franklyn JA. 2007. Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med 167:928–934 [DOI] [PubMed] [Google Scholar]
- 16. Duntas LH, Wartofsky L. 2007. Cardiovascular risk and subclinical hypothyroidism: focus on lipids and new emerging risk factors. What is the evidence? Thyroid 17:1075–1084 [DOI] [PubMed] [Google Scholar]
- 17. Kim B. 2008. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid 18:141–144 [DOI] [PubMed] [Google Scholar]
- 18. Jumpertz R, Hanson RL, Sievers ML, Bennett PH, Nelson RG, Krakoff J. 2011. Higher energy expenditure in humans predicts natural mortality. J Clin Endocrinol Metab 96:E972–E976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Surks MI, Hollowell JG. 2007. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92:4575–4582 [DOI] [PubMed] [Google Scholar]
- 20. Bauer DC, Rodondi N, Stone KL, Hillier TA. 2007. Thyroid hormone use, hyperthyroidism and mortality in older women. Am J Med 120:343–349 [DOI] [PubMed] [Google Scholar]
- 21. Petersen K, Bengtsson C, Lapidus L, Lindstedt G, Nyström E. 1990. Morbidity, mortality, and quality of life for patients treated with levothyroxine. Arch Intern Med 150:2077–2081 [PubMed] [Google Scholar]
- 22. Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, Braverman LE. 2002. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 87:3221–3226 [DOI] [PubMed] [Google Scholar]
- 23. Díez JJ, Iglesias P. 2004. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab 89:4890–4897 [DOI] [PubMed] [Google Scholar]
- 24. Figge J, Leinung M, Goodman AD, Izquierdo R, Mydosh T, Gates S, Line B, Lee DW. 1994. The clinical evaluation of patients with subclinical hyperthyroidism and free triiodothyronine (free T3) toxicosis. Am J Med 96:229–234 [DOI] [PubMed] [Google Scholar]