Abstract
Context:
Endometriosis is characterized by progesterone resistance and associated with infertility. Krüppel-like factor 9 (KLF9) is a progesterone receptor (PGR)-interacting protein, and mice null for Klf9 are subfertile. Whether loss of KLF9 expression contributes to progesterone resistance of eutopic endometrium of women with endometriosis is unknown.
Objective:
The aims were to investigate 1) KLF9 expression in eutopic endometrium of women with and without endometriosis, 2) effects of attenuated KLF9 expression on WNT-signaling component expression and on WNT inhibitor Dickkopf-1 promoter activity in human endometrial stromal cells (HESC), and 3) PGR and KLF9 coregulation of the stromal transcriptome network.
Methods:
Transcript levels of KLF9, PGR, and WNT signaling components were measured in eutopic endometrium of women with and without endometriosis. Transcript and protein levels of WNT signaling components in HESC transfected with KLF9 and/or PGR small interfering RNA were analyzed by quantitative RT-PCR and Western blot. KLF9 and PGR coregulation of Dickkopf-1 promoter activity was evaluated using human Dickkopf-1-luciferase promoter/reporter constructs and by chromatin immunoprecipitation. KLF9 and PGR signaling networks were analyzed by gene expression array profiling.
Results:
Eutopic endometrium from women with endometriosis had reduced expression of KLF9 mRNA together with those of PGR-B, WNT4, WNT2, and DKK1. KLF9 and PGR were recruited to the DKK1 promoter and modified each other's transactivity. In HESC, KLF9 and PGR coregulated components of the WNT, cytokine, and IGF gene networks that are implicated in endometriosis and infertility.
Conclusion:
Loss of KLF9 coregulation of endometrial stromal PGR-responsive gene networks may underlie progesterone resistance in endometriosis.
Endometriosis is an estrogen-dependent disorder commonly associated with infertility in reproductive-aged women (1). Of the 6–10% of women affected with endometriosis, 35–50% are found to be infertile (2). Defective implantation is considered to be an underlying cause of endometriosis-related infertility in women (3) and mouse models (4). Patients undergoing in vitro fertilization and diagnosed with endometriosis have poor pregnancy outcomes (5, 6). Furthermore, patients with endometriosis showed higher rates of pregnancy loss and pregnancy-associated complications (7, 8). Nonetheless, a definitive mechanistic association between endometriosis and infertility remains lacking.
Progesterone (P) resistance is considered to underlie endometriosis (1). Genes affected by P are dysregulated during the window of uterine receptivity for embryo implantation in eutopic endometrium of women with endometriosis (9, 10). Regulators of P receptor (PGR) expression and transactivation constitute major determinants of successful implantation and pregnancy (11). Thus, the loss of PGR activity due to reduced PGR (12, 13) and/or inappropriate PGR coactivator (14, 15) expression, cumulatively leading to deregulated downstream effector signaling (9, 10, 14, 16), may link endometriosis with endometriosis-associated infertility.
Dissecting the mechanisms by which PGR regulates gene networks for establishment of a successful pregnancy is complicated by the presence of a large repertoire of PGR coregulator proteins that function under distinct contexts (17). Accordingly, a systematic evaluation of the functional consequence of individual PGR coregulators under physiological and pathophysiological conditions is warranted to delineate their coordinate, opposing, and compensatory functions in PGR-mediated responses. In the present study, we examined the contribution of the transcription factor Krüppel-like factor 9 (KLF9) to the PGR network in human endometrial stromal cells and how it may be associated with the pathological condition of endometriosis. KLF9 is likely participatory to PGR function linking endometriosis and endometriosis-associated infertility, given our previous findings that KLF9 is a PGR-B-interacting protein (18); loss of KLF9 expression in mice leads to subfertility, P-resistance, and uterine hypoplasia (19); and in human endometrial stromal cells, premature expression of a key decidualizing factor, bone morphogenetic protein 2, leading to compromised stromal function is a consequence of deregulated KLF9 activity (20).
Materials and Methods
Tissues
Endometrial tissue samples were obtained from women without (control) and with diagnosed endometriosis undergoing endometrial biopsy, following protocols approved by the University of California San Francisco Committee on Human Research (14, 15). Participants (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org) were documented to be nonpregnant and not to have undergone hormone treatments for at least 3 months before surgery.
Cell culture and treatments
The human endometrial stromal cell (HESC) line was treated with a cocktail of 8-bromo-cAMP (0.5 mm), 1 μm progestin [medroxyprogesterone acetate (MPA)] and 10 nm estradiol-17β (E2) (Sigma-Aldrich, St. Louis, MO), henceforth designated 8-bromo-cAMP+MPA+E2 (cAME) (20).
RNA isolation and analyses
Total RNA, isolated using RNeasy Plus minikit (QIAGEN, Valencia, CA), was reverse transcribed (iScript; Bio-Rad Laboratories, Hercules, CA) and used for SYBR green-based real-time quantitative PCR (QPCR) (20). Primer sequences are provided in Supplemental Table 2.
Western blot analyses
Nuclear and cytoplasmic proteins were resolved by SDS-PAGE. Proteins were incubated with rabbit antirat KLF9 (18), monoclonal goat antihuman Dickkopf-1 (DKK1) (R&D Systems, Minneapolis, MN), and mouse antihuman PGR (Pgr-1294; Dako, Carpinteria, CA). Protein-antibody complexes were detected as described (20). Lamin A and β-actin were used as normalizing controls (20).
RNA interference
Transfection was performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) in OPTI-MEM reduced serum-containing medium (20). Small interfering RNA (siRNA) targeting human KLF9 and PGR (siGeNOME SMART pool) and nontargeting (siCONTROL) siRNA (Dharmacon, Waltham, MA) were used at 50 nm concentration. When appropriate, final siRNA concentrations were made up to 100 nm with the addition of nontargeting siRNA. The transfected groups and treatments were 1) nontargeting siRNA plus vehicle alone (vehicle), 2) nontargeting siRNA plus cAME (NT); 3) KLF9 siRNA plus 0.5cAME (siKLF9), 4) PGR siRNA plus 0.5cAME (siPGR), and 5) KLF9 plus PGR siRNA plus 0.5cAME (siKP). Six hours after transfection, cells were washed, incubated for 24 h in phenol red-free DMEM-F12 containing 2% charcoal-stripped bovine calf serum, and then exposed for 48 h in medium containing 0.5cAME or vehicle. Collected cells were subjected to Western blot or RNA expression analyses.
DKK1 promoter-driven luciferase assay
The DKK1 promoter and 5′-flanking regions were amplified (by PCR) from human genomic DNA and subcloned into the NheI and Xho sites of pGL3-basic vector containing the luciferase reporter gene (Promega, Madison, WI). Reporter constructs and pGL3-basic (0.5 μg per well) were transiently transfected with appropriate siRNA using Lipofectamine 2000 in OPTI-MEM. Six hours after transfection, cells were washed, transferred to phenol red-free DMEM-F12 medium containing 2% charcoal-stripped bovine calf serum and after 24 h, treated with 0.5cAME. Cells harvested after 48 h were processed for luciferase activity (luciferase reagent kit; Promega).
Chromatin immunoprecipitation (ChIP)
Cells were processed for ChIP using the ChIP-IT Express Kit (Activ Motif, Carlsbad, CA). Chromatin was immunoprecipitated with goat antihuman KLF9 polyclonal antibody (sc-12994; Santa Cruz Biotechnology, Santa Cruz, CA), mouse antihuman PGR clone 636 monoclonal antibody (M3569; Dako), or goat antirat specificity protein 1 (SP1) polyclonal antibody (sc-59G; Santa Cruz). Preimmune control antibodies were normal goat IgG (sc-2028; Santa Cruz) or normal mouse IgG (X0931; Dako). Antibody-bound protein-DNA complexes were recovered using protein G-coated magnetic beads, and the final DNA were analyzed by PCR. The oligonucleotide primers used for amplification of the proximal [−190 to −12 nucleotides (nt)] and distal (−1850 to −1250 nt) regions of the DKK1 promoter were as follows: proximal, 5′-CCAGCC GAGCGACTAAGCAA-3′ and 5′-ACCGCGGCTGCCTTTATACC-3′ (forward and reverse, 179 bp), and distal, 5′-TGGAATTTGGGATGGGAAGGACAC-3′ and 5′-CTGCCCTCTGGGTTGTTACCTTAT-3′ (forward and reverse, 601 bp).
Gene expression profiling with microarrays
Gene expression analyses used Human Genome U133 Plus version 2.0 high-density oligonucleotide arrays (Affymetrix Inc., Santa Clara, CA), with 54,120 probe sets to interrogate 38,500 well-characterized human transcripts. cRNA preparation, hybridization, washes, and detection followed the manufacturer's recommendations. Samples were HESC from the five treatment groups described under RNA interference. RNA were prepared using GeneChip 3′ IVT Express Kit (Affymetrix). RNA pooled from triplicate samples per treatment group constituted one biological replicate and were hybridized to an array; two independent replicates were evaluated per treatment group. Expression data were analyzed using GeneSpring GX version 11 software (Agilent Technologies, Santa Clara, CA). The .CEL files containing probe level intensities were processed using the GeneChip robust multiarray analysis (GC-RMA) with quantile normalization. The normalized data were subjected to pairwise comparisons: 1) NT vs. vehicle, 2) siKLF9 vs. NT, 3) siPGR vs. NT, and 4) siKP vs. NT. Gene sets were subjected to genome set enrichment analyses (21) and biological function and ontology analyses using Affymetrix NetAffx and GeneSpring, and genes with at least 1.5-fold change and P ≤ 0.05 were analyzed for functional gene annotations using Ingenuity pathway analysis (IPA; Ingenuity Systems, Redwood City, CA). Data discussed herein were deposited in Gene Expression Omnibus (GSE31683).
Statistical analysis
Data (mean ± sem) were analyzed by one-way ANOVA or two-tailed Student's t test using SigmaStat version 3.5 software (SPSS, Chicago, IL). P ≤ 0.05 was considered significant.
Results
Aberrant WNT signaling and deregulated KLF9 expression in eutopic endometrium of women with endometriosis
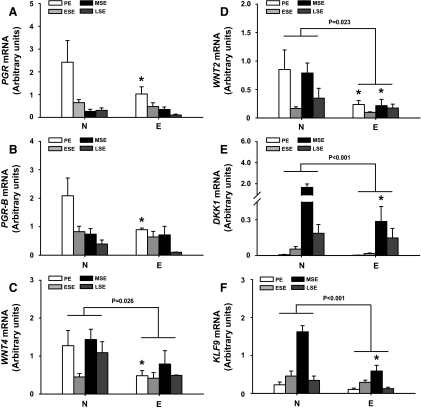
Eutopic endometria from women with (E) and without (N) endometriosis at different menstrual cycle phases [proliferative endometrium (PE), early secretory endometrium, midsecretory endometrium (MSE), and late secretory endometrium] were evaluated for expression of KLF9 and other genes functionally relevant to endometriosis and endometriosis-associated infertility (1, 9, 14), by QPCR. Levels of total PGR, PGR-B, WNT4, WNT2, DKK1, and KLF9 transcripts were reduced in eutopic endometria of women with vs. without endometriosis (Fig. 1). Reductions in PGR, PGR-B, and WNT4 transcripts for PE (Fig. 1, A–C) and of DKK1 and KLF9 transcripts for MSE (Fig. 1, E and F) were significant for women with vs. without endometriosis. Similarly, women with endometriosis had lower (P < 0.05) WNT2 expression in PE and MSE than women without disease (Fig. 1D).
Fig. 1.
Differentially expressed genes in eutopic endometrium of women with endometriosis. Transcript levels of PGR (panel A), PGR-B (panel B), WNT4 (panel C), WNT2 (panel D), DKK1 (panel E), and KLF9 (panel F) in eutopic endometrium of patients with (E) and without (N) endometriosis at different phases of the menstrual cycle were quantified by QPCR and normalized to that of 18S RNA. Each bar represents the mean ± sem of at least four samples per type per menstrual phase: PE, early secretory endometrium (ESE), MSE, and late-secretory endometrium (LSE). Significant differences (P < 0.05) between N and E were determined using two-way ANOVA followed by Tukey's test. *, P < 0.05 between N and E for each menstrual cycle phase by Student's t test.
KLF9 participates in PGR regulation of WNT signaling components in endometrial stromal cells
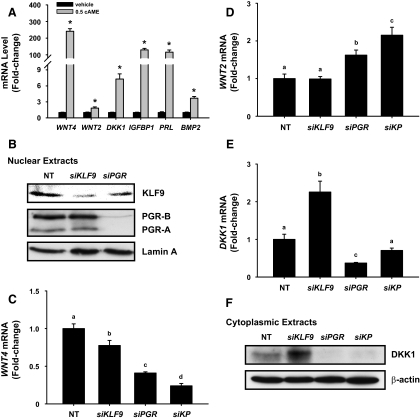
Because KLF9 is predominantly expressed in endometrial stromal cells (19), we used HESC treated with cAME for 48 h to investigate KLF9 and PGR regulation of WNT signaling under conditions that mimicked the uterine receptive phase (MSE). cAME-treated cells showed increased transcript levels for IGFBP1 (130-fold), PRL (116-fold), and BMP2 (4-fold) relative to untreated cells. The WNT components WNT4, WNT2, and DKK1 were highly up-regulated by cAME, with WNT4 showing the greatest response (Fig. 2A).
Fig. 2.
Effects of KLF9 and PGR on expression of WNT signaling components in human endometrial stromal cells. A, Expression levels of genes in decidualizing HESC. Control (vehicle) and cAME-treated cells (2 d) were analyzed for gene expression by QPCR. Values (n = 6 wells per treatment group) were normalized to 18S and renormalized to vehicle-treated group. Transcript levels (mean ± sem) are expressed as fold change. *, P < 0.05 between vehicle- and cAME-treated groups using Student's t test. B, Representative Western blots of nuclear KLF9 and PGR protein levels from HESC treated with cAME for 2 d in the presence of nontargeting siRNA (NT), KLF9 siRNA (siKLF9) and PGR siRNA (siPGR). Lamin A served as loading control. C–E, Transcript levels (mean ± sem) of WNT4, WNT2, and DKK1 were analyzed by QPCR, normalized to 18S, and renormalized to the NT group. Values with different letters are significantly different at P < 0.05 by one-way ANOVA. F, Representative Western blots of cytoplasmic DKK1 protein from HESC treated with cAME for 2 d in the presence of nontargeting RNA (NT), KLF9 siRNA (siKLF9), PGR siRNA (siPGR), or si(KLF9+PGR) (siKP). β-Actin served as loading control.
To evaluate whether loss of KLF9 in the background of P resistance, conditions found in eutopic endometrium of women with endometriosis (Fig. 1), contributes to deregulated WNT signaling associated with endometriosis and infertility (9, 22), we used siRNA to decrease KLF9 and PGR expression before cAME treatment. siRNA to KLF9 and PGR reduced their respective transcript levels by more than 75%, relative to control siRNA (data not shown). Nuclear KLF9 protein levels were reduced to a comparable extent with siKLF9 (Fig. 2B) without affecting PGR isoform levels. With PGR siRNA, nuclear PGR-A and PGR-B levels were drastically decreased, whereas KLF9 levels showed a modest reduction (Fig. 2B).
Knockdowns of KLF9, PGR, and KLF9+PGR (KP) progressively reduced WNT4 transcript levels in cAME-treated HESC (Fig. 2C). WNT2 transcript levels were unchanged and increased, respectively, with knockdown of KLF9 and PGR, whereas coaddition of both siRNA augmented the increase above that of siPGR (Fig. 2D). DKK1 transcript levels were increased and decreased, respectively, with siRNA to KLF9 and to PGR (Fig. 2E); these changes were mirrored by cytoplasmic DKK1 protein (Fig. 2F). KP co-knockdown led to total loss of DKK1 protein, similar to that found with siPGR (Fig. 2, E and F).
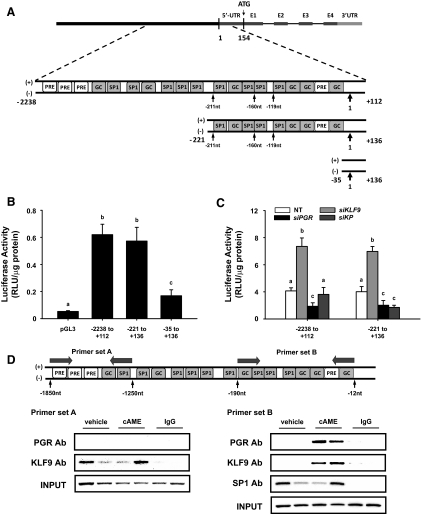
KLF9 and PGR regulate DKK1 promoter activity in endometrial stromal cells
Because DKK1 and KLF9 transcript levels are coincidentally reduced in MSE of women with vs. without endometriosis, when PGR levels are already low (Fig. 1) and because HESC had drastically reduced DKK1 expression in KP vs. KLF9 knockdowns, we examined whether loss of KLF9+PGR regulation of DKK1 promoter activity underlies attenuated DKK1 expression in endometriosis. A region of the human DKK1 promoter (−2238/+136 nt) containing multiple GC-rich, SP1/KLF binding sites (23) and several half-P response element/glucocorticoid response element (PRE/GRE) sites (24) (Fig. 3A) was evaluated for activity by transfection of promoter-reporter constructs containing the entire (−2238/+112 nt) or truncated (−221/+136 nt; −35/+136 nt) regions (translation initiation site ATG, +1). The −2238/+112 and −221/+136 DKK1-Luc constructs demonstrated comparable luciferase activities, indicating DKK1 promoter activity to mostly reside between −221 to +112 nt (Fig. 3B). To determine which sequences within the DKK1 promoter region are KLF9 and/or PGR responsive, HESC cotransfected with KLF9, PGR, or KP siRNA and the longer and shorter DKK1-Luc constructs were evaluated for reporter activity. KLF9 siRNA increased, whereas PGR siRNA decreased the activities of both promoter/reporter constructs to similar extents (Fig. 3C).
Fig. 3.
KLF9 and PGR regulate DKK1 gene promoter activity and are recruited to the DKK1 promoter region. A, Schematic representation of human DKK1 promoter region showing the locations of the generated promoter-reporter constructs: −2238 to +112, −221 to +136, and −35 to +136; 1 refers to the translation initiation site. B, HESC cotransfected with 0.5 μg promoter-reporter plasmids or pGL3-basic (negative control) were treated with cAME. Luciferase activity of cell lysates (mean ± sem from two independent experiments, n = 4 per group per experiment) is expressed as relative luminescence units (RLU) normalized to the protein content. Significant differences among groups were identified by one-way ANOVA. Means with different letters differed at P < 0.05. C, Luciferase activity of the two promoter-reporter constructs (−2238/+112 and −221/+136) containing regions of the DKK1 promoter were cotransfected in HESC in the presence or absence of siRNA for KLF9, PGR, or siKLF9+PGR (siKP) and then treated with cAME. Luciferase activities were expressed as relative luminescence units (RLU) normalized to protein content. Significant differences were identified by one-way ANOVA, followed by Tukey's test. Means with different letters differed at P < 0.05. D, Map representation (not drawn to scale) of the DKK1 promoter region containing GC-rich (GGGAGG, CCTCCC), SP1 (GGGCGG, CACCC, GGGTG) and possible half-PRE/GRE (TGTTGT, TGTTTT, AGAACA) sites amplified by primer sets A and B (denoted by arrows, → ←). ChIP assays were performed with chromatin prepared from vehicle and cAME-treated HESC using anti-KLF9, anti-PGR, and anti-SP1 antibodies. Precipitated DNA was analyzed by PCR. Representative gels using primer set A (left) and primer set B (right) from one of three independent experiments using cells of similar passages are shown. Preimmune IgG was evaluated similar to the other antibodies and served as negative control.
KLF9 and PGR are recruited to the DKK1 promoter
To evaluate the molecular basis for KLF9 and PGR regulation of DKK1, we examined the recruitment of both transcription factors to the DKK1 promoter region using ChIP assay. Chromatin isolated from vehicle and cAME-treated HESC was precipitated using KLF9 or PGR antibodies, and precipitated DNA was amplified using primers flanking two regions of the DKK1 promoter. Primer set A (−1850/−1250 nt) amplified a region containing two GC/SP1 and three possible half-PRE/GRE sites, whereas primer set B (−190/−12 nt) amplified a region containing seven GC/SP1 sites and one half-PRE/GRE site (Fig. 3D). Amplification of the precipitated DNA yielded the expected product sizes of 601 and 179 bp, respectively (data not shown). The region amplified by primer set A showed KLF9 but undetectable PGR binding in vehicle- and cAME-treated HESC (Fig. 3D, left). The region amplified by primer set B bound both KLF9 and PGR in cAME- but not in vehicle-treated cells (Fig. 3D, right). SP1 ubiquitously bound to the latter site, irrespective of cAME treatment. Preimmune sera did not precipitate the DKK1 promoter regions containing KLF9 or PGR binding sites.
Microarray analyses
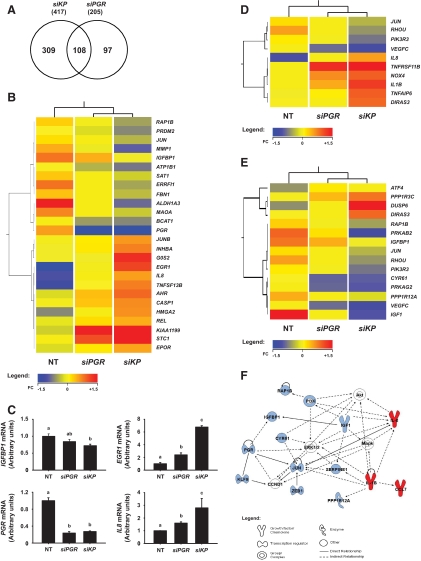
Gene expression profiling was conducted in decidualizing HESC, perturbed by KLF9 and PGR knockdowns, to identify additional KLF9 and PGR coregulated genes. Samples for the microarray analyses were those used for the QPCR analyses (Fig. 2, C–E), and non-cAME-treated HESC transfected with NT siRNA, added as a control group. Unsupervised hierarchical clustering of expression profiles indicated that cAME-treated HESC grouped together and separately from the non-cAME-treated group (data not shown). A total of 1740 annotated genes were significantly regulated by at least 1.5-fold with cAME treatment; the top 100 genes (50 up- and 50 down-regulated) are presented in Supplemental Table 3. The list included genes reportedly regulated in decidualizing stromal cells (25), such as somatostatin (SST), IGF-binding protein 1 (IGFBP1), IGF1, WNT4, prolactin (PRL), and forkhead box O1 (FOXO1). Of the cAME-regulated genes, expression of 34 (2.0%), including IGFBP1, was perturbed with KLF9 knockdown (Supplemental Table 3). With siPGR, expression of 109 (6.3%) genes were modified; these included IGF1, IL8, monoamine oxidase (MAOA), and RAR-related orphan receptor B (RORB).
Analyses of common and unique genes perturbed by siPGR and by siKP in decidualizing HESC (Fig. 4A) indicated that more genes were uniquely regulated by siKP than by siPGR alone (309 vs. 97). Table 1 lists siKP up- and down-regulated genes whose expression were either unaffected by siPGR or affected by greater than 25% over that of siPGR alone. Genes unaffected by siPGR but that were either up- or down-regulated with siKP included epiregulin (EREG), IL-1β (IL1B), dual-specificity phosphatase 10 (DUSP10), matrix metallopeptidase I (MMP1), caspase 1 (CASP1), KLF4, and tissue inhibitor of metalloproteinase 3 (TIMP3). Genes whose expression was affected by siPGR and additionally affected by siKP included early growth response (EGR1), stanniocalcin 1 (STC1), amphiregulin (AREG), and DUSP6.
Fig. 4.
Expression profiling of genes in HESC with PGR knockdown and with KLF9+PGR co-knockdowns. A, Venn diagram showing shared and unique genes in cAME-treated HESC between the pair-wise comparisons: 1) si(KLF9+PGR) (siKP) vs. nontargeting siRNA (NT) and 2) siPGR alone vs. NT. Only genes differing by at least 1.5-fold (P < 0.05) are included in the analyses. B, Heat map representation of transcripts perturbed by siKP that were previously reported to be deregulated in patients with endometriosis. Red indicates up-regulated; blue, down-regulated; and yellow, unchanged. C, QPCR analyses of representative genes differentially regulated by PGR (siPGR) and KLF9+PGR (siKP). D and E, Heat map representation of several transcripts associated with IL-6/IL-8/IL-17 (D) and IGF/ERK/MAPK (E) signaling pathways and whose expression levels are distinctly deregulated by siKP relative to siPGR. F, Molecular network from pathway analyses of dataset indicating perturbations in expression of molecules involved in cellular growth, proliferation, and apoptosis with loss of functional KLF9+PGR interactions. Red indicates up-regulated; blue, down-regulated; and white, other genes identified as part of the gene network.
Table 1.
List of selected up- and down-regulated genes in cAME-treated HESC with knockdown of PGR ± KLF9
| Gene symbol | Gene name | Fold change |
hESFendo
vs. hESFnonendo |
||
|---|---|---|---|---|---|
| siKP vs. NT | siPGR vs. NT | EPa,c | cAMPb,c | ||
| Up-regulated | |||||
| INHBE | Inhibin, β E | 13.01 | 18.59 | ||
| CNIH3 | Cornichon homolog 3 (Drosophila) | 9.40 | 5.89 | ↑ | |
| KIAA1199 | KIAA1199 | 8.52 | 3.31 | ||
| EGR1 | Early growth response 1 | 7.85 | 3.55 | ||
| C2orf88 | Chromosome 2 open reading frame 88 | 6.27 | 3.05 | ||
| TMEM100 | Transmembrane protein 100 | 6.20 | 2.05 | ||
| STC1 | Stanniocalcin 1 | 5.97 | 2.09 | ↑ | |
| PTHLH | PTH-like hormone | 5.49 | 3.70 | ↓ | |
| RXFP1 | Relaxin/insulin-like family peptide receptor 1 | 4.74 | 3.86 | ↓ | ↓ |
| DUSP6 | Dual-specificity phosphatase 6 | 4.56 | 1.56 | ↑ | |
| TNFRSF11B | TNF receptor superfamily, member 11b | 4.04 | 2.49 | ||
| TNFSF13B | TNF (ligand) superfamily, member 13b | 4.03 | 2.44 | ||
| IER3 | Immediate early response 3 | 3.84 | 2.08 | ↑ | |
| LOC100131897 | Uncharacterized protein LOC100131897 | 3.76 | 2.05 | ||
| AREG | Amphiregulin | 3.71 | 2.31 | ↑ | |
| RGS18 | Regulator of G-protein signaling 18 | 3.65 | 2.40 | ||
| FAM84A | Family with sequence similarity 84, member A | 3.56 | 2.08 | ||
| ETV1 | ETS variant 1 | 3.45 | 2.10 | ||
| FJX1 | Four jointed box 1 (Drosophila) | 3.37 | ↑ | ||
| HK2 | Hexokinase 2 | 3.27 | 2.06 | ↑ | |
| FAM20A | Family with sequence similarity 20, member A | 3.14 | 2.27 | ↓ | |
| IL8 | IL-8 | 3.11 | 2.11 | ↓ | |
| SERPINB2 | Serpin peptidase inhibitor, clade B (ovalbumin), member 2 | 3.08 | 1.62 | ||
| SHC4 | SHC (Src homology 2 domain containing) family, member 4 | 3.02 | 2.28 | ↑ | |
| TMSB15A | Thymosin β 15a | 3.01 | 2.28 | ||
| TNFSF10 | TNF (ligand) superfamily, member 10 | 2.98 | 2.26 | ↓ | |
| FABP5 | Fatty acid binding protein 5 (psoriasis-associated) | 2.95 | 1.93 | ↓ | |
| GEM | GTP binding protein overexpressed in skeletal muscle | 2.90 | 2.24 | ||
| ETV5 | ETS variant 5 | 2.79 | 1.58 | ↑ | |
| KYNU | Kynureninase (l-kynurenine hydrolase) | 2.77 | ↓ | ||
| SPRY1 | Sprouty homolog 1, antagonist of FGF signaling (Drosophila) | 2.73 | |||
| OAS1 | 2′,5′-oligoadenylate synthetase 1, 40/46 kDa | 2.73 | 1.92 | ↓ | |
| KCNE4 | Potassium voltage-gated channel, Isk-related family, member 4 | 2.69 | 2.00 | ↓ | |
| VWA5A | von Willebrand factor A domain containing 5A | 2.67 | |||
| IFIT2 | Interferon-induced protein with tetratricopeptide repeats 2 | 2.60 | 2.08 | ↓ | |
| CCL7 | Chemokine (C-C motif) ligand 7 | 2.59 | 1.60 | ||
| G0S2 | G0/G1switch 2 | 2.59 | |||
| ARSG | Arylsulfatase G | 2.58 | 1.70 | ||
| KRTAP1–5 | Keratin-associated protein 1–5 | 2.55 | ↑ | ||
| HMGA2 | High-mobility group AT-hook 2 | 2.53 | 1.61 | ||
| GDF15 | Growth differentiation factor 15 | 2.52 | 1.77 | ↑ | |
| RGL1 | RAL guanine nucleotide dissociation stimulator-like 1 | 2.48 | 1.99 | ↑ | |
| TMEM158 | Transmembrane protein 158 | 2.45 | |||
| EREG | Epiregulin | 2.44 | |||
| KCND2 | Potassium voltage-gated channel, Shal-related subfamily, member 2 | 2.41 | 1.60 | ||
| IL1B | IL-1, β | 2.33 | ↓ | ||
| PPFIBP2 | PTPRF interacting protein, binding protein 2 (liprin β 2) | 2.31 | 1.50 | ||
| NR4A2 | Nuclear receptor subfamily 4, group A, member 2 | 2.29 | 1.55 | ↓ | |
| SLC22A4 | Solute carrier family 22 (organic cation/ergothioneine transporter), member 4 | 2.29 | 1.60 | ||
| TSPAN13 | Tetraspanin 13 | 2.26 | 1.68 | ||
| LOC729345 | Hypothetical LOC729345 | 2.19 | 1.17 | ||
| TMEM106C | Transmembrane protein 106C | 2.18 | 1.67 | ||
| C21orf7 | Chromosome 21 open reading frame 7 | 2.18 | 1.54 | ↑ | |
| AHR | Aryl hydrocarbon receptor | 2.16 | 1.50 | ||
| LOC154761 | Hypothetical LOC154761 | 2.09 | |||
| SLC45A4 | Solute carrier family 45, member 4 | 2.01 | |||
| MYLIP | Myosin regulatory light chain interacting protein | 2.01 | 1.61 | ↓ | |
| ITPRIP | Inositol 1,4,5-triphosphate receptor interacting protein | 1.99 | |||
| TNFAIP6 | TNF, α-induced protein 6 | 1.99 | ↓ | ||
| DHCR7 | 7-Dehydrocholesterol reductase | 1.99 | |||
| TPBG | Trophoblast glycoprotein | 1.98 | |||
| TNFRSF12A | TNF receptor superfamily, member 12A | 1.96 | |||
| ARRDC3 | Arrestin domain containing 3 | 1.95 | |||
| IGF2BP3 | IGF-II mRNA binding protein 3 | 1.93 | |||
| EWSR1/ FLI1 | Ewing sarcoma breakpoint region 1/friend leukemia virus integration 1 | 1.92 | |||
| ZFP36L2 | Zinc finger protein 36, C3H type-like 2 | 1.92 | |||
| MAFF | v-MAF musculoaponeurotic fibrosarcoma oncogene homolog F (avian) | 1.91 | |||
| PDE4B | Phosphodiesterase 4B, cAMP-specific (phosphodiesterase E4 dunce homolog, Drosophila) | 1.90 | ↑ | ||
| NPC1 | Niemann-Pick disease, type C1 | 1.90 | |||
| LOC387763 | Hypothetical protein LOC387763 | 1.90 | 1.51 | ||
| FAM102B | Family with sequence similarity 102, member B | 1.86 | |||
| SGPL1 | Sphingosine-1-phosphate lyase 1 | 1.86 | |||
| NRM | Nurim (nuclear envelope membrane protein) | 1.85 | |||
| SP4 | Sp4 transcription factor | 1.84 | |||
| RGMB | RGM domain family, member B | 1.83 | ↑ | ||
| CD97 | CD97 molecule | 1.83 | |||
| DIRAS3 | DIRAS family, GTP-binding RAS-like 3 | 1.83 | |||
| RNF144B | Ring finger protein 144B | 1.82 | |||
| vTSTA3 | Tissue-specific transplantation antigen P35B | 1.81 | |||
| HEY1 | Hairy/enhancer-of-split related with YRPW motif 1 | 1.78 | ↑ | ||
| ALG1 | Asparagine-linked glycosylation 1, β-1,4-mannosyltransferase homolog (Saccharomyces cerevisiae) | 1.78 | |||
| C14orf109 | Chromosome 14 open reading frame 109 | 1.78 | |||
| PITPNC1 | Phosphatidylinositol transfer protein, cytoplasmic 1 | 1.78 | |||
| RNF24 | Ring finger protein 24 | 1.78 | |||
| ADAM17 | ADAM metallopeptidase domain 17 | 1.78 | |||
| RAB3D | RAB3D, member RAS oncogene family | 1.77 | |||
| ZNF75D | Zinc finger protein 75D | 1.76 | |||
| HSD17B6 | Hydroxysteroid (17-β) dehydrogenase 6 homolog (mouse) | 1.75 | |||
| PNPLA4 | Patatin-like phospholipase domain containing 4 | 1.75 | |||
| PPP1R3C | Protein phosphatase 1, regulatory (inhibitor) subunit 3C | 1.75 | |||
| SCD | Stearoyl-CoA desaturase (δ-9-desaturase) | 1.74 | |||
| LPCAT2 | Lysophosphatidylcholine acyltransferase 2 | 1.74 | ↑ | ||
| INHBA | Inhibin, β A | 1.73 | |||
| S1PR2 | Sphingosine-1-phosphate receptor 2 | 1.73 | |||
| CSRNP1 | Cysteine-serine-rich nuclear protein 1 | 1.72 | |||
| SDC1 | Syndecan 1 | 1.72 | |||
| PUSL1 | Pseudouridylate synthase-like 1 | 1.70 | |||
| ZC3HAV1L | Zinc finger CCCH-type, antiviral 1-like | 1.70 | |||
| ZNRF1 | Zinc and ring finger 1 | 1.70 | |||
| GPR89B/GPR89C/GPR89A | G protein-coupled receptor 89B/G protein-coupled receptor 89A/G protein-coupled receptor 89C | 1.69 | |||
| ILVBL | ILVB (bacterial acetolactate synthase)-like | 1.68 | |||
| CRABP2 | Cellular retinoic acid binding protein 2 | 1.68 | |||
| PLCL2 | Phospholipase C-like 2 | 1.67 | |||
| ALG5 | Asparagine-linked glycosylation 5, dolichyl-phosphate β-glucosyltransferase homolog (S. cerevisiae) | 1.67 | |||
| DUSP10 | Dual-specificity phosphatase 10 | 1.66 | ↑ | ||
| FAM158A | Family with sequence similarity 158, member A | 1.66 | |||
| EPOR | Erythropoietin receptor | 1.66 | |||
| STEAP1 | Six transmembrane epithelial antigen of the prostate 1 | 1.65 | |||
| PAICS | Phosphoribosylaminoimidazole carboxylase, phosphoribosylaminoimidazole succinocarboxamide synthetase | 1.64 | |||
| GK/GK3P | Glycerol kinase/glycerol kinase 3 pseudogene | 1.64 | |||
| HERC6 | Hect domain and RLD 6 | 1.64 | |||
| RIN2 | Ras and Rab interactor 2 | 1.64 | |||
| MXI1 | MAX interactor 1 | 1.64 | |||
| CD47 | CD47 molecule | 1.63 | |||
| SLC41A3 | Solute carrier family 41, member 3 | 1.63 | |||
| UCHL3 | Ubiquitin carboxyl-terminal esterase L3 (ubiquitin thiolesterase) | 1.63 | |||
| IFI35 | Interferon-induced protein 35 | 1.63 | |||
| RNF145 | Ring finger protein 145 | 1.63 | |||
| GOLPH3L | Golgi phosphoprotein 3-like | 1.62 | |||
| TBL2 | Transducin (β)-like 2 | 1.61 | |||
| IER2 | Immediate early response 2 | 1.61 | |||
| PKM2 | Pyruvate kinase, muscle | 1.61 | |||
| LAP3 | Leucine aminopeptidase 3 | 1.61 | |||
| ADFP | Adipose differentiation-related protein | 1.61 | ↓ | ||
| CASP1 | Caspase 1, apoptosis-related cysteine peptidase (IL-1, β, convertase) | 1.60 | ↑ | ||
| TM4SF1 | Transmembrane 4L six family member 1 | 1.60 | ↑ | ||
| KIAA1715 | KIAA1715 | 1.59 | |||
| UAP1L1 | UDP-N-acteylglucosamine pyrophosphorylase 1-like 1 | 1.59 | |||
| POLD3 | Polymerase (DNA-directed), δ 3, accessory subunit | 1.57 | |||
| GAS2L3 | Growth arrest-specific 2 like 3 | 1.57 | |||
| LOC100129973 | Hypothetical protein LOC100129973 | 1.57 | |||
| MAD1L1 | MAD1 mitotic arrest deficient-like 1 (yeast) | 1.57 | |||
| EXTL3 | Exostoses (multiple)-like 3 | 1.56 | |||
| YPEL2 | Yippee-like 2 (Drosophila) | 1.55 | |||
| GMDS | GDP-mannose 4,6-dehydratase | 1.55 | |||
| DTX3L | Deltex 3-like (Drosophila) | 1.55 | |||
| SP110 | SP110 nuclear body protein | 1.54 | |||
| PHF1 | PHD finger protein 1 | 1.53 | |||
| ATP13A3 | ATPase type 13A3 | 1.53 | |||
| XYLT2 | Xylosyltransferase II | 1.53 | |||
| SOAT1 | Sterol O-acyltransferase 1 | 1.53 | |||
| SAC3D1 | SAC3 domain containing 1 | 1.53 | |||
| FAM46A | Family with sequence similarity 46, member A | 1.53 | |||
| FAM57A | Family with sequence similarity 57, member A | 1.53 | |||
| FAM162A | Family with sequence similarity 162, member A | 1.53 | |||
| REL | v-Rel reticuloendotheliosis viral oncogene homolog (avian) | 1.52 | |||
| JUNB | Jun B protooncogene | 1.52 | |||
| AKAP2 | A kinase (PRKA) anchor protein 2 | 1.52 | |||
| NTN4 | Netrin 4 | 1.52 | |||
| PDXK | Pyridoxal (pyridoxine, vitamin B6) kinase | 1.52 | |||
| IFI44L | Interferon-induced protein 44-like | 1.52 | ↓ | ||
| TMEM60 | Transmembrane protein 60 | 1.52 | |||
| C19orf66 | Chromosome 19 open reading frame 66 | 1.52 | |||
| NEU1 | Sialidase 1 (lysosomal sialidase) | 1.51 | |||
| DOCK10 | Dedicator of cytokinesis 10 | 1.51 | ↓ | ||
| SLC15A3 | Solute carrier family 15, member 3 | 1.51 | |||
| SRPK2 | SFRS protein kinase 2 | 1.51 | |||
| SUSD3 | Sushi domain containing 3 | 1.51 | |||
| DHRS7 | Dehydrogenase/reductase (SDR family) member 7 | 1.51 | |||
| SLC31A1 | Solute carrier family 31 (copper transporters), member 1 | 1.51 | |||
| RQCD1 | RCD1 required for cell differentiation1 homolog (S. pombe) | 1.50 | |||
| TAF1A | TATA box binding protein (TBP)-associated factor, RNA polymerase I, A, 48 kDa | 1.50 | |||
| Down-regulated | |||||
| RORB | RAR-related orphan receptor B | 9.19 | 5.67 | ↓ | |
| ALDH1A3 | Aldehyde dehydrogenase 1 family, member A3 | 7.69 | 4.23 | ||
| IGF1 | IGF-I (somatomedin C) | 7.68 | 3.78 | ↓ | |
| KLF9 | Kruppel-like factor 9 | 5.97 | |||
| RASD1 | RAS, dexamethasone-induced 1 | 5.91 | 4.64 | ||
| PGR | Progesterone receptor | 5.36 | 5.88 | ↓ | |
| NPR3 | Natriuretic peptide receptor C/guanylate cyclase C (atrionatriuretic peptide receptor C) | 4.98 | 2.61 | ↓ | |
| CNTN3 | Contactin 3 (plasmacytoma associated) | 4.56 | 3.59 | ↓ | |
| DHRS3 | Dehydrogenase/reductase (SDR family) member 3 | 4.53 | 2.48 | ↓ | ↑ |
| C5orf23 | Chromosome 5 open reading frame 23 | 4.30 | 2.85 | ↓ | |
| PRKAB2 | Protein kinase, AMP-activated, β 2 non-catalytic subunit | 4.16 | 1.68 | ||
| SDPR | Serum deprivation response (phosphatidylserine binding protein) | 3.51 | 2.31 | ||
| FERMT2 | Fermitin family homolog 2 (Drosophila) | 3.27 | 2.58 | ↓ | |
| PLCXD3 | Phosphatidylinositol-specific phospholipase C, X domain containing 3 | 3.18 | 2.16 | ||
| FAM116A | Family with sequence similarity 116, member A | 3.09 | 1.73 | ||
| ITGBL1 | Integrin, β-like 1 (with EGF-like repeat domains) | 2.78 | 2.24 | ||
| CCDC69 | Coiled-coil domain containing 69 | 2.74 | 1.57 | ||
| MMP1 | Matrix metallopeptidase 1 (interstitial collagenase) | 2.72 | |||
| ODC1 | Ornithine decarboxylase 1 | 2.63 | |||
| GLT8D3 | Glycosyltransferase 8 domain containing 3 | 2.62 | 1.97 | ||
| ST6GALNAC5 | ST6 (α-N-acetyl-neuraminyl-2, 3-β-galactosyl-1, 3)-N-acetylgalactosaminide α-2, 6-sialyltransferase 5 | 2.55 | 1.98 | ||
| ERRFI1 | ERBB receptor feedback inhibitor 1 | 2.54 | 1.78 | ↓ | |
| ADAM19 | ADAM metallopeptidase domain 19 (meltrin β) | 2.45 | |||
| ZBTB47 | Zinc finger and BTB domain containing 47 | 2.43 | 1.60 | ||
| QDPR | Quinoid dihydropteridine reductase | 2.42 | |||
| CDKN1C | Cyclin-dependent kinase inhibitor 1C (p57, Kip2) | 2.42 | |||
| NEXN | Nexilin (F actin-binding protein) | 2.40 | |||
| PDK4 | pyruvate dehydrogenase kinase, isozyme 4 | 2.39 | 1.88 | ||
| TGFBR3 | TGF, β receptor III | 2.33 | 1.71 | ||
| NBN | Nibrin | 2.31 | |||
| RHOU | Ras homolog gene family, member U | 2.26 | ↓ | ||
| MESDC2 | Mesoderm development candidate 2 | 2.26 | |||
| C1orf9 | Chromosome 1 open reading frame 9 | 2.23 | 1.52 | ||
| ZNF675 | Zinc finger protein 675 | 2.22 | 1.56 | ||
| KIAA0317 | KIAA0317 | 2.22 | |||
| SLC11A2 | Solute carrier family 11 (proton-coupled divalent metal ion transporters), member 2 | 2.21 | |||
| NBLA00301 | Nbla00301 | 2.18 | 1.59 | ↓ | |
| PLBD1 | Phospholipase B domain containing 1 | 2.16 | |||
| UST | Uronyl-2-sulfotransferase | 2.16 | ↓ | ||
| C17orf80 | Chromosome 17 open reading frame 80 | 2.16 | |||
| SUCLA2 | Succinate-CoA ligase, ADP-forming, β subunit | 2.14 | |||
| SATB1 | SATB homeobox 1 | 2.12 | |||
| SLC40A1 | Solute carrier family 40 (iron-regulated transporter), member 1 | 2.10 | ↓ | ||
| MRVI1 | Murine retrovirus integration site 1 homolog | 2.06 | 1.56 | ||
| LPHN3 | Latrophilin 3 | 2.04 | |||
| MAOA | Monoamine oxidase A | 2.03 | 1.64 | ||
| RASL11B | RAS-like, family 11, member B | 2.03 | 1.53 | ||
| ZNF91 | Zinc finger protein 91 | 1.98 | |||
| ARNT | Aryl hydrocarbon receptor nuclear translocator | 1.97 | |||
| TIMP3 | TIMP metallopeptidase inhibitor 3 | 1.95 | ↓ | ||
| TGOLN2 | Trans-golgi network protein 2 | 1.95 | |||
| INPP5A | Inositol polyphosphate-5-phosphatase, 40 kDa | 1.95 | ↓ | ||
| HSP90B1 | Heat-shock protein 90 kDa β (Grp94), member 1 | 1.94 | |||
| TBCEL | Tubulin folding cofactor E-like | 1.93 | |||
| STAG3L4 | Stromal antigen 3-like 4 | 1.91 | |||
| TSHZ1 | Teashirt zinc finger homeobox 1 | 1.91 | |||
| CD55 | CD55 molecule, decay accelerating factor for complement (Cromer blood group) | 1.91 | |||
| CFL2 | Cofilin 2 (muscle) | 1.89 | |||
| PIK3R3 | Phosphoinositide-3-kinase, regulatory subunit 3 (γ) | 1.88 | |||
| FAM167A | Family with sequence similarity 167, member A | 1.88 | |||
| GAS1 | Growth arrest-specific 1 | 1.88 | ↓ | ||
| HIGD1A | HIG1 hypoxia inducible domain family, member 1A | 1.86 | |||
| EXOSC3 | Exosome component 3 | 1.85 | |||
| MASP1 | Mannan-binding lectin serine peptidase 1 (C4/C2 activating component of Ra-reactive factor) | 1.83 | |||
| SYNJ2BP | Synaptojanin 2 binding protein | 1.82 | |||
| IGFBP1 | IGF-binding protein 1 | 1.81 | ↓ | ||
| KIAA0146 | KIAA0146 | 1.81 | |||
| MCEE | Methylmalonyl CoA epimerase | 1.79 | |||
| ACP5 | Acid phosphatase 5, tartrate resistant | 1.78 | |||
| TCEAL1 | Transcription elongation factor A (SII)-like 1 | 1.78 | |||
| ACER3 | Alkaline ceramidase 3 | 1.78 | |||
| ZADH2 | Zinc binding alcohol dehydrogenase domain containing 2 | 1.78 | |||
| AKAP7 | A kinase (PRKA) anchor protein 7 | 1.78 | |||
| RAP1B | RAP1B, member of RAS oncogene family | 1.77 | |||
| ATP1B1 | ATPase, Na+/K+ transporting, β 1 polypeptide | 1.77 | ↑ | ||
| AGPS | Alkylglycerone phosphate synthase | 1.76 | |||
| LYRM7 | Lyrm7 homolog (mouse) | 1.75 | |||
| ANKRD13C | Ankyrin repeat domain 13C | 1.75 | |||
| LOC253039 | Hypothetical LOC253039 | 1.74 | |||
| DNMBP | Dynamin binding protein | 1.73 | ↑ | ||
| TP53INP1 | Tumor protein p53 inducible nuclear protein 1 | 1.71 | |||
| RUNX1T1 | Runt-related transcription factor 1; translocated to, 1 (cyclin d-related) | 1.71 | |||
| KLF4 | Kruppel-like factor 4 (gut) | 1.71 | |||
| PTN | Pleiotrophin | 1.70 | |||
| VAPA | VAMP (vesicle-associated membrane protein)-associated protein A, 33 kDa | 1.70 | |||
| PRDM2 | PR domain containing 2, with ZNF domain | 1.69 | |||
| EARS2 | Glutamyl-tRNA synthetase 2, mitochondrial (putative) | 1.68 | |||
| LOC100129387 | Hypothetical LOC100129387 | 1.68 | |||
| DIABLO | Diablo homolog (Drosophila) | 1.67 | |||
| FBN1 | Fibrillin 1 | 1.66 | |||
| JAZF1 | JAZF zinc finger 1 | 1.66 | |||
| PLA2G16 | Phospholipase A2, group XVI | 1.66 | |||
| CGA | Glycoprotein hormones, α polypeptide | 1.64 | ↓ | ||
| TMED8 | Transmembrane emp24 protein transport domain containing 8 | 1.64 | |||
| C15orf17 | Chromosome 15 open reading frame 17 | 1.64 | |||
| C1orf124 | Chromosome 1 open reading frame 124 | 1.63 | |||
| ZCCHC6 | Zinc finger, CCHC domain containing 6 | 1.63 | |||
| PCYOX1 | Prenylcysteine oxidase 1 | 1.63 | |||
| OBFC2A | Oligonucleotide/oligosaccharide-binding fold containing 2A | 1.63 | |||
| KCNMA1 | Potassium large conductance calcium-activated channel, subfamily M, α member 1 | 1.63 | |||
| COX15 | COX15 homolog, cytochrome c oxidase assembly protein (yeast) | 1.62 | |||
| C1orf2 | Chromosome 1 open reading frame 2 | 1.62 | |||
| BCAT1 | Branched chain aminotransferase 1, cytosolic | 1.62 | ↓ | ||
| COL8A1 | Collagen, type VIII, α 1 | 1.62 | |||
| TPM1 | Tropomyosin 1 (α) | 1.62 | |||
| TES | Testis derived transcript (3 LIM domains) | 1.61 | |||
| ABHD14B | Abhydrolase domain containing 14B | 1.60 | |||
| CDC14B/CDC14C | CDC14 cell division cycle 14 homolog B (S. cerevisiae)/CDC14 cell division cycle 14 homolog C (S. cerevisiae) | 1.59 | |||
| RPGR | Retinitis pigmentosa GTPase regulator | 1.59 | |||
| GADD45B | Growth arrest and DNA-damage-inducible, β | 1.59 | |||
| PPP1R12A | Protein phosphatase 1, regulatory (inhibitor) subunit 12A | 1.59 | |||
| PCDH19 | Protocadherin 19 | 1.58 | |||
| CDC37L1 | Cell division cycle 37 homolog (S. cerevisiae)-like 1 | 1.57 | |||
| MDFIC | MyoD family inhibitor domain containing | 1.57 | |||
| KCTD12 | Potassium channel tetramerization domain containing 12 | 1.57 | |||
| TNPO1 | Transportin 1 | 1.56 | |||
| RHOBTB2 | ρ-Related BTB domain containing 2 | 1.55 | |||
| SRPR | Signal recognition particle receptor (docking protein) | 1.54 | |||
| TRMT11 | tRNA methyltransferase 11 homolog (S. cerevisiae) | 1.53 | |||
| RAB6A/RAB6C | RAB6A, member RAS oncogene family/RAB6C, member RAS oncogene family | 1.53 | |||
| C11orf70 | Chromosome 11 open reading frame 70 | 1.53 | |||
| TCEAL4 | Transcription elongation factor A (SII)-like 4 | 1.53 | |||
| JUN | Jun oncogene | 1.53 | |||
| MAP7D3 | MAP7 domain containing 3 | 1.53 | |||
| CKS2 | CDC28 protein kinase regulatory subunit 2 | 1.53 | |||
| SEMA5A | SEMA domain, seven thrombospondin repeats (type 1 and type 1-like), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 5A | 1.52 | |||
| SAT1 | Spermidine/spermine N1-acetyltransferase 1 | 1.52 | |||
| GLUL | Glutamate-ammonia ligase (glutamine synthetase) | 1.51 | |||
| MST150 | MSTP150 | 1.51 | |||
| CHN1 | Chimerin (chimaerin) 1 | 1.51 | |||
| LGMN | Legumain | 1.51 | |||
Results are expressed as fold change vs. nontargeting (NT) siRNA (≥1.5-fold change cutoff). ↑, Up-regulated; ↓, down-regulated.
Genes significantly regulated in hESFendoEP vs. hESFnonendoEP (27); E2+MPA (EP).
Genes significantly regulated in hESFendocAMP vs. hESFnonendocAMP (26).
Similar entities (overlaps) were determined using Genespring software based on the hypergeometric distribution with P < 0.001 (siKP vs. NT and hESFendoEP vs. hESFnonendoEP) and P < 0.001 (siKP vs. NT and hESFendocAMP vs. hESFnonendocAMP), respectively.
KLF9 and PGR coregulate endometriosis-associated genes
We compared our transcriptome data with previously reported expression profiles for human endometrial stromal fibroblasts (hESF) isolated from women with endometriosis and showing deregulated P or cAMP responsiveness (26, 27). Of the P-resistant genes in hESF of women with endometriosis, 48 (e.g. STC1, DUSP6, AREG, CASP1, IGF1, ERRFI1, and TIMP3) were also found in our list of up- or down-regulated genes with KP knockdown [Table 1; hESFendo vs. hESFnonendo (EP)]. Of these, 36 showed deregulated expression in the same direction as those for disrupted KLF9+PGR expression. A number of cAMP-responsive genes (e.g. IL8, IL1B, and IGFBP1) whose expression was deregulated in hESF of women with endometriosis, also showed overlap with genes altered by KP knockdown [Table 1; hESFendo vs. hESFnonendo (cAMP)]. Dysregulated genes in the siKP-HESC dataset and those with deregulated P or cAMP responses were highly correlated (P < 0.001).
Analyses using genome set enrichment analyses upon KLF9, PGR, or KLF9+PGR knockdowns indicated that KLF9 can function independently of PGR (Table 2). The gene functions regulated by KLF9+PGR (enriched or diminished with siKP) did not overlap with those identified for KLF9 alone (siKLF9). Although there was overlap in the gene functions regulated by siKP and siPGR (compare Diminished in siKP vs. Diminished in siPGR), KLF9+PGR loss significantly exacerbated the effect of siPGR alone, as indicated by the false discovery rate values (Table 2).
Table 2.
Summary of GSEA with FDR no more than 0.25
| Gene set | FDR |
|---|---|
| Enriched in siKP | |
| Chemokine activity | 0.154 |
| G-protein coupled receptor binding | 0.158 |
| Cytokine activity | 0.209 |
| Defense response | 0.235 |
| Response to biotic stimulus | 0.237 |
| Negative regulation of MAP kinase activity | 0.249 |
| Diminished in siKP | |
| Proteinaceous extracellular matrix | <0.001 |
| Extracellular matrix | <0.001 |
| Collagen proteins | <0.001 |
| Basement membrane components | <0.001 |
| Actin cytoskeleton | 0.025 |
| Structural constituents of muscle | 0.026 |
| Basal lamina | 0.044 |
| Peptidase activity | 0.120 |
| Integrin binding | 0.125 |
| Intramolecular oxidoreductase activity | 0.190 |
| Metallopeptidase activity | 0.197 |
| Enriched in siKLF9 | |
| Microtubule cytoskeleton organization and biogenesis | 0.008 |
| Transcription factors | 0.223 |
| Mitotic cell cycle | 0.238 |
| DNA polymerase activity | 0.243 |
| Cell cycle process | 0.248 |
| Diminished in siKLF9 | |
| Protein phosphorylation | 0.043 |
| Jak-stat cascade | 0.171 |
| Diminished in siPGR | |
| Proteinaceous extracellular matrix | 0.002 |
| Extracellular matrix | 0.006 |
| Collagen proteins | 0.051 |
| Structural constituents of muscle | 0.111 |
| Peptidase activity | 0.210 |
| Basement membrane components | 0.250 |
| Intramolecular oxidoreductase activity | 0.250 |
Refer to Supplemental Table 8 for the complete list that includes gene ontology, functional processes, and genes involved. FDR, False discovery rate.
We further analyzed by Ingenuity pathway analysis the 417 annotated genes deregulated by siKP (≥1.5-fold change). The top gene networks (Supplemental Table 4), functional processes (Supplemental Table 5), and canonical pathways (Supplemental Table 6) deregulated with KLF9+PGR over PGR knockdown include cell proliferation, angiogenesis, apoptosis, and immune signaling. Predictably, uterine cancer (34 of 417) and other reproductive system diseases (57 of 417), notably endometriosis, were associated with disruption of KLF9+PGR expression (Supplemental Table 7).
The heat map representation of identified genes affected by siKP relative to siPGR that were previously reported to be deregulated in patients with endometriosis (9, 22, 26, 27) is shown in Fig. 4B. Selected genes were confirmed for differential expression by QPCR (Fig. 4C). EGR1 and IL8 levels were increased by siPGR alone and further increased by siKP. IGFBP1 levels were significantly decreased by siKP but not by siPGR. PGR mRNA levels were not additionally affected by siKP.
With respect to physiological functions, immune cell trafficking was highly affected by KP loss (Supplemental Table 5). Correlation-based hierarchical clustering showed enrichment of selected cytokine (Fig. 4D) and IGF/MAPK (Fig. 4E) signaling component genes with siKP, relative to siPGR alone. Figure 4F illustrates representative overlapping gene networks impacted by loss of functional KLF9+PGR interactions.
Discussion
This study addressed whether endometriosis, a uterine pathology characterized by attenuated PGR expression and transactivity, involves the coordinate loss of KLF9 function. Here, we report that eutopic endometrium of women with vs. without endometriosis, had significantly reduced KLF9 expression, coincident with reductions in PGR, PGR-B, WNT4, WNT2, and DKK1 levels. We demonstrate a causal relationship between loss of KLF9+PGR and reduced expression of WNT4 and DKK1 using the siRNA approach in cAME-treated HESC. Furthermore, we show that KLF9/PGR coregulation of DKK1 expression involves their corecruitment to the DKK1 proximal promoter region to regulate DKK1 gene transcription. Finally, we establish the global aspects of KLF9/PGR coregulation of human endometrial stromal genes by gene expression array and identify unique stromal KLF9/PGR gene targets that previously showed resistance to P action in hESF of women with endometriosis (27). Together, our results implicate KLF9 loss of expression as a participatory event in the pathogenesis of endometriosis.
Our study revealed for the first time that although KLF9 and PGR can function independently of each other, their interaction modifies PGR transactivity such that their coincident loss of expression elicits biological responses distinct from those caused by their individual losses. This was supported by our candidate gene approach for DKK1, WNT2, and WNT4 and, on a more global scale, by our identification of genes and biological functions distinctly deregulated by siKP vs. siPGR alone. KLF9+PGR-regulated genes include predominant candidate contributors to the pathology of endometriosis such as IGF1, IL8, AREG, MMP1, STC1, DUSP6, MAOA, EGR1, and CASP1 (10, 13, 22, 26, 27, 28). Importantly, we showed the potential validity of identified KLF9+ PGR-regulated genes as clinically relevant to endometriosis by demonstrating significant overlaps in expression with those exhibiting abnormal response to P in hESF isolated from women with endometriosis (27). Although our studies were carried out using total PGR rather than specific PGR-B isoform knockdown, raising the potential contribution of PGR-A to the effects shown here, this is not likely because KLF9 preferentially interacts with PGR-B (18, 29) and endometriosis is largely associated with decreased PGR-B expression (12, 13).
Our studies also established a mechanistic underpinning for how KLF9 and PGR cooperate, using DKK1 as a paradigm target in stromal cells. DKK1 is a potent inhibitor of the canonical WNT signaling pathway (30), and disruption of its expression leads to abnormal uterine cellular proliferation, differentiation, and death (31). In the decidualizing stroma, KLF9 and PGR are recruited to the DKK1 proximal promoter, and although KLF family member SP1 can bind to these same promoter sequences, only KLF9 interacted with PGR under the proper decidual stimulus. Interestingly, loss of KLF9 alone in HESC enhanced DKK1 promoter activity in contrast to the noted reduction with the coincident loss of KLF9+PGR. Data suggest that KLF9 may provide specificity to and/or fine-tune the promoter response to P/PGR and that KLF9 effects may involve gene networks distinct from those perturbed by KLF9/PGR together. The latter possibility is consistent with the universal loss of DKK1 expression in eutopic endometrium of women with endometriosis (14, 26, 27) and the distinct enrichment of functional gene sets with KLF9 vs. KLF9+PGR loss of expression (this study).
Our results identified the WNT signaling pathway as a major target of KLF9/PGR interactions. Another signaling pathway potentially influenced by KLF9+PGR in stromal cells is the IGF system, with IGF-I and IGF-binding protein 1 as major candidate targets of KLF9/PGR coregulation. The immune system component IL-8, whose increased expression in eutopic endometria of women with endometriosis has been suggested to facilitate disease progression (32), is also distinctly regulated by KLF9+PGR. Furthermore, the pronounced up-regulated expression of EGR1, STC1, AREG, and EREG, all of which are hallmarks of escape from normal growth regulation, with loss of KLF9+PGR expression, provides additional proof to the biological importance of their interactions. Nevertheless, it remains unclear whether these genes are direct targets of KLF9/PGR, as we have shown here for DKK1 or whether other PGR coregulators may also contribute. Given that the Klf9-null mouse exhibits a subfertile phenotype (19) and the recent data suggesting deregulated expression of several KLF family members in endometriosis (22), it is possible that perturbations of the KLF family regulatory network (20, 33–35) contributes to the pathogenesis of this disease.
An important question is what links endometriosis and infertility. Our work confirms other previously published studies (9, 22) that disruption of WNT signaling contributes to this association. Mutant mice lacking Wnt4 are subfertile, due partly to defects in endometrial cell survival, differentiation, and P sensitivity (36). Targeted Wnt2 ablation in mice also resulted in placentation defects (37). Thus, studies on how appropriate expression of WNT signaling components is maintained by PGR and its coregulatory proteins, one of which is KLF9, may present viable options for the treatment of these disorders. An equally important question is the underlying mechanism for decreased KLF9 expression in eutopic endometrium of women with endometriosis. Although PGR may contribute to KLF9 regulation, because PGR knockdown in HESC modestly reduced KLF9 protein levels (this study), it is likely that other, more predominant mechanisms for the dramatic loss of KLF9 expression in endometriosis exist. Given that PGR hypermethylation leading to reduced PGR expression is found in both endometrial cancer (38) and in endometriosis (39) and the recent report that KLF9 is subject to epigenetic regulation (40), it is tempting to speculate that aberrant epigenetic mechanisms may cause loss of KLF9 and PGR expression in endometriosis, thereby initiating a cascade of deregulated transcriptional events.
In conclusion, our study demonstrates that KLF9, by modifying PGR action, plays a functional role in uterine PGR sensitivity, the loss of which may underlie the pathogenesis of endometriosis. The breadth of the KLF9+PGR network shown here suggests that further investigations into these linkages may provide therapeutic targets for endometriosis and endometriosis-associated infertility.
Supplementary Material
Acknowledgments
We thank members of our laboratories for helpful discussions during the course of this work.
This work was supported by the National Institutes of Health (HD21961 to R.C.M.S.) and Eunice Kennedy Shriver National Institutes of Child Health and Human Development Specialized Cooperative Centers Program in Reproduction and Infertility Research (HD055764 Human Endometrial Tissue Bank to L.C.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- cAME
- 8-bromo-cAMP+MPA+E2
- ChIP
- chromatin immunoprecipitation
- DKK1
- Dickkopf-1
- E2
- estradiol-17β
- HESC
- human endometrial stromal cell
- hESF
- human endometrial stromal fibroblasts
- KLF9
- Krüppel-like factor 9
- MPA
- medroxyprogesterone acetate
- MSE
- midsecretory endometrium
- nt
- nucleotides
- P
- progesterone
- PE
- proliferative endometrium
- PGR
- P receptor
- PRE/GRE
- P response element/glucocorticoid response element
- QPCR
- quantitative PCR
- siRNA
- small interfering RNA
- SP1
- specificity protein 1.
References
- 1. Giudice LC, Kao LC. 2004. Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- 2. Eskenazi B, Warner ML. 1997. Epidemiology of endometriosis. Obstet Gynecol Clin North Am 24:235–258 [DOI] [PubMed] [Google Scholar]
- 3. Hahn DW, Carraher RP, Foldesy RG, McGuire JL. 1986. Experimental evidence for failure to implant as a mechanism of infertility associated with endometriosis. Am J Obstet Gynecol 155:1109–1113 [DOI] [PubMed] [Google Scholar]
- 4. Illera MJ, Juan L, Stewart CL, Cullinan E, Ruman J, Lessey BA. 2000. Effect of peritoneal fluid from women with endometriosis on implantation in the mouse model. Fertil Steril 74:41–48 [DOI] [PubMed] [Google Scholar]
- 5. Arici A, Oral E, Bukulmez O, Duleba A, Olive DL, Jones EE. 1996. The effect of endometriosis on implantation: results from the Yale University in vitro fertilization and embryo transfer program. Fertil Steril 65:603–607 [DOI] [PubMed] [Google Scholar]
- 6. Barnhart K, Dunsmoor-Su R, Coutifaris C. 2002. Effect of endometriosis on in vitro fertilization. Fertil Steril 77:1148–1155 [DOI] [PubMed] [Google Scholar]
- 7. Brosens I. 2004. Endometriosis and the outcome of in vitro fertilization. Fertil Steril 81:1198–1200 [DOI] [PubMed] [Google Scholar]
- 8. Stephansson O, Kieler H, Granath F, Falconer H. 2009. Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Hum Reprod 24:2341–2347 [DOI] [PubMed] [Google Scholar]
- 9. Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. 2003. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 144:2870–2881 [DOI] [PubMed] [Google Scholar]
- 10. Osteen KG, Bruner-Tran KL, Eisenberg E. 2005. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril 83:529–537 [DOI] [PubMed] [Google Scholar]
- 11. Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. 2004. Molecular cues to implantation. Endocr Rev 25:341–373 [DOI] [PubMed] [Google Scholar]
- 12. Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, Osteen KG. 2005. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril 84:67–74 [DOI] [PubMed] [Google Scholar]
- 13. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. 2000. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 85:2897–2902 [DOI] [PubMed] [Google Scholar]
- 14. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. 2007. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148:3814–3826 [DOI] [PubMed] [Google Scholar]
- 15. Aghajanova L, Velarde MC, Giudice LC. 2009. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology 150:3863–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor HS, Bagot C, Kardana A, Olive D, Arici A. 1999. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 14:1328–1331 [DOI] [PubMed] [Google Scholar]
- 17. Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. 2008. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 19:178–186 [DOI] [PubMed] [Google Scholar]
- 18. Zhang D, Zhang XL, Michel FJ, Blum JL, Simmen FA, Simmen RC. 2002. Direct interaction of the Kruppel-like family (KLF) member, BTEB1, and PR mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology 143:62–73 [DOI] [PubMed] [Google Scholar]
- 19. Simmen RC, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman L, Jr, Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP. 2004. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem 279:29286–29294 [DOI] [PubMed] [Google Scholar]
- 20. Pabona JM, Zeng Z, Simmen FA, Simmen RC. 2010. Functional differentiation of uterine stromal cells involves cross-regulation between bone morphogenetic protein 2 and Kruppel-like factor (KLF) family members KLF9 and KLF13. Endocrinology 151:3396–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aghajanova L, Giudice LC. 2011. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod Sci 18:229–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suske G, Bruford E, Philipsen S. 2005. Mammalian SP/KLF transcription factors: bring in the family. Genomics 85:551–556 [DOI] [PubMed] [Google Scholar]
- 24. Strähle U, Klock G, Schütz G. 1987. A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proc Natl Acad Sci USA 84:7871–7875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, Ishihara O, Lam EW, Unterman TG, Brosens JJ, Kim JJ. 2007. Transcriptional cross-talk between the forkhead transcription factor forkhead box 01A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 21:2334–2349 [DOI] [PubMed] [Google Scholar]
- 26. Aghajanova L, Horcajadas JA, Weeks JL, Esteban FJ, Nezhat CN, Conti M, Giudice LC. 2010. The protein kinase A pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology 151:1341–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aghajanova L, Tatsumi K, Horcajadas JA, Zamah AM, Esteban FJ, Herndon CN, Conti M, Giudice LC. 2011. Unique transcriptome, pathways and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol Reprod 84:801–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuzaki S, Canis M, Vaurs-Barriere C, Boespflug-Tanguy O, Dastugue B, Mage G. 2005. DNA microarray analysis of gene expression in eutopic endometrium from patients with deep endometriosis using laser capture microdissection. Fertil Steril 84(Suppl 2):1180–1190 [DOI] [PubMed] [Google Scholar]
- 29. Zhang XL, Zhang D, Michel FJ, Blum JL, Simmen FA, Simmen RC. 2003. Selective interactions of Kruppel-like factor 9/basic transcription element-binding protein with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. J Biol Chem 278:21474–21482 [DOI] [PubMed] [Google Scholar]
- 30. Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, Kraus MH, Aaronson SA. 1999. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem 274:19465–19472 [DOI] [PubMed] [Google Scholar]
- 31. Li J, Liu WM, Cao YJ, Peng S, Zhang Y, Duan EK. 2008. Roles of Dickkopf-1 and its receptor Kremen1 during embryonic implantation in mice. Fertil Steril 90:1470–1479 [DOI] [PubMed] [Google Scholar]
- 32. Ulukus M, Ulukus EC, Tavmergen Goker EN, Tavmergen E, Zheng W, Arici A. 2009. Expression of interleukin-8 and monocyte chemotactic protein 1 in women with endometriosis. Fertil Steril 91:687–693 [DOI] [PubMed] [Google Scholar]
- 33. Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. 2008. Kruppel-like transcription factors: a functional family. Int J Biochem Cell Biol 40:1996–2001 [DOI] [PubMed] [Google Scholar]
- 34. Simmons CD, Pabona JM, Heard ME, Friedman TM, Spataro MT, Godley AL, Simmen FA, Burnett AF, Simmen RC. 2011. Krüppel-like factor 9 loss-of-expression in human endometrial carcinoma links altered expression of growth-regulatory genes with aberrant proliferative response to estrogen. Biol Reprod 85:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simmen FA, Su Y, Xiao R, Zeng Z, Simmen RC. 2008. The Krüppel-like factor 9 (KLF9) network in HEC-1-A endometrial carcinoma cells suggests the carcinogenic potential of dys-regulated KLF9 expression. Reprod Biol Endocrinol 6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, DeMayo FJ. 2011. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 25:1176–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. 1996. Targeted disruption of the Wnt2 gene results in placentation defects. Development 122:3343–3353 [DOI] [PubMed] [Google Scholar]
- 38. Ren Y, Liu X, Ma D, Feng Y, Zhong N. 2007. Down-regulation of the progesterone receptor by the methylation of progesterone receptor gene in endometrial cancer cells. Cancer Genet Cytogenet 175:107–116 [DOI] [PubMed] [Google Scholar]
- 39. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. 2006. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 1:106–111 [DOI] [PubMed] [Google Scholar]
- 40. Wolf I, Bose S, Desmond JC, Lin BT, Williamson EA, Karlan BY, Koeffler HP. 2007. Unmasking of epigenetically silenced genes reveals DNA promoter methylation and reduced expression of PTCH in breast cancer. Breast Cancer Res Treat 105:139–155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.