An environment enriched with an excessive supply of food and lacking demand for physical activity requires adaptive response to “buffer” against metabolic imbalance. If utilization of excessive calories fails to fulfill this goal, deposition of triglyceride in adipose tissue is the natural barrier against the toxic effects of lipid and glucose in virtually all cell types (1, 2). Normal adipose tissue functions will guarantee protection against lipo- and glucotoxicity. This is accomplished through adipocyte differentiation from precursor cells and maturation to triglyceride-loaded adipocytes of various sizes. If unchanged, this condition will determine progressive increase in body fat content and weight gain. Different control mechanisms of adipocyte differentiation and maturation in various adipose tissue areas are likely responsible for variability in fat distribution between genders and among individuals.
For decades, research has focused on the role of body fat mass excess, and then fat distribution, on metabolic complications typically associated with obesity. We have witnessed a proliferation of literature in support of the detrimental effects of increased abdominal/truncal fat mass on glucose and lipid metabolism. Both visceral and sc abdominal/truncal fat deposition have been associated with systemic insulin resistance (3) and enhanced risk for associated health complications, such as type 2 diabetes and cardiovascular disease (3–6). Unfortunately, the clinical implications of this research have been rather limited. Although body mass index (BMI) and waist circumference are widely used as measures of fat mass and distribution and have a role in predicting risk for type 2 diabetes and cardiovascular disease, their clinical value is evident only when combined with other metabolic abnormalities, such as those defining the metabolic syndrome (7). Metabolically healthy obese persons are frequent in our clinics, and intervention for prevention of cardiovascular disease and type 2 diabetes in this group is questionable. More importantly, metabolically unhealthy nonobese persons are not easily identifiable as candidates for preventive treatment, based on fat mass and distribution. This is particularly evident in ethnic minorities of the U.S. population, such as the African-Americans, Hispanics, and Asians. Metabolic abnormalities, including insulin resistance, are more common in these groups at lower BMI and waist circumferences, compared with the European descent group (8, 9). Consequently, use of specific BMI and waist circumference targets have been suggested for different populations (10), but the overall result seems to be more confusion both for patients and physicians on the real value of these measurements. Given the growing need to optimize our health care resource allocation into prevention of chronic diseases, the lack of appropriate tools to identify patients at risk for the two major health complications of insulin resistance, type 2 diabetes and cardiovascular disease, is of significance. Recent trends toward research focusing on adipose tissue function and metabolic complications of obesity are promising. Adipose tissue function is now recognized to play a major role in metabolic homeostasis of both lipids and glucose. Identification of biomarkers of adipose tissue dysfunction may provide the much needed tools to identify a disease status (adiposopathy) that precedes and is the cause of metabolic complications, such as insulin resistance. Defining an adipose tissue dysfunction as a disease would be of value for regulatory agencies, pharmaceutical companies, and clinicians who could better focus therapy to the appropriate patient for prevention of adverse health consequences of glucose and lipid metabolism abnormalities, such as type 2 diabetes and cardiovascular disease.
Increasing numbers of investigators are refocusing obesity research along these lines. In this issue of the JCEM, Rogers et al. (11) identified epidermal growth factor receptor-1 (ErbB1) as a modulator of adipose tissue function. Using a combination of in vitro and clinical studies, their results suggest that ErbB1 is involved in promoting triglyceride storage in adipocytes, perhaps through maintenance of adequate peroxisome proliferator-activated receptor-γ activity. Decreased ErbB1 protein in sc abdominal adipose tissue was shown in insulin-resistant subjects and in those who developed type 2 diabetes. The possibility that ErbB1 is part of a network of regulators of adipocyte maturation is intriguing and opens an opportunity to better understand the role of defective adipocyte triglyceride synthesis in fat partitioning, lipid toxicity, and systemic insulin resistance to glucose disposal. Among other candidate mechanisms of adipocyte maturation, the ectonucleotide pyrophosphatase/phosphodiesterase-1 was recently shown to induce abnormalities in fat partitioning in association with systemic insulin resistance (12). In both instances, adipocyte maturation arrest can be viewed as a specific type of adipose tissue dysfunction or “adiposopathy” (13) linked to systemic insulin resistance. Other known types of adiposopathy, which have been associated with systemic insulin resistance, may or may not be exclusively related to adipocyte maturation arrest and include abnormal adipokine production, adipocyte leptin resistance, and adipose tissue inflammation.
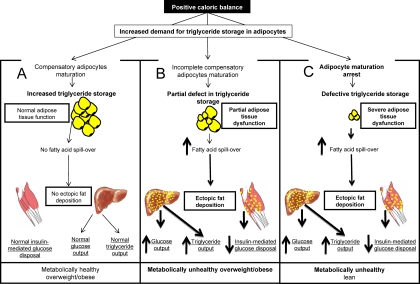
As schematically depicted in Fig. 1, adipocyte maturation arrest can mechanistically provide an explanation for the apparent dissociation between metabolic health and fat mass/distribution. In the presence of positive caloric balance, increased demand for triglyceride storage is normally met by compensatory triglyceride synthesis in maturing adipocytes. In scenario A, adipocytes are fully capable of accommodating fatty acids derived from lipolysis of triglycerides in circulating lipoproteins; no spillover of fatty acid will occur. Persisting adipocyte triglyceride synthesis unmatched by lipolysis will lead to progressive increase in body fat storage until a new level of caloric balance is accomplished. The patient could become overweight, obese, or even morbidly obese. In these circumstances, plasma fatty acids are not expected to be elevated, and substrate supply for ectopic fat deposition would be minimal. This scenario explains the coexistence of obesity and normal glucose/lipid metabolism. It is worthwhile mentioning that this is not an uncommon circumstance and that about 25% of obese adults are estimated to be insulin sensitive (14). Additionally, according to the National Health and Nutrition Examination Survey data, 32% of obese adults have one or less metabolic abnormalities carrying risk for cardiovascular complications (15).
Fig. 1.
Adipocyte maturation regulation can modulate overall caloric “buffering” function of adipose tissue.
The other extreme is shown in scenario C of Fig. 1. In this case, adipocyte maturation arrest will reduce triglyceride storage capacity in adipose tissue. Consequent increase of fatty acid spillover into plasma will increase substrate availability for triglyceride synthesis in other tissues, such as liver, skeletal muscle, myocardium, or even pancreas (16, 17). The absence of acquired resistance to the lipogenic effects of insulin in the liver (18) makes this organ a major target of ectopic fat deposition. Consequent fatty liver actively contributes to systemic abnormalities of glucose metabolism, dyslipidemia, and various components of the metabolic syndrome (19–24). Elevated circulating fatty acid contributes to systemic insulin resistance also by promoting skeletal muscle switch from glucose to fatty acid utilization (25). Therefore, a common root for the metabolic cluster of abnormalities we observe in systemic insulin resistance could be identified in the inability to store new triglyceride (adipocyte maturation arrest) in conditions of persisting caloric excess. In this scenario, patients are expected to have insulin resistance shortly after positive caloric balance has begun, even in the absence of significant weight gain. This would explain the observation of metabolically unhealthy lean persons.
Most of our patients likely belong to scenario B depicted in Fig. 1. According to this scenario, a defect in adipocyte maturation can occur at different stages of adipocyte growth. One can envision the possibility of genetic and metabolic regulator of the threshold at which triglyceride storage may come to a halt in a given adipocyte or group of adipocytes. The predicted result will be heterogeneity in adipocyte size distribution and degree of fat mass increase. Once this threshold is achieved, metabolic complications similar to scenario C will ensue. Importantly, metabolic complications will be observed at any level of either total or regional fat mass.
Clearly, the studies of Rogers et al. (11) together with the growing literature on mechanisms of adipose tissue dysfunction and its link with the pathogenesis of systemic insulin resistance beg the need for a shift in the focus of clinical research from fat mass/distribution to adipose tissue function. Translational applications of the proposed mechanisms of adipocyte maturation arrest have the potential to help clinicians in better identifying patients at risk for type 2 diabetes and cardiovascular disease. Better understanding of these mechanisms in relation to systemic insulin resistance will certainly lead to new therapeutic opportunities for prevention of these major causes of morbidity and mortality in our population.
Acknowledgments
This work is supported by National Institutes of Health Grants RO1-DK072158 and UL1-RR-029876 and Shriners Hospitals for Children Grant 71007.
Disclosure Summary: The author has nothing to declare.
For article see page E329
- BMI
- Body mass index
- ErbB1
- epidermal growth factor receptor-1.
References
- 1. Del Prato S. 2009. Role of glucotoxicity and lipotoxicity in the pathophysiology of type 2 diabetes mellitus and emerging treatment strategies. Diabet Med 26:1185–1192 [DOI] [PubMed] [Google Scholar]
- 2. Unger RH, Clark GO, Scherer PE, Orci L. 2010. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801:209–214 [DOI] [PubMed] [Google Scholar]
- 3. Abate N. 1996. Insulin resistance and obesity. The role of fat distribution pattern. Diabetes Care 19:292–294 [DOI] [PubMed] [Google Scholar]
- 4. DeFronzo RA. 2010. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grundy SM. 2004. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89:2595–2600 [DOI] [PubMed] [Google Scholar]
- 6. Zavaroni I, Bonora E, Pagliara M, Dall'Aglio E, Luchetti L, Buonanno G, Bonati PA, Bergonzani M, Gnudi L, Passeri M, Reaven G. 1989. Risk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose tolerance. N Engl J Med 320:702–706 [DOI] [PubMed] [Google Scholar]
- 7. Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. 2004. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109:433–438 [DOI] [PubMed] [Google Scholar]
- 8. Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkänen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE. 1996. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 45:742–748 [DOI] [PubMed] [Google Scholar]
- 9. Chandalia M, Lin P, Seenivasan T, Livingston EH, Snell PG, Grundy SM, Abate N. 2007. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS ONE 2:e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alberti KG, Zimmet P, Shaw J. 2005. The metabolic syndrome—a new worldwide definition. Lancet 366:1059–1062 [DOI] [PubMed] [Google Scholar]
- 11. Rogers C, Moukdar F, McGee MA, Davis B, Buehrer BM, Daniel KW, Collins S, Barakat H, Robidoux J. 2012. EGF receptor (ERBB1) abundance in adipose tissue is reduced in insulin-resistant and type 2 diabetic women. J Clin Endocrinol Metab 97:E329–E340 [DOI] [PubMed] [Google Scholar]
- 12. Pan W, Ciociola E, Saraf M, Tumurbaatar B, Tuvdendorj D, Prasad S, Chandalia M, Abate N. 2011. Metabolic consequences of ENPP1 over-expression in adipose tissue. Am J Physiol Endocrinol Metab 301:E901–E911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bays H, Abate N, Chandalia M. 2005. Adiposopathy: sick fat causes high blood sugar, high blood pressure and dyslipidemia. Future Cardiol 1:39–59 [DOI] [PubMed] [Google Scholar]
- 14. Reaven G. 2005. All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diab Vasc Dis Res 2:105–112 [DOI] [PubMed] [Google Scholar]
- 15. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. 2008. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 168:1617–1624 [DOI] [PubMed] [Google Scholar]
- 16. Perseghin G. 2011. Lipids in the wrong place: visceral fat and nonalcoholic steatohepatitis. Diabetes Care 34(Suppl 2):S367–S370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van der Zijl NJ, Goossens GH, Moors CC, van Raalte DH, Muskiet MH, Pouwels PJ, Blaak EE, Diamant M. 2011. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab 96:459–467 [DOI] [PubMed] [Google Scholar]
- 18. Li S, Brown MS, Goldstein JL. 2010. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA 107:3441–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aly FZ, Kleiner DE. 2011. Update on fatty liver disease and steatohepatitis. Adv Anat Pathol 18:294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cali AM, Caprio S. 2009. Ectopic fat deposition and the metabolic syndrome in obese children and adolescents. Horm Res 71(Suppl 1):2–7 [DOI] [PubMed] [Google Scholar]
- 21. Fabbrini E, Sullivan S, Klein S. 2010. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51:679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samuel VT, Petersen KF, Shulman GI. 2010. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375:2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volovelsky O, Weiss R. 2011. Fatty liver disease in obese children—relation to other metabolic risk factors. Int J Pediatr Obes 6(Suppl 1):59–64 [DOI] [PubMed] [Google Scholar]
- 24. Yki-Järvinen H. 2010. Liver fat in the pathogenesis of insulin resistance and type 2 diabetes. Dig Dis 28:203–209 [DOI] [PubMed] [Google Scholar]
- 25. Boden G, Chen X. 1995. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest 96:1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]