Abstract
Context:
Despite increased understanding of the pathogenesis and targets for thyroid cancer and other cancers, developing a new anticancer chemical agent remains an expensive and long process. An alternative approach is the exploitation of clinically used and/or bioactive compounds.
Objective:
Our objective was to identify agents with an anticancer effect in thyroid cancer cell lines using quantitative high-throughput screening (qHTS).
Design:
We used the newly assembled National Institutes of Health Chemical Genomic Center's pharmaceutical collection, which contains 2816 clinically approved drugs and bioactive compounds to perform qHTS.
Results:
Multiple agents, across a variety of therapeutic categories and with different modes of action, were found to have an antiproliferative effect. We found the following therapeutic categories were the most enriched categories with antiproliferative activity: cardiotonic and antiobesity agents. Sixteen agents had an efficacy of greater than 60% and a 50% inhibitory concentration (IC50) in the nanomolar range. We validated the results of the qHTS using two agents (bortezomib and ouabain) in additional cell lines representing different histological subtypes of thyroid cancer and with different mutations (BRAF V600E, RET/PTC1, p53, PTEN). Both agents induced apoptosis, and ouabain also caused cell cycle arrest.
Conclusions:
To our knowledge, this is the first study to use qHTS of a large drug library to identify candidate drugs for anticancer therapy. Our results indicate such a screening approach can lead to the discovery of novel agents in different therapeutic categories and drugs with nonclassic chemotherapy mode of action. Our approach could lead to drug repurposing and accelerate clinical trials of compounds with well-established pharmacokinetics and toxicity profiles.
The discovery of new chemical entities capable of modulating a disease remains to be a long and expensive process (1, 2). An alternative approach that is just beginning to be explored is the exploitation of compound collections composed of already established drugs that have been approved for clinical use for one or more indications. Although many of these compounds may have been optimized for specificity against a molecular target, drugs are known to show polypharmacology, which is oftentimes essential to the efficacy of the drug (3). Drug polypharmacology can also be used to discover new molecular targets for drugs and, when clinically relevant assays are used, potential new drug indications. This drug repositioning, repurposing, or indication switching has several important advantages over other drug discovery approaches (4–6). Because the pharmacokinetic, pharmacodynamic, and toxicity profiles of approved drugs are well known, immediate translation into a clinical phase II/III trial to test efficacy can occur. In addition, identifying approved drugs with previously uncharacterized anticancer properties has the potential to reveal new mechanisms of drug action and/or biological processes involved in carcinogenesis. Also, drugs found to have an anticancer effect may be used for chemoprevention of common cancers in high-risk populations (7–10).
Thyroid cancer is the most common endocrine malignancy and has an increasing incidence and dramatically different mortality rates based on specific histological subtypes (11). Approximately 10–15% of patients present or develop distant and locoregional metastases, extrathyroidal invasion, and poorly differentiated tumors. These patients have a high risk of recurrent disease and mortality (∼30–40%) (12). The use of cytotoxic drugs and external radiation has been palliative, at best, in patients with advanced thyroid cancer. In fact, the only drug approved for thyroid cancer of follicular cell origin, by the U.S. Food and Drug Administration, is doxorubicin, which has poor efficacy (13). Thus, new effective therapeutic alternatives are needed.
In this study, we used a high-throughput screening (HTS) approach of clinically approved drugs in thyroid cancer cells. To obtain pharmacological information from the screening data, we employed a titration-based screening paradigm, quantitative HTS (qHTS) where compounds are screened at multiple concentrations (14). In this way, concentration-response curves were derived directly from the HTS, allowing both potency and efficacy values to be used to select compounds and create robust bioactivity profiles (15, 16). By employing this approach and the available information associated with drug annotations, we found numerous agents across different therapeutic categories and mode of action that had an antiproliferative effect. Sixteen agents showed an efficacy of greater than 60% with a 50% inhibitory concentration (IC50) in the nanomolar range. We further validated the anticancer effect of two agents (bortezomib and ouabain), identified by our qHTS, in additional thyroid cancer cell lines representing different histological subtypes of thyroid cancer and with different mutations (BRAF V600E, RET/PTC1, p53, and PTEN) common in thyroid cancer.
Materials and Methods
Thyroid cell culture
Human papillary thyroid cancer cell line TPC-1, follicular thyroid cancer cell line FTC-133, and Hürthle cell carcinoma cell line XTC-1 were maintained in DMEM supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 μg/ml), fungizone (250 ng/ml), TSH (10 IU/liter), and insulin (10 μg/ml) in a 5% CO2 atmosphere at 37 C. The undifferentiated human thyroid cancer cell line 8505C was maintained in Eagle's MEM supplemented with 10% FCS, 2 mm glutamine, and 1% nonessential amino acids. The TPC-1 cell line was provided by Dr. Nabuo Satoh (Japan), the FTC-133 cell line by Dr. Peter Goretzki (Germany), and the XTC-1 cell line by Dr. Orlo H. Clark (California). The 8505C cell line was obtained from European Collection of Cell Culture (St. Louis, MO), and the NFκb, Mrc5, Messangial, Uch, ccl4b, and Lam cell lines were from American Type Culture Collection (Manassas, VA). The European Collection of Cell Culture and American Type Culture Collection characterize cell lines by short-tandem repeat profiling, cell morphology, and karyotyping. All experiments were performed using cells at 25 passages or fewer.
National Institutes of Health Chemical Genomic Center Pharmaceutical library screening
The National Institutes of Health Chemical Genomic Center Pharmaceutical Collection (NPC) consists of 2816 small-molecule compounds with 52% of the drugs approved for human or animal use by the U.S. Food and Drug Administration (17). The remaining drugs are either approved for use in other countries, such as Europe, Canada, or Japan, or are compounds that have been tested in clinical trials. Additional detailed information on the drugs can be found at http://spotlite.nih.gov/npc/.
The NPC library was prepared as 15 interplate titrations, which were serially diluted 1:2.236 in dimethylsulfoxide (DMSO) (Thermo Fisher Scientific, Waltham, MA) in 384-well plates. The stock concentrations of the test compounds ranged from 10 mm to 0.13 μm. Transfer of the diluted compounds from 384-well plates to 1536-well plates was performed using an Evolution P3 system (PerkinElmer Life and Analytical Sciences, Waltham, MA). Each treatment plate included concurrent DMSO and positive control wells and concentration-response titrations of controls, all occupying columns 1–4. During screening, the compound plates were sealed and kept at room temperature, whereas other copies were maintained at −80 C for storage.
Quantitative high-throughput proliferation assay
Cell viability after compound treatment was measured using a luciferase-coupled ATP quantitation assay (CellTiter-Glo; Promega, Madison, WI) in TPC-1 cells. The change of intracellular ATP content indicates the number of metabolically competent cells after compound treatment. TPC-1 cells were harvested from T225 flask and resuspended in 2% FCS DMEM/F12 media at 120,000 cells/ml. Then 5 μl of resuspended cells was dispensed into each well of white, solid-bottom, 1536-well tissue culture-treated plates using a Multidrop Combi dispenser. After overnight culture at 37 C with 5% CO2, a total of 23 nl of compounds at eight selected concentrations from the NPC or positive control (10 mm stock of doxorubicin hydrochloride) in DMSO was transferred to each well of the assay plate using a pintool (Kalypsys, San Diego, CA), and the plates were further incubated at 37 C with 5% CO2 for 48 h. Then 4 μl of CellTilter-Glo luminescent substrate mix (Promega) was added to each well. The plate was incubated at room temperature for 15 min. The plates were measured on a ViewLux plate reader (PerkinElmer) with clear filter. The final concentration of the compounds in the 5-μl assay volume ranged from 0.5 nm to 46 μm.
Validation cell proliferation assay
Cell proliferation assays were performed in quadruplicates. Cells were plated in 96-well black plates at a concentration of 2 × 103 to 4× 103 cells per well depending on the cell line in 100 μl culture medium. After 24 h (d 0), 100 μl fresh culture medium containing the indicated drugs or corresponding vehicles was added into each well. CyQuant (Invitrogen, Carlsbad, CA) proliferation assays were performed at d 0, 1, 2, 3, 4, and 6 according to the manufacturer's instructions. The cell densities were determined using a 96-well fluorescence microplate reader (Molecular Devices, Sunnyvale, CA) at 485 nm/538 nm. For each drug and cell line tested, the experiment was repeated at least three times.
Drug testing on tumor spheroids
FTC-133 and XTC-1 form tumor spheroids in suspension culture. To generate spheroids, FTC-133 and XTC-1 cells were plated in 24-well Ultra Low Cluster plates (Costar, Corning, NY) at a concentration of 4 × 104 cells per well and incubated at 37 C in 5% CO2 for 2 wk with media changed every 2–3 d. After 2 wk culture, tumor spheroids were photographed under the microscope and treated with the indicated concentrations of drugs or their corresponding vehicle. For each round of testing, quadruplicate wells were used for each testing concentration. The tumor spheroids were continuously treated for 2–3 wk and photographed under the microscope. For each compound and each cell line tested, the experiments were repeated at least three times.
Apoptosis assay
To determine whether drug treatment resulted in cell death by inducing apoptosis, we used the Caspase-Glo 3/7 assay (Promega) to measure caspase activity. Cells were plated in 96-well white plates at 2.2 × 103 to 4 × 103 cells per well depending on the cell line in 200 μl culture medium. After 2 d (d 0), 100 μl culture medium was removed from each well, and 100 μl fresh culture medium containing the double concentration of the indicated drugs (40 nm bortezomib or 100 nm ouabain) or corresponding vehicles was added into each well. After another 48 h, caspase 3/7 activity was determined using the Caspase-Glo 3/7 assay kit (Promega) according to manufacturer's instruction. The relative luminescence unit (which is proportional to caspase 3/7 activity) was calculated based on the cell numbers.
Cell cycle analysis
Cells were plated in six-well plates at 6 × 104 to 12 × 104 cells per well depending on the cell line in 3 ml culture medium. Two days later (d 0), 1.5 ml culture medium was removed from each well, and 1.5 ml fresh culture medium (for control wells) or the fresh medium containing 100 nm ouabain (for treatment wells) was added into each well. Three days later, cells were harvested, washed, resuspended with PBS, and fixed with ice-cold 70% ethanol at 4 C. After washing with PBS, ribonuclease A was added to the cell suspension and incubated at 37 C for 20 min. Then, propidium iodide (PI) (50 μg/ml in PBS) was added, and samples were stored at 4 C until analysis.
Flow cytometric analysis for cell cycle was performed on a FACScan using CellQuest software (BD Biosciences, San Jose, CA). Data files were generated for more than 10,000 events (cells) per sample gated on single cells. Doublets, cell clumps, and debris were excluded by PI fluorescence pulse width and pulse area measurements. Cell cycle analysis on the gated PI distribution was performed using Modfit software (Verity Software House, Inc., Topsham, ME).
Data analysis
To determine compound activity in the qHTS assay, the titration-response data for each sample was plotted and modeled by a four-parameter logistic fit yielding IC50 (concentration of half-maximal inhibition) and efficacy (maximal response) values. Raw plate reads for each titration point were first normalized relative to positive control (doxorubicin hydrochloride, 100% inhibition) and DMSO only wells (basal, 0%). Curve fits were then classified by the criteria previously described (14). In brief, class 1.1 and 1.2 were the highest-confidence curves containing upper and lower asymptotes with efficacy of 80% or higher and lower than 80%, respectively. Class 2.1 and 2.2 were incomplete curves having only one asymptote with efficacy of 80% or higher and lower than 80%, respectively. Class 3 curves showed activity at only the highest concentration or were poorly fit. Class 4 curves were inactive, having a curve fit of insufficient efficacy or lacking a fit altogether.
There were a total of 22 plates in the primary qHTS screen, which included 16 plates corresponding to NPC library set and four DMSO plates. Compounds from the primary qHTS screen were classified into three categories according to the quality of curve fit and efficacy: active, including compounds in curve class 1.1, 1.2, 2.1, and 2.2 curves with efficacy higher than 60%; inactive, including compounds with class 4 curves; and inconclusive, including all other compounds including those shallow curves and curves with single point activity.
Compounds were clustered hierarchically with Spotfire DecisionSite version 8.2 based on their activity outcomes from the primary screen across a wide variety of cell viability assays against different cell lines or primary culture cells from patients. Each compound was converted into an integer that represents its activity outcome in each cell viability assay. Integer 1 represents compounds in the active category that correspond to red in the heatmap (Fig. 1). Integer 2 represents compounds in inconclusive category that corresponds to light red in the heatmap. If a compound didn't show any activity in an assay, integer 3 was assigned to such category, and it was indicated as white in the heatmap. Compounds not tested in a cell line assay were labeled as 4 and are represented as gray in the heatmap. Different activity outcomes were observed from each cell viability assay, and the compounds were categorized based on the similarity metric derived from the activity profiles.
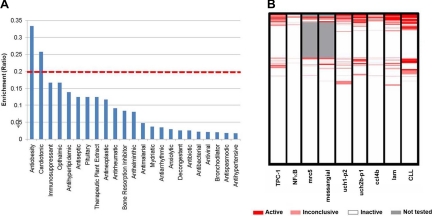
Fig. 1.
qHTS of NPC drug library. A, Enrichment analysis of active agents in each drug category. To determine whether the active compounds were predominantly identified from a certain therapeutic category, we performed an enrichment analysis of the drug library. The 2816 compounds in the NPC collection were classified into different therapeutic categories based on their pharmaceutical indications. The red dashed line indicates enrichment ratio of greater than 20% in the category of active drugs. B, The comparison of antiproliferative activity of each drug in normal and cancer cell lines and primary culture of chronic lymphocytic leukemia cells. NFκB is a cervical cancer cell line; Mrc5 is a lung tissue cell line, fetus, normal; messangial is a kidney cell line, normal; Uch and ccl4b is a chordoma, tumor from bone in skull base or spine; Lam is a lung smooth muscle proliferation, benign tumor, human patient cell line; and CLL is a chronic lymphocytic leukemia. Each compound was converted into an integer that represents its activity outcome in each cell viability assay. Integer 1 represents compounds in the active category, which corresponds to red in the heatmap. Integer 2 represents compounds in the inconclusive category, which corresponds to light red in the heatmap. If a compound didn't show any activity in an assay, integer 3 was assigned to such category, indicated as white in the heatmap. Compounds not tested in a cell line assay were labeled as 4 and are represented as gray in the heatmap. Different activity outcomes were observed from each cell viability assay, and the compounds were categorized based on the similarity metric derived from the activity profiles.
To determine whether the active compounds were predominantly identified from a certain therapeutic category, an enrichment analysis was implemented against the drug library. The 2816 compounds in the NPC collection were classified into different therapeutic categories based on their pharmaceutical indications. The enrichment score was calculated from the following formula: E = a/n, where a is the number of actives and n is the total number of drugs in each therapeutic category.
For validation of cell proliferation assays, t test was used for statistical analysis.
Results
HTS of clinical drug library
To identify small-molecule drugs that can act as inhibitors of thyroid cancer cell proliferation, we screened the NPC using qHTS. This strategy used concentration-response curve information to identify 244 active compounds in the TPC-1 thyroid cancer cell line. Of these, 40 compounds had high-confidence activity (Table 1). Sixteen of the 40 compounds with high-confidence activity had efficacy higher than 60% and a potency (IC50) less than 1.7 μm (Table 2). The 40 compounds with high-confidence activity were distributed across various therapeutic categories and have different modes of drug action. Doxorubicin, a drug currently approved for advanced thyroid cancer therapy, was not among the 40 drugs identified to have high-confidence activity.
Table 1.
Compounds with high-confidence antiproliferative activity in thyroid cancer cells
| Drug | Efficacy | Curve classa | IC50 (μm) |
|---|---|---|---|
| 6-Mercaptopurine monohydrate | −66 | −1.2 | 2.11 |
| Acivicin | −62 | −2.2 | 13.34 |
| Adapalene | −122 | −2.1 | 18.84 |
| Alazanini triclofenas | −114 | −2.1 | 21.14 |
| Amsacrine hydrochloride | −120 | −2.1 | 23.72 |
| Anazolenum natricum | −62 | −2.2 | 14.96 |
| a-Solanine | −130 | −2.1 | 26.61 |
| Atorvastatin | −62 | −2.2 | 14.96 |
| Auranofin | −93 | −2.3 | 2.66 |
| Azacitidine | −72 | −2.2 | 13.34 |
| Azathioprine | −61 | −1.2 | 4.22 |
| Benzylhydrochlorothiazide | −115 | −2.1 | 23.72 |
| Bortezomib | −94 | −1.1 | 0.02 |
| Broquinaldol | −93 | −2.2 | 21.14 |
| Bufogenin | −66 | −1.2 | 0.75 |
| C.I. basic violet 14, free base | −65 | −2.2 | 21.14 |
| Cantharidin | −69 | −1.2 | 1.28 |
| Carboquone | −81 | −1.1 | 1.50 |
| Carminomycin | −61 | −1.2 | 0.47 |
| Chlorure de methylrosanilinum | −120 | −2.1 | 21.14 |
| Clofoctol | −104 | −2.1 | 21.14 |
| Compactin | −67 | −2.2 | 6.68 |
| Deslanoside | −65 | −1.2 | 0.13 |
| Deslorelin acetate | −94 | −2.1 | 11.89 |
| Dithiazanine iodide | −122 | −2.1 | 23.72 |
| Ebastine | −126 | −2.1 | 23.72 |
| Floxuridine | −67 | −1.2 | 0.60 |
| Fluvastatina | −68 | −1.2 | 1.19 |
| Gramicidin | −95 | −2.2 | 18.84 |
| Homoharringtonine | −78 | −2.2 | 3.76 |
| Idarubicin hydrochloride | −93 | −2.1 | 0.94 |
| Ivermectin | −119 | −2.1 | 23.72 |
| Lanatoside A | −63 | −1.2 | 0.09 |
| Lanatoside C | −78 | −1.2 | 0.17 |
| Lestaurtinib | −112 | −2.1 | 13.34 |
| Lissamine green B | −77 | −2.2 | 11.89 |
| Lovastatin | −68 | −1.2 | 3.35 |
| Mecetronium ethylsulfate | −101 | −2.1 | 18.84 |
| Mercufenol chloride | −101 | −2.1 | 6.68 |
| Methyl violet | −108 | −2.1 | 16.79 |
| Mibefradil dihydrochloride | −118 | −2.1 | 23.72 |
| Mitomycin C | −93 | −2.2 | 23.72 |
| Mitoxantrone dihydrochloride | −90 | −2.2 | 8.05 |
| Niclosamide | −64 | −1.2 | 0.27 |
| Nitrovin | −93 | −2.1 | 7.50 |
| Nitroxoline | −80 | −2.2 | 13.34 |
| Ouabain | −71 | −1.1 | 0.05 |
| Para−chloromercuriphenol | −98 | −2.1 | 9.44 |
| Phenylmercuric acetate | −99 | −1.1 | 2.66 |
| Phenylmercuric borate | −104 | −2.1 | 9.44 |
| Potassium dichromate | −126 | −2.1 | 21.14 |
| Proflavine hemisulfate | −75 | −2.2 | 21.14 |
| Proscillaridin A | −60 | −1.2 | 0.01 |
| Puromycin hydrochloride | −94 | −1.1 | 5.31 |
| Pyrvinium pamoate | −109 | −2.1 | 9.03 |
| Quinaldine blue | −122 | −2.1 | 26.61 |
| Raloxifene hydrochloride | −121 | −2.1 | 23.72 |
| Rimonabant | −89 | −2.2 | 23.72 |
| Sanguinarine | −91 | −2.1 | 18.84 |
| Simvastatin | −77 | −2.2 | 8.05 |
| Sodium Dichromate Dihydrate | −119 | −2.1 | 23.72 |
| Sorafenib tosylate | −97 | −2.2 | 26.61 |
| Suberoylanilide hydroxamic acid | −65 | −2.2 | 11.89 |
| Tannic acid | −87 | −2.2 | 21.14 |
| Teniposide | −106 | −2.1 | 4.73 |
| Tetradonii bromidum | −83 | −2.2 | 26.61 |
| Thimerosal | −93 | −1.1 | 2.66 |
| Thionosine | −68 | −1.2 | 1.68 |
| Tomatine | −92 | −2.1 | 7.50 |
| Trequinsin hydrochloride | −123 | −2.1 | 26.61 |
| Tribromsalan | −69 | −2.2 | 11.89 |
| Trypan blue | −94 | −2.2 | 10.59 |
| Tyrothricin | −107 | −2.1 | 7.50 |
| Valrubicin | −73 | −2.2 | 16.79 |
| Vandetanib | −70 | −2.2 | 14.96 |
| Vinblastine sulfate | −64 | −1.2 | 0.21 |
| Zinc pyrithione | −91 | −1.1 | 2.37 |
Highest-confidence curve classes (see also Materials and Methods for definition).
Table 2.
Candidate agent preparation, route of administration, half-maximal inhibitory concentration (IC50), maximal and sustained serum concentrations after systemic delivery, and drug half-life in humans
| Drug | Efficacy | Route | IC50 (μm) | Maximal serum concentration | Sustained serum concentration | Elimination half-life | Therapeutic category | Mode of action |
|---|---|---|---|---|---|---|---|---|
| Bortezomib | −94.38 | iv | 0.02 | 0.16–0.31 μm | 0.078–0.156 mm | 9–15 h | Antineoplastic | Proteasome inhibitor |
| Bufogenin | −65.83 | NA | 0.75 | NA | NA | NA | Cardiotonic, respiratory stimulants | Steroids |
| Cantharidin | −69.03 | Topical | 1.28 | NA | NA | NA | Antiviral | PP-1 and PP-2A inhibitor |
| Carboquone | −81.00 | NA | 1.5 | NA | No available | NA | Antineoplastic | Alkylating agents |
| Carminomycin | −61.50 | iv | 0.47 | NA | NA | NA | Antineoplastic, anthracycline antibiotics | DNA synthesis inhibitor |
| Deslanoside | −64.83 | NA | 0.13 | 0.16 μm | 0.0021 mm | 51 h | Cardiotonic | NA+/K+ ATPase inhibition |
| Floxuridine | −66.83 | Arterial | 0.6 | NA | NA | NA | Antineoplastic | Pyrimidine antagonists |
| Fluvastatin | −67.78 | PO | 1.19 | 0.51–1.07 μm | 0.022–0.03 μm | 2.5–3 h | Antilipid | Apoptosis inducer |
| HMG-CoA reductase inhibitor | ||||||||
| Idarubicin | −92.87 | iv | 0.94 | 0.04–0.08 μm | 0.004–0.01 μm | 16–17.5 h | Antineoplastic | DNA polymerase inhibitor, Antimetabolites |
| Lanatoside A | −62.74 | NA | 0.09 | NA | NA | NA | Cardiotonic | NA+/K+ ATPase inhibition |
| Lanatoside C | −78.28 | NA | 0.17 | NA | NA | NA | Cardiotonic | NA+/K+ ATPase inhibition |
| Niclosamide | −64.31 | PO/iv | 0.27 | NA | NA | NA | Anthelmintic | Niclosamide uncouples oxidative phosphorylation |
| Ouabain | −70.50 | iv | 0.05 | 0.128 μm | 0.0004 μm | 18 h | Cardiotonic | NA+/K+ ATPase inhibition |
| Proscillaridin A | −60.34 | iv/PO | 0.01 | 0.019 μm by iv; 0.0019 μm by PO | NA | 23–33 h | Cardiotonic | Cardiac glycoside |
| Thioinosine | −67.79 | NA | 1.68 | NA | NA | NA | Antineoplastic | Immunosuppressant |
| Vinblastine sulfate | −64.24 | iv | 0.21 | NA | NA | 24–25 h | Antineoplastic | Antimitotic drugs, tubulin polymerization inhibitor |
NA, Not available; PO, by mouth; HMG-CoA, 3-hydroxyl-methyl-glutaryl-CoA.
Enrichment analysis of active agents in each drug category indicated that only a small proportion (12%) of antineoplastic drugs showed activity in the thyroid cancer cells (Fig. 1A). In contrast, a higher percentage (>25%) of drugs that belonged to antiobesity and cardiotonic groups had potent activity (P < 0.05). To test whether these drugs have specific anticancer activity, we compared the antiproliferative activity of the same drug library in several normal and cancer cell lines and primary culture of chronic lymphocytic leukemia cells from patients. As shown in Fig. 1B, many compounds with activity against TPC-1 cells showed no cytotoxicity when tested in normal cells (Mrc5, Messangial). In addition, when compared with other cancer types, a distinct activity pattern was observed for TPC-1 cells; specifically, many drugs that showed antiproliferative effect in other cancer cell types tested did not have activity in TPC-1 cells and vice versa (Fig. 1B).
Validation of candidate anticancer drugs from qHTS
Those drugs from the qHTS that had an antiproliferative efficacy of more than 60% and IC50 in the nanomolar concentration range, which were clinically achievable serum concentrations, were chosen for further exploration. We analyzed the available pharmacokinetic data for these candidate agents, including the currently available preparation and route of administration, the IC50, the maximal and sustained serum concentrations after systemic delivery, and the drug's half-life in humans (Table 2). Based on this analysis, we selected two drugs, bortezomib and ouabain, for validation of the qHTS results.
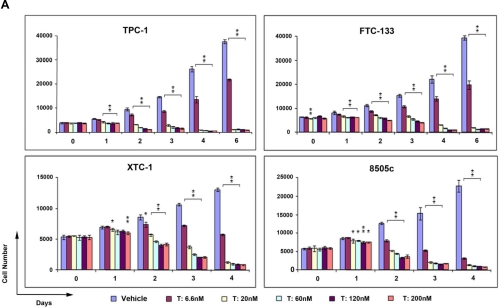
To confirm bortezomib's antiproliferative effect in thyroid cancer cells from the qHTS, we validated this drug using four different cell lines representing all of the major histological and aggressive subtypes of thyroid cancer and with different somatic mutations (8505c for anaplastic cancer with BRAF V600E and p53 mutation, TPC-1 for papillary thyroid cancer with RET/PTC1 rearrangement, XTC-1 for Hürthle cell carcinoma, and FTC-133 for follicular thyroid carcinoma with p53 and PTEN mutation) (18–20). As shown in Fig. 2, treatment with bortezomib had a dramatic effect on cellular proliferation in all of the cell lines. Bortezomib, as low as 6.6 nm, resulted in 40–50% growth inhibition. At 20 nm, bortezomib not only inhibited cancer cell growth but also caused cell death of more than 90%.
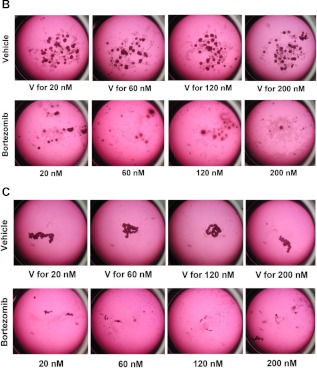
Fig. 2.
Bortezomib treatment resulted in thyroid cancer cell death. A, Cancer cells representing different histological subtypes of thyroid cancer were treated with bortezomib at the indicated concentrations (T). Cell proliferation rates were determined using CyQuant proliferation assay kit. Error bars indicate mean ± sd. *, P values between 0.05 and 0.01; **, P < 0.01. B and C, Tumor spheroids generated from FTC-133 cells (B) or XTC-1 cells (C) were treated with bortezomib at the indicated concentrations or incubated with the medium containing the same concentrations of vehicle (V). The photographs were taken after 2–3 wk of treatment. Magnification, ×6.3.
Monolayer cell cultures can provide cell-specific response to drugs but does not account for tissue-related functions. On the other hand, three-dimensional multicellular tumor spheroids (MCTS) are thought to be better models of the in vivo behavior of tumor cells, and therefore this assay can provide a better estimation for anticancer efficacy of drugs (21). Therefore, we also tested the effect of bortezomib on thyroid cancer MCTS. FTC-133 and XTC-1 cell lines form MCTS, and these spheroids continuously grew when treated with vehicle control medium. Strikingly, bortezomib treatment not only inhibited growth of tumor spheroids but also resulted in the cell death of preexisting MCTS (Fig. 2).
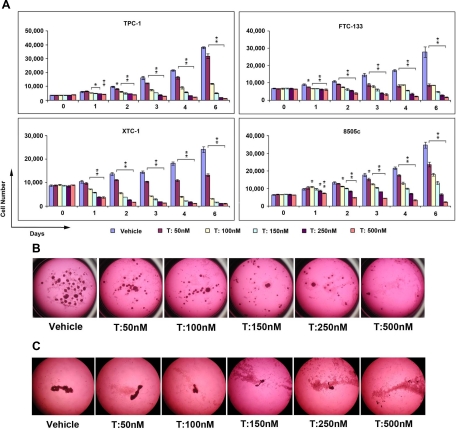
Another drug selected for validation of the qHTS results was ouabain. We also validated the effect of ouabain in the four different thyroid cancer cell lines. As shown in Fig. 3, ouabain inhibited cell growth in all four cell lines. The BRAF mutant cell line, 8505c, showed less growth-inhibitory effect to ouabain treatment. The effect of ouabain was further validated by using MCTS generated from FTC-133 and XTC-1 cells. Ouabain at clinical achievable concentrations also inhibited cancer cell spheroid growth and caused cell death in FTC-133 cells and XTC-1 cells (Fig. 3).
Fig. 3.
The effect of ouabain on thyroid cancer cell proliferation. A, Cells were treated with ouabain at the indicated concentrations (T), and proliferation rates were tested using CyQuant proliferation assay kit. Error bars indicate mean ± sd. *, P values between 0.05 and 0.01; *, P < 0.01. Ouabain treatment inhibited thyroid cancer cell spheroid growth. B and C, Tumor spheroids from FTC-133 (B) and XTC-1 (C) cells were treated with ouabain at the indicated concentrations. The photographs were taken after 2–3 wk of treatment. Magnification, ×6.3.
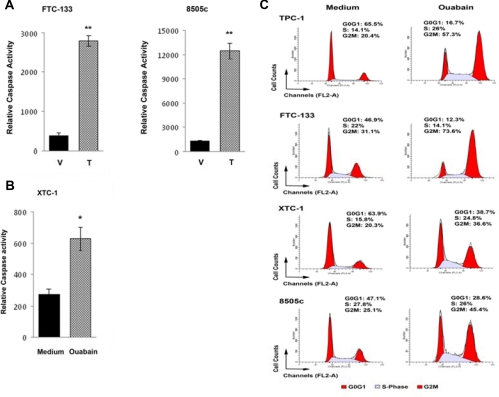
Because we saw bortezomib- and ouabain-induced cell death in both the monolayer and MCTS validation of the qHTS results, we determined whether this effect was mediated through apoptosis. We measured caspase activity in the thyroid cancer cell lines with and without bortezomib and ouabain treatment and found 4- to 8-fold and 2-fold increase in caspase activity, respectively, with treatment (Fig. 4, A and B). We also tested the effect of bortezomib and ouabain on cell cycle progression. We found ouabain caused cell cycle arrest by increasing the number of cells in G2M (Fig. 4C).
Fig. 4.
Effect of bortezomib and ouabain treatment on caspase activity. A and B, Caspase 3/7 activity in thyroid cancer cells was increased with bortezomib (A) and ouabain (B) treatment. FTC-133 and 8505c cells were plated in 96-well plates and then treated with 20 nm bortezomib (T) or vehicle (V). XTC-1 cells were plated in 96-well plates and treated with ouabain at 50 nm concentration. At 48 h later, caspase 3/7 activity was determined. Error bars indicate mean ± sd. **, Mean P < 0.01. C, Ouabain induces cell cycle arrest in thyroid cancer cell lines by increasing the number of cells in G2M.
Discussion
In this study, we used a qHTS approach in a large collection of clinically used drugs for those that had antiproliferative activity in thyroid cancer cells. This screen provided potency and efficacy information that allowed identification of multiple agents across different therapeutic categories having various modes of action. Unexpectedly, the active agents were enriched for drugs in the antiobesity and cardiotonic therapeutic categories. Of the 16 agents that had efficacy higher than 60% and an IC50 in the nanomolar range, we selected two agents to validate the qHTS results: bortezomib (a proteasome inhibitor) used to treat treatment-refractory multiple myeloma and ouabain (a Na+/K+ ATPase inhibitor) previously used for cardiovascular disease (heart failure and myocardial infarction) (22, 23). In multiple thyroid cancer cell lines, representing different histological subtypes of thyroid cancer and having different mutations (BRAF V600E, RET/PTC1, p53, PTEN), both of these drugs significantly inhibited proliferation, reduced MCTS, and caused cell death by inducing apoptosis and/or cell cycle arrest.
To our knowledge, this is the first study to use such a large collection of clinical drugs to test antiproliferative effect in cancer cells. The identification of novel anticancer drugs using this approach has several important ramifications. First, the compounds found to have potent activity in our screen represent possible opportunities to repurpose these drugs for the treatment of patients with aggressive recurrent or metastatic thyroid cancer that is refractory to standard therapy. Moreover, implementing such a strategy allows for the selection of drugs with known and minimal toxicity but with high anticancer activity. Second, the drug therapeutic categories and drug mode of action of the active compounds represent a unique window on formulating a hypothesis as to whether these factors influence tumor cell biology both in the pathogenesis of cancer and its treatment and for chemoprevention of common cancers in high-risk populations as has been done for example with COX2 inhibitors (24–28).
It is interesting that a high proportion of drugs not known to have an antineoplastic effect (therapeutic category) had potent activity in our screening. The two most enriched categories were antiobesity and cardiotonic drugs. There have been some recent studies suggesting that drugs with antiobesity and cardiotonic (Na+/K+-ATPase) activity may have anticancer effects (29–31). Thus, these drug categories represent novel classes of drugs that warrant further study to test their anticancer effect in other types of malignancy. Moreover, the mode of action of the drug may suggest pathways altered in carcinogenesis. For example, because there is an association between obesity (higher body mass index) and a higher risk of thyroid cancer (32), the identification of anticancer agents with well-established antiobesity activity provides an opportunity to explore whether the pathways affected by these drugs are involved in cancer initiation and/or progression.
We used clinically relevant filter criteria to identify drugs that could readily be considered for clinical trials in patients with incurable thyroid cancer. We also selected two drugs to validate the results of the qHTS in four thyroid cancer cell lines with different histological subtypes, aggressiveness, and genetic alterations common in patients with aggressive thyroid cancer (33). Both drugs, bortezomib and ouabain, were validated to have an antiproliferative effect. Moreover, in the MCTS model, which more closely recapitulates an in vivo solid tumor, we observed potent anticancer effect of both of these agents. In fact, there is a clinical trial currently open to test the efficacy of bortezomib in patients with advanced and metastatic thyroid cancer (34). These findings suggest that our clinical drug library and high-throughput assay are likely to be a viable approach for screening drug activity in other cancer cells from different tumor types. Drug toxicity is one of the most common reasons for the failure of a drug candidate. Screening existing drugs for new activity may be helpful because the toxicity of these drugs is well characterized and the agents with the lowest toxicity profile can be selected. Furthermore, agents with clinical achievable concentrations can be determined after HTS and the selection of those agents with potent activity well below the clinical sustained and peak concentrations of a drug is also a very attractive approach to use for cancer therapeutics.
In summary, we have identified, using qHTS, multiple agents across different therapeutic categories and mode of action with potent anticancer activity. We validated the results of the qHTS using two agents (bortezomib and ouabain) in additional thyroid cancer cell lines representing different histological subtypes of thyroid cancer and with different mutations. We believe our results support the use of qHTS to obtain pharmacological information rapidly from specialized libraries such as the NPC library. Employing a panel of different cancer cell lines or primary tumor cultures with a common assay format can identify drugs with potent and selective activity that could be translated into clinical trials for patients with incurable malignancies or those with cancers refractory to standard therapy. We believe qHTS of clinically approved drugs has important clinical ramifications. These types of reproducible assays could be used for identifying therapeutics not only for cancer but also for many other diseases such as diabetes, obesity, and infectious disease. Clinicians can more readily translate these findings into therapy given the drug characteristics are well known be it in developing clinical trials or in some cases for off-label use.
Acknowledgments
This research was supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Disclosure Summary: The authors have no conflict of interest to declare.
Footnotes
- DMSO
- Dimethylsulfoxide
- FCS
- fetal calf serum
- HTS
- high-throughput screening
- qHTS
- quantitative HTS
- MCTS
- multicellular tumor spheroids
- NPC
- National Institutes of Health Chemical Genomic Center Pharmaceutical Collection
- PI
- propidium iodide.
References
- 1. Fojo T, Grady C. 2009. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst 101:1044–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munos B. 2009. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov 8:959–968 [DOI] [PubMed] [Google Scholar]
- 3. Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R, Glennon RA, Hert J, Thomas KL, Edwards DD, Shoichet BK, Roth BL. 2009. Predicting new molecular targets for known drugs. Nature 462:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller SC, Huang R, Sakamuru S, Shukla SJ, Attene-Ramos MS, Shinn P, Van Leer D, Leister W, Austin CP, Xia M. 2010. Identification of known drugs that act as inhibitors of NF-κB signaling and their mechanism of action. Biochem Pharmacol 79:1272–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shahinas D, Liang M, Datti A, Pillai DR. 2010. A repurposing strategy identifies novel synergistic inhibitors of Plasmodium falciparum heat shock protein 90. J Med Chem 53:3552–3557 [DOI] [PubMed] [Google Scholar]
- 6. Shum D, Smith JL, Hirsch AJ, Bhinder B, Radu C, Stein DA, Nelson JA, Früh K, Djaballah H. 2010. High-content assay to identify inhibitors of dengue virus infection. Assay Drug Dev Technol 8:553–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Platz EA, Yegnasubramanian S, Liu JO, Chong CR, Shim JS, Kenfield SA, Stampfer MJ, Willett WC, Giovannucci E, Nelson WG. 2011. A novel two-stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer Discovery 1:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan XL, Reid-Lombardo KM, Bamlet WR, Oberg AL, Robinson DP, Anderson KE, Petersen GM. 2011. Aspirin, nonsteroidal anti-inflammatory drugs (NSAID), acetaminophen, and pancreatic cancer risk: a clinic-based case-control study. Cancer Prev Res (Phila) 4:1835–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang F, Lv ZS, Fu YK. 2010. Nonsteroidal anti-inflammatory drugs and esophageal inflammation - Barrett's esophagus - adenocarcinoma sequence: a meta-analysis. Dis Esophagus 10.1111/j.1442-2050.2010.01153.x [DOI] [PubMed] [Google Scholar]
- 10. Harris RE. 2009. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology 17:55–67 [DOI] [PubMed] [Google Scholar]
- 11. Robbins J, Merino MJ, Boice JD, Jr, Ron E, Ain KB, Alexander HR, Norton JA, Reynolds J. 1991. Thyroid cancer: a lethal endocrine neoplasm. Ann Intern Med 115:133–147 [DOI] [PubMed] [Google Scholar]
- 12. Treseler PA, Clark OH. 1997. Prognostic factors in thyroid carcinoma. Surg Oncol Clin N Am 6:555–598 [PubMed] [Google Scholar]
- 13. Sherman SI. 2010. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin Oncol (R Coll Radiol) 22:464–468 [DOI] [PubMed] [Google Scholar]
- 14. Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. 2006. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci USA 103:11473–11478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shukla SJ, Sakamuru S, Huang R, Moeller TA, Shinn P, Vanleer D, Auld DS, Austin CP, Xia M. 2011. Identification of clinically used drugs that activate pregnane X receptors. Drug Metab Dispos 39:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Veith H, Southall N, Huang R, James T, Fayne D, Artemenko N, Shen M, Inglese J, Austin CP, Lloyd DG, Auld DS. 2009. Comprehensive characterization of cytochrome P450 isozyme selectivity across chemical libraries. Nat Biotechnol 27:1050–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang R, Southall N, Wang Y, Yasgar A, Shinn P, Jadhav A, Nguyen DT, Austin CP. 2011. The NCGC Pharmaceutical Collection: a comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci Transl Med 3:80ps16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weng LP, Gimm O, Kum JB, Smith WM, Zhou XP, Wynford-Thomas D, Leone G, Eng C. 2001. Transient ectopic expression of PTEN in thyroid cancer cell lines induces cell cycle arrest and cell type-dependent cell death. Hum Mol Genet 10:251–258 [DOI] [PubMed] [Google Scholar]
- 19. Jossart GH, Epstein HD, Shaver JK, Weier HU, Greulich KM, Tezelman S, Grossman RF, Siperstein AE, Duh QY, Clark OH. 1996. Immunocytochemical detection of p53 in human thyroid carcinomas is associated with mutation and immortalization of cell lines. J Clin Endocrinol Metab 81:3498–3504 [DOI] [PubMed] [Google Scholar]
- 20. Meireles AM, Preto A, Rocha AS, Rebocho AP, Máximo V, Pereira-Castro I, Moreira S, Feijão T, Botelho T, Marques R, Trovisco V, Cirnes L, Alves C, Velho S, Soares P, Sobrinho-Simões M. 2007. Molecular and genotypic characterization of human thyroid follicular cell carcinoma-derived cell lines. Thyroid 17:707–715 [DOI] [PubMed] [Google Scholar]
- 21. Ho WJ, Pham EA, Kim JW, Ng CW, Kim JH, Kamei DT, Wu BM. 2010. Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs. Cancer Sci 101:2637–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludwig H, Khayat D, Giaccone G, Facon T. 2005. Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer 104:1794–1807 [DOI] [PubMed] [Google Scholar]
- 23. Fürstenwerth H. 2010. Ouabain: the insulin of the heart. Int J Clin Pract 64:1591–1594 [DOI] [PubMed] [Google Scholar]
- 24. Zhu B, Bai R, Kennett MJ, Kang BH, Gonzalez FJ, Peters JM. 2010. Chemoprevention of chemically induced skin tumorigenesis by ligand activation of peroxisome proliferator-activated receptor-β/δ and inhibition of cyclooxygenase 2. Mol Cancer Ther 9:3267–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim YY, Lee EJ, Kim YK, Kim SM, Park JY, Myoung H, Kim MJ. 2010. Anti-cancer effects of celecoxib in head and neck carcinoma. Mol Cells 29:185–194 [DOI] [PubMed] [Google Scholar]
- 26. Kim DJ, Prabhu KS, Gonzalez FJ, Peters JM. 2006. Inhibition of chemically induced skin carcinogenesis by sulindac is independent of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ). Carcinogenesis 27:1105–1112 [DOI] [PubMed] [Google Scholar]
- 27. Mann JR, Backlund MG, DuBois RN. 2005. Mechanisms of disease: inflammatory mediators and cancer prevention. Nat Clin Pract Oncol 2:202–210 [DOI] [PubMed] [Google Scholar]
- 28. Li N, Sood S, Wang S, Fang M, Wang P, Sun Z, Yang CS, Chen X. 2005. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventive effects of zileuton and celecoxib. Clin Cancer Res 11:2089–2096 [DOI] [PubMed] [Google Scholar]
- 29. Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. 2007. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res 67:1262–1269 [DOI] [PubMed] [Google Scholar]
- 30. Mijatovic T, Jungwirth U, Heffeter P, Hoda MA, Dornetshuber R, Kiss R, Berger W. 2009. The Na+/K+-ATPase is the Achilles heel of multi-drug-resistant cancer cells. Cancer Lett 282:30–34 [DOI] [PubMed] [Google Scholar]
- 31. Mijatovic T, Ingrassia L, Facchini V, Kiss R. 2008. Na+/K+-ATPase α-subunits as new targets in anticancer therapy. Expert Opin Ther Targets 12:1403–1417 [DOI] [PubMed] [Google Scholar]
- 32. Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, Schairer C, Schatzkin A, Shikany JM, Berrington de González A. 2011. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev 20:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suh I, Kebebew E. 2010. The biology of thyroid oncogenesis. Cancer Treat Res 153:3–21 [DOI] [PubMed] [Google Scholar]
- 34. National Cancer Institute 2010. Bortezomib in treating patients with metastatic thyroid cancer that did not respond to radioactive iodine therapy. http://www.clinicaltrials.gov/ct2/show/NCT00104871?term=thyroid+cancer&intr=%22Protease+Inhibitors%22&rank=1