Abstract
Context:
Leptin replacement therapy improves metabolic complications in patients with lipodystrophy and severe hypoleptinemia (SH), but whether the response is related to the degree of hypoleptinemia remains unclear.
Objective:
The aim of the study was to compare efficacy of leptin therapy in familial partial lipodystrophy, Dunnigan variety (FPLD) patients with SH (serum leptin <7th percentile of normal) vs. those with moderate hypoleptinemia (MH; serum leptin in 7th to 20th percentiles).
Design, Setting, and Patients:
We conducted an open-label, parallel group, observational study in 14 SH (mean ± sd, serum leptin, 1.9 ± 1.1 ng/ml) and 10 MH (serum leptin, 5.3 ± 1.0 ng/ml) women with FPLD.
Intervention:
Patients received 0.08 mg/kg · d of metreleptin by twice daily sc injections for 6 months.
Main Outcome Measures:
The primary outcome variable was change in fasting serum triglycerides. Other secondary variables were fasting plasma glucose and insulin, insulin sensitivity, hemoglobin A1c, and hepatic triglyceride content.
Results:
Median fasting serum triglycerides decreased from 228 to 183 mg/dl in the SH group (P = 0.04) and from 423 to 339 mg/dl in the MH group (P = 0.02), but with no difference between the groups (P value for interaction = 0.96). Hepatic triglyceride levels similarly declined significantly from 8.8 to 4.9% in the SH group and from 23.7 to 9.2% in the MH group (P value for interaction = 0.9). Loss of body weight and body fat occurred in both groups. Fasting glucose, insulin, glucose tolerance, and hemoglobin A1c levels did not change. K value on insulin tolerance test improved slightly in the SH group (0.98 to 1.24%; P = 0.01), but not in the MH group (1.1 to 1.27%; P = 0.4).
Conclusion:
Metreleptin replacement therapy is equally effective in FPLD patients with both SH and MH in reducing serum and hepatic triglyceride levels, but did not improve hyperglycemia.
Leptin replacement therapy has been shown to be very effective in ameliorating metabolic complications and decreasing ectopic fat deposition in the liver and skeletal muscles in patients with generalized lipodystrophy and severe hypoleptinemia (SH) (1–6). However, whether the metabolic response to leptin therapy is related to the degree of hypoleptinemia is not clear. Patients with familial partial lipodystrophy, Dunnigan variety (FPLD), a rare autosomal dominant disorder due to heterozygous missense mutations in lamin A/C (LMNA) gene, have wide ranging serum leptin levels: some are severely or moderately hypoleptinemic, whereas others have normal serum leptin values (7). Only limited data about the efficacy of leptin therapy in FPLD patients (8) are available, and most of the previous patients studied had SH. The available data further suggest that response to leptin therapy in FPLD patients is not as robust as that observed in patients with generalized lipodystrophy (1–6). The cause for this discrepancy is not clear, but it must be noted that patients with generalized lipodystrophy have uniformly low leptin levels, unlike FPLD patients. Therefore, to test the hypothesis that response to leptin replacement therapy is dependent on the degree of hypoleptinemia, we compared efficacy and safety of leptin replacement therapy in FPLD patients with SH (serum leptin levels <7th percentile) and those with moderate hypoleptinemia (MH; serum leptin levels in 7th to 20th percentiles) (9).
Patients and Methods
Patients
A total of 24 female patients with clinical features of FPLD and serum leptin levels less than 7 ng/ml (<20th percentile of normal) were recruited for the study. Seventeen patients had heterozygous p.R482W LMNA mutation, three each had p.R482Q and p.L515E mutations, and one patient had p.K486N mutation. All of them had at least one of the following metabolic abnormalities: diabetes mellitus, fasting serum triglycerides of at least 200 mg/dl, or fasting serum insulin of at least 30 μU/ml. Fourteen women with SH (serum leptin concentrations of 0.38 to 3.69 ng/ml; mean ± sd, 1.9 ± 1.1 ng/ml), and 10 women with MH (serum leptin, 4.1 to 6.9 ng/ml; 5.3 ± 1.0 ng/ml) were enrolled. The study protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, and all patients signed an informed consent. The study protocol has been reported at www.clinicaltrials.gov (NCT 00457938).
Study design
A parallel group, open-label, observational study was conducted with both the groups receiving recombinant human leptin (metreleptin; Amylin Pharmaceuticals Inc., San Diego, CA) for 6-month duration. Patients were admitted to the Clinical and Translational Research Center at baseline and after 3 and 6 months of therapy for 3 d for evaluation.
Study medication
Metreleptin was administered at a dose of 0.08 mg/kg · d by twice daily sc injection as used previously in female patients with lipodystrophy (1).
Biochemical analyses
Fasting blood samples were obtained on 3 consecutive days during the inpatient evaluation, and the average of the three measurements was used for data analysis. Serum glucose and lipids were measured as part of the systematic multichannel analysis using automated equipment (Synchron CX9ALX clinical system; Beckman, Fullerton, CA). Serum insulin and leptin levels were determined using RIA kits (Linco Research Inc., St. Charles, MO). Glycosylated hemoglobin (HbA1c) levels were measured by an immunoturbidimetric colorimetric assay (Quest Diagnostics, Irving, TX).
Oral glucose tolerance test (OGTT)
An OGTT for 120 min using 75-g glucose was performed in nondiabetic subjects, and glucose and insulin areas under the curve (AUC) were calculated.
Intravenous insulin tolerance test
A high-dose insulin tolerance test using regular human insulin 0.2 U/kg was performed as described before (1) to measure K value, an index of insulin sensitivity.
Anthropometry and resting energy expenditure (REE)
Height, body weight, waist and hip circumference, and skinfold thickness were measured as described previously (10). REE was measured by indirect calorimetry (Deltatrac II; Sensormedics Corp., Yorba Linda, CA) for 30 min in the fasting state with the patients resting in a recumbent position.
Dual-energy x-ray absorptiometry
Total body fat and skeletal muscle mass were measured by whole body dual-energy x-ray absorptiometry scan using Hologic QDR-2000 densitometer (Hologic Inc., Waltham, MA) as described before (10).
1H Magnetic resonance spectroscopy (MRS)
Intrahepatic lipid concentration was determined by 1H MRS using 1.5 T Gyroscan NT whole body system (Philips Medical Systems, Best, The Netherlands) as reported earlier (2).
Statistical methods
The primary endpoint of the study was to compare change in serum triglycerides between the two groups. Sample size calculations were based on previously reported serum triglyceride reductions of approximately 60% from a median level of 600 mg/dl with a sd of the differences of approximately 150 mg/dl (1). Based on these values, 10 subjects in each group would be required for 80% power to detect a 200 mg/dl difference, assuming α = 0.05. Accordingly, we had planned on recruiting approximately 12 subjects in each group to accommodate dropouts. Data were analyzed according to intention-to-treat principle using all available data. Treatment responses between and within the two groups were compared using mixed model repeated measures analysis. Spearman correlation coefficients were calculated to measure the association between basal serum leptin levels and serum and hepatic triglyceride changes in response to leptin therapy. SAS version 9.2 statistical software (SAS Institute, Cary, NC) was used for performing statistical analyses.
Results
Baseline characteristics of the patients
All participants were female, and the baseline characteristics of the two groups are summarized in Table 1. Age, body weight, and body fat percentage did not differ between the two groups. At baseline, all subjects had been diagnosed with hypertriglyceridemia, and patients in the MH group had higher serum triglyceride levels (P = 0.04). There was no difference in the fasting glucose and HbA1c levels between the two groups. However, eight patients in the SH group had been diagnosed with diabetes mellitus, of whom four were on insulin therapy, whereas only four patients in the MH group had diabetes mellitus, and of those, one was on insulin therapy. In the SH group, three patients were on combination therapy with fibrates and fish oil, two were on fibrates and statin, whereas one was on fish oil alone. In the MH group, one patient was on fibrate, one on statin, two on fibrate and statin, one on fish oil and statin, and one on fibrate and fish oil. Patients were asked to make no changes in their medications during the study, except for reduction or discontinuation of insulin and/or oral hypoglycemic medications in the event of hypoglycemia. One patient in the SH group reduced her daily insulin dose from 70 to 25 U, and two others, also in the SH group, discontinued their statins and fibrates themselves. Three patients in the SH group and one in the MH group dropped out of the study at 3 months for personal reasons.
Table 1.
Baseline characteristics of severely hypoleptinemic and moderately hypoleptinemic women with FPLD enrolled in the study
| Variable | SH group (n = 14) | MH group (n = 10) |
|---|---|---|
| Age (yr) | 40.8 ± 13.1 | 36.3 ± 16.6 |
| Weight (kg) | 63.2 ± 11.4 | 71.2 ± 5.9 |
| Body fat (% of body mass)c | 17.2 ± 3.4 | 18.8 ± 2.9 |
| Fasting serum triglycerides (mg/dl) | 321 ± 238 | 614 ± 476a |
| Fasting plasma glucose (mg/dl) | 111 ± 39 | 111 ± 36 |
| HbA1c (%) | 6.6 ± 1.7 | 6.4 ± 1.8 |
| Patients with hypertriglyceridemia (n)d | 14 | 10 |
| Patients with diabetes mellitus (n) | 8 | 4 |
| Insulin dose (U/d)b | 75 ± 38 | 50 |
Data shown as mean ± sd.
P < 0.05.
Data from four patients in SH group and one patient in MH group.
Measured by dual-energy x-ray absorptiometry.
Fasting serum TG >200 mg/dl.
Changes in serum leptin levels
Metreleptin replacement therapy increased serum leptin levels in both groups to high-normal physiological levels at 3 months, and these levels were maintained at the end of 6 months. By the end of the study, the median (range) serum leptin level in the SH group was 33.4 (7.4 to 101.3) ng/ml, compared with 49.1 (12.2 to 78.3) ng/ml in the MH group, with the increase in leptin levels not being different between the two groups (P value for interaction between group and time = 0.08).
Changes in serum lipids
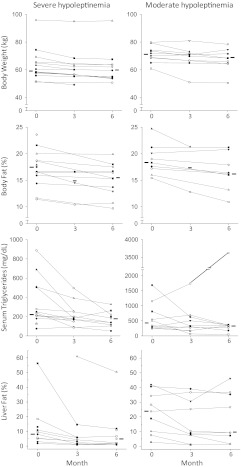
The median fasting serum triglycerides decreased from 228 to 183 mg/dl in the SH group (P = 0.04) and from 423 to 339 mg/dl in the MH group (P = 0.02) after 6 months of leptin replacement therapy (Fig. 1). The magnitude of decline was similar in both groups (19 vs. 17%; P = 0.96). Serum total cholesterol and high-density lipoprotein cholesterol levels did not change with leptin therapy.
Fig. 1.
Effect of metreleptin replacement therapy on different anthropometric and metabolic variables in FPLD patients with SH and MH. Month 0 values indicate data before metreleptin administration, and the month 3 and 6 values indicate data during metreleptin therapy. One patient in the MH group was not compliant with metreleptin or hypoglycemic therapy, and a marked increase was noted in serum triglyceride concentrations.
Changes in glucose metabolism
Fasting plasma glucose, plasma glucose AUC during OGTT, and HbA1c levels did not change significantly with leptin replacement therapy in both groups (Table 2 and Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). K value, which is the rate of glucose disappearance during the insulin tolerance test, increased from 0.98 ± 0.63 to 1.24 ± 0.49% in the SH group (P = 0.01), and from 1.1 ± 0.4 to 1.27 ± 0.66% in the MH group (P = 0.4), with no difference between the two groups (P = 0.7).
Table 2.
Effect of leptin replacement therapy on glucose metabolism in FPLD patients with SH and MH
| Variable | SH |
MH |
P value | ||||
|---|---|---|---|---|---|---|---|
| 0 months | 3 months | 6 months | 0 months | 3 months | 6 months | ||
| Fasting plasma glucose (mg/dl) | 111 ± 40 (14) | 111 ± 23 (11) | 106 ± 32 (11) | 111 ± 36 (10) | 109 ± 49 (10) | 97 ± 24 (9) | 0.7 |
| Fasting serum insulin (μU/ml) | 39.7 ± 32.1 (6) | 26.0 ± 15.8 (6) | 30.1 ± 23.9 (4) | 62.2 ± 45.3 (5) | 52.9 ± 56.2 (6) | 42.9 ± 52.7 (5) | 0.67 |
| HbA1c (%) | 6.7 ± 1.7 (14) | 6.3 ± 1.6 (11) | 6.3 ± 1.5 (10) | 6.4 ± 1.8 (10) | 6.3 ± 2 (10) | 6.7 ± 2.6 (9) | 0.16 |
| KITT (%) | 0.98 ± 0.63 (13) | 1.26 ± 0.55 (9) | 1.24 ± 0.49 (10)a | 1.10 ± 0.35 (7) | 1.25 ± 0.4 (5) | 1.27 ± 0.66 (5) | 0.7 |
| GlucoseAUC (mg/dl · min)b | 17,112 ± 2,067 (6) | 16,597 ± 1,670 (6) | 18,208 ± 1787 (4) | 20,319 ± 3,717 (7) | 18,845 ± 4,991 (7) | 18,229 ± 5,031 (7) | 0.27 |
| InsulinAUC (mg/dl · min)b | 42,212 ± 29,681 (6) | 33,167 ± 17,036 (6) | 32,908 ± 23,223 (4) | 54,586 ± 45,737 (5) | 31,987 ± 24,313 (6) | 33,073 ± 34,443 (5) | 0.7 |
Numbers in parentheses indicate total number of subjects for whom the data were available. P values in the last column represent interaction between the two groups using mixed model repeated measures ANOVA. KITT, K value (rate of glucose disappearance) on iv glucose tolerance test; AUC, area under the curve during 2-h OGTT.
P < 0.05 compared to baseline.
Measured only in those not on insulin or oral hypoglycemic therapy.
Changes in hepatic steatosis
The median intrahepatic lipid content declined from 8.8 to 4.9% in the SH group and from 23.7 to 9.2% in the MH group after 6 months of leptin therapy (Fig. 1 and Table 3). This decrease was statistically significant in both groups (P < 0.005), but there was no difference between the two groups (P = 0.86). Serum alanine aminotransferase and aspartate aminotransferase did not change much with the intervention, except for a modest reduction in the latter from 24.2 ± 9.0 to 20.0 ± 8.2 IU/liter in the SH group only (P = 0.02).
Table 3.
Effect of leptin replacement therapy on select anthropometric and metabolic variables in FPLD patients with SH and MH
| Variable | SH |
MH |
P value | ||||
|---|---|---|---|---|---|---|---|
| 0 months | 3 months | 6 months | 0 months | 3 months | 6 months | ||
| Body weight (kg) | 63.2 ± 11.4 | 62.1 ± 12.0b | 61.8 ± 12.3c | 71.2 ± 5.9 | 68.7 ± 7.6b | 67.4 ± 7.9c | 0.97 |
| Body fat (%) | 17.2 ± 3.4 | 13.8 ± 3.2 | 15.1 ± 3.1b | 18.8 ± 2.9 | 17.1 ± 6.0 | 16.6 ± 3.5b | 0.88 |
| Waist circumference (cm) | 78.9 ± 7.4 | 77.8 ± 7.2 | 77.3 ± 8.5 | 85.6 ± 8.0 | 80.6 ± 5.6 | 82.3 ± 10.4 | 0.68 |
| Hip circumference (cm) | 89.9 ± 5.4 | 88.7 ± 5.7a | 88.5 ± 5.5a | 94.6 ± 3.0 | 92.0 ± 4.1c | 91.8 ± 5.2c | 0.23 |
| Skeletal muscle mass (kg) | 28.5 ± 5.4 | 26.9 ± 1.3 | 29.1 ± 5.4 | 31.7 ± 2.9 | 27.1 ± 4.2 | 30.6 ± 4.1 | 0.65 |
| Total cholesterol (mg/dl) | 185 ± 46 | 190 ± 34 | 169 ± 28 | 237 ± 66 | 197 ± 71a | 242 ± 134 | 0.15 |
| Serum triglycerides (mg/dl) | 228 (180–506) | 181 (160–253) | 183 (116–220)a | 423 (295–813) | 319 (177–630) | 339 (275–359)a | 0.97 |
| HDL cholesterol (mg/dl) | 34.0 ± 8.3 | 37.0 ± 9.0 | 35.4 ± 9.3 | 33.2 ± 6.5 | 32.7 ± 2.8 | 33.8 ± 5.0 | 0.48 |
| Liver fat (%) | 8.8 (5.2–11.9) | 3.4 (1.6–5.9)c | 4.9 (1.6–11.6)c | 23.7 (10.2–34.2) | 9.7 (7.4–30.6)b | 9.2 (7.3–35.3)b | 0.86 |
| ALT (U/liter) | 25.0 ± 11.3 | 22.1 ± 10.3 | 20.6 ± 8.0 | 34.4 ± 17.1 | 29.9 ± 20.2 | 35.2 ± 29.1 | 0.39 |
| AST (U/liter) | 24.2 ± 9.0 | 21.5 ± 8.1 | 20.0 ± 8.2a | 26.3 ± 8.0 | 23.6 ± 10.1 | 24.8 ± 13.6 | 0.74 |
Values are expressed as mean ± sd or median (25th–75th percentile). P values in the last column represent interaction between the two groups using mixed model repeated measures ANOVA. ALT, Alanine aminotransferase; AST, aspartate aminotransferase; HDL, high-density lipoprotein.
P < 0.05;
P < 0.01;
P < 0.001, compared to baseline values.
Changes in anthropometry and energy expenditure
Body weight decreased by 2.4% in the SH group, whereas in the MH group, it decreased by 5.3% (P < 0.001 for both groups) (Fig. 1). Similarly, total body fat (Fig. 1) decreased by 12.3% in the SH group and by 11.7% in the MH group after leptin therapy (P < 0.01). However, the declines in body weight or body fat were not different between the two groups (P = 0.9). Skeletal muscle mass did not change in either group (Table 3). Similarly, waist circumference and waist-to-hip circumference ratio did not show any significant changes, but the hip circumference decreased in both groups. Skinfold thickness measurements at various central and peripheral sites did not change with leptin therapy in the MH group but tended to decrease in the SH group at the chin, subscapular, and hip region, but the measurements did not achieve statistical significance (Supplemental Table 2). Consistent with the decline in weight, REE decreased from 1331 ± 344 to 1215 ± 105 kcal/d at the end of 6 months (P = 0.01) in the SH group, whereas in the MH group, it decreased from 1618 ± 310 to 1302 ± 229 kcal/d during the same period (P = 0.13). There was no change in the respiratory quotient measured after a 12-h fast in both groups.
Combined group results
Because there was no significant difference in the response to leptin therapy among the two groups for the primary or secondary variables, we combined the results and found a significant decline in plasma triglycerides, hepatic steatosis, fasting insulin, insulin AUC during OGTT, K value, body weight, body fat, hip circumference, abdominal skinfold thickness, REE, and aspartate aminotransferase values (Supplemental Tables 1 and 2).
Correlation of basal serum leptin to serum triglyceride changes
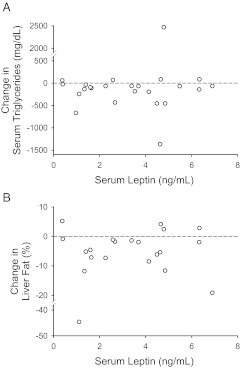
As shown in Fig. 2A, basal serum leptin levels did not correlate with reduction in serum triglycerides in either group, or when both the groups were analyzed together (r = 0.05; P = 0.8). Similarly, basal leptin levels did not correlate with reductions in either hepatic triglyceride content (r = 0.01; P = 0.97; Fig. 2B) or body weight (r = −0.03; P = 0.9).
Fig. 2.
Correlation between basal serum leptin levels and reduction in serum triglycerides (A) and hepatic triglyceride concentrations (B) after metreleptin-replacement therapy.
Adverse effects of metreleptin therapy
Mild injection site reactions were reported by two patients and subsided without any therapeutic intervention. Hypoglycemia in one patient necessitated reduction in insulin dosage.
Discussion
Leptin replacement therapy is emerging as a promising option for the treatment of metabolic complications related to lipodystrophy (11, 12). However, most of the earlier studies have focused on patients with congenital or acquired generalized lipodystrophy and SH (1, 5, 6, 13). To test the hypothesis of whether response to metreleptin therapy is dependent on the severity of leptin deficiency, we designed this trial to enroll a homogenous group of patients with typical FPLD due to LMNA mutation only. Patients with typical FPLD have a marked loss of sc fat from the extremities and trunk accompanied by a variable amount of excess fat deposition in the nonlipodystrophic areas such as the face, chin, back, and intraabdominal regions (14–16). This results in marked variation in serum leptin levels in FPLD patients (7, 17), making this group ideal to test the hypothesis. In the first study of leptin replacement therapy in patients with lipodystrophy (1), all subjects had baseline serum leptin levels less than 4 ng/ml, and in the previous trial of leptin therapy in FPLD patients (8), the mean ± sd baseline serum leptin level was 3.8 ± 0.3 ng/ml. We therefore designated patients with serum leptin levels less than 4 ng/ml as having SH, and those above this level but less than the 20th percentile (7 ng/ml), who have not been studied before as a group, as having MH. Despite conventional hypoglycemic and hypolipidemic therapies, some FPLD patients continue to have extreme hypertriglyceridemia, hepatic steatosis, and poorly controlled diabetes (18), thus justifying the need to develop novel therapies such as metreleptin for them. These would be useful adjuncts to dietary fat restriction and other lifestyle changes to avoid weight gain, which are critical for effective management of metabolic complications in patients with lipodystrophy.
Most importantly, we successfully enrolled the required number of patients for this study, despite rare prevalence of FPLD (14–16). Although both the genders could participate, only female patients were enrolled. This reduced the heterogeneity among the study subjects because gender differences in the prevalence of metabolic complications have been previously reported among FPLD patients (19). This is also the first report of efficacy of metreleptin therapy in patients with serum leptin levels above the 7th percentile of normal values. The mean serum leptin level in the large National Institutes of Health (NIH) cohort of 48 patients with different types of lipodystrophy treated with metreleptin was 2.53 ± 0.4 ng/ml (13). Although a large number of patients in this cohort had generalized lipodystrophy, the nine patients with FPLD had a mean ± sd serum leptin level of 4.62 ± 0.79 ng/ml, and of these, only two had levels greater than 4.5 ng/ml. Other studies in patients with FPLD (8) or familial partial lipodystrophy due to PPARG mutation (20) also reported markedly low baseline serum leptin levels (<4 ng/ml) in most patients.
We noted similar and significant reductions in serum triglycerides and intrahepatic lipid content after 6 months of leptin replacement therapy in patients with either MH or SH. However, it must be noted that, whereas at baseline there were six patients in both groups who were on one or more hypolipidemic medications, two subjects in the SH group had stopped these by the end of the study. Furthermore, baseline serum triglycerides were lower in the SH group. Although both these confounders could potentially mask a greater effect in SH patients, it is very unlikely that they would significantly affect the overall results, given a P value of 0.97 for the difference between the two groups.
The decline in serum triglycerides, however, was not as dramatic as seen in earlier studies (1, 5, 13). In the initial NIH-University of Texas Southwestern collaborative study (1), we had observed a nearly 60% decline in serum triglycerides with a similar dose of metreleptin (1), and Chong et al. (13) confirmed a similar response in the larger cohort of patients followed for 1 yr. Park et al. (8) did report a 65% reduction in serum triglycerides in six patients with FPLD after 4 months of therapy. However, at the end of 1 yr, the decline in five of them was about 40%. Overall, we observed only a 20% decline, which could be due to the lower baseline serum triglycerides (by 300 mg/dl) in our cohort than in patients reported by Park et al. (8). Post hoc analysis of our data, however, revealed a 56% decline in serum triglycerides in patients with severe hypertriglyceridemia (serum triglycerides ≥500 mg/dl), compared with 19% in others with serum triglycerides below 500 mg/dl (P for interaction = 0.04).
The effects of leptin replacement therapy on glucose metabolism were not very striking, with no change in either HbA1c or glucose tolerance. These findings are similar to the earlier study of metreleptin therapy in FPLD patients (8) but are strikingly different from marked reduction in HbA1c and the use of antidiabetic medication noted in patients with generalized lipodystrophy after metreleptin therapy (1). A post hoc analysis revealed a significant lowering of HbA1c (from 8.7 ± 1.5 to 8.0 ± 3.1%; P = 0.03) in the subgroup of patients with HbA1c above 6.5% compared with the other subgroup with HbA1c of 6.5% or less (from 5.6 ± 0.5 to 5.8 ± 0.6%; P = 0.4). Significant decline in fasting insulin, insulin AUC, and K value suggests improvement in insulin sensitivity in the combined group. Thus, overall data suggest that metreleptin therapy is likely to be beneficial in the management of insulin resistance and hyperglycemia in FPLD patients.
The reduction in hepatic triglycerides (43 vs. 63% in the SH and MH groups, respectively) were of similar magnitude as previously seen in patients with generalized lipodystrophy after metreleptin therapy (2, 3). Furthermore, Javor et al. (4) have shown metreleptin-induced improvement in both steatosis and histological scores of steatohepatitis in 10 patients with severe lipodystrophy; two of these patients had FPLD, and one of them showed a reduction in hepatic fat as measured by magnetic resonance imaging (4). Whether reduction in hepatic steatosis as estimated by 1H MRS will lead to histological improvement in those with steatohepatitis needs to be addressed in future studies.
Similar magnitude of reduction in serum triglycerides and hepatic lipids in the two groups and the lack of correlation between baseline serum leptin concentrations and reduction in serum triglycerides and hepatic lipids suggest that response to metreleptin therapy may not be dependent upon the degree of hypoleptinemia and that metreleptin therapy is likely to be useful even in lipodystrophy patients who do not have marked reductions in serum leptin levels. Whether our conclusions will extend to lipodystrophy patients without hypoleptinemia will require further studies of the efficacy of metreleptin therapy in FPLD patients with normoleptinemia.
The mechanisms by which leptin therapy improves metabolic abnormalities in patients with lipodystrophy are not completely clear. Whether leptin exerts its effect primarily on the central regulation of food intake, or by amelioration of lipotoxicity in the peripheral tissues, or both is not fully understood (21). Marked hyperphagia is a feature of hypoleptinemic patients with generalized lipodystrophy (18), and leptin replacement therapy reduces energy consumption (1, 22) and improves measures of satiety (23). Significant loss of body weight and body fat occurred in our patients with no changes in skeletal muscle mass. The loss of body fat seen in our study is reflective of higher body fat in patients with FPLD compared with those with generalized lipodystrophy where a greater reduction in lean body mass has been noted (22). Consistent with decreased energy intake and weight loss, REE tended to decrease as observed previously (1, 22).
We did not observe any serious side effects of metreleptin therapy. Previously, besides local injection site reactions, metreleptin therapy has been associated with development of T-cell lymphoma in two patients with acquired generalized lipodystrophy and proteinuria (13), although it also ameliorated proteinuria in many patients with lipodystrophy (24). Recently, the lack of efficacy of metreleptin therapy in patients with congenital generalized lipodystrophy was attributed to development of neutralizing antibodies in two patients (25). Antibody-related laboratory findings were reported in two obese patients who received metreleptin, and the ongoing trial of metreleptin in obese subjects was voluntarily halted (26). Thus, careful monitoring of immune responses to metreleptin in future trials in lipodystrophy patients may be warranted.
In conclusion, leptin therapy in FPLD patients is safe and equally efficacious in patients with both MH and SH in reducing serum triglycerides and hepatic steatosis. Finally, metabolic response to leptin therapy may not depend upon the degree of hypoleptinemia in lipodystrophy patients with low leptin levels. Whether leptin therapy would be efficacious in FPLD patients with normal leptin levels needs to be determined.
Supplementary Material
Acknowledgments
We thank Sarah Masood and Crystal Kittisopikul for help with illustrations and hormone assays.
This work was supported by grants from the National Institutes of Health (R01-DK74959, M01-RR00633, and UL1-RR-024982), the Southwest Medical Foundation, and the Amylin Pharmaceuticals Inc.
Trial Registration: www.clinicaltrials.gov identifier: NCT00457938.
Disclosure Summary: A.G. is coholder of a patent for use of metreleptin in patients with lipodystrophy but does not receive any monetary benefits. All other authors have nothing to declare.
Footnotes
- AUC
- Area under the curve
- FPLD
- familial partial lipodystrophy, Dunnigan variety
- HbA1c
- glycosylated hemoglobin
- MH
- moderate hypoleptinemia
- MRS
- magnetic resonance spectroscopy
- OGTT
- oral glucose tolerance test
- REE
- resting energy expenditure
- SH
- severe hypoleptinemia.
References
- 1. Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. 2002. Leptin-replacement therapy for lipodystrophy. N Engl J Med 346:570–578 [DOI] [PubMed] [Google Scholar]
- 2. Simha V, Szczepaniak LS, Wagner AJ, DePaoli AM, Garg A. 2003. Effect of leptin replacement on intrahepatic and intramyocellular lipid content in patients with generalized lipodystrophy. Diabetes Care 26:30–35 [DOI] [PubMed] [Google Scholar]
- 3. Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. 2002. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 109:1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, Kleiner DE, Gorden P. 2005. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology 41:753–760 [DOI] [PubMed] [Google Scholar]
- 5. Ebihara K, Kusakabe T, Hirata M, Masuzaki H, Miyanaga F, Kobayashi N, Tanaka T, Chusho H, Miyazawa T, Hayashi T, Hosoda K, Ogawa Y, DePaoli AM, Fukushima M, Nakao K. 2007. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 92:532–541 [DOI] [PubMed] [Google Scholar]
- 6. Beltrand J, Beregszaszi M, Chevenne D, Sebag G, De Kerdanet M, Huet F, Polak M, Tubiana-Rufi N, Lacombe D, De Paoli AM, Levy-Marchal C. 2007. Metabolic correction induced by leptin replacement treatment in young children with Berardinelli-Seip congenital lipoatrophy. Pediatrics 120:e291–e296 [DOI] [PubMed] [Google Scholar]
- 7. Haque WA, Shimomura I, Matsuzawa Y, Garg A. 2002. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab 87:2395. [DOI] [PubMed] [Google Scholar]
- 8. Park JY, Javor ED, Cochran EK, DePaoli AM, Gorden P. 2007. Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metabolism 56:508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruhl CE, Everhart JE. 2001. Leptin concentrations in the United States: relations with demographic and anthropometric measures. Am J Clin Nutr 74:295–301 [DOI] [PubMed] [Google Scholar]
- 10. Simha V, Garg A. 2002. Body fat distribution and metabolic derangements in patients with familial partial lipodystrophy associated with mandibuloacral dysplasia. J Clin Endocrinol Metab 87:776–785 [DOI] [PubMed] [Google Scholar]
- 11. Gorden P, Park JY. 2006. The clinical efficacy of the adipocyte-derived hormone leptin in metabolic dysfunction. Arch Physiol Biochem 112:114–118 [DOI] [PubMed] [Google Scholar]
- 12. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. 2010. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 152:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chong AY, Lupsa BC, Cochran EK, Gorden P. 2010. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia 53:27–35 [DOI] [PubMed] [Google Scholar]
- 14. Garg A. 2004. Acquired and inherited lipodystrophies. N Engl J Med 350:1220–1234 [DOI] [PubMed] [Google Scholar]
- 15. Chan JL, Oral EA. 2010. Clinical classification and treatment of congenital and acquired lipodystrophy. Endocr Pract 16:310–323 [DOI] [PubMed] [Google Scholar]
- 16. Garg A, Peshock RM, Fleckenstein JL. 1999. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab 84:170–174 [DOI] [PubMed] [Google Scholar]
- 17. Hegele RA, Kraw ME, Ban MR, Miskie BA, Huff MW, Cao H. 2003. Elevated serum C-reactive protein and free fatty acids among nondiabetic carriers of missense mutations in the gene encoding lamin A/C (LMNA) with partial lipodystrophy. Arterioscler Thromb Vasc Biol 23:111–116 [DOI] [PubMed] [Google Scholar]
- 18. Simha V, Garg A. 2006. Lipodystrophy: lessons in lipid and energy metabolism. Curr Opin Lipidol 17:162–169 [DOI] [PubMed] [Google Scholar]
- 19. Garg A. 2000. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab 85:1776–1782 [DOI] [PubMed] [Google Scholar]
- 20. Guettier JM, Park JY, Cochran EK, Poitou C, Basdevant A, Meier M, Clément K, Magré J, Gorden P. 2008. Leptin therapy for partial lipodystrophy linked to a PPAR-γ mutation. Clin Endocrinol (Oxf) 68:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farooqi IS, O'Rahilly S. 2009. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr 89:980S–984S [DOI] [PubMed] [Google Scholar]
- 22. Moran SA, Patten N, Young JR, Cochran E, Sebring N, Reynolds J, Premkumar A, Depaoli AM, Skarulis MC, Oral EA, Gorden P. 2004. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism 53:513–519 [DOI] [PubMed] [Google Scholar]
- 23. McDuffie JR, Riggs PA, Calis KA, Freedman RJ, Oral EA, DePaoli AM, Yanovski JA. 2004. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. J Clin Endocrinol Metab 89:4258–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Javor ED, Moran SA, Young JR, Cochran EK, DePaoli AM, Oral EA, Turman MA, Blackett PR, Savage DB, O'Rahilly S, Balow JE, Gorden P. 2004. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J Clin Endocrinol Metab 89:3199–3207 [DOI] [PubMed] [Google Scholar]
- 25. Beltrand J, Lahlou N, Le Charpentier T, Sebag G, Leka S, Polak M, Tubiana-Rufi N, Lacombe D, de Kerdanet M, Huet F, Robert JJ, Chevenne D, Gressens P, Lévy-Marchal C. 2010. Resistance to leptin-replacement therapy in Berardinelli-Seip congenital lipodystrophy: an immunological origin. Eur J Endocrinol 162:1083–1091 [DOI] [PubMed] [Google Scholar]
- 26. 16 March 2011. Amylin and Takeda voluntarily suspend clinical activities in obesity trial. Amylin Pharmaceuticals Inc. and Takeda Pharmaceutical Company Limited:, San Diego, CA, and Osaka, Japan: PRNewswire via COMTEX (press release) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.