Abstract
Co-stimulation blockade can be used to modulate the immune response for induction of organ transplantation tolerance, treatment of autoimmune disease as well as cancer treatment. Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4), also known as CD152, is an important co-stimulatory molecule which serves as a negative regulator for T cell proliferation and differentiation. CTLA-4/CD28-CD80/CD86 pathway is a critical co-stimulatory pathway for adaptive immune response. T cell activation through the T cell receptor and CD28 leads to increased expression of CTLA-4, an inhibitory receptor for CD80 and CD86. MGH MHC-defined miniature swine provide a unique large animal model useful for preclinical studies of transplantation tolerance and immune regulation. In this study, we have expressed the codon-optimized soluble porcine CTLA-4 in the yeast Pichia pastoris system. The secreted porcine CTLA-4 was captured using Ni-Sepharose 6 fast flow resin and further purified using strong anion exchange resin Poros 50HQ. Glycosylation analysis using PNGase F demonstrated the N-linked glycosylation on Pichia pastoris expressed soluble porcine CTLA-4. To improve the expression level and facilitate the downstream purification we mutated the two potential N-linked glycosylation sites with non-polarized alanines by site-directed mutagenesis. Removal of the two N-glycosylation sites significantly improved the production level from ~2 mg/L to ~8 mg/L. Biotinylated glycosylated and non-N-glycosylated soluble porcine CTLA-4 both bind to a porcine CD80-expressing B-cell lymphoma cell line (KD = 13 nM) and competitively inhibit the binding of an anti-CD80 monoclonal antibody. The availability of soluble porcine CTLA-4, especially the non-N-glycosylated CTLA-4, will provide a very valuable tool for assessing co-stimulatory blockade treatment for translational studies in the clinically relevant porcine model.
Keywords: Porcine CTLA-4, Pichia pastoris expression, purification, porcine CD80, glycosylation
Introduction
T-cell proliferation and function depends on signals from the antigen-receptor complex (TCR/CD3) and by various co-stimulatory receptors such as CD28 and CTLA-4. The balance of positive and negative signals determines the outcome of the T-cell response of foreign and self-antigen. The most well studied co-stimulatory pairs are CD28/CTLA-4-CD80/CD86. CD28 is constitutively expressed on native and activated CD4 and CD8 positive T cells. CTLA-4 is expressed on activated T cells and plays a negative regulatory role in T cell response. CD80 and CD86 are induced on antigen presenting cells (APC) with their activation [1, 2]. The discovery of co-stimulatory molecules introduced the possibility of therapeutic intervention at the level of the costimulatory signal without interference with an antigen-receptor (TCR/CD3) signal. One could dampen the co-receptor signal without needing to know the exact nature of the antigen involved in the antigen-receptor complex of T-cell activation cascade [1]. Therefore co-stimulation blockade has been broadly used to modulate the immune response for organ transplantation, autoimmune diseases as well as cancer treatment [3]. The higher affinity of CTLA-4 for CD80/CD86 has allowed the use of a CTLA-4-Ig fusion protein to out-compete CD28-CD80/CD86 binding in the treatment of autoimmune disorders [1]. It was also reported that the suppressive function of natural regulatory T cells is dependent on CTLA-4 [2, 4].
Recombinant soluble CTLA-4 has been dominantly expressed as a commercial Fc-fusion protein (CTLA4-Ig) in mammalian cell systems and insect cells. So far CTLA4-Ig has been developed for mouse, human, swine, monkey and cannine [5]. To avoid the Fc domain interaction, it is necessary to express the CTLA-4 extracellular domain alone. The commercial recombinant soluble human and mouse CTLA-4 has been mainly expressed in insect cells such as sf9, mammalian cells and E. coli. They are very useful for in vitro assays in research. However, it is very difficult to meet the in vivo need for large animal models because the dose requirement is high and the price is too expensive. Therefore, it is necessary to develop an alternative eukaryotic expression system such as Pichia pastoris to produce functional and cost-effective recombinant soluble CTLA-4.
Pichia pastoris has been widely utilized to express heterologous recombinant proteins. As a eukaryotic expression system, Pichia pastoris can process post-translational modification [6, 7]. Since the expressed soluble porcine CTLA-4 will be used in a swine model, cost-effectiveness, high production level, high purity, easy purification are needed. Based on the literature and our experience, Pichia pastoris should be the best choice to meet those requirements.
In this study we expressed and purified the glycosylated and non-N-glycosylated soluble porcine CTLA-4 in Pichia pastoris to generate a reagent that could be used to study co-stimulatory blockade protocols for transplantation studies in swine models. The binding function to porcine CD80 was assessed by biotinylating glycosylated or non-N-glycosylated soluble porcine CTLA-4 and FACS analysis using a porcine CD80-expressing B-cell lymphoma cell line.
Materials and Methods
Plasmid construction
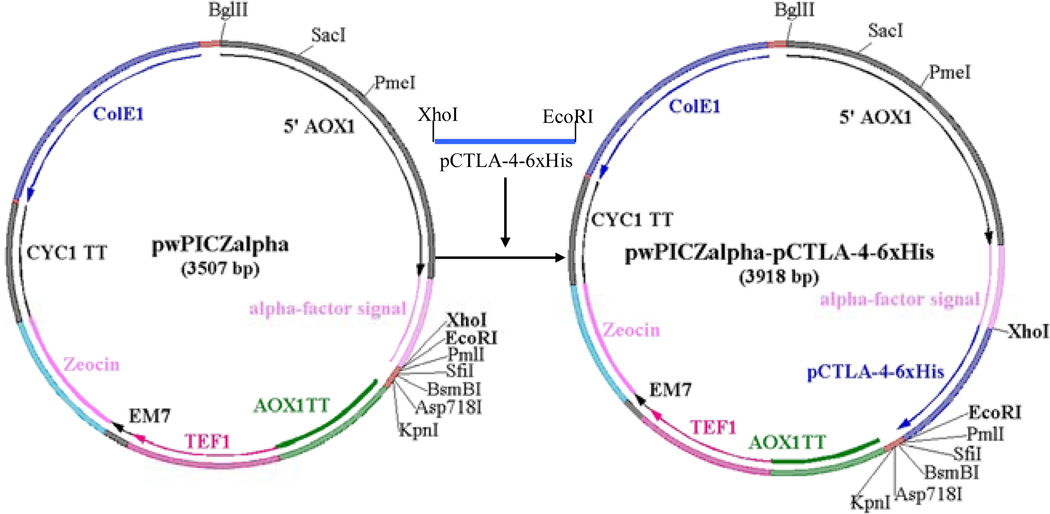
According to the published amino acid sequence (UniProtKB/Swiss-Prot accession number: Q9MYX7), soluble porcine CTLA-4 DNA was synthesized using the Pichia pastoris preferred codons [8]. Ten primers (Table 1) were designed to cover the full length of the soluble porcine CTLA-4 gene as well as its 6xHis tag in the C-terminus (130 aa). There was a 21 base overlap between any of the neighboring primers. Ten pmol of the first and the last primer, and 2 pmol for the rest of the primers were used. The PCR program was conducted at 95°C for 5 min, 25 cycles of 95°C for 30 sec, 55°C for 30 sec, 72°C for 1 min and an extension at 72°C for 10 min. The PCR products were analyzed with 1% agarose gel electrophoresis, and the band with the correct size cut out and extracted with QIAquick Gel Extraction Kit. The synthesized DNA was digested using XhoI and EcoRI and cloned into pwPICZα (Figure 1) [9]. To facilitate the downstream purification, six histidines (6x His tag) were added at the C-terminus. To construct the non-N-glycosylated soluble porcine CTLA-4 two asparagines (potential N-linked glycosylation sites: N76A, N108A) were replaced with two non-polarized alanines using site-directed mutagenesis kit (Stratagene). The site-directed mutagenesis primers are listed in Table 1.
Table 1.
PCR primers used to synthesize glycosylated and non-N-glycosylated soluble porcine CTLA-4
| CTL1 |
| 5’ CCG CTC GAG AAG AGA GAG GCT GAA GCT ATG CAC GTT GCT CAA CCA GCT GTT GTC TTG 3’ |
| CTL2 |
| 5’ ACC GTA CTC ACA AAC GAA AGA AGC AAC ACC TCT AGA GTT AGC CAA GAC AAC AGC TGG TTG AGC 3’ |
| CTL3 |
| 5’ TCT TTC GTT TGT GAG TAC GGT TCT GCT GGT AAG GCT GCT GAG GTT AGA GTT ACT GTT TTG AGA 3’ |
| CTL4 |
| 5’ GTA AGT AGC AGC ACA AAC CTC AGT CAT TTG AGA ACC AGC TCT TCT CAA AAC AGT AAC TCT AAC 3’ |
| CTL5 |
| 5’ GAG GTT TGT GCT GCT ACT TAC ACT GTT GAG GAC GAG TTG ACT TTC TTG GAC GAC TCT ACT TGT 3’ |
| CTL6 |
| 5’ TTG AAT AGT CAA GTT AAC CTT GTT CTC AGT AGA AGT ACC AGT ACA AGT AGA GTC GTC CAA GAA 3’ |
| CTL7 |
| 5’ AAG GTT AAC TTG ACT ATT CAA GGT TTG AGA GCT GTC GAC ACC GGT TTG TAC ATC TGT AAG GTC 3’ |
| CTL8 |
| 5’ GTT ACC CAT ACC AAC GTA GTA TGG AGG TGG GTA CAA CAA TTC GAC CTT ACA GAT GTA CAA ACC 3’ |
| CTL9 |
| 5’ TAC TAC GTT GGT ATG GGT AAC GGT ACT CAA ATT TAC GTT ATT GAC CCT GAA CCA TGT CCT GAC 3’ |
| CTL10 |
| 5’ CCG GAA TTC TTA GTG GTG GTG GTG GTG GTG GTC AGA GTC AGG ACA TGG TTC AGG GTC 3’ |
| For non-N-glycosylated soluble porcine CTLA4 DNA by site-directed mutagenesis: |
| N113A For |
| 5’ ACT TCT ACT GAG AAC AAG GTT GCT TTG ACT ATT CAA GGT TTG AGA 3 |
| N113A Rev |
| 5’ TCT CAA ACC TTG AAT AGT CAA AGC AAC CTT GTT CTC AGT AGA AGT 3’ |
| N145A For |
| 5’ CCA TAC TAC GTT GGT ATG GGT GCT GGT ACT CAA ATT TAC GTT ATT 3’ |
| N145A Rev |
| 5’ AAT AAC GTA AAT TTG AGT ACC AGC ACC CAT ACC AAC GTA GTA TGG 3’ |
Figure 1.
Construction of the glycosylated or non-N-glycosylated soluble porcine CTLA4-6xHis (pCTLA4-6xHis) into pwPICZalpha between XhoI and EcoRI sites. pwPICZalpha was derived from the pPICZalpha vector (Invitrogen). pwPICZalpha contains a unique XhoI site and the original BamHI and NcoI sites were removed by mutation. AOX1: AOX1 region; AOX1 TT: AOX1 trascription termination region; TEF1: TEF1 promoter region; EM7: EM7 promoter region; Zeocin: Zeocin resistance gene (Sh ble ORF); CYC1: CYC1 transcription termination region; ColE1: ColE1 origin (pUC-derived).
Protein expression in Pichia pastoris
Approximately 5–10 µg of the above constructed plasmid DNA was linearized by SacI digestion for 3 h at 37°C, treated with Qiagen PCR purification kit, and transformed into Pichia pastoris strain X33 using Gene Pulser Xcell Electroporation system (Bio-Rad). Cells were spread on YPD agar plates (1% Bacto™ yeast extract, 2% Bacto™ peptone, 1.5% Bacto™ agar, 2% dextrose) containing 100 µg/mL of Zeocin and incubated at 30°C for 3–4 days. Six colonies were randomly picked and cultivated in test tubes containing 5 mL YPD (1% Bacto™ yeast extract, 2% Bacto™ peptone, 2% dextrose) at 30°C at 250 rpm for 24 h as growth phase I, then in YPG (1% Bacto™ yeast extract, 2% Bacto™ peptone, 1% glycerol) at 30°C at 250 rpm for another 24 h as growth phase II. The cultures were induced in 2 mL BMMYC [1% Bacto™ yeast extract, 2% Bacto™ peptone, 100 mM potassium phosphate, pH 7.0, 1.34% yeast nitrogen base without amino acids (MP), 4 × 10−5 % biotin, 0.5% methanol and 1% Bacto™ casamino acids] for 48 h at 25°C at 225 rpm. 0.5% methanol was added twice daily (at 5 h, 24 h, and 30 h after initial induction) to sustain the methanol level. Antifoam (Emerald Performance Materials, Cat# KFO673) was added in all of the growth and induction media at 0.02%. 1mM phenylmethanesulfonyl fluoride (PMSF, Sigma) was added to inhibit the protein degradation during the induction phase. Additionally, 100 units/mL of penicillin and 100 µg/mL of streptomycin were also added to all of the growth and induction media to suppress bacterial contamination. The culture supernatants were analyzed using 12% NuPAGE SDS gel under non-reducing conditions.
One clone was selected for large-scale expression. The large-scale expression is scaled-up from the small tube expression described previously. The Excella E24 incubator shaker (New Brunswick) was employed to express both glycosylated and non-N-glycosylated soluble porcine CTLA-4 on a large-scale. The seed culture was prepared by inoculating a single colony into YPD medium, then incubating at 25°C, 225 rpm over the weekend. 5% of this seed culture was transferred to 1L PYREX shake flasks containing 250 mL YPD medium and cultured at 30°C, 250 rpm for 24 h. Subsequently, cells were centrifuged at 1500 rpm for 5 minutes and the cell pellet was re-suspended in 250 mL YPG medium, and cultured at 30°C, 250 rpm for 24 h. For induction, cells were centrifuged at 1500 rpm for 5 minutes and the cell pellet was re-suspended in 125 mL BMMYC induction medium and induced at 25°C, 225 rpm. Following induction, 0.5% methanol was added twice daily (at 5 h, 24 h, and 30 h after initial induction) to sustain the methanol level. After 48 h induction, yeast cells were pelleted by centrifugation at 3000 rpm, 4°C for 10 minutes. The supernatant, containing the soluble porcine CTLA-4 could thereafter be purified. Antifoam, PMSF and penicillin/streptomycin were added to the media at the same concentrations as mentioned previously for the small-scale expression.
Protein Purification
Ni-Sepharose™ 6 fast flow resin was packed in a 5 cm × 20 cm XK50 column (GE healthcare Cat#18-1000-71) for the first step purification. The column was equilibrated with 10 column volumes (CV) of 20 mM sodium phosphate pH 7.4, 0.5 M NaCl, and 5 mM imidazole. The sample was prepared by adding 0.5 M NaCl, 20 mM sodium phosphate pH 7.4, and 5 mM imidazole, filtered through crepe fluted filter paper (VWR) and loaded onto the equilibrated column. The column was washed using 6 CV of 20 mM sodium phosphate pH 7.4, 0.5 M NaCl, and 5 mM imidazole. The bound proteins were eluted with 20 mM sodium phosphate pH 7.4, 0.5 M NaCl, 100 then 200 mM imidazole into eight fractions each. The purification fractions were analyzed using 12% NuPAGE® Bis-Tris gel and stained with GelCode Blue stain reagent (Thermo Scientific). The fractions containing the bound protein of interest were pooled and dialyzed using a 3.5 kDa cut off Spectra/Por® membrane tubing (Spectrumlabs) against 4L of 20 mM Tris-HCl pH 8.0, 1 mM EDTA pH 8.0, 5% glycerol at 4°C with constant stirring. The dialysis buffer was replaced once.
Strong anion exchange resin Poros® 50 HQ (Applied Biosystems) in a XK16/20 column (GE Healthcare) was applied for the second step purification. The column was equilibrated with 20 mM Tris-HCl pH 8.0, 1 mM EDTA, 5% glycerol (10 CV). The above dialyzed sample was loaded into the column and washed with 20 mM Tris-HCl pH 8.0, 1 mM EDTA, 5% glycerol (8 CV). The bound protein was eluted with 100 then 200 mM sodium borate in 20 mM Tris-HCl pH 8.0, 1 mM EDTA, 5% glycerol into eight fractions each. The purified fractions were analyzed using 12% NuPAGE Bis-Tris SDS gel. Protein concentration was determined using Pierce BCA protein assay kit (Thermo Scientific). The endotoxin level was measured using The Endosafe-PTS Portable Test System (Charles River Laboratories).
Western blotting
Protein samples were separated by electrophoresis using NuPAGE 12% Bis-Tris Gel and the gel electro-transferred at 35 V onto a nitrocellulose membrane filter paper using 1xNuPAGE® transfer buffer (Invitrogen). The membrane was thereafter blocked in 5% non-fat dry milk (Bio-Rad) in 1xPBS, 0.02% Tween 20 for 1 h with shaking and then washed once with 1xPBS, pH7.4, 0.02% Tween 20 at room temperature with shaking. The soluble porcine CTLA-4 was detected with mouse anti-His monoclonal antibody (1.11 µg/mL) (Invitrogen) and rat anti-mouse IgG-HRP (1.25 µg/mL) (Invitrogen) in 5% non-fat dry milk in 1xPBS, 0.02% Tween 20. Detection of the proteins was done by TMB membrane peroxidase substrate (KPL Cat#:50-77-02), and color development was stopped with dH2O.
Biotin-labeling of the soluble porcine CTLA-4
Glycosylated or non-N-glycosylated soluble porcine CLTA-4 was labeled with EZ-Link Sulfo-NHS Biotin (Pierce, Cat# 21217). One milligram of NHS-Biotin was added to one milligram of glycosylated or non-N-glycosylated soluble porcine CTLA-4 then the reaction was incubated for 2 hours at 4°C with rocking. The reaction was transferred to a Slide-a-lyzer dialysis cassette, 10K MWCO, 0.5–3 mL (Thermo Fisher Cat# 66380) and dialyzed against 2 L of 1xPBS for 24 hours at 4°C with constant stirring, changed once.
Screening porcine CD80-expressing B- cell lymphoma cell line
Porcine B-cell lymphoma tumor cell lines available in our laboratory [10] were cultured in T75 culture flasks (Corning, Cat# 10-126-31) with 1x RPMI 1640 media supplemented with 12% fetal bovine serum, 10 mM hepes (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid), 1x nonessential amino acids, 1mM sodium pyruvate, 2 mM glutamine, and 2.5 × 10−5 M 2-mercaptoethanol. Cultures were incubated at 37°C with 5% CO2 until a cell density of 1.0 × 106 cells/mL was achieved. Cells were spun down and re-suspended to a concentration of 1.0 × 107 cells/mL in FACS media containing 1x Hanks Balanced Salt Solution with Ca+ and Mg+, 0.1% Bovine serum albumin and 0.1% sodium azide and aliquotted into FACS tubes at 1.0 × 106 cells/tube. Biotin-labeled hamster anti-mouse CD80 mAb clone 16-10A1 (BioLegend, Cat#104703) was then added to each cell line at a final concentration of 17 nM and cells were incubated at 4°C for 30 minutes. Cells were washed twice with 2 mL FACS media and centrifuged at 1200 rpm, 4°C for 5 minutes. Streptavidin-PE was added to each tube at a final concentration of 1.5 ng/uL and incubated in the dark at 4°C for 15 minutes. Cells were then washed once as described above then re-suspended in 500 µL of FACS media and flow cytometry was carried out using a Becton Dickinson FACScan. Flow cytometry data were analyzed using Winlist analysis software (Verity Software House, Topsham, ME). Human CD80-expressing leukemia cell line THP-1 (kindly provided by Dr. Fabrice Porcheray and Dr. Emmanuel Zorn, MGH) was used as the hamster anti-mouse CD80 mAb positive control cell line.
FACS analysis of soluble porcine CTLA-4 binding to porcine CD80
The screened porcine CD80-expressing B-cell lymphoma cell line LCL13271-5E4 as well as the control human CD80-expressing leukemia cell line THP-1 were cultured and stained as described above. Cells were stained with biotinylated glycosylated or non-N-glycosylated soluble porcine CTLA-4 at a range of dilutions from 3.5 nM, 35 nM and 350 nM. Biotin-labeled hamster anti-mouse CD80 mAb clone 16-10A1 (BioLegend, Cat# 104703) used at a final concentration of 17 nM as positive staining control. Negative control cells were stained only with streptavidin-PE at a final concentration of 1.5 ng/uL (without biotylated porcine CTLA-4). Flow cytometry was carried out using a Becton Dickson FACScan. Flow cytometry data were analyzed using Winlist analysis software (Verity Software House, Topsham, ME).
Porcine CTLA-4 competitively inhibits anti-CD80 binding to porcine B-cell lymphoma cell line
The screened porcine B cell lymphoma line 13271-5E4 as well as the control human CD80-expressing leukemia cell line THP-1was cultured as described above. The cells were resuspended to a concentration of 1.0 × 107 cells/mL in FACS media and aliquotted into FACS tubes at 1.0 × 106 cells / tube. Glycosylated or non-N-glycosylated soluble porcine CTLA-4 was then added to cells at a range of concentrations including 0.7 nM, 7 nM, 70 nM, 700 nM, and maximum concentration (2800 nM for glycosylated version and 2600 nM for non-N-glycosylated version), and allowed to incubate for 5 minutes at 4°C in the dark. After five minutes, biotin-labeled hamster anti-mouse CD80 mAb clone 16-10A1 (BioLegend, Cat#104703) was added to each tube at a final concentration of 17 nM and the cell suspensions were incubated at 4°C in the dark for 30 minutes. Cells were washed twice with 2 mL FACS media and centrifuged at 1200 rpm, 4°C for 5 minutes. Streptavidin-PE was added to each tube at a final concentration of 1.5 ng/uL and incubated in the dark at 4°C for 15 minutes. Cells were then washed once as described above and then resuspended in 500 µL of FACS media. FACS analysis was carried out using a Becton Dickson FACScan. Flow cytometry data were analyzed using Winlist analysis software (Verity Software House, Topsham, ME).
KD determination
FACS binding analysis of porcine CTLA-4 was performed using the porcine CD80-expressing porcine B cell lymphoma line LCL13271-5E4 with wide range concentrations of biotinylated soluble porcine CTLA-4. The mean fluorescence intensity (MFI) values were tabulated and plotted on a linear scale versus each biotinylated soluble porcine CTLA-4 concentration. This plot was fitted using non-linear least-squares by KaleidaGraph software (Synergy Software, Reading PA) using the hyperbolic equation y=m1+m2*m0/(m3+m0), where y = MFI at the given biotinylated soluble porcine CTLA-4 concentration, m0 = biotinylated soluble porcine CTLA-4 concentration, m1 = MFI of zero biotinylated soluble porcine CTLA-4 control, m2 = MFI at saturation and m3 = KD, the equilibrium dissociation constant (Yeast Display scFv Antibody Library User’s Manual, Pacific Northwest National Laboratory, Richland, WA; http://www.sysbio.org/dataresources/index.stm)
Results
Expression and purification of glycosylated soluble porcine CTLA-4 in Pichia pastoris
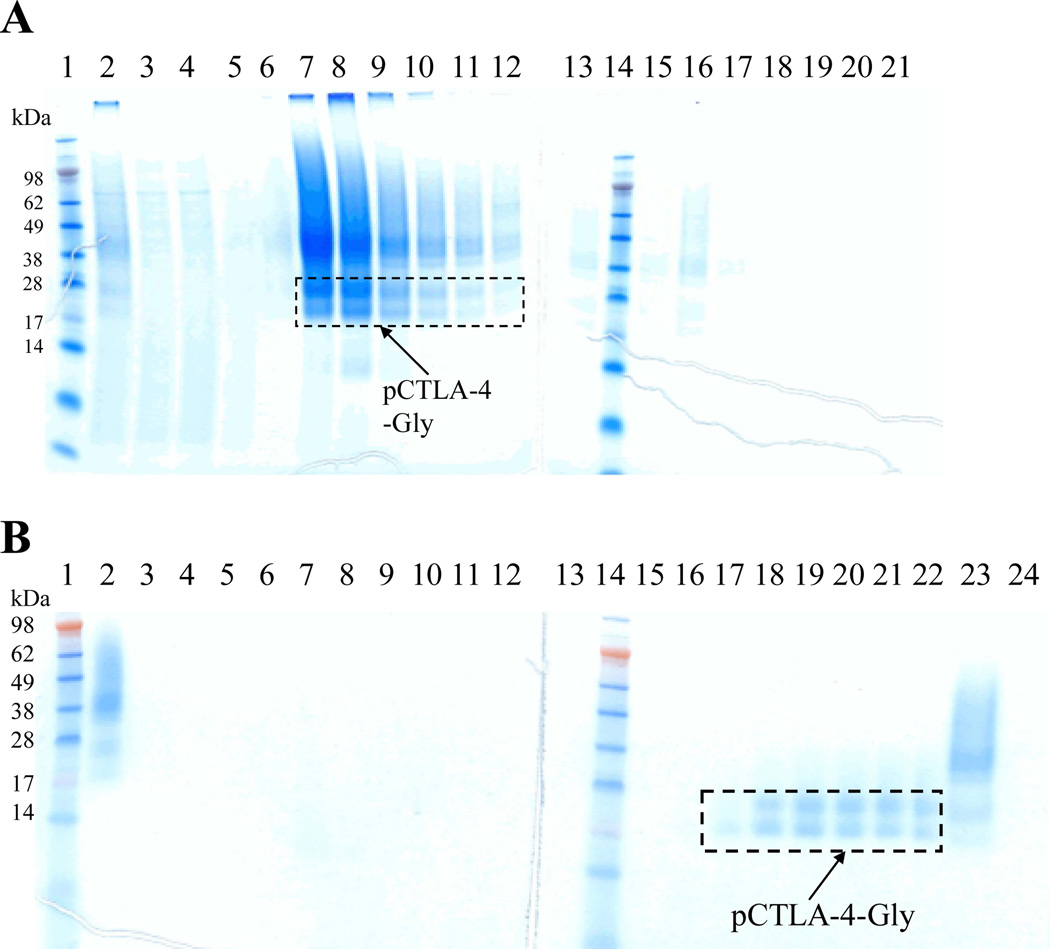
The codon-optimized soluble porcine CTLA-4 DNA carrying 6xHis tag (130aa) in the C-terminus was synthesized and cloned into Pichia pastoris expression vector pwPICZα (Figure 1) [9]. The 6xHis tag in the C-terminus was added to facilitate the downstream purification. The DNA construct was linearized and transformed into Pichia pastoris strain X33. Soluble porcine CTLA-4 carrying 6xHis in the C-terminus was expressed using the large-scale shake-flask system as described in materials and methods. Western blotting analysis confirmed the expression using mouse anti-His monoclonal antibody (data not shown). The secreted soluble porcine CTLA-4 was captured directly by Ni-Sepharose 6 fast flow resin through its 6xHis-tag in the C-terminus (Figure 2A). The eluted fractions from the capturing step were pooled, concentrated with Centricon Plus-70 (5 kDa cut off) and dialyzed to remove the salts for the second step purification. Based on the PI value (5.33) strong anion exchange resin Poros 50 HQ was used for the second step purification. In which, sodium borate was applied to separate the porcine CTLA-4 from the glycosylated yeast host protein and aggregates [11]. As shown in Figure 2B (elution fractions with 200 mM sodium borate) soluble porcine CTLA-4 was obtained after this two-step purification. The final product level was ~ 2 mg/L (Table 2) with an assessed endotoxin level of <13.5 EU/mg.
Figure 2.
Purification for glycosylated soluble porcine CTLA-4: A) First step purification using Ni-Sepharose 6 fast flow resin. Lane 1 and 14: protein markers; Lane 2: sample; Lane 3: flowthrough; Lane 4: washing; Lane 5–12: eight 50 mL elution fractions using 100 mM imidazole; Lane 13 and 15–21: eight 50 mL elution fractions using 200 mM imidazole. B) Second step purification using strong anion-exchange resin Poros 50HQ. Lane 1 and 14: protein markers; Lane 2: sample; Lane 3: flowthrough; Lane 4–5: two 40 mL washing fractions; Lane 6–13: eight 10 mL elution fractions using 100 mM sodium borate; Lane 15–22: eight 10 mL elution fractions using 200 mM sodium borate; Lane 23-24: flushing using 1 M NaCl.
Table 2.
Comparison of the expression and purification level for the glycosylated and non-N-glycosylated soluble porcine CTLA-4
| Purification step | Glycosylated (Recovery %) | Non-N-Glycosylated (Recovery %) |
|---|---|---|
| Supernatant | ~5 mg/L (100%) | ~20 mg/L (100%) |
| Ni-Sepharose 6 fast flow | ~3 mg/L (60%) | ~16 mg/L (80%) |
| Poros 50 HQ | ~2 mg/L (40%) | ~8 mg/L (40%) |
Expression and purification of non-N-glycosylated soluble porcine CTLA-4 in Pichia Pastoris
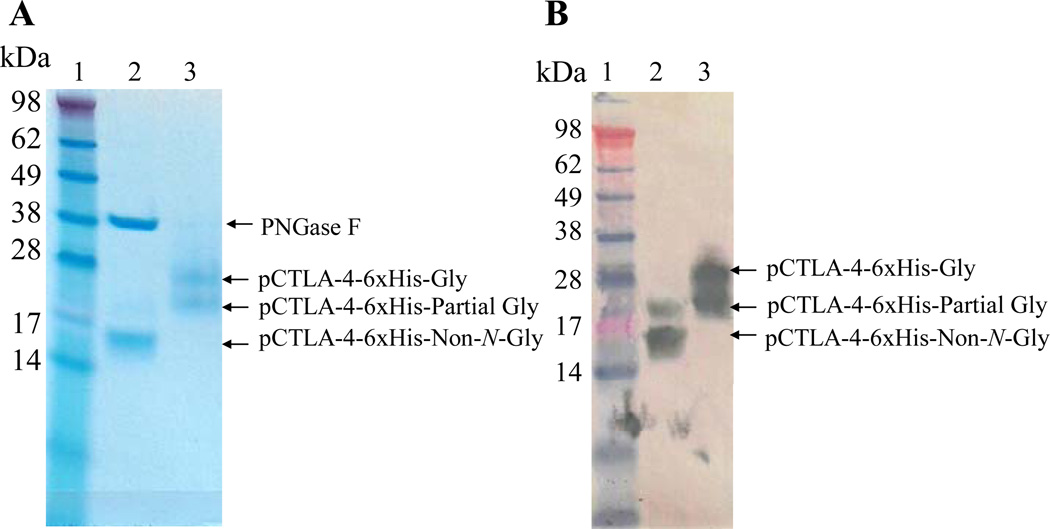
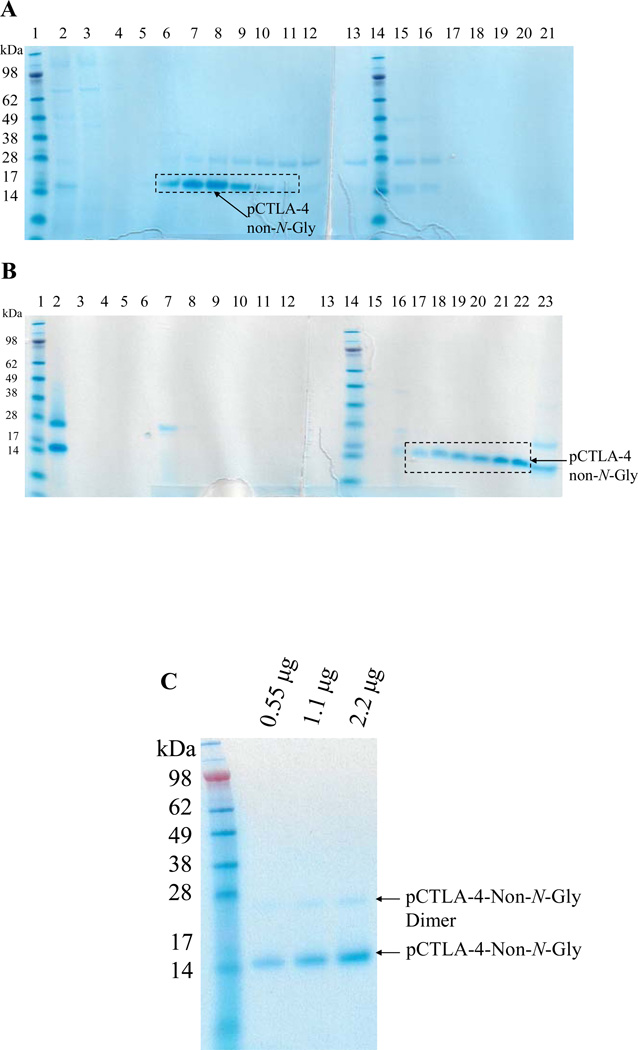
As shown in Figure 3, N-linked glycosylation analysis using PNGase F digestion demonstrated that the Pichia pastoris expressed soluble porcine CTLA-4 was N-glycosylated completely or partially. The protein expression level is low and the final product is not well purified as shown in Figure 2B (elution fractions with 200 mM sodium borate). Using this approach it would be difficult to scale up to produce enough pure and cost-effective soluble porcine CTLA-4 for our porcine transplantation model. We hypothesized that N-glycosylation may be responsible for the low expression level and the difficulty of purification. If the potential N-glycosylation sites were mutated and the soluble porcine CTLA-4 still maintained its original binding function, it may be possible to improve the expression level and facilitate a more effective purification. The two potential N-glycosylation sites were replaced with non-polarized alanines by site-directed mutagenesis (Stratagene). As shown in Fig. 4A, B and C, a very pure non-N-glycosylated soluble porcine CTLA-4 was obtained after two step purification and the production level was improved to ~8 mg/L (Table 2). The endotoxin concentration is <14.2 EU/mg. This facilitates large scale production of pure non-N-glycosylated soluble porcine CTLA-4 suitable for use in our porcine transplantation model.
Figure 3.
N-linked glycosylation analysis for soluble porcine CTLA-4 using PNGase F.: A) 12% NuPAGE SDS gel analysis. B) Western blot analysis using mouse anti-His mAb (1.1µg/mL, Invitrogen) and HRP-rat anti-mouse IgG (1.25µg/mL, Invitrogen). Lane 1: protein marker; Lane 2: with PNGase F enzyme to remove N-linked glycosylation; Lane 3: without PNGase F enzyme. Soluble porcine CTLA-4 is approximately 14.1 kDa.
Figure 4.
Purification for non-N-glycosylated soluble porcine CTLA-4: A) First step purification using Ni-Sepharose 6 fast flow resin. Lane 1 and 14: protein markers; Lane 2: sample; Lane 3: flowthrough; Lane 4: washing; Lane 5–12: eight 50 mL elution fractions using 100 mM imidazole; Lane 13 and 15–21: eight 50 mL elution fractions using 200 mM imidazole. B) Second step purification using strong anion-exchange resin Poros 50HQ. Lane 1 and 14: protein markers; Lane 2: sample; Lane 3: flowthrough; Lane 4–5: two 40 mL washing fractions; Lane 6–13: eight 10 mL elution fractions using 100 mM sodium borate; Lane 15–22: eight 10 mL elution fractions using 200 mM sodium borate. Lane 23: flushing using 1 M NaCl. C) 12% NuPAGE SDS gel analysis for the final product of non-N-glycosylated soluble porcine CTLA-4 (14.1 kDa). The weak band seen at approximately 28 kDa is a covalent homodimer of the non-N-glycosylated soluble porcine CTLA-4 generated by disulfide bond formation (analyzed under reduced conditions using DTT and beta-mercaptoethanol, data not shown).
FACS analysis for binding to porcine CD80 using a porcine CD80-expressing B-cell lymphoma cell line
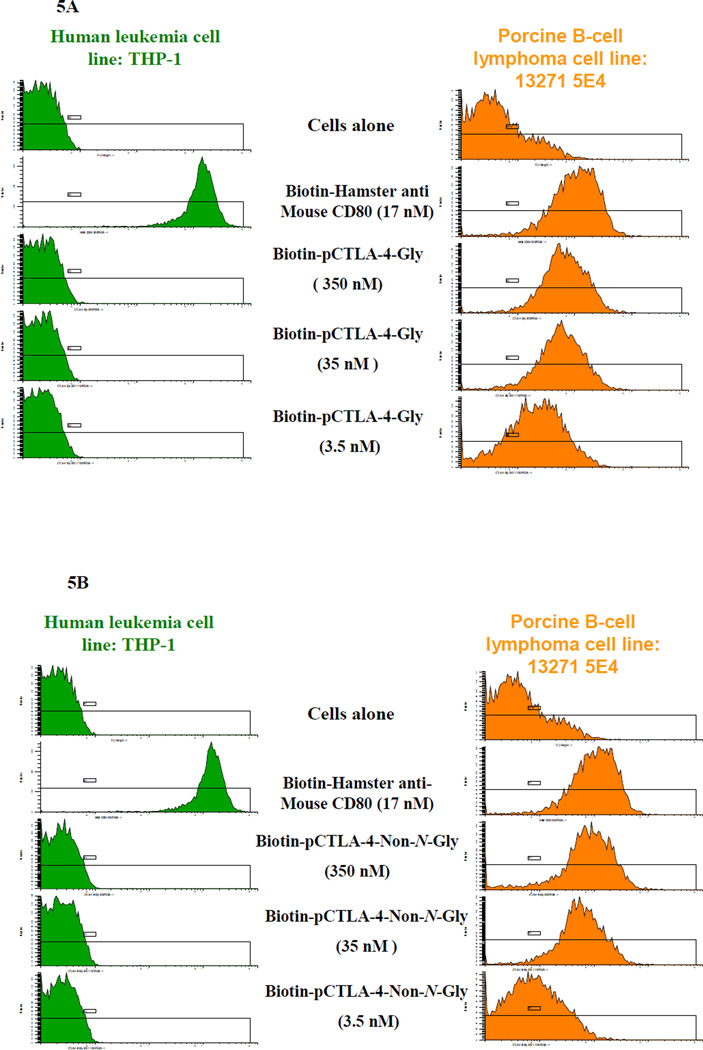
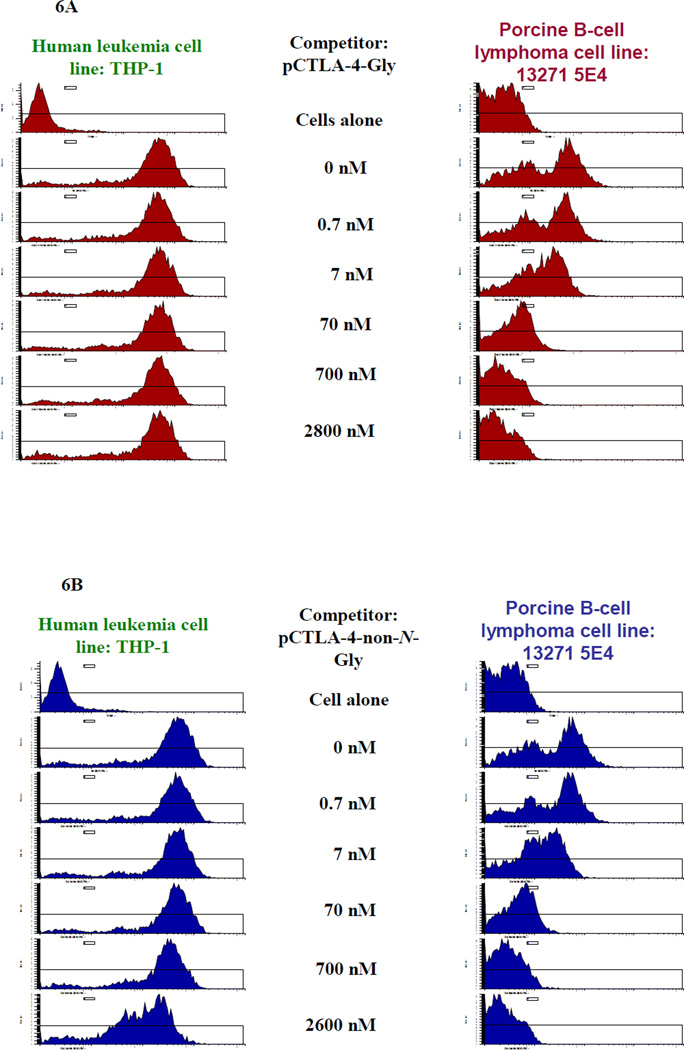
Porcine CTLA-4 binds to porcine CD80 and CD86 in nature. Therefore a CD80- or CD86-expressing porcine antigen presenting cell line is required in order to assess the binding function of the soluble porcine CTLA-4 in vitro. Using FACS analysis with hamster anti-mouse CD80 mAb (clone 16-10A1) (BioLegend, Cat#104703) a porcine CD80-expressing B-cell lymphoma cell line (LCL13271-5E4) was successfully screened from our available potential porcine B-cell lymphoma cell lines [10]. As shown in Figure 5A and 5B, FACS binding analysis demonstrated that both biotinylated glycosylated and non-N-glycosylated soluble porcine CTLA-4 bound to the porcine CD80-expressing B-cell lymphoma cell line LCL13271-5E4 in a dose-dependent manner. No binding to the human CD80-expressing leukemia cell line THP-1 was detected indicating that binding is species specific. To confirm the specific binding of the soluble porcine CTLA-4 to porcine CD80, a dose-dependent blocking/competition assay for the binding of hamster anti-mouse CD80 mAb (clone 16-10A1) to the porcine CD-80 expressing B-cell lymphoma cell line (LCL13271-5E4) was also performed using the non-biotinylated glycosylated or non-N-glycosylated soluble porcine CTLA-4. As shown in Figure 6A and 6B, both glycosylated and non-N-glycosylated soluble porcine CTLA-4 blocked the binding in a dose-dependent manner. Therefore we conclude that both glycosylated and non-N-glycosylated soluble porcine CTLA-4 are biologically functional in vitro. These results also indicate that the binding epitope/domain of the soluble porcine CTLA-4 and the hamster anti-mouse CD80 mAb on the porcine B-cell lymphoma cell line is same or overlapped.
Figure 5.
FACS binding analysis for biotinylated A) glycosylated or B) non-N-glycosylated soluble porcine CTLA-4 using porcine CD80-expressing B-cell lymphoma cell line (LCL13271-5E4) shown in the column on the right. Human CD80-expressing leukemia cell line THP-1 was included as positive control cell line for the hamster anti-mouse CD80 mAb (clone 16-10A1) shown in the column on the left.
Figure 6.
Blocking/competition analysis for the binding of hamster anti-mouse CD80 mAb (clone 16-10A1) to the porcine CD-80 expressing B-cell lymphoma cell line (LCL13271-5E4), shown in the column on the right, using non-biotinylated A) glycosylated or B) non-N-glycosylated porcine soluble CTLA-4. Human CD80-expressing leukemia cell line THP-1, shown in the column on the left, was included as positive control cell line for the hamster anti-mouse CD80 mAb clone 16-10A1. Concentrations listed represent the concentration of the non-biotinylated soluble porcine CTLA-4 competitor. The concentration of the biotinylated hamster anti-mouse CD80 mAb is 17 nM.
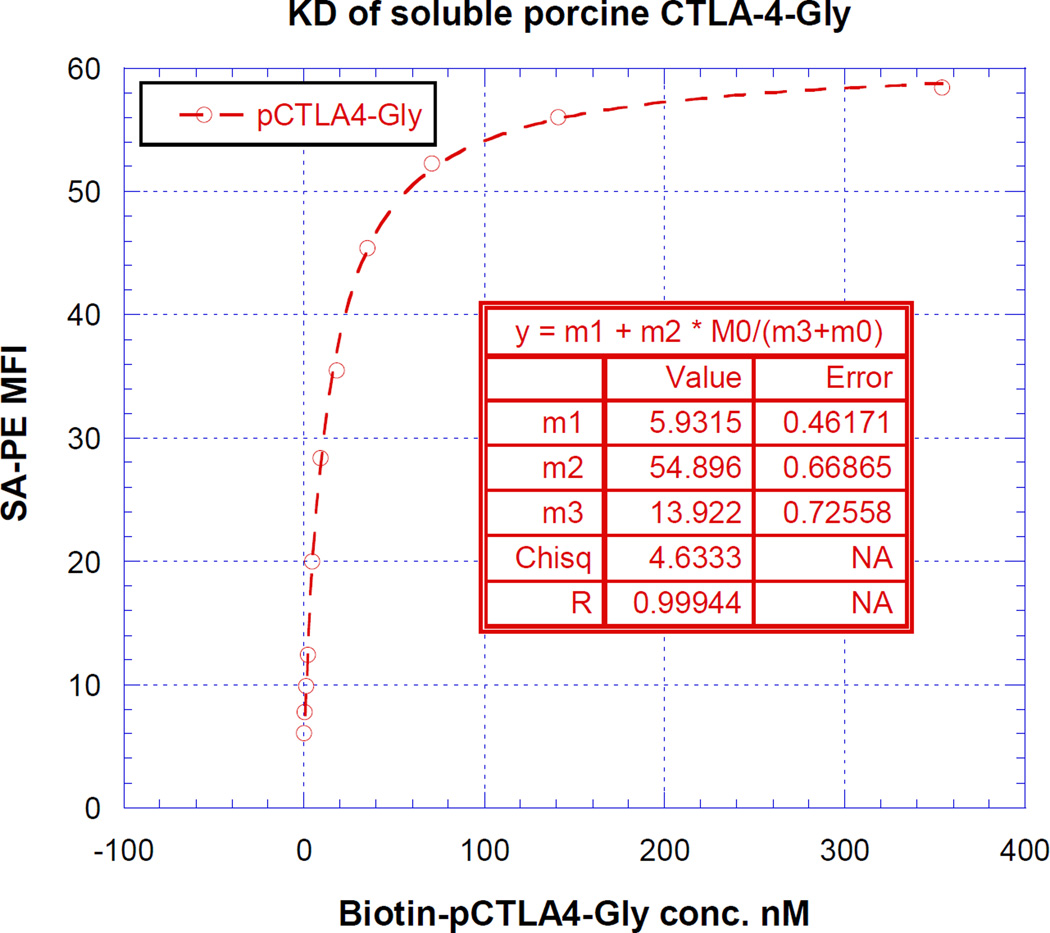
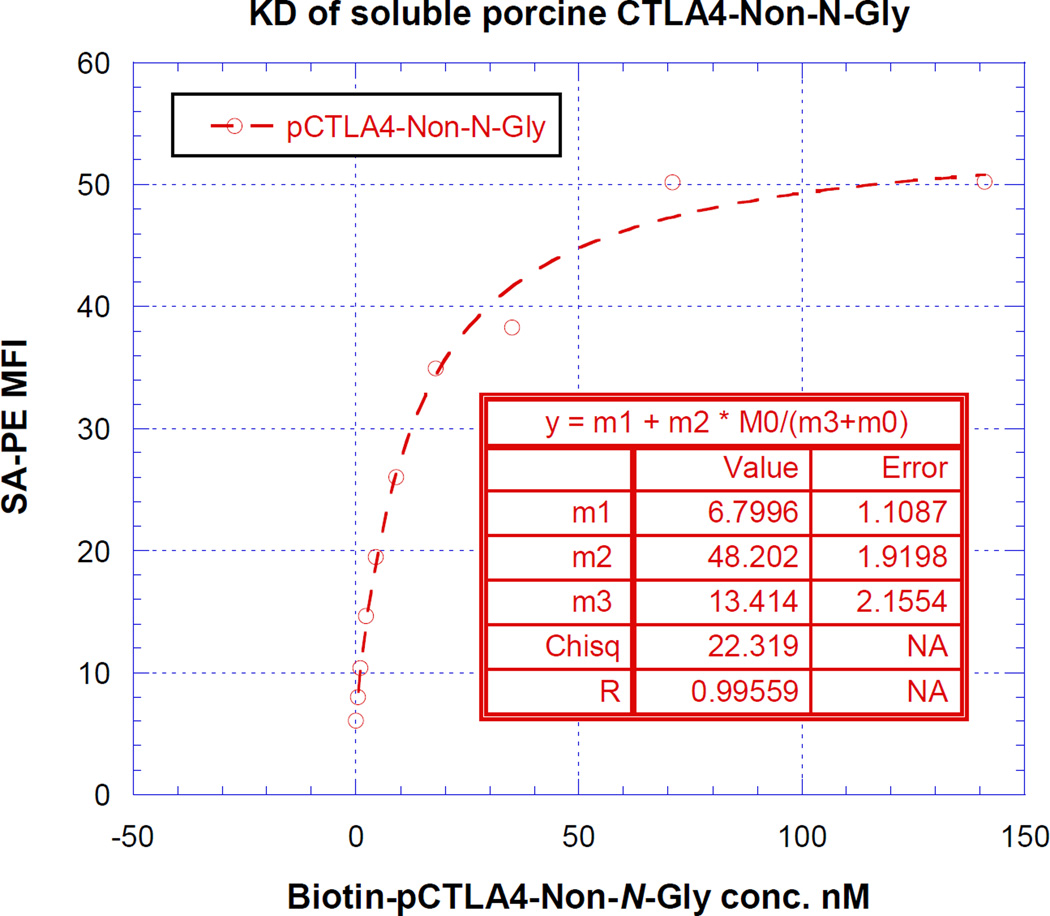
As shown in Figure 7, the KD value was determined by plotting the FACS- MFI values versus a wide range of concentrations of the biotinylated glycosylated or non-N-glycosylated soluble porcine CTLA-4. The non-linear least-squares curve fitting revealed a KD of 13 nM (m3 value of the inset table in Figure 7A and 7B) for both glycosylated and non-N-glycosylated soluble porcine CTLA-4.
Figure 7.
KD determination using flow cytometry and nonlinear least squares fit. MFI was plotted over a wide range concentration of biotinylated A) glycosylated or B) non-N-glycosalated soluble porcine CTLA-4. The accompanying least-squares fits and the parameters are shown based on the hyperbolic equation y = m1 + m2 * m0/(m3 + m0) where y = MFI at the given biotinylated soluble porcine CTLA-4 concentration, m0 = biotinylated soluble porcine CTLA-4 concentration, m1 = MFI of zero biotinylated soluble porcine CTLA-4 control, m2 = MFI at saturation and m3 = KD. The inset table in A) showed a fitted KD of 13 nM for glycosalated soluble porcine CTLA-4. The inset table in B) showed a fitted KD of 13 nM for non-N-glycosalated soluble porcine CTLA-4.
Discussion
In this study we have successfully expressed and purified the functional glycosylated and non-N-glycosylated soluble porcine CTLA-4 in yeast Pichia pastoris system. The mutation of the N-glycosylation sites of soluble porcine CTLA-4 improved the expression and purification in the yeast Pichia pastoris system, which facilitated large scale production. We speculate that mutating the N-glycosylation sites may be a useful approach to improve the Pichia pastoris expression and especially the purification as long as the N-glycosylation is not associated with the biological function for the recombinant protein in question. Given that expression and purification of non-N-glycosylated soluble porcine CTLA-4 proved to be more straightforward than that of glycosylated soluble procine CTLA-4, we plan to produce this non-N-glycosylated version for our in vivo application. We believe that this soluble porcine CTLA-4, especially the non-N-glycosylated version, could be a very valuable and cost-effective costimulatory blockade module in our porcine model for transplantation, autoimmune disease and cancer treatment studies.
Theoretically porcine soluble CTLA-4 should also bind to porcine CD86. However we could not provide the direct evidence for this since at this time we do not have access to a porcine CD86-expressing cell line.
It has been reported that CTLA-4 binds to CD80/CD86 using the conserved signature binding motif “MYPPPY” for mammalian species [3], however for pig “LYPPPY” is used [12]. In this study we demonstrated that soluble porcine CTLA-4 can not bind to human CD80-expressing leukemia cell line THP-1. This provides additional direct evidence for the importance of the methionine to leucine substitution at the signature motif to abolish the binding of porcine CTLA-4 to human CD80 as reported by Vaughan et al., 2000 [12]. We speculate that this soluble porcine CTLA-4 could be used in pig to non-human primate transplantation to specifically inhibit the donor APC-costimulated T cell response. As expected, the mutation of the N-linked glycosylation sites did not influence the binding of the non-N-glycosylated porcine soluble CTLA-4 to CD80-expressing porcine B-cell lymphoma cell line LCL13271-5E4.
In this study we identified a porcine B-cell lymphoma line (LCL13271-5E4) expressing high levels of porcine CD80 using hamster anti-mouse CD80 mAb clone 16-10A1. The availability of this CD80 expressing porcine cell line provides a useful tool for studies of immune modulation in pigs. Biotinylated glycosylated and non-N-glycosylated porcine CTLA-4 both bind LCL13271-5E4 cells. The soluble porcine CTLA-4 competitively inhibits the binding of the hamster anti-mouse CD80 mAb 16-10A1 to LCL13271-5E4 cells indicating that the porcine CTLA-4 and the hamster anti-mouse CD80 mAb bind to the same domain. We are currently developing a diphtheria toxin-based porcine CTLA-4 immunotoxin for the purpose of depleting CD80 expressing cells including antigen presenting cells in vivo in pigs. The porcine CD80-expressing lymphoma cell line LCL13271-5E4 will be useful for assessing function of this immunotoxin in vitro.
Glycosylated and non-N-glycosylated soluble porcine CTLA-4 bind to the porcine CD80 expressing B-cell lymphoma cell line LCL13271-5E4 with a KD value of 13 nM. This is comparable to the binding affinity of human CTLA-4 to human CD80 at KD value of approximately 12 nM [13, 14]. It has been reported that CTLA-4 homodimer binds to bivalent CD80 in vivo [13]. We speculate that the porcine soluble CTLA-4 dimer will further improve the binding affinity to porcine CD80 further.
Increased expression of CD80 on podocytes has been identified as a likely cause of proteinuria in minimal change disease [15]. CTLA-4 appears to regulate CD80 expression in podocytes, and to be altered in minimal change disease patients. The availability of soluble porcine CTLA-4 may allow us to devise new treatment regimens for proteinuria in our pig-to-non-human primate renal transplant studies [16] and experiments directed toward this goal are in preparation.
Highlights.
Soluble porcine CTLA-4 was successfully expressed and purified in P. pastoris system.
Removing N-glycosylation sites improved the soluble porcine CTLA-4 expression and purification.
Soluble porcine CTLA-4 can bind to a porcine CD80-expressing B-cell lymphoma cell line.
Acknowledgement
The work was supported by National Institutes of Health (2P01AI45897 to DHS) and Dana Farber/Harvard Cancer Center Core development grant. We thank Kazuhiko Yamada and David Leonard for intensive manuscript review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Riha P, Rudd CE. CD28 co-signaling in the adaptive immune response. Self Nonself. 2010;1:231–240. doi: 10.4161/self.1.3.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sansom DM. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology. 2000;101:169–177. doi: 10.1046/j.1365-2567.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 5.Zhu S, Liu S, Wan L, Yang G, Yang H, Cheng J, Lu X. Molecular cloning, expression and characterization of the functional domain of CTLA4 from the rhesus monkey, Macaca mulatta. Dev Comp Immunol. 2011;35:736–744. doi: 10.1016/j.dci.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Cregg JM. Introduction: distinctions between Pichia pastoris and other expression systems. Methods Mol Biol. 2007;389:1–10. doi: 10.1007/978-1-59745-456-8_1. [DOI] [PubMed] [Google Scholar]
- 7.Brondyk WH. Selecting an appropriate method for expressing a recombinant protein. Methods Enzymol. 2009;463:131–147. doi: 10.1016/S0076-6879(09)63011-1. [DOI] [PubMed] [Google Scholar]
- 8.Sreekrishna K. Strategies for optimizing protein expression and secretion in the methylotrophic yeast Pichia pastoris. In: Baltz RH, Hegeman GD, Skatrud PL, editors. Industrial Microorganism: Basic and Applied Molecular Genetics. Washington, DC: American Society of Microbiology; 1993. pp. 119–126. [Google Scholar]
- 9.Woo JH, Liu YY, Mathias A, Stavrou S, Wang Z, Thompson J, Neville DM., Jr Gene optimization is necessary to express a bivalent anti-human anti-T cell immunotoxin in Pichia pastoris. Protein Expr. Purif. 2002;25:270–282. doi: 10.1016/s1046-5928(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 10.Cho PS, Lo DP, Wikiel KJ, Rowland HC, Coburn RC, McMorrow IM, Goodrich JG, Arn JS, Billiter RA, Houser SL, Shimizu A, Yang YG, Sachs DH, Huang CA. Establishment of transplantable porcine tumor cell lines derived from MHC-inbred miniature swine. Blood. 2007;110:3996–4004. doi: 10.1182/blood-2007-02-074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo JH, Neville DM., Jr Separation of bivalent anti-T cell immunotoxin from Pichia pastoris glycoproteins by borate anion exchange. BioTechniques. 2003;35:392–398. doi: 10.2144/03352pt04. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan AN, Malde P, Rogers NJ, Jackson IM, Lechler RI, Dorling A. Porcine CTLA4-Ig lacks a MYPPPY motif, binds inefficiently to human B7 and specifically suppresses human CD4+ T cell responses costimulated by pig but not human B7. J Immunol. 2000;165:3175–3181. doi: 10.4049/jimmunol.165.6.3175. [DOI] [PubMed] [Google Scholar]
- 13.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;29:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garin EH, Mu W, Arthur JM, Rivard CJ, Araya CE, Shimada M, Johnson RJ. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010;78:296–302. doi: 10.1038/ki.2010.143. [DOI] [PubMed] [Google Scholar]
- 16.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O'Malley P, Nobori S, Vagefi PA, Patience C, Fishman J, Cooper DK, Hawley RJ, Greenstein J, Schuurman HJ, Awwad M, Sykes M, Sachs DH. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat. Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]