SUMMARY
The IκB kinase (IKK) pathway is an essential mediator of inflammatory, oncogenic, and cell stress pathways. Recently IKK was shown to be essential for autophagy induction in mammalian cells independent of its ability regulate NF-κB, but the mechanism by which this occurs is unclear. Here we demonstrate that the p85 regulatory subunit of PI3K is an IKK substrate, phosphorylated at S690 in vitro and in vivo in response to cellular starvation. Cells expressing p85 S690A or inhibited for IKK activity exhibit increased Akt activity following cell starvation, demonstrating that p85 phosphorylation is required for starvation-induced PI3K feedback inhibition. S690 is in a conserved region of the p85 cSH2 domain, and IKK-mediated phosphorylation of this site results in decreased affinity for tyrosine-phosphorylated proteins and decreased PI3K membrane localization. Finally, leucine deprivation is shown to be necessary and sufficient for starvation-induced, IKK-mediated p85 phosphorylation and PI3K feedback inhibition.
Keywords: cellular starvation, IKK, PI3K, p85, SH2 domain
INTRODUCTION
Phosphatidylinositol 3-kinases (PI3Ks) belong to a conserved family of lipid kinases that phosphorylate the 3’ hydroxyl group of phosphoinositides and phosphatidylinositol. Class IA PI3K consists of a heterodimer of a p110 catalytic subunit and a p85 regulatory subunit. The class IA p85 regulatory proteins (p85α, p85β, p55γ, p55α and p50α) are characterized by a p110-binding domain flanked by two Src-homology 2 (SH2) domains. p85 SH2 domains control activation of PI3K by receptor tyrosine kinases (RTKs) via interactions with tyrosine-phosphorylated receptor/adaptor proteins (Engelman et al., 2006). SH2-phosphotyrosine interactions promote lipid kinase activity by both properly localizing PI3K to the plasma membrane and by relieving inhibitory effects of p85 (Yu et al., 1998).
Phosphatidylinositol-3,4,5-triphophosphate (PIP3) generated by PI3K serves as a second messenger to recruit and activate pleckstrin homology (PH)-containing proteins (Cantley, 2002). A critical target of PIP3 is the serine-threonine kinase Akt. Upon binding of PIP3, Akt localizes to the cellular membrane where it is activated by PDK1 and mTORC2 phosphorylation at Thr308 and Ser473 respectively (Alessi et al., 1997; Sarbassov et al., 2005). Activated Akt regulates cell growth, survival and metabolism through phosphorylation of a number of effector molecules including Foxo transcription factors, the Ser/Thr kinase GSK-3α/β, and the tuberous sclerosis complex 2 (TSC2) protein (Manning and Cantley, 2007). Phosphorylation of TSC2 suppresses the inhibition of Rheb, leading to the activation of mTORC1, a key regulator of cell metabolism and protein synthesis (Inoki et al., 2002; Manning et al., 2002; Potter et al., 2002). Consistent with its role in promoting cell growth, mTOR negatively regulates autophagy, an intracellular bulk degradation process used by cells to consume proteins and organelles under conditions of cell stress such as nutrient deprivation (He and Klionsky, 2009). The ability to positively regulate cell growth and metabolism and negatively regulate catabolic programs in response to nutrients and growth factors (GFs) has made the PI3K/Akt/mTOR signaling axis a central focus of numerous studies investigating molecular mechanisms of cancer and metabolic disorders.
Recently, an unbiased small molecule screen identified an unexpected role for the inflammatory kinase complex IKK in regulating stress-induced autophagy (Criollo et al., 2010). IKK is a Ser/Thr kinase widely studied as the cytokine- and stress-induced regulator of NF-κB transcription factors and pro-inflammatory gene expression. In order to activate NF-κB, IKK phosphorylates the inhibitor IκBα leading to its ubiquitination and ultimate degradation (Hayden and Ghosh, 2004). Interestingly, control of autophagy by IKK occurs independently of its ability to regulate NF-κB (Criollo et al., 2010; Comb et al., 2010). IKK is rapidly activated in response to starvation but substrates and downstream signaling events remain undefined, suggesting a unique role for IKK in coordinating the cellular response to metabolic stress.
In this study we have focused on control of the PI3K/Akt pathway due to its importance in driving nutrient-sensitive metabolic pathways. We find that IKK activity is essential for feedback inhibition of PI3K/Akt in response to nutrient depletion and identify the p85α regulatory subunit of PI3K as an IKK substrate phosphorylated in both cell-based and animal models in response to this stress. p85α is phosphorylated by IKK in the C-terminal SH2 domain, suppressing interactions between p85α and tyrosine-phosphorylated proteins, as well as PI3K membrane localization. We further show that leucine deprivation is necessary and sufficient for both p85 phosphorylation and PI3K feedback inhibition. These data reveal a mechanism by which IKK downregulates PI3K/Akt signaling in response to nutrient deprivation and have significant implications for the development of diseases associated with cell metabolism and inflammation.
RESULTS
IKK promotes starvation-induced feedback inhibition of Akt
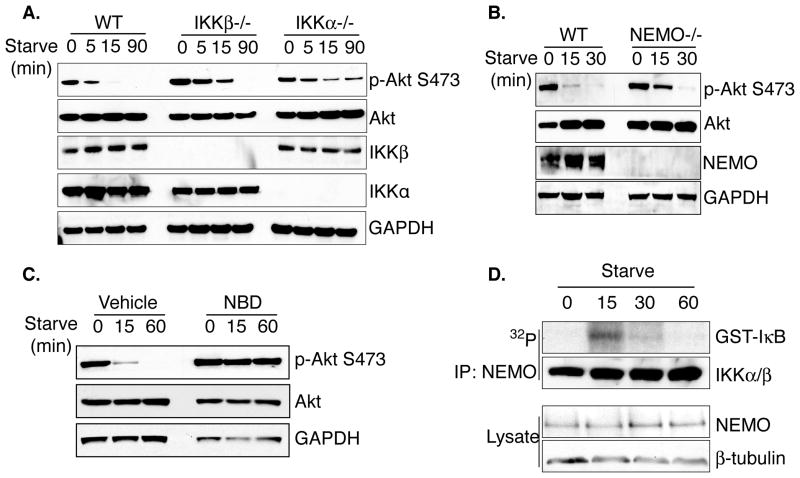
In order to assess mechanisms by which IKK promotes the cellular response to starvation, phosphorylation of Akt at Ser473 was analyzed in WT and IKK-deficient mouse embryonic fibroblasts. Cells lacking IKK subunits displayed increased Akt activity under starvation conditions, but interestingly, have slightly distinct requirements. Specifically, IKKβ−/− mEFs displayed increased basal Akt phosphorylation and slower kinetics of feedback inhibition while IKKα−/− mEFs demonstrated sustained Akt phosphorylation following both brief and extended starvation (Figure 1A). NEMO−/− mEFs phenocopied IKKβ−/− mEFs, demonstrating sustained Akt phosphorylation following 15 minutes of starvation, thus confirming that the canonical IKK holoenzyme contributes to starvation-induced PI3K/Akt inhibition (Figure 1B). To ensure that these effects are dependent on IKK activity, WT mEFs were treated with the NEMO Binding Domain (NBD) peptide, which disrupts association between IKK regulatory and catalytic subunits and inhibits stimulus-induced activity, prior to starvation (May et al., 2000). Consistent with data from the null cell lines, IKK inhibition with the NBD peptide disrupted starvation-induced feedback inhibition of Akt activity (Figure 1C). Notably, cells treated with the IKK inhibitor display a more penetrant phenotype than NEMO−/− cells, as Akt activity is completely maintained following one hour of nutrient deprivation (Figure 1C). This suggests the possibility that transient inhibition of IKK leads to a more dramatic effect than sustained genetic loss of activity. Finally, IKK activity is maximal following 15 minutes of starvation and returns to basal levels following one hour of starvation, as measured by an in vitro kinase assay (Figure 1D). The requirement for IKK to suppress Akt activity following 5–15 minutes of starvation therefore correlates with increased IKK kinase activity following starvation. These data suggest that IKK activity, induced in response to cellular starvation, is important for inhibition of the Akt pathway.
Figure 1. IKK is required for starvation-induced feedback inhibition of Akt.
A. WT, IKKβ−/−, and IKKα−/− mouse embryonic fibroblasts (mEFs) were grown in starvation media (Hank’s Balanced Salt Solution; HBSS) for the indicated times and whole cell extracts were harvested for western blot analysis
B. WT mEFs or NEMO−/− mEFs were grown in starvation media for the indicated times and whole cell extracts were harvested for western blot analysis.
C. WT mEFs were treated with vehicle control or 200μM NEMO Binding Domain (NBD) peptide for 1 hour prior to starvation time course. Cells were harvested and WCEs were examined by western blot analysis.
D. WT mEFs were treated with starvation media for the indicated times. IKK was immunopurified with an antibody against NEMO and was used to phosphorylate a GST-IκBα fragment in an in vitro kinase assay. Kinase reactions were analyzed for incorporated 32P. See also Figure S6.
The PI3K regulatory subunit p85α is an IKK substrate
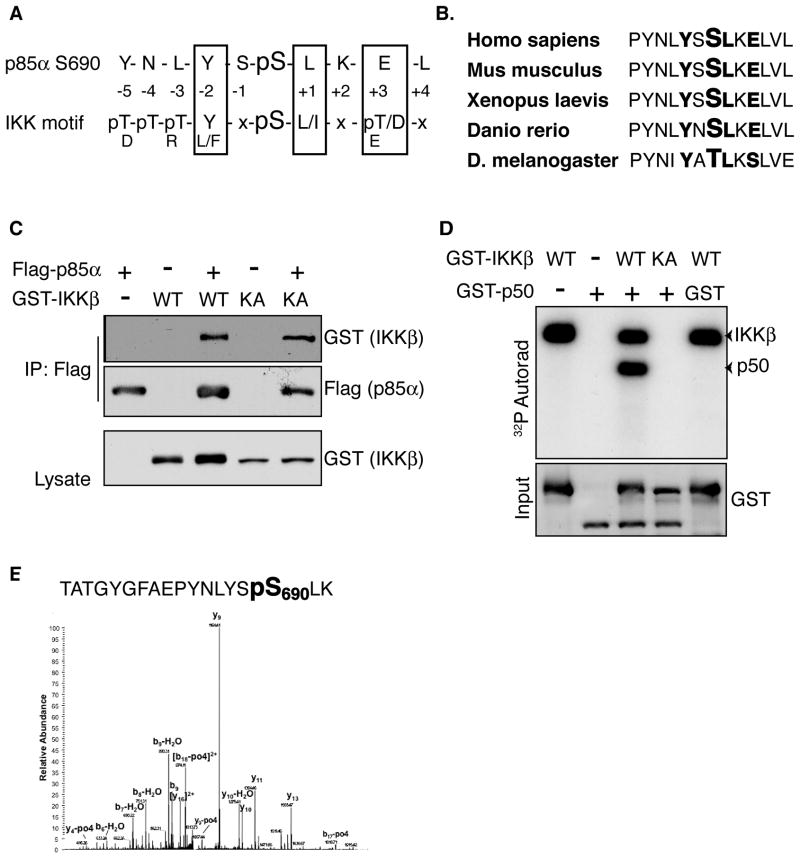
The rapidness by which starvation induces IKK activity and Akt inhibition indicates that the effect of IKK is likely to be direct and occurring through modulation of an unknown substrate. Components of the PI3K/Akt signaling pathway were screened for amino acid sequence similarity with the published IKKβ substrate phosphorylation motif using Scansite (http://scansite.mit.edu) in order to identify candidate substrates involved this activity (Yaffe et al., 2001; Obenauer, 2003). This bioinformatic analysis identified S690 in the cSH2 domain of p85α as a likely IKK phosphorylation site: the +3 acidic, +1 hydrophobic, and -2 aromatic correlate well with the published motif (Figure 2A) (Hutti et al., 2007). Importantly, S690 and the surrounding motif are evolutionarily conserved (Figure 2B) prompting further investigation of p85α as a putative IKK substrate. The ability of IKK subunits to physically interact with p85α was tested by co-expression of Flag-p85 and either GST-IKKβ or -IKKα in HEK293T cells. Flag immune complexes co-precipitate both IKK subunits, but not an unrelated kinase VRK1 (Figure 2C, S1A, and data not shown), demonstrating the basis for a functional interaction.
Figure 2. PI3K regulatory subunit p85α is a putative IKK substrate.
A. Alignment of p85α S690 with the IKK preferred substrate phosphorylation motif.
B. Evolutionary conservation of p85α S690 and the surrounding IKK phosphorylation motif (bold).
C. HEK293T cells were co-transfected with Flag-p85α and GST-IKKβ (WT or K44A) as indicated. Flag immunoprecipitates were blotted with an anti-GST antibody.
D. A bacterially purified GST-p85 C-terminal fragment (p50) containing the p85 cSH2 was incubated with recombinant IKKβ (WT or K44A). Kinase reactions were analyzed for incorporated 32P.
E. Mass spectra from an in vitro IKK kinase reaction with p85-cSH2 substrate. The recovered phosphorylated tryptic peptide fragment corresponding to S690 is indicated. See also Figure S1.
In order to determine if IKK can directly phosphorylate p85α, an in vitro kinase assay was performed using recombinant IKKβ and p50, an alternatively spliced p85α isoform consisting of the C-terminal region including S690 (Engelman et al., 2006), as a substrate. Incubation of WT, but not kinase-dead IKKβ, resulted in robust incorporation of 32P into p50 (Figure 2D). Similar results were also obtained using purified IKKα (Figure S1B). These results indicate that p85α interacts with, and is phosphorylated by, both catalytic subunits of the IKK complex in vitro. The site of phosphorylation was mapped by phosphorylating the GST-cSH2 substrate with IKK in vitro and resolving the reaction by SDS-PAGE. The substrate was gel-excised, subjected to trypsin digestion, and analyzed by microcapillary LC/MS/MS. A phosphopeptide consistent with phosphorylation at S690 was identified, confirming that the site predicted using bioinformatic analysis is phosphorylated by IKK in vitro (Figure 2E).
IKK phosphorylates p85 S690 in response to nutrient deprivation
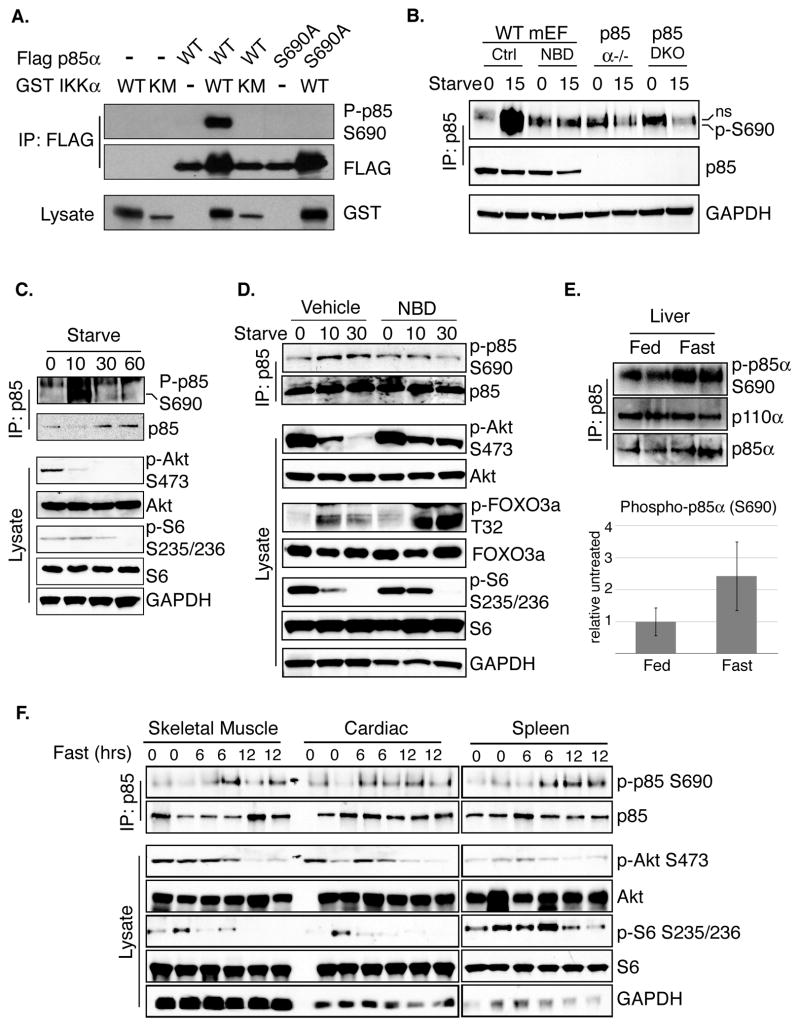
A phosphospecific antibody was raised against a phosphopeptide encompassing p85 S690 in order to determine whether IKK can phosphorylate p85 in cultured cells. Coexpression of WT p85, but not S690A, and IKKα led to dramatically increased signal with the p85 S690 phosphospecific antibody, indicating that the antibody is highly specific for phosphorylated S690 (Figure 3A). The ability of IKK to phosphorylate endogenous p85 in response to starvation was next addressed by treating WT mEFs with starvation media in the presence or absence of the NBD peptide. Strong induction of S690 phosphorylation was observed in cells treated with vehicle control but not cells treated with the IKK inhibitor (Figure 3B, lanes 1–4). The p85 phospho-S690 specific antibody recognizes a non-specific species that co-purifies following immunoprecipitation of p85 from lysates prepared in 1% NP40 lysis buffers but not under more stringent conditions (ie: RIPA buffer or sonication) explaining the presence of the band in Figure 3B and 3C but not in 3D. Interestingly, TNFα and LPS, well-established inflammatory stimuli that activate IKK, also induce phosphorylation of p85 S690, suggesting that many IKK-dependent signals can induce this post-translational modification (Figure S2).
Figure 3. IKK phosphorylates S690 in vitro and in vivo.
A. HEK293T cells were cotransfected with Flag-p85α (WT or S690A) and GST-IKKα (WT or K44M) as indicated. Flag immunoprecipitates were analyzed with the phospho-p85 S690 antibody.
B. WT mEFs were pretreated for 1 hour with vehicle control or NBD peptide (200μM) (lanes 1–4). WT (lanes 1–4), p85α null (5–6), or p85α/β double knock out cells (7–8) were then left untreated or starved for 10 minutes. p85 immunoprecipitates were analyzed for phosphorylated S690. A nonspecific band recognized by the P-p85 S690 antibody is indicated with “ns.”
C. WT mEFs were starved for the indicated times and p85 immunoprecipitates were analyzed for phosphorylated S690. Whole cell extracts were also blotted with the indicated antibodies.
D. WT mEFs were pretreated with vehicle control or NBD peptide (200μM) for 1 hour and starved for the indicated times. Lysates were subjected to immunoprecipitation with an anti-p85 antibody. Immunoprecipitates and whole cell extracts were evaluated with the indicated antibodies.
E. C57/B6 WT mice were fed or fasted for 24 hours. Livers were harvested as described previously (Criollo et al., 2010). Tissue extracts were subjected to immunoprecipitation with an anti-p85 antibody. Immunoprecipitates were evaluated by western blot for phosphorylation of p85 at S690 (upper panel). Tissue extracts were also analyzed by western blot with the indicated antibodies (upper panel). Quantitation of phosphorylation is shown in the lower panel. Phospho-p85 levels were normalized to co-precipitating p110 levels and 4 mice per group were analyzed. Error bars represent standard deviation.
F. C57/B6 WT mice were fed or fasted for 6 or 12 hours. Animals were sacrificed and the indicated organs were harvested. Tissue extracts were subjected to immunoprecipitation with an anti-p85 antibody. Immunoprecipitates were evaluated by western blot for phosphorylation of p85 at S690. Tissue extracts were also analyzed by western blot with the indicated antibodies. See also Figure S2.
The kinetics of starvation-induced p85 phosphorylation were next compared to markers of PI3K/Akt pathway activation status. Induction of p85 phosphorylation inversely correlated with markers of PI3K activation including Akt phosphorylation at Ser473 and phosphorylation of the S6K substrate ribosomal protein S6 (Ser235/236) (Figure 3C). These data indicate that both upstream (Akt) and downstream (mTOR effector arm, S6) pathway activity was inhibited following p85 phosphorylation. Inhibition of IKK with the NBD peptide blocked starvation-induced p85 S690 phosphorylation and PI3K pathway feedback inhibition, confirming that both of these events are dependent on IKK activity. Cells treated with an IKK inhibitor displayed increased Akt phosphorylation, phosphorylation of the Akt substrate FOXO3a at Thr32, and signaling through mTORC1 (as measured by S6 phosphorylation), following 10 minutes of starvation compared to control treated cells (Figure 3D). An unexpected and transient increase in FOXO phosphorylation was observed following nutrient depletion but the level of phosphorylation was higher and sustained in IKK-inhibited cells, concordant with IKK inhibiting Akt. The data above confirm that IKK phosphorylates p85 in response to cellular starvation and this event corresponds with loss of PI3K/Akt signaling.
p85 S690 phosphorylation is increased in animal tissues in response to fasting
Having demonstrated that incubation of cultured cells with nutrient-depleted media results in IKK-dependent PI3K/Akt feedback inhibition, this pathway was explored in living organisms. WT C57/B6 mice were fasted for 24 hours and p85 was immunoprecipitated from liver extracts. Consistent with data from cell lines, S690 phosphorylation was increased in liver tissue from fasted animals compared to fed controls (Figure 3E). Interestingly, Criollo et al. demonstrated that hepatocytes lacking IKKβ display sustained phospho-S6K activity following 24 hours of fasting, consistent with data presented herein demonstrating that IKK is required for feedback inhibition of PI3K/Akt/mTOR in response to starvation (2009). No change in p85 S690 phosphorylation was detected in skeletal muscle, kidney, spleen and heart following 24 hours of fasting (data not shown). Cell-based studies indicate that induction of IKK activity is an early event in response to nutrient deprivation. Therefore, p85 phosphorylation in vivo was explored in response to acute fasting. Indeed, S690 phosphorylation was induced in skeletal muscle, cardiac, and spleen extracts following six and twelve hours of fasting and this increase coincided with loss of Akt effector activity (Figure 3F). These results demonstrate that p85 phosphorylation occurs in a variety of tissues in response to metabolic restriction.
S690A expression suppresses starvation-induced Akt feedback
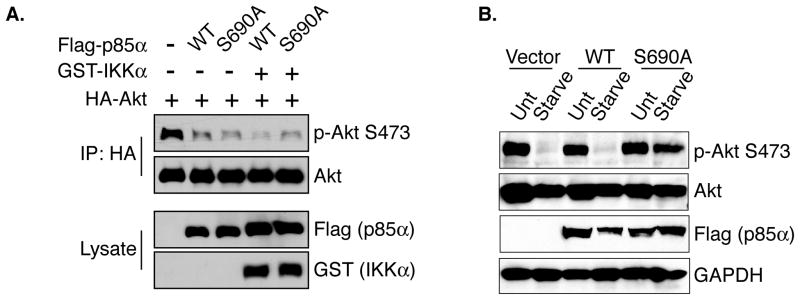
If IKK-dependent phosphorylation of p85 S690 is required for starvation-induced feedback inhibition of PI3K then mutation of S690 to alanine (S690A) should abrogate these effects. This hypothesis was tested by performing an ectopic expression experiment to determine the role of S690 in IKK-dependent PI3K inhibition. Overexpression of p85 WT or S690A resulted in decreased PI3K activity, consistent with the long-understood role of p85 in inhibiting PI3K activity when overexpressed (Figure 4A) (Luo and Cantley, 2005). Co-expression of IKK and WT p85 led to a further loss of PI3K activity as measured by Ser473 phosphorylation, supporting the hypothesis that IKK-mediated phosphorylation of p85 inhibits PI3K function (Figure 4A). Importantly, co-expression of IKK with the S690A mutant did not lead to the decrease in Akt Ser473 phosphorylation observed in cells co-transfected with IKK and WT p85, indicating that this site is important for the IKK-mediated effects on PI3K activity (Figure 4A). Expression of IKK alone leads to slight downregulation of Akt (Supplemental Figure S3). The importance of S690 in IKK-dependent PI3K feedback was next assessed in the context of the biological stimulus cellular starvation. Starved cells expressing WT p85α displayed efficient inhibition of PI3K pathway as measured by phospho-Akt Ser473. Expression of p85α S690A, however, resulted in impaired starvation-induced PI3K feedback inhibition (Figure 4B), demonstrating that S690 phosphorylation is important for IKK-mediated and starvation-induced PI3K feedback inhibition.
Figure 4. Phosphorylation of p85 S690 is required for IKK-mediated and starvation-induced PI3K feedback inhibition.
A. Cos7 cells were transfected with Flag p85α (WT or S690A), GST-IKKα, and HA-Akt. Anti-HA immunoprecipitates were analyzed for Akt Ser473 phosphorylation.
B. Cos7 cells were transfected with p85α WT or S690A for 48 hours. Cells were then untreated (Unt) or starved for 1 hour. Whole cell extracts were analyzed by western blotting as indicated. See also Figure S3.
Phosphorylation of p85 by IKK results in reduced SH2-phosphotyrosine interaction
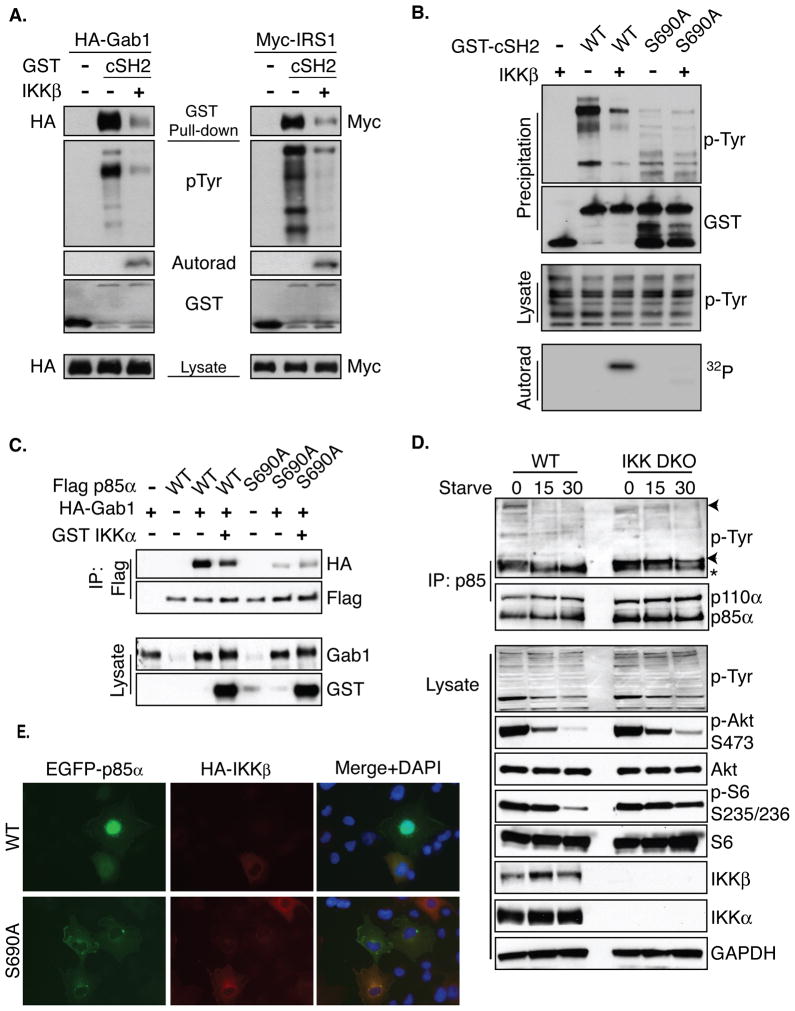
The presence of S690 in a conserved region of the cSH2 domain led to the hypothesis that phosphorylation could disrupt the ability of p85 to interact with tyrosine-phosphorylated proteins. To evaluate this hypothesis, the effect of S690 phosphorylation on SH2 function was assessed by performing an in vitro SH2-phosphotyrosine affinity experiment. Briefly, bacterially purified GST-p85-cSH2 peptide was incubated in the presence or absence of recombinant IKK in order to achieve maximal S690 phosphorylation in vitro. The SH2 domains were used to precipitate known interacting proteins IRS1 and Gab1 from transfected, pervanadate-treated HEK293T cell lysates. As expected, the unphosphorylated cSH2 domain efficiently precipitates IRS1, Gab1, and other tyrosine phosphorylated proteins (Figure 5A). In contrast, IKK-dependent SH2 domain phosphorylation in vitro resulted in a markedly reduced affinity for phosphotyrosine-containing proteins (Figure 5A).
Figure 5. S690 phosphorylation disrupts p85-phosphotyrosine binding.
A. GST-p85 cSH2 was precipitated from E. coli with GSH conjugated beads and precipitates were incubated in the presence or absence of recombinant IKKβ in an in vitro kinase assay. Intrinsic phosphotyrosine affinity of the phosphorylated or unphosphorylated cSH2 domain was assessed using lysates prepared from pervanadate-treated HEK293T cells expressing either HA-Gab1 or Myc-IRS1.
B. GST-p85 cSH2 WT or S690A precipitates were prepared and evaluated as described in A.
C. Flag-p85 WT or S690A was transfected into Cos7 cells with HA-GAB1 and GST-IKKα. Flag immunoprecipitates were evaluated by western blot for coprecipitating HA-GAB1.
D. IKK WT or DKO cells were starved for the indicated times and p85 immunoprecipitates were analyzed by western blot to identify coprecipitating tyrosine phosphorylataed proteins. Whole cell extracts were also analyzed by western blotting for the indicated signaling markers.
E. Cos7 cells were transfected with HA-IKKβ and either GFP-p85 WT or S690A. Cells were treated with pervanadate for 10 minutes prior to being fixed, permeabilized, and stained with an Alexa 647-conjugated anti-HA antibody (red). See also Figures S4 and S5.
To determine the importance of S690 phosphorylation on p85 cSH2/P-Tyr interaction, a similar experiment was performed using a bacterially-purified GST-cSH2 S690A fragment. As expected, the WT cSH2 domain is phosphorylated and demonstrates dramatically decreased phosphotyrosine affinity following phosphorylation. p85-cSH2 S690A, however, is not phosphorylated and is insensitive to IKK-mediated decreases in phosphotyrosine binding (Figure 5B). While mutation of p85 S690 to alanine results in an overall decrease in phospho-tyrosine affinity, this mutant maintains significant binding compared to a phospho-tyrosine binding deficient mutant R649A (Supplemental Figure S4A).
In order to determine if S690 phosphorylation disrupts the interaction between p85 and tyrosine-phosphorylated proteins in cells, the ability of GAB1 to interact with p85 was assessed. In accordance with in vitro data, p85 is able to interact with GAB1, but this interaction is diminished by expression of IKKα (Figure 5C). The interaction between p85 S690A and GAB1 is maintained in the presence of IKKα, confirming that S690 phosphorylation decreases SH2-dependent interactions in cells. These results led to the hypothesis that starvation-induced IKK-dependent PI3K phosphorylation may serve to actively remove PI3K from tyrosine-phosphorylated RTKs and adaptors. Pulldown experiments were used to analyze IKK-dependent effects on PI3K-pTyr interactions in starved cells as described previously (Engelman et al., 2005). In response to starvation, WT mEFs displayed decreased association between p85 and tyrosine-phosphorylated proteins (marked with arrows in Figure 5D, quantified in Figure S4B), supporting the hypothesis that starvation-induced PI3K phosphorylation results in loss of pTyr binding. Importantly, cells lacking IKK subunits displayed sustained pTyr binding following 15 minutes of nutrient starvation compared to WT cells, demonstrating that loss of PI3K-pTyr associations is dependent on IKK activity in response to starvation (Figure 5D).
Interactions between p85 SH2 domains and pTyr proteins serve to recruit PI3K to the cell membrane where it phosphorylates lipid substrates. The ability of p85 phosphorylation to affect PI3K membrane localization was next examined by fluorescence microscopy. These experiments revealed that cells expressing low levels of IKKβ have clear p85 membrane localization, while cells with high IKKβ expression consistently showed low levels of p85 membrane localization (Figure 5E upper panels and S5A). However, cells expressing GFP-p85 S690A displayed dramatic membrane localization, even in cells expressing high levels of IKKβ (Figure 5E lower panels and S5B). Notably, increased membrane co-localization of IKKβ and p85 S690A was observed suggesting that IKK interacts with p85 S690A, but is unable to phosphorylate and displace PI3K from membrane complexes. p85 membrane localization and IKK expression were then scored from a large number of images in a blinded manner. A weighted kappa statistical analysis confirmed that there is a statistically significant agreement between IKK expression and decreased WT p85 membrane localization (weighted kappa=0.23, P=0.001). However, consistent with the other data in Figure 5, there is no agreement between IKK expression and membrane localization of p85 S690A (weighted kappa=0.09, p=0.50) (Figure S5C). Together, data in Figure 5 point to a model in which starvation-induced phosphorylation of p85 by IKK promotes dissociation of PI3K from P-Tyr containing membrane complexes, effectively inhibiting PI3K/Akt signal transduction.
Amino acid deprivation activates IKK
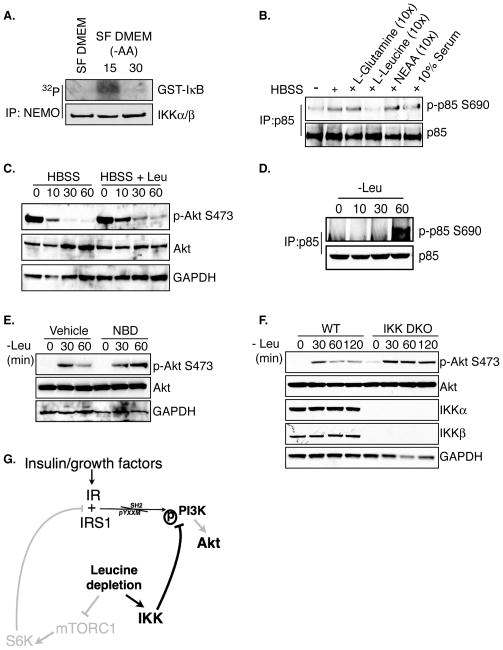
Individual nutrients dictate distinct signaling events within the cell. For example, glucose levels indirectly coordinate AMPK activity and leucine regulates subcellular localization and activity of mTOR (Hardie, 2011; Sengupta et al., 2010). Complete starvation therefore results in major signal transduction changes and understanding the significance and contribution of IKK-specific effects becomes difficult. Preliminary experiments indicated that serum and glucose were not involved in IKK-dependent PI3K feedback (data not shown). Amino acids have previously been shown to restrict PI3K activity and AA withdrawal leads to increases in PI3K/Akt activity, but it is unknown if IKK is affected by amino acids. MEFs were grown in serum free (SF) DMEM or SF DMEM lacking amino acids and IKK complexes were immunoprecipitated with an anti-NEMO antibody. Purified IKK complexes from cells treated for 15 minutes in AA free DMEM displayed increased in vitro kinase activity compared cells in grown in the presence of amino acids (Figure 6A).
Figure 6. Leucine deprivation induces IKK-dependent p85 phosphorylation.
A. WT mEFs were grown in serum-free media overnight and then were treated with amino acid-free media for either 15 or 30 minutes. Anti-NEMO immunoprecipitates were subjected to an in vitro kinase assay using a GST-IκBα fragment as a substrate. Kinase reactions were analyzed for incorporated 32P.
B. WT mEFs were treated with HBSS or HBSS supplemented with the indicated factors for 15 minutes. p85 immunoprecipitates were evaluated by western blot for phosphorylation of p85 at S690.
C. WT mEFs were treated with either HBSS or HBSS supplemented with leucine for the indicated times and lysates were blotted with the indicated antibodies
D. WT mEFs were treated with complete media lacking leucine for the indicated times. p85 Immunoprecipitates were evaluated by western blot for phosphorylation of p85 at S690.
E. Cells were grown in low serum DMEM for 12 hours and then pretreated with vehicle control or 200μM NBD for 1h prior to starvation. Cells were then treated with low serum DMEM lacking leucine for 30 or 60 minutes and WCEs were blotted with the indicated antibodies.
F. WT or IKKα/β−/− (DKO) cells were grown in low serum DMEM for 12 hours and then treated with 0.02% serum DMEM lacking leucine for the indicated times and WCEs were blotted with the indicated antibodies.
G. Leucine deprivation leads to loss of mTORC1-dependent PI3K/Akt feedback resulting in reactivation of Akt phosphorylation. Compensatory IKK activity restricts this Akt reactivation through phosphorylation of p85 S690 in the absence of leucine.
Leucine deprivation is necessary and sufficient for starvation-induced IKK-dependent PI3K/Akt feedback inhibition
Glutamine and leucine are two important amino acids that dictate metabolism and signaling in proliferating cells and both have been demonstrated to have anti-inflammatory properties (Singleton and Wischmeyer, 2008; Sengupta et al., 2010). The requirement for these amino acids in starvation-induced PI3K phosphorylation was next tested by stimulating WT mEFs with HBSS or HBSS supplemented with L-glutamine, L-leucine, a mixture of non-essential amino acids (NEAA), or serum. Of these various treatments, only leucine-supplemented starvation media failed to induce p85 phosphorylation (Figure 6B). If leucine deprivation is important for starvation-induced p85 phosphorylation then Akt feedback inhibition should also be disrupted by addition of leucine. Indeed, addition of leucine blunted Akt feedback in the absence of all other nutrients (Figure 6C). Concordantly, growing mEFs in complete media lacking leucine was sufficient to induce p85 phosphorylation (Figure 6D). Together these data indicate that leucine deprivation is both necessary and sufficient for starvation-induced p85 phosphorylation.
Finally, the role of IKK in leucine deprivation-induced Akt feedback was examined. The data presented above suggest a model whereby IKK becomes activated to restrict PI3K/Akt activity in the absence of leucine. Leucine-dependent activation of mTORC1 has been demonstrated to inhibit PI3K as leucine withdrawal leads to increased Akt activity through loss of mTOR-dependent feedback (Sancak et al., 2008; Harrington et al., 2004). Consistent with previously published studies, WT mEFs displayed reactivation of Akt in response to leucine deprivation but S473 phosphorylation remained relatively low over time (Figure 6E–F). If leucine deprivation activates IKK-dependent PI3K feedback, then cells lacking IKK should display faster and greater reactivation of Akt activity compared to WT cells in response to leucine withdrawal. Indeed, one hour of leucine deprivation resulted in enhanced Akt activity in mEFs treated with IKK inhibitor NBD peptide compared to vehicle control (Figure 6E) or in IKK DKO mEFs (Figure 6F). Together these data suggest a model in which IKK-dependent PI3K/Akt feedback compensates for loss of mTORC1-dependent feedback in the absence of leucine (Figure 6G).
DISCUSSION
The regulation and control of IKK continues to be a widely studied area. Given the cardinal role IKK plays in coordinating stress-induced immune and inflammatory responses the identification of substrates has been mostly limited to modulators of NF-κB activity (Häcker and Karin, 2006). The discovery that IKK is required for mammalian autophagy, independent of NF-κB activation, however, suggests that IKK influences cellular function beyond simply controlling inflammatory transcription networks (Criollo et al., 2010; Comb et al., 2010). These findings also highlight the need to identify novel IKK substrates involved in crosstalk with signaling networks that regulate cellular metabolism.
The mechanism by which cellular starvation activates and promotes IKK-dependent changes in metabolism are not well-understood but this stress often induces cessation of signaling through the PI3K/Akt pathway. Cells lacking IKK subunits or treated with an IKK-specific inhibitor display persistent Akt activation under periods of starvation, suggesting that both IKKα and IKKβ play important roles in controlling PI3K-dependent inhibition following nutrient deprivation (Figure 1). Interestingly, loss of IKKβ in vivo appears to have a greater effect in response to fasting than it does in immortalized null MEFs (data not shown).
Starvation-induced IKK activity has not been well-studied. Increased IKK activity is observed following just 15 minutes of nutrient depletion (Figure 1D), consistent with our previous findings that starvation-induced NF-κB DNA binding is maximal at 30 minutes, and data reported by Criollo, et al. demonstrating that IKK plays an early role in the initiation of autophagy (Criollo et al., 2010; Comb et al., 2010) . Induction of IKK activity corresponds well with the kinetics of PI3K/Akt feedback in response to starvation in cultured cells (Figure 1A), indicating that the effect of IKK on PI3K feedback is likely direct. The mechanism by which IKK is rapidly activated in response to nutrient deprivation is an important question and a topic of current investigation. Our data indicate that core components of the IKK holoenzyme (IKKα, IKKβ, NEMO) are required for starvation-induced Akt inhibition, but that the IKK activating kinase Tak1 is not. These data therefore suggest the interesting possibility that activation of IKK by nutrient deprivation differs from activation by inflammatory stimuli such as TNFα (Supplemental Figure S6).
The PI3K regulatory subunit p85α contains a strong consensus IKK phosphorylation motif in the C-terminal SH2 domain, and we show that p85 Ser690 is indeed phosphorylated in vitro and in vivo in response to cellular starvation (Figures 2D and 3B). In addition, p85 is phosphorylated in animals in response to whole-body fasting (Figure 3F). Starvation-induced p85 Ser690 phosphorylation inversely correlates with markers of PI3K/AKT pathway activation (such as pAKT and pS6) (Figure 3C) and mutation of Ser690 to alanine abrogates IKK-mediated, or starvation-induced, PI3K feedback inhibition (Figure 4). Taken together these data confirm that p85α is a bona fide IKK substrate.
In order to elucidate the mechanism by which IKK promotes PI3K feedback inhibition we analyzed the crystal structure of a p85 SH2 domain bound to a phosphotyrosine peptide. Serine 690 resides in a conserved region of the second α-helix that stabilizes the β-sheets that form the phospho-tyrosine binding pocket. The hydroxyl side-chain, which makes direct contact with the +3 acidic residue, faces the outside surface of the phosphotyrosine pocket, and is thus accessible (Booker et al., 1992; Hoedemaeker et al., 1999). This structure suggests that S690 phosphorylation may disrupt the alpha helix causing destabilization of the beta-sheets and loss of phosphotyrosine binding. Consistent with this hypothesis, data in Figures 5A–B show that a phosphorylated p85α cSH2 domain fails to bind tyrosine-phosphorylated proteins as efficiently as an unphosphorylated SH2 domain, and this is dependent on Serine690. We also find that interaction between Gab1 and WT p85, but not p85 S690A, is decreased in Cos7 cells overexpressing IKK (Figure 5C). Additionally, IKK-deficient cells displayed prolonged pTyr binding following starvation compared to WT cells (Figure 5D and S4B), which is consistent with the inability of IKK deficient cells to induce p85 phosphorylation and attenuate Akt signaling in response to cellular starvation (Figure 1D and 3C). Our data therefore indicate that phosphorylation of p85 disrupts GF-dependent interactions with the cSH2 domain. Interestingly, a recent report demonstrated that PKC family members phosphorylate p85-cSH2 domain in response to phorbol ester stimulation and this also negatively regulates pTyr binding (Lee et al., 2011). Intriguingly, phorbol esters are known activators of IKK/NF-κB signaling. It would therefore be interesting to determine whether PKD- and IKK-dependent cSH2 phosphorylation events are coordinated.
Identification of the PI3K regulatory subunit p85 as an important molecule for starvation-induced PI3K feedback inhibition is consistent with a number of previous studies that have also defined roles for p85 subunits in negatively regulating PI3K activity. For example, mutations in p110 catalytic subunits are thought to induce cell transformation via abrogating the ability of p85 to negatively regulate PI3K activity (Wu et al., 2009), and monomeric p85 can form sequestration complexes in response to insulin stimulation to restrict phospho-tyrosine from PI3K p85/p110 dimers (Luo et al., 2005). In addition, p85 has been shown to bind to and promote the activity of PTEN following stimulus-induced activation of PI3K as a means of feedback inhibition (Taniguchi et al., 2006). Mammalian cells have also evolved further mechanisms to allow precise regulation of PI3K activity, including an important leucine-dependent feedback loop involving S6K phosphorylation and inhibition of IRS proteins (Harrington et al., 2004; Shah et al., 2004). We demonstrate that Akt reactivation in response to leucine deprivation is restricted by IKK. Furthermore, we show that AA-withdrawal is both necessary and sufficient to induce IKK kinase activity and phosphorylation of p85 S690 (Figure 6). These studies reveal an IKK-dependent compensatory feedback loop that prevents activation of PI3K in amino acid-deprived environments.
While the ancient mTOR pathway regulates cell size and growth in all eukaryotes, multi-system organisms also possess components of the PI3K/Akt pathway to allow the additional layer of regulation provided by hormones and GFs. It is therefore exciting, though perhaps not surprising, that inflammatory signaling networks converge on metabolic pathways in higher eukaryotes to influence growth in response to stress. Indeed, TNFα and LPS, well-known inflammatory signals and IKK stimuli, induce phosphorylation of p85 at S690 (Supplemental Figure S2). TNF also activates mTOR in an IKKβ-dependent manner involving phosphorylation and inhibition of the upstream inhibitor TSC1 to control growth and angiogenic potential in a breast cancer model (Lee et al., 2007). Moreover, our lab has demonstrated that constitutive activation of Akt drives an interaction between IKKα and mTORC1 in PTEN-null prostate cancer cells which is important for control of mTOR phosphorylation of substrates S6K and 4EBP1 (Dan et al., 2007). Thus, under oncogenic conditions, where Akt is constitutively activated, IKKα can function to promote mTORC1 activaiton. Conversely, TNFα and IKK family members are essential inflammatory mediators that promote insulin resistance in response to high fat diet (Hotamisligil et al., 1993; Yuan et al., 2001; Chiang et al., 2009; Baker et al., 2011). These studies indicate that under pathological conditions chronic inflammatory signaling pathways converge with and modulate GF responsive pathways that control metabolism. Further understanding the mechanistic underpinnings of inflammatory and metabolic pathway intersections will be important to understand how cell stress influences metabolic activity.
EXPERIMENTAL PROCEDURES
Antibodies, Plasmids, and Reagents
Anti-phospho-Akt S473, Akt, anti-HA (rabbit), IKKβ, GST, p85, phospho-S6 S235/236, phospho-FOXO3a T32, FOXO3a, S6, p110α, phosphotyrosine, Myc, and anti-HA Alexa 647 conjugated antibodies were obtained from Cell Signaling Technology. Anti-IKKα was from Millipore. GAPDH was obtained from Santa Cruz. Anti-HA-11 was from Covance, and Flag (M2) was from Sigma. Expression plasmids encoding GST-IKKβ, GST-IKKβ K44A, GST-IKKα, GST-IKKα K44M, Flag-p85α, HA-p110α, GST-p50, GST-p85 cSH2, and HA-Akt were generous gifts from the Cantley lab. Myc-IRS1 was obtained from Addgene (plasmid 11374). A GAB1 Creator donor plasmid was obtained from the Harvard Institute of Proteomics and Creator recombination-based cloning was used to generate HA-GAB1. GFP-p85 was generated by PCR cloning from Flag-p85. p85 S690A was generated using a modification of the QuickChange Site-directed mutagenesis protocol (Stratagene).
Cell Culture, Transfections, Immunoprecipitations, and Westernblotting
Cos7, HEK293T, and mEFs were grown in DMEM supplemented with 10% FBS. Cos7 and HEK293T cells were obtained from ATCC and transfected according to manufacturers protocol with FuGENE (Roche). IKKα−/− and IKKβ−/− mEFs were gifts from Inder Verma. NEMO−/− mEFs were a gift from Dr. Derek Abbott. TAK1−/− mEFs were a gift from Dr. Jun Ninomiya-Tsuji. Whole cell extracts were prepared on ice with RIPA buffer supplemented with protease inhibitor mix (Roche, IN, USA) and phosphatase inhibitor mix (Sigma, MO, USA). For immunoprecipitations and kinase assays cells were lysed as previously described (Hutti et al., 2007).
Analysis of Phospho-S690 by Western blotting
A Phospho-p85 S690 antibody was generated by Cell Signaling Technology Inc. Immunoprecipitated p85 must be used to evaluate phosphorylation of S690. Briefly, whole cell lysates were prepared with either RIPA or 1% NP-40 buffer described above. Total p85 antibody (4292, Cell Signaling Technology, Inc.) (1:100) was used to IP PI3K from 250–500μg of lysate.
In vitro IKK kinase assay
Endogenous IKK complexes were immunoprecipitated with an antibody against NEMO (BD Biosciences) and an in vitro kinase assay was performed with GST-IκB fragment (1–54) purified from bacteria and analyzed as previously described (Steinbrecher et al., 2005).
In vitro p85 and cSH2 phosphorylation with recombinant IKK
Recombinant IKKβ was prepared from GST-IKKβ transfected 293T cells and kinase buffer and 10μCi-γ-32Phosphate/reaction and were performed as previously described (Hutti et al., 2007). GST-p50 substrate was purified from bacteria by GSH pulldown.
Immunofluorescence
Cos7 cells were grown on cover slips and transfected as indicated. Cells were fixed, permeabilized, blocked, and stained according to manufacturers instructions (Cell Signaling Technology Inc). Coverslips were mounted on slides with Slowfade Gold antifade reagent with DAPI (Invitrogen). All immunofluorescence was performed on an Olympus BX61 Upright Fluorescence Microscope in collaboration with the UNC School of Medicine Microscopy Services Laboratory.
Animal Experiments
Mice were grown in a pathogen free facility according to the protocols established by the University of North Carolina Institutional Animal Care and Use Committee. WT c57/B6 (Jackson Labs) were fasted as previously described (Criollo et al., 2010).
In vitro SH2 Affinity
E. coli BL-21 expressing GST-cSH2 domain were induced in log phase by 0.5mM IPTG for 16h at RT. PrecipitatedGST-cSH2 was subjected to an in vitro kinase reaction in the presence or absence of recombinant IKKβ. The kinase reaction was allowed to proceed for sixteen hours and the reaction was spiked with new kinase once in order to achieve maximal phosphorylation of the substrate. SH2 domains were washed extensively and incubated with pervanadate-treated HEK293T lysate expressing HA-Gab1 or Myc-IRS1 for 2 hours at 4ºC.
In vivo PI3K-pTyr pulldowns
5mg of whole cell lysates were prepared with NP-40 lysis buffer and PI3K was immunopurified with anti-p85. Experimental design and analysis was performed as previously described (Engelman et al., 2005).
Supplementary Material
Acknowledgments
We thank John Asara of the BIDMC Mass Spectrometry Core for performing mass spectrometry, LeShara Fulton for technical assistance, and Dominic Moore for statistical analysis. We are grateful to Jeff Engelman and members of the Baldwin Lab for helpful discussions. Research support is provided by NIH grants CA75080 (A.S.B), AI35098 (A.S.B), CA73756 (A.S.B), and R01 GM041890-21 (L.C.C). Additional support is provided by the Samuel Waxman Cancer Research Foundation (A.S.B) and Department of Defense CDMRP BCRP grant W81XWH-10-1-0342 (J.E.H) J.E.H. is a Lilly Research Labs fellow of the Life Sciences Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker GW, Breeze AL, Downing AK, Panayotou G, Gout I, Waterfield MD, Campbell ID. Structure of an SH2 domain of the p85 alpha subunit of phosphatidylinositol-3-OH kinase. Nature. 1992;358:684–687. doi: 10.1038/358684a0. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, Ma JT, Zhou J, Qi N, Westcott D, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comb WC, Cogswell P, Sitcheran R, Baldwin AS. IKK-dependent, NF-κB-independent control of autophagic gene expression. Oncogene. 2010;30:1727–1732. doi: 10.1038/onc.2010.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, et al. The IKK complex contributes to the induction of autophagy. EMBO J. 2010;29:619–631. doi: 10.1038/emboj.2009.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan HC, Adli M, Baldwin AS. Regulation of mammalian target of rapamycin activity in PTEN-inactive prostate cancer cells by I kappa B kinase alpha. Cancer Research. 2007;67:6263–6269. doi: 10.1158/0008-5472.CAN-07-1232. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Jänne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, Cichowski K, Johnson BE, Cantley LC. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci USA. 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase--an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, et al. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoedemaeker FJ, Siegal G, Roe SM, Driscoll PC, Abrahams JP. Crystal structure of the C-terminal SH2 domain of the p85alpha regulatory subunit of phosphoinositide 3-kinase: an SH2 domain mimicking its own substrate. Journal of Molecular Biology. 1999;292:763–770. doi: 10.1006/jmbi.1999.3111. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hutti JE, Turk BE, Asara JM, Ma A, Cantley LC, Abbott DW. IkappaB kinase beta phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-kappaB pathway. Mol Cell Biol. 2007;27:7451–7461. doi: 10.1128/MCB.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Lee JY, Chiu YH, Asara J, Cantley LC. Inhibition of PI3K binding to activators by serine phosphorylation of PI3K regulatory subunit p85 Src homology-2 domains. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1107747108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Cantley LC. The negative regulation of phosphoinositide 3-kinase signaling by p85 and it's implication in cancer. Cell Cycle. 2005;4:1309–1312. doi: 10.4161/cc.4.10.2062. [DOI] [PubMed] [Google Scholar]
- Luo J, Field SJ, Lee JY, Engelman JA, Cantley LC. The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signaling via the formation of a sequestration complex. J Cell Biol. 2005;170:455–464. doi: 10.1083/jcb.200503088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- May MJ, D'Acquisto F, Madge LA, Glöckner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- Obenauer JC. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Singleton KD, Wischmeyer PE. Glutamine attenuates inflammation and NF-kappaB activation via Cullin-1 deneddylation. Biochemical and Biophysical Research Communications. 2008;373:445–449. doi: 10.1016/j.bbrc.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher KA, Wilson W, Cogswell PC, Baldwin AS. Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol. 2005;25:8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Tran TT, Kondo T, Luo J, Ueki K, Cantley LC, Kahn CR. Phosphoinositide 3-kinase regulatory subunit p85alpha suppresses insulin action via positive regulation of PTEN. Proc Natl Acad Sci USA. 2006;103:12093–12097. doi: 10.1073/pnas.0604628103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Shekar SC, Flinn RJ, El-Sibai M, Jaiswal BS, Sen KI, Janakiraman V, Seshagiri S, Gerfen GJ, Girvin ME, et al. Regulation of Class IA PI 3-kinases: C2 domain-iSH2 domain contacts inhibit p85/p110alpha and are disrupted in oncogenic p85 mutants. Proc Natl Acad Sci USA. 2009;106:20258–20263. doi: 10.1073/pnas.0902369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MB, Leparc GG, Lai J, Obata T, Volinia S, Cantley LC. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat Biotechnol. 2001;19:348–353. doi: 10.1038/86737. [DOI] [PubMed] [Google Scholar]
- Yu J, Wjasow C, Backer JM. Regulation of the p85/p110alpha phosphatidylinositol 3'-kinase. Distinct roles for the n-terminal and c-terminal SH2 domains. J Biol Chem. 1998;273:30199–30203. doi: 10.1074/jbc.273.46.30199. [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.