Abstract
We determined the spatio-temporal dynamics of intracranially-recorded gamma-oscillations modulated by spontaneous cooing and babbling, which are considered to embody pre-linguistic language behaviors during infancy. Electrocorticographic (ECoG) signals were recorded from 110 cortical sites in the right hemisphere of a 10-month-old girl with focal epilepsy. ECoG signals were time-locked to the onset of cooing or babbling. The amplitudes of gamma-oscillations during vocalizations were compared to those during preceding silent reference periods. Cooing and babbling elicited significant gamma-augmentation at 30–100 Hz at distinct sites of the inferior Rolandic region, whereas both forms of vocalizations elicited gamma-augmentation at an identical superior temporal site. The spatial, temporal and spectral characteristics of gamma-augmentation elicited by cooing and babbling were similar to those elicited by phoneme vocalization in older children and adults. Differential activation within the right inferior Rolandic region during cooing and babbling may reflect the mechanical or developmental difference between these two forms of vocalizations. The right superior temporal gyrus may participate in an auditory feedback system during vocalization.
Keywords: Pediatric epilepsy surgery, High-frequency oscillations (HFOs), Event-related synchronization, Speech, Spontaneous vocalization
INTRODUCTION
Cooing and babbling are considered to embody the pre-linguistic language behaviors of infancy, with humans beginning to voluntarily coo and babble before imitating or speaking discernible words [1]. Cooing is defined as vocalization of long pure vowel sounds with variations (e.g.: “ah-ah-ah”) other than those observed during crying or yawning, and normally occurs by the age of 3 months [1, 2]. Babbling, vocalization of syllables containing consonants (e.g.: “ba-ba-ba”), normally occurs around the age of 6 to 10 months [1, 2].
We hypothesize that both cooing and babbling are executed by the Rolandic cortices bilaterally and that such self-generated phonetic sounds are processed in the superior temporal gyri bilaterally. This hypothesis is based on the substantial evidence from lesioning, imaging, and electrophysiological studies of speech in adults and older children [3–13]. Here, we obtained a unique opportunity to monitor electrocorticographic (ECoG) signals directly sampled from the right-sided cortical surface in a 10-month-old girl with focal epilepsy. We determined the spatio-temporal dynamics of gamma-oscillations modulated by spontaneous cooing and babbling, and determined whether such pre-linguistic behaviors would elicit augmentation of gamma-oscillations at >30 Hz in the Rolandic and superior temporal regions. It has been generally accepted that event-related cortical activation is reflected by gamma-augmentation, which is tightly correlated to increased blood oxygen level dependent (BOLD) responses on functional MRI (fMRI) [14, 15] and increased firing rate on single unit recording [16, 17]. ECoG can provide neural measures with a much better signal-to-noise ratio and anatomical specificity, compared to scalp electroencephalography (EEG).
METHODS
Patient
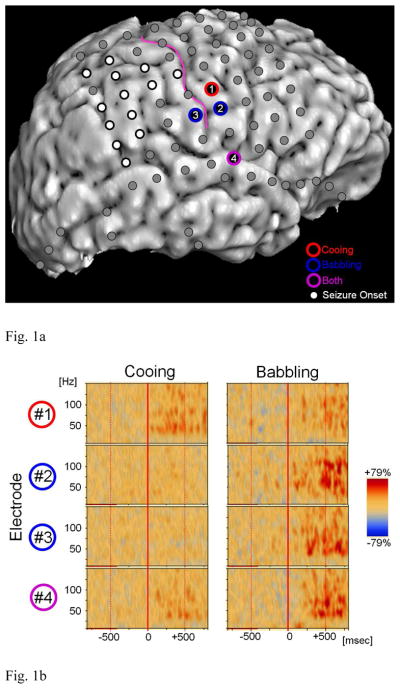
The study has been approved by the Institutional Review Board of Wayne State University, and written informed consent was obtained from the parents. We studied a 10-month-old girl with medically-uncontrolled epilepsy, who underwent a two-stage epilepsy surgery following extraoperative ECoG recording. The patient was born full-term to English-speaking parents. Her developmental milestones were normal and there was no medical issue other than seizures at the time of surgery. Preoperative brain MRI suggested the presence of focal cortical dysplasia in the right parietal region. Scalp video-EEG recording localized the seizure onset zone in the right hemisphere. Subsequently, a total of 110 platinum subdural electrodes (intercontact distance: 10 mm; diameter: 4 mm) were placed over the widespread regions in the right hemisphere (Figure 1), to localize the presumed seizure focus to be surgically removed.
Figure 1. Locations of subdural electrodes and the results of time–frequency analysis.
(A) White circles indicate sites over seizure-onset tissue. The patient has been seizure-free following surgical resection involving the seizure onset tissue and structural lesion (follow-up period: 23 months). (B) Electrode #1 (inferior pre-central gyrus) showed significant gamma-augmentation during cooing only (red circle); babbling-related gamma-augmentation in Electrode #1 failed to reach statistical significance. Electrode #2 (inferior pre-central gyrus) and Electrode #3 (inferior post-central gyrus) showed significant gamma-augmentation during babbling but not during cooing (blue circle). Electrode #4 (superior temporal gyrus) showed significant gamma-augmentation during both cooing and babbling (purple circle).
Measurement of gamma-modulations elicited by cooing and babbling
Expanded methodological detail of ECoG recording and subsequent time-frequency analysis are described in the supplementary document on the website (Supplementary Document S1) as well as in our previous studies [10, 18, 19]. In short, spontaneous vocalizations of the patient were recorded with a digital voice recorder and the amplified audio waveform was concurrently integrated into the Digital ECoG Recording System. The onsets of cooing and babbling events were manually marked directly on ECoG.
Each ECoG epoch containing the onset of each vocalization was transformed into the time-frequency domain using a complex demodulation technique [20, 21]. We determined ‘when’ and ‘where’ the amplitudes of gamma-oscillations (30–150 Hz) were augmented relative to that during the reference period (i.e.: the quiet and resting baseline) as well as statistical significance of vocalization-related augmentation of gamma-oscillations.
RESULTS
A total of 64 events of spontaneous cooing and 36 events of spontaneous babbling were identified. Immediately following the onset of cooing, significant gamma-augmentation at 30–100 Hz was elicited in the right inferior pre-central gyrus (electrode #1). Immediately following the onset of babbling, significant gamma-augmentation at 30–100 Hz was elicited at a more inferior portion of the right pre-central gyrus (electrodes #2) as well as in the right inferior post-central gyrus (electrode #3). Babbling-related gamma-augmentation in electrode #1 failed to reach significance; this finding can be explained by a less number of trials for babbling. During both cooing and babbling, significant gamma-augmentation at 30–100 Hz was noted in a single, identical site of the right superior temporal gyrus (electrode #4).
DISCUSSION
The present study, for the first time, demonstrated that gamma-oscillations were augmented in the right Rolandic and superior temporal regions during cooing and babbling in an infant. The spatial, temporal and spectral characteristics of such gamma-augmentations were similar to those elicited by phoneme vocalization in older children and adults. Previous studies of older children and adults using ECoG showed that gamma-oscillations at 30–150 Hz in the Rolandic regions (including bilateral pre- and post-central gyri) were augmented during overt vocalization of phonemes or words [8–10, 12]. Studies of children and adults using fMRI showed that overt and covert speech increased BOLD responses in the Rolandic regions, bilaterally [6, 7, 11]. Studies of humans and monkeys demonstrated that not unilateral but bilateral lesions involving the lower Rolandic regions resulted in complete loss of control of voluntary articulation [3, 4]. Taken together, the right inferior-Rolandic gamma-augmentations observed in the present study may reflect the dynamic involvement of sensorimotor cortex in the execution of cooing and babbling as pre-linguistic language behaviors.
In the present study, cooing and babbling elicited significant gamma-augmentation at different sites of the right inferior Rolandic region. Differential activation within the inferior Rolandic region during cooing and babbling might be explained by the mechanical difference between these two forms of vocalizations. Cooing is produced by vibration of the vocal cord in the throat while the oral cavity remained open with only minimal neuromuscular controls, whereas babbling requires a more complex modulation of the oral cavity either by lips or involvement of the tongue and palate that may be accompanied by dynamic movements of the jaw [22, 23]. Previous studies using electrical stimulation as well as fMRI showed that motor functions of different portions of the mouth are represented by different Rolandic sites [6, 24, 25]. Our previous ECoG study showed that articulation of the fricative phoneme [f] more than [h] elicited differential gamma-augmentation in subsets of the inferior Rolandic area in each individual [10]. Phoneme [f] is produced with the upper teeth placed on the lower lip, whereas phoneme [h] is produced with the upper and lower lips apart. Another potential explanation of differential gamma-augmentation in the right inferior Rolandic region between cooing and babbling is that different stages of linguistic development involve different portions of the cortex according to its maturation.
What is the developmental significance of gamma-augmentation in the right superior temporal gyrus during cooing and babbling? It is possible, even in infants, that the superior temporal gyrus participates in an auditory feedback system during vocalization [26]. A behavioral study of deaf and normal hearing infants showed that well-formed syllable production is established in the first 10 months of life by normal hearing infants but not by deaf infants, indicating the important role of acoustic analysis in vocal development [27]. A study of infants using single photon emission computed tomography failed to show a left-right difference in cerebral blood flow during the resting period [28]. An fMRI study of healthy 3-month-old infants reported that BOLD responses to passive listening of phonemes were larger on the left superior temporal gyrus compared to the right [29]. Studies of older children and adults showed that the superior temporal gyri in both hemispheres were activated by all of the following tasks: (i) discrimination of pitch and duration, (ii) categorical perception of syllables, and (iii) lexical decision [30–32]. Thereby, greater activation in the right more than left superior temporal gyrus was noted during discrimination of pitch and duration, while greater activation in the left more than right superior temporal gyrus was noted during categorical perception of syllables, and lexical decision. Another study of adults using fMRI showed that the right superior temporal gyrus was highly activated during a word recognition task in loud noise, a condition demanding greater acoustic analyses compared to during the same task in silence [33]. Previous ECoG studies demonstrated that externally-delivered vocal sounds and self-vocalized sounds differentially elicited gamma-augmentation within the superior temporal gyrus on either side, suggesting bilateral involvement [9, 12].
The major limitations of this case study include limited numbers of vocalization events and sampling limitations. It was not feasible to obtain a larger number of cooing or babbling events in silence, since the recording period was determined clinically. Failure to find significant gamma-augmentation at sites other than those reported could be attributed to insufficient numbers of vocalization events; thus, absence of gamma-augmentation does not necessarily indicate the lack of function at a given site. The left hemisphere was not sampled with subdural electrodes in the present study, since phase-I presurgical evaluation did not suggest the presence of a seizure focus there. Further ECoG studies of both left and right hemispheres are warranted to better understand the dynamics of neural activations during pre-linguistic behaviors.
Supplementary Material
Acknowledgments
This work was supported by NIH grant NS64033 (to E. Asano), Scholarship from Japan Foundation for Neuroscience and Mental Health (to Y. Cho-Hisamoto and K. Kojima) and Scholarship from Japan-North America Medical Exchange Foundation (to K. Kojima). We are grateful to Harry T. Chugani, MD, Csaba Juhász, MD, PhD, Sarah Minarik, RN, BSN, Carol Pawlak, REEG/EPT, and the staff of the Division of Electroneurodiagnostics at Children’s Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coplan J, Gleason JR. Quantifying language development from birth to 3 years using the Early Language Milestone Scale. Pediatrics. 1990;86:963–971. [PubMed] [Google Scholar]
- 2.Capute AJ, Accardo PJ. Linguistic and auditory milestones during the first two years of life: a language inventory for the practitioner. Clin Pediatr. 1978;17:847–853. doi: 10.1177/000992287801701108. [DOI] [PubMed] [Google Scholar]
- 3.Kirzinger A, Jürgens U. Cortical lesion effects and vocalization in the squirrel monkey. Brain Res. 1982;233:299–315. doi: 10.1016/0006-8993(82)91204-5. [DOI] [PubMed] [Google Scholar]
- 4.Mao CC, Coull BM, Golper LA, Rau MT. Anterior operculum syndrome. Neurology. 1989;39:1169–1172. doi: 10.1212/wnl.39.9.1169. [DOI] [PubMed] [Google Scholar]
- 5.Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, Lenz FA, Crone NE. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128:1556–1570. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- 6.Pulvermüller F, Huss M, Kherif F, Moscoso del Prado Martin F, Hauk O, Shtyrov Y. Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci USA. 2006;103:7865–7870. doi: 10.1073/pnas.0509989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhanjal NS, Handunnetthi L, Patel MC, Wise RJ. Perceptual systems controlling speech production. J Neurosci. 2008;28:9969–9975. doi: 10.1523/JNEUROSCI.2607-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, Spire JP, Kohrman MH. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–2027. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flinker A, Chang EF, Kirsch HE, Barbaro NM, Crone NE, Knight RT. Single-trial speech suppression of auditory cortex activity in humans. J Neurosci. 2010;30:16643–16650. doi: 10.1523/JNEUROSCI.1809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuda M, Rothermel R, Juhász C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by listening and overt repetition of phonemes. Neuroimage. 2010;49:2735–2745. doi: 10.1016/j.neuroimage.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golfinopoulos E, Tourville JA, Guenther FH. The integration of large-scale neural network modeling and functional brain imaging in speech motor control. Neuroimage. 2010;52:862–874. doi: 10.1016/j.neuroimage.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenlee JD, Jackson AW, Chen F, Larson CR, Oya H, Kawasaki H, Chen H, Howard MA., 3rd Human auditory cortical activation during self-vocalization. PLoS One. 2011;6:e14744. doi: 10.1371/journal.pone.0014744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei X, Leuthardt EC, Gaona CM, Brunner P, Wolpaw JR, Schalk G. Spatiotemporal dynamics of electrocorticographic high gamma activity during overt and covert word repetition. Neuroimage. 2011;54:2960–2972. doi: 10.1016/j.neuroimage.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 15.Koch SP, Werner P, Steinbrink J, Fries P, Obrig H. Stimulus-induced and state-dependent sustained gamma activity is tightly coupled to the hemodynamic response in humans. J Neurosci. 2009;29:13962–13970. doi: 10.1523/JNEUROSCI.1402-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–11536. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, Sood S, Chugani HT, Asano E. In vivo animation of auditory-language-induced gamma-oscillations in children with intractable focal epilepsy. Neuroimage. 2008;41:1120–1131. doi: 10.1016/j.neuroimage.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu HC, Nagasawa T, Brown EC, Juhasz C, Rothermel R, Hoechstetter K, Shah A, Mittal S, Fuerst D, Sood S, Asano E. Gamma-oscillations modulated by picture naming and word reading: intracranial recording in epileptic patients. Clin Neurophysiol. 2011;122:1929–1942. doi: 10.1016/j.clinph.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–145. [PubMed] [Google Scholar]
- 21.Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- 22.Stark RE. Features of infant sounds: the emergence of cooing. J Child Lang. 1978;5:379–390. doi: 10.1017/s0305000900002051. [DOI] [PubMed] [Google Scholar]
- 23.Wilson EM, Green JR, Yunusova Y, Moore CA. Task specificity in early oral motor development. Semin Speech Lang. 2008;29:257–266. doi: 10.1055/s-0028-1103389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 25.Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtheim L. On aphasia. Brain. 1885;7:433– 484. [Google Scholar]
- 27.Oller DK, Eilers RE. The role of audition in infant babbling. Child Dev. 1988;59:441–449. [PubMed] [Google Scholar]
- 28.Chiron C, Jambaque I, Nabbout R, Lounes R, Syrota A, Dulac O. The right brain hemisphere is dominant in human infants. Brain. 1997;120:1057–1065. doi: 10.1093/brain/120.6.1057. [DOI] [PubMed] [Google Scholar]
- 29.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 30.Poeppel D, Guillemin A, Thompson J, Fritz J, Bavelier D, Braun AR. Auditory lexical decision, categorical perception, and FM direction discrimination differentially engage left and right auditory cortex. Neuropsychologia. 2004;42:183–200. doi: 10.1016/j.neuropsychologia.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Reiterer SM, Erb M, Droll CD, Anders S, Ethofer T, Grodd W, Wildgruber D. Impact of task difficulty on lateralization of pitch and duration discrimination. Neuroreport. 2005;16:239–242. doi: 10.1097/00001756-200502280-00007. [DOI] [PubMed] [Google Scholar]
- 32.Hyde KL, Peretz I, Zatorre RJ. Evidence for the role of the right auditory cortex in fine pitch resolution. Neuropsychologia. 2008;46:632–639. doi: 10.1016/j.neuropsychologia.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Wong PC, Uppunda AK, Parrish TB, Dhar S. Cortical mechanisms of speech perception in noise. J Speech Lang Hear Res. 2008;51:1026–1041. doi: 10.1044/1092-4388(2008/075). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.