Abstract
Purpose
BOLD fMRI, an important research and clinical tool, depends on relatively greater transient increases in (cerebral blood flow) rCBF than CMRO2 during neural activity. We investigated whether reduced resting rCBF in patients with TLE affects BOLD signal during fMRI language mapping.
Methods
We used [15O] water PET to measure rCBF, and 3T EPI BOLD fMRI with an auditory description decision task in 33 patients with temporal lobe epilepsy (16 men; age 33.6±10.6 years; epilepsy onset 14.8±10.6 years; mean duration 18.8±13.2 years; 23 left focus, 10 right focus). Anatomical regions drawn on structural MRI, based on the Wake Forest PickAtlas, included Wernicke’s area (WA), inferior frontal gyrus (IFG), middle frontal gyrus (MFG), and hippocampus (HC)]. Laterality indices (LI), and Asymmetry Indices (AI), were calculated on co-registered fMRI and PET.
Key findings
Twelve patients had mesial temporal sclerosis (7 left), two a tumor or malformation of cortical development (both left), one a right temporal cyst and 18 normal MRI (14 left). Decreasing relative left WA CBF correlated with decreased left IFG voxel activation and decreasing left IFG LI. However, CBF WA AI was not related to left WA voxel activation itself or WA LI. There was a weak positive correlation between absolute CBF and fMRI activation in left IFG, right IFG, and left WA. Patients with normal and abnormal MRI did not differ in fMRI activation or rCBF AI.
Significance
Reduced WA rCBF is associated with reduced fMRI activation in IFG but not WA itself, suggesting distributed network effects, but not impairment of underlying BOLD response. Hypoperfusion in TLE does not affect fMRI clinical value.
Keywords: PET, CBF, fMRI, TLE, language, BOLD
Introduction
Functional magnetic resonance imaging (fMRI) is an important procedure for pre-surgical evaluation of epilepsy patients, particularly for language mapping. The most common measure used is Blood Oxygen Level Dependent contrast (BOLD) fMRI. BOLD depends on relatively greater transient increases in regional blood flow (rCBF) than oxygen metabolism during neural activity, leading to increased oxygenated blood levels in brain regions activated by a task (Ogawa et al 1990).
Patients with temporal lobe epilepsy (TLE) may have focal cerebral dysfunction associated with reduced rCBF despite the absence of overt lesions, even after correcting for partial volume effects due to structural atrophy (Giovacchini et al 2007). The effect of disrupted rCBF on the BOLD response and its impact on fMRI is unclear. In rats, inhibiting nitric oxide-mediated neurovascular coupling abolished rCBF and BOLD signal increases during electrical stimulation, but somatosensory evoked potentials were still detectable, suggesting that BOLD signal may not reflect neural activity under pathological conditions (Burke and Buhrle 2006). In man, BOLD signal changes can be affected by CNS tumors (Giussani et al 2010) and arteriovenous malformations (Pouratian et al 2002, Lehericy et al 2002). Altered BOLD responses may be modulated by perfusion changes in evolving stroke stages (Saur et al 2006). One report suggested that structural pathology may affect activation during language fMRI in patients with epilepsy (Wellmer et al 2009).
Although CBF is only one factor influencing BOLD, fMRI results might be affected by altered blood flow ipsilateral to epileptogenic zones. Reduced CBF might be a risk factor for a diminished BOLD response, increasing the risk of falsely lateralized language dominance on fMRI. In a previous study, hypometabolism was a marker for diseased temporal lobe and language reorganization, but we did not find evidence of impaired blood oxygenation level-dependent response in hypometabolic cortex (Gaillard et al 2011). However, we were not able to perform precise regional comparison of PET and fMRI data. Moreover, cerebral glucose metabolism is not linked as closely to the BOLD response as is CBF. In this study, we used more rigorous co-registration methods to test the hypothesis that reduced resting rCBF affects the BOLD signal response during language tasks in patients with TLE.
Methods
Participants
We studied 33 patients with TLE confirmed by clinical characteristics, ictal video-EEG monitoring, neurological examination, and an epilepsy imaging protocol including high resolution structural MRI (Table 1). The cohort represented all TLE patients from 2004 to 2010 who had both fMRI and positron emission tomography (PET) measurement of rCBF as part of presurgical evaluation for intractable localization-related epilepsy. Patients with extra-temporal foci, or clear epilepsy etiologies such as metabolic disorders, metastatic disease, or large structural lesions were excluded.
Table 1.
Demographic, fMRI Activation and Cerebral Blood Flow Asymmetry Data
| subject | Gender | handedness | Study Age | onset age | Epilepsy duration | focus | MRI | fMRI IFG LI |
fMRI MFG LI |
fMRI WA LI |
CBF PET IFG AI |
CBF PET MFG AI |
CBF PET WA AI |
Resection volume (cc) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.00 | F | atypical | 18.00 | 12.00 | 6.00 | left | normal | 0.73 | 0.38 | 0.48 | 0.06 | 0.01 | 0.04 | |
| 2.00 | M | right | 37.00 | 2.50 | 35.00 | left | MTS | 0.45 | 0.40 | −0.48 | −0.03 | 0.17 | 0.02 | 13343.00 |
| 3.00 | M | right | 51.00 | 4.00 | 47.00 | right | MTS | 0.98 | 0.65 | 0.98 | 0.03 | 0.05 | 0.02 | 12257.00 |
| 4.00 | M | right | 26.00 | 14.00 | 12.00 | left | normal | 0.83 | 0.87 | 0.70 | 0.06 | 0.09 | 0.03 | |
| 5.00 | F | right | 46.00 | 9.00 | 37.00 | left | MTS | 0.36 | 0.26 | 0.22 | 0.08 | 0.06 | −0.01 | 11869.00 |
| 6.00 | M | right | 32.00 | 19.00 | 13.00 | right | normal | 0.94 | 0.74 | 0.72 | −0.02 | 0.00 | −0.13 | |
| 7.00 | M | right | 25.00 | 22.00 | 3.00 | left | normal | 0.14 | 0.47 | 0.74 | 0.11 | 0.05 | 0.15 | |
| 8.00 | M | right | 18.00 | 14.00 | 4.00 | left | normal | 0.94 | 0.72 | 0.74 | −0.01 | −0.09 | 0.02 | 24500.00 |
| 9.00 | M | right | 38.00 | 27.00 | 11.00 | right | MTS | 0.98 | 0.71 | 0.61 | 0.07 | 0.08 | 0.05 | 31351.00 |
| 10.00 | F | atypical | 23.00 | 20.00 | 3.00 | right | normal | −0.01 | −0.03 | 0.80 | −0.10 | 0.05 | 0.04 | |
| 11.00 | F | atypical | 43.00 | 38.00 | 5.00 | left | normal | −0.49 | −0.59 | −0.53 | −0.04 | −0.10 | 0.05 | 21074.00 |
| 12.00 | M | right | 24.00 | 4.00 | 20.00 | left | normal | 0.40 | 0.40 | 0.92 | 0.02 | 0.07 | 0.05 | |
| 13.00 | F | right | 27.00 | 15.00 | 12.00 | left | normal | 0.44 | 0.07 | 0.71 | 0.06 | 0.01 | 0.01 | 11985.00 |
| 14.00 | M | right | 55.00 | 28.00 | 27.00 | left | MTS | −0.11 | −0.06 | 0.64 | 0.03 | −0.01 | 0.04 | |
| 15.00 | F | right | 37.00 | 4.00 | 33.00 | left | MTS | 0.72 | 0.47 | 0.57 | 0.13 | 0.09 | 0.10 | 17041.00 |
| 16.00 | F | right | 45.00 | 29.00 | 16.00 | left | normal | −0.54 | −0.33 | −0.17 | 0.06 | 0.05 | 0.03 | 15500.00 |
| 17.00 | F | right | 22.00 | 13.00 | 9.00 | left | normal | 0.49 | 0.42 | 0.01 | 0.09 | 0.09 | 0.11 | |
| 18.00 | F | atypical | 36.00 | 10.00 | 26.00 | left | normal | 0.24 | 0.61 | 0.94 | 0.03 | 0.05 | 0.10 | |
| 19.00 | F | atypical | 28.00 | 8.00 | 21.00 | right | normal | 0.85 | 0.63 | 0.89 | −0.12 | −0.17 | −0.24 | |
| 20.00 | F | right | 50.00 | 13.00 | 37.00 | left | Hamartoma | −0.07 | 0.16 | 0.89 | 0.04 | 0.01 | 0.09 | |
| 21.00 | M | right | 38.00 | 37.00 | 1.00 | right | MTS | 0.92 | 0.33 | 0.65 | −0.04 | −0.16 | −0.02 | |
| 22.00 | M | right | 39.00 | 1.00 | 38.00 | left | MTS | 0.52 | −0.45 | 0.81 | 0.06 | 0.03 | 0.06 | |
| 23.00 | F | right | 46.00 | 40.00 | 6.00 | right | cyst | 0.79 | 0.79 | 0.18 | −0.02 | 0.03 | −0.01 | |
| 24.00 | M | right | 22.00 | 7.00 | 15.00 | left | normal | 0.94 | 0.60 | 0.88 | −0.09 | −0.06 | 0.00 | 2683.00 |
| 25.00 | M | atypical | 38.00 | 9.00 | 29.00 | right | normal | 0.73 | 0.64 | 0.74 | 0.02 | −0.09 | −0.01 | |
| 26.00 | M | right | 23.00 | 6.00 | 17.00 | left | normal | 0.49 | 0.52 | 0.75 | −0.03 | 0.04 | 0.01 | |
| focal | 13775.00 | |||||||||||||
| 27.00 | F | atypical | 23.00 | 21.00 | 2.00 | left | dysplasia | −0.56 | −0.14 | −0.02 | −0.09 | −0.08 | 0.01 | |
| 28.00 | M | right | 48.00 | 13.00 | 35.00 | left | MTS | 0.95 | 0.77 | 0.96 | 0.15 | 0.07 | 0.02 | |
| 29.00 | F | right | 19.00 | 16.00 | 3.00 | left | normal | −0.60 | 0.42 | 0.05 | 0.06 | −0.01 | 0.15 | |
| 30.00 | M | right | 30.00 | 1.70 | 28.00 | left | MTS | −0.69 | −0.38 | 0.01 | 0.04 | −0.02 | 0.02 | 21025.00 |
| 31.00 | F | right | 34.00 | 13.00 | 21.00 | left | normal | 0.96 | 0.62 | 0.45 | −0.04 | −0.14 | −0.05 | |
| 32.00 | F | atypical | 41.00 | 4.00 | 37.00 | right | MTS | 0.72 | 0.61 | −0.40 | −0.07 | 0.02 | −0.07 | 11143.00 |
| 33.00 | F | right | 26.00 | 13.00 | 14.00 | right | MTS | 0.77 | 0.14 | 0.59 | 0.02 | 0.02 | −0.09 | 9914.00 |
Twenty-one have had temporal lobectomy. Two had subdural monitoring with deferred surgery due to concerns about potential cognitive deficits; ten are awaiting further evaluation.
Patients underwent 3T structural MRI (General Electric, Milwaukee WI), EPI BOLD language, and [15O] water PET to measure rCBF. The protocol was approved by the NIH Division of Intramural Research Combined Neuroscience IRB and patients provided informed consent before the study.
Neuroimaging protocol
fMRI
fMRI data were acquired at 3.0 T (General Electric Medical Systems, Milwaukee, WI) using EPI blood oxygen level dependent (BOLD) techniques. Acquisition parameters, paradigm presentation and analysis methods have been described previously (Gaillard et al 2007. We used an Auditory Description Decision Task (ADDT, word definition decision) with a five block design (30 second hemicycle). During the experimental condition subjects heard a sentence that described, then named, an object (“a large pink bird is a flamingo”); 70% were correct definitions, 30 % were foils. The control condition was reverse speech with appended tone identification for 70% of reverse items. After normalization into the Montreal Neurological Institute (MNI) anatomic atlas, language dominance was established with a bootstrap method using the LI toolbox adapted for Statistical Parametric Mapping 2 (Wilke and Schmithorst 2006). Regions of interest were based on the Wake Forest Pick Atlas, and selected because of their functional role in processing language (Berl et al 2010), including Wernicke’s area (WA) and Broca’s area (Inferior Frontal Gyrus, IFG) as well as middle frontal gyrus (MFG) and hippocampus (Figure 1). A Laterality Index (LI) was calculated for each region where LI = (L−R)/(L+R). Left language dominance for each region was defined as LI ≥ 0.20; atypical language dominance was defined as regional LI < 0.20 (Gaillard et al 2007). Patients were classified as having atypical language dominance overall when either WA or IFG was right dominant (LI < −0.20), or when both regions were bilateral (LI between −0.20 and + 0.20). Regional activation voxel counts were recorded at individual mean LI threshold levels used in the bootstrap method. Average percent signal change was calculated at the individual level in left and right WA and IFG to compare activation magnitude in the task to the control condition.
Figure 1.
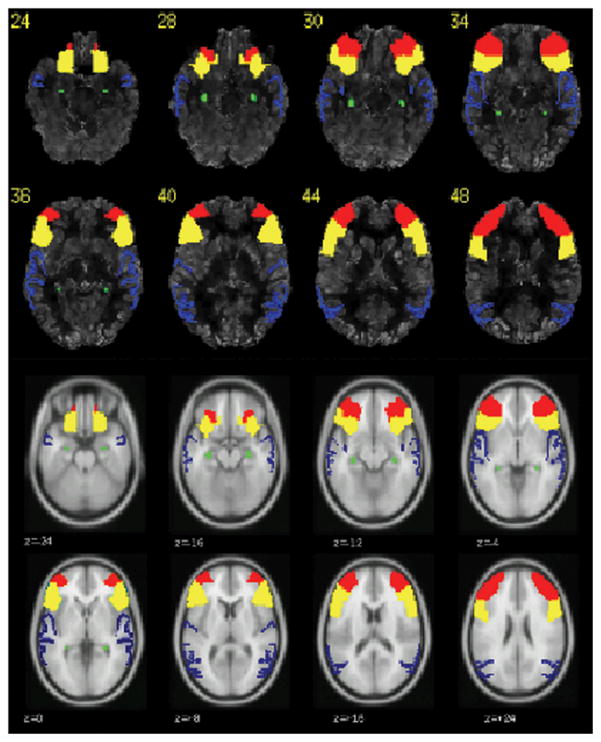
Co-registered PET and fMRI showing regions chosen for data analysis. Inferior frontal gyrus (IFG): Yellow; Middle frontal gyrus (MFG): red, Wernicke’s area (BA 21,22,39): blue; Hippocampus: green
[15O] Water PET
[15O] Water PET acquisition and analysis have been described previously (Giovacchini et al 2007). Briefly, [15O] water PET was performed in the awake state, in a quiet, dim room with eyes closed and ears occluded. Indwelling radial vein and artery catheters were inserted before scanning, and the subject’s head was secured in a thermoplastic facemask fixed to the scanner bed. Following transmission scans, 10mCi [15O] water were injected intravenously. During the scan, continuous arterial blood sampling was acquired with an automatic blood counter to produce quantitative maps of CBF (arterial blood sampling was not obtained from one subject, who was excluded from analysis of the relation between absolute CBF and fMRI activation). CBF PET images were processed using MEDx software and normalized to the MNI template using statistical parametric mapping.. CBF values were determined for regions of interest co-registered and identical to those used in the fMRI analyses (WA and IFG). A CBF Asymmetry Index, (AI) was calculated for each region of interest where AI=200*(R−L/L+R), following historical PET convention. A positive AI indicates relatively decreased left-sided CBF. Thus, laterality index refers to fMRI and asymmetry index to PET data. In addition, quantitative measurements of the rate of tissue blood flow in units of milliliters per minute per 100 grams (‘quantitative CBF’) was measured in all regions, and compared to previous studies (Raichle et al 1983, Giovacchini et al 2007), in order to assess further the relationship between functional activation and blood flow.
Data Analysis
Statistical analysis was performed with SPSS: Edition 12. Descriptive statistics were used to characterize demographic, seizure-related, fMRI, and CBF variables. The primary analysis focused on the relationship between rCBF and fMRI activation in frontal and temporal language regions. Pearson r correlations were calculated to examine this relationship and the Dunn-Sidak correction (Dunn 1961) was applied to account for multiple comparisons. To test two independent hypotheses (frontal and temporal effects) on the same data at 0.05 significance level, a more conservative p value of 0.025 instead of 0.05 was determined. fMRI variables included extent (number of voxels and LI) and magnitude of activation (percent signal change). T-tests evaluated the differences between patients with typical and atypical language dominance, left and right seizure focus, and positive and negative MRI findings.
Results
Clinical Data
Seventeen women and 16 men participated; mean age was 33.6±10.6 years (range 18–55 years); mean epilepsy onset age was 14.8±10.6 years (range 0.8–40 years); and mean epilepsy duration was 18.8±13.2 years (range 1–47 years) (Table 1). Twenty-three subjects had a left and ten a right focus. Twelve patients had mesial temporal sclerosis (MTS) (7 left, 5 right), two a tumor or malformation of cortical development (MCD) (both left). One had a 7–8 mm fluid filled cyst in the right medial temporal lobe, and 18 had a normal MRI (14 left, 4 right). Handedness was established clinically in 20 patients through demonstration of everyday tasks (i.e., handwriting, eating); 13 had the Edinburgh Handedness Inventory.
Cerebral Blood Flow and Functional Activation
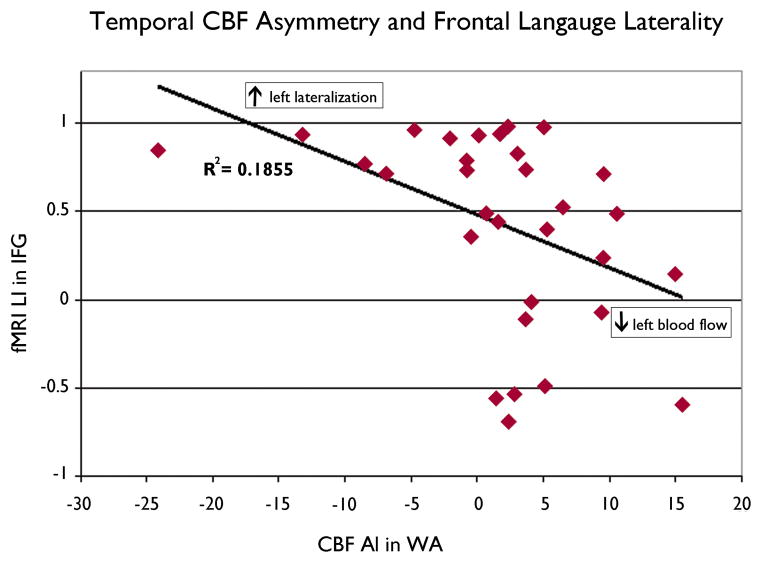
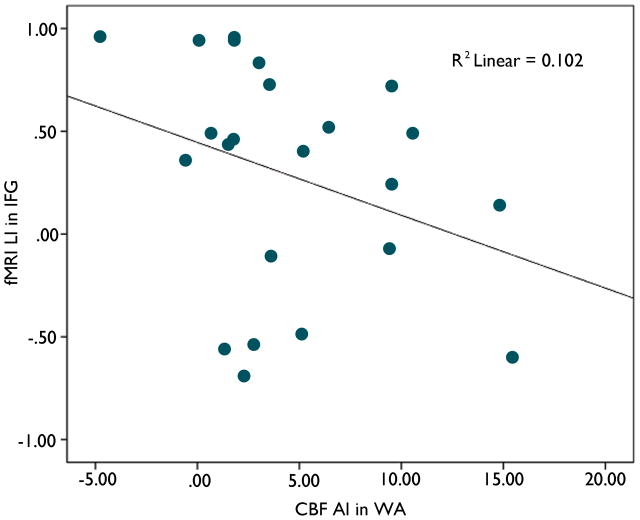
Asymmetry of CBF in the temporal region was related to activation in the frontal area but not to activation in the temporal area. Relatively lower CBF in left WA (positive CBF WA AI) was correlated with fewer activated voxels in left IFG (F= 5.7; r=−0.40, p=0.02) and thus, lower language laterality (LI) in left IFG (F= 7.1; r=−0.43, p=0.01) (Figure 2) However, examination of the data suggests there may be two separate populations. For patients with left temporal foci, a non-significant trend was present (Figure 3). The relationship was reversed when patients with a left temporal focus alone were examined; higher left WA CBF correlated with greater extent of Left IFG activation (F=5.37; r=0.45, p=0.03) The magnitude of activation did not correlate with CBF asymmetry (p >0.10). IFG CBF asymmetry was not related to functional activation in any region (p>0.10).
Figure 2.
Degree of CBF asymmetry in WA and fMRI language laterality in IFG. Decreasing left blood flow in WA was correlated with decreasing left lateralization in IFG (F= 7.1; r=−0.43, p=0.01).
Figure 3.
Degree of CBF asymmetry in WA and fMRI language laterality in IFG for patient with left temporal foci (F=2.4; r=−0.32, p=0.14)
Cerebral blood flow values were in the normal range (mean Left WA CBF=59.7±17.6; mean Left IFG CBF=62.7±16.3). There was a weak correlation between absolute rCBF and fMRI activation. Left IFG activation was positively correlated with mean absolute CBF at a trend level for left IFG (r=0.35, p=0.05), right IFG (r=0.35, p=0.05), and left WA (r=0.33, p=0.06) but not for right WA (r=0.24, p>0.10). Regional voxel activation in the other regions (right IFG, left WA, and right WA) was not correlated with quantitative CBF values in any region (p>0.10).
Impact of language dominance and patient characteristics
Overall, subjects showed left-lateralized language (mean WA LI=0.51±0.42; mean IFG LI=0.43±0.53) with six (18%) patients having atypical language dominance (LI<0.2). There was no difference in regional absolute CBF between subjects with typical versus atypical language dominance (p>0.10).
Seizure Focus: Left vs. Right
Subjects with a left seizure focus showed decreased left language lateralization in IFG compared to those with a right seizure focus (t=−3.28, p<0.01), with a similar trend in WA (t=−1.95, p=0.06). Eight of 23 (35%) left seizure focus subjects had atypical language dominance (two only temporal, four only frontal, two in both regions), compared to no patients with a right focus (χ2=2.89, p=0.09). Regional average percent signal change during the fMRI task was similar for subjects with a left and right seizure focus (p>0.10).
CBF reductions ipsilateral to epileptic foci were modest (Table 2). Mean absolute asymmetries were: IFG 0.06 ± 0.04; MFG 0.06 ± 0.05; WA 0.06 ± 0.05; hippocampus 0.11 ± 0.07. Subjects with a left seizure focus had decreased left regional cerebral blood flow in WA (t=2.00, p=0.01) and IFG (t=2.15, p=0.04) compared to subjects with a right focus. While the right greater than left asymmetry was significant in these areas, the mean absolute blood flow was similar for both groups in these regions on the left and right (p>0.10). Subjects with a left compared to right seizure focus did not differ by age, age of onset, duration of epilepsy, gender, or handedness (p>0.10).
Table 2. ABSOLUTE CBF VALUES.
Absolute values of mean regional cerebral blood flow
| Region | Left Focus (n=23) | Right focus (n=9) |
|---|---|---|
| Left IFG | 60.7 ± 13.3 | 60.7 ± 7.1 |
| Right IFG | 62.5 ± 13.8 | 59.1 ± 6.4 |
| Left MFG | 61.2 ± 13.2 | 60.8 ± 6.1 |
| Right MFG | 62.1 ± 12.5 | 59.8 ± 8.8 |
| Left WA | 56.6 ± 12.2 | 58.9 ± 7.5 |
| Right WA | 59.3 ± 12.6 | 56.7 ± 9.8 |
| Left HF | 43.7 ± 9.0 | 46.8 ± 6.4 |
| Right HF | 46.7 ± 9.6 | 46.1 ± 9.3 |
MRI: Normal vs. Lesion
In an analysis of subjects with a normal MRI (n=18) compared to those with a lesion (n=15; MTS, dysplasia), subjects with a lesion tended to be older (t=4.05, p<0.001) and have longer epilepsy duration (t=3.11, p<0.01) than those with normal MRI. The two groups had similar amounts of voxel activation in all measured ROIs and similar regional percent signal change during the task condition (p>0.10). Subjects with structural lesions and normal MRI did not differ by age of onset, gender, side of focus, handedness, CBF AI, absolute CBF or fMRI LI in any region (p>0.10). There was no relation between resection volume in 14 patients and fMRI LI.
Assessment of Outlier Effects
One subject had L WA and LIFG CBF 3–4 standard deviations higher than the rest of the sample. This patient was omitted from calculation of absolute CBF results, but not AI, as their AI was within 1 standard deviation of the overall sample. All other subjects were within 1.5 SD of the overall distribution. Calculations based on omission of all subjects with data greater than 1 standard deviation outside the overall range did not change significance of the results. Six subjects were ’outliers ’ on the basis of atypical language dominance. Omitting these, the negative correlation between CBF WA AI and L voxel activation in IFG still holds. Omitting the two subjects with the highest number of activated voxels activated in IFG did not affect correlations.
Discussion
Our study shows that reduced rCBF in Wernicke’s area is associated with reduced fMRI activation in left IFG, but not WA itself, suggesting effects of temporal lobe epilepsy on a distributed language processing network, but not on the underlying BOLD response. Thus, regional hypoperfusion in patients with TLE does not impair the value of fMRI for language localization.
In our study, CBF was reduced only modestly ipsilateral to epileptic foci, consistent with previous observations (Giovacchini et al 2007). As we used partial volume correction for cortical atrophy, our results reflect true CBF reduction. Several studies support our finding that the CBF reductions seen in temporal lobe foci would not be large enough to affect BOLD signal. Relative left hemisphere CBF reductions associated with altered BOLD response in patients with stroke were 20% or greater (Bonakdarpour et al 2007, Krainik et al 2005). Modeling studies also suggest that greater CBF reduction would be needed to affect BOLD signal (Aubert et al 2002). We used PET to assess resting CBF, since we wished to study the effect of reduced baseline CBF on the potential for activation. Moreover, absolute CBF increases associated with functional activation are small (Bookheimer et al 1997). Thus, limitations to brain water permeability would not lead to underestimation of values (Raichle et al 1983).
None of our patients had large structural lesions that might have disrupted CBF. As expected, we found no effect of resection volume, which has only a weak relation to the size of the pathology or epileptogenic zone, on LI. Some studies in patients with vascular malformations, large infarcts, or high-grade gliomata suggested that altered rCBF may affect the BOLD response (Giussani et al 2010, Hou et al 2006). However, studies in patients with low-grade gliomata showed that tumors in language regions did not alter activation, suggesting that the CBF component of the BOLD response was unaffected, and that fMRI language localization was accurate (Thiel et al 1998, Stippich et al 2007, Ruff et al 2008).
Signal artifacts, as well as physiologic alterations, may compromise fMRI, particularly in patients with high flow lesions (Lehericy et al 2002). Comparison of fMRI with ESM in patients with vascular lesions found 90–100% sensitivity but lower specificity for eloquent cortex (Pouratian et al 2002); effects may depend on lesion size and contiguity to language areas (Lee et al 2010, Cannestra et al 2004). Tumor infiltration, neovascularity, inflammation, or hemodynamic effects may suggest fMRI language dominance contralateral to ESM results, probably due to reduced left hemisphere BOLD signal, and apparent increased prominence of normal homologous right-sided activation in relation to diminished Left BOLD signal (Ulmer et al 2004).
Acute physiologic alterations could affect BOLD contrast. fMRI performed after a cluster of left temporal lobe seizures falsely lateralized language cortex in a child (Jayakar et al 2002). Multiple language tasks revealed no activation over the left temporal lobe despite a normal neurologic exam at the time of the study. A second fMRI performed two weeks later activated sites predominantly over the left, which were confirmed by extra-operative functional language mapping. fMRI may be unreliable after frequent seizures (Jayakar et al 2002).
In a previous study, we found that atypical language dominance was not more common in patients with structural lesions than those with normal MRI, unless an early left hemispheric stroke had occurred (Gaillard et al 2007). The structural lesions commonly found in epilepsy, such as low grade tumors, small malformations of cortical development, and MTS appear to have little impact on the BOLD response. Patients with seizures due to lesions such as Rasmussen’s encephalitis, some tumors, porencephaly, or large malformations of cortical development, but not MTS, focal dysplasia, or gangliogliomata had reduced left lateralization in Broca’s area but not parieto-temporal regions (Wellmer et al 2009). Three of 35 children with a variety of structural lesions showed discordant results when fMRI language lateralization was compared to clinical (mainly ictal or postictal language disturbance) or ESM data (Anderson et al 2006). An O-15 water PET study of 92 patients with seizures due to structural lesions (58 had tumors) showed no consistent effects on laterality indices, compared to controls; patients with both frontal lesions had lower MTG LI than controls (Tanriverdi et al 2009).
Our study showed that decreased relative left WA rCBF reduced functional activation in Left IFG, but not WA itself, although two subpopulations may be present, and there was only a non-significant trend when patients with left temporal foci were considered as a separate group. Fronto-temporal connections have long been known to subserve language function. In addition to the arcuate fasiculus pathway connecting IFG and WA, there is a parallel indirect lateral pathway with an anterior segment connecting IFG with inferior parietal lobe and posterior segment connecting inferior parietal lobe to WA (Catani et al 2005). Moreover, fronto-temporal-parietal connections including arcuate and superior longitudinal fasiculus, may vary between Broca’s areas 44 and 45 (Frey et al 2008). Additional dorsal and ventral pathways probably are present (Friederici et al 2009). Consistent with these reports, combined studies using ESM and diffusion tensor imaging showed good co-localization of language-sensitive grid contacts and arcuate fasiculus; better agreement in anterior regions suggested a more dispersed posterior network (Diehl et al 2010). Studies using evoked potentials during ESM show bidirectional activation between frontal and temporal language regions, supporting our finding that IFG activation may be modulated by WA (Matsumoto et al 2004).
Temporal lobe epilepsy has wide ranging structural and physiologic effects on cortical and subcortical regions beyond the epileptogenic zone (Meador and Hermann 2010), and on cognitive function (Gaillard et al 2007, Woermann et al 2003). Left mesial temporal foci, though remote from neocortical language processing cortex, are associated with altered temporal neocortical and frontal language laterality (Gaillard et al 2007, Weber et al 2006). Reduced IFG activation may be related both to impaired input from WA and greater susceptibility to reorganization and developmental neuroplasticity, with partial language transfer to right homologues (Berl et al 2010). Frontal regions appear to have a longer structural and functional brain development trajectory and greater capacity for bilateral language processing recruitment (Chugani et al 1987, Mbwana et al 2009). Frontal lobe myelination continues into the early 20s (Paus et al 1999, Gogtay et al 2004). fMRI verbal fluency data suggest later maturation of anterior than temporal language processing networks. (Gaillard et al 2000, Holland et al 2001).
In patients with TLE, reduced regional fMRI activation reflects alterations in underlying language organization. CBF reductions do not impair the underlying BOLD response, or affect the clinical value of fMRI for language mapping.
Acknowledgments
Supported by NINDS NIH Division of Intramural Research
Footnotes
We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
None of the authors has any conflict of interest to disclose.
References
- Anderson DP, Harvey AS, Saling MM, Anderson V, Kean M, Abbott DF, Wellard RM, Jackson GD. FMRI lateralization of expressive language in children with cerebral lesions. Epilepsia. 2006;47:998–1008. doi: 10.1111/j.1528-1167.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R, Duffau H, Benali H. Modeling of pathophysiological coupling between brain electrical activation, engery metabolism and hemodynamics: insights for the interpretation of intracerebral tumor imaging. Acta Biotheoretica. 2002;50:281–295. doi: 10.1023/a:1022620818701. [DOI] [PubMed] [Google Scholar]
- Berl MM, Duke ES, Mayo J, Rosenberger LR, Moore EN, VanMeter J, Ratner NB, Vaidya CJ, Gaillard WD. Functional anatomy of listening and reading comprehension during development. Brain Lang. 2010;114:115–25. doi: 10.1016/j.bandl.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B, Parrish TB, Thompson CK. Hemodynamic response function in patients with stroke-induced aphasia: Implications for fMRI data analysis. NeuroImage. 2007;36:322–331. doi: 10.1016/j.neuroimage.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Malow BA, Gaillard WD, Sato S, Kufta C, Fedio P, Theodore WH. A direct comparison of PET activation and electrocortical stimulation mapping for language localization. Neurology. 1997;48:1056–65. doi: 10.1212/wnl.48.4.1056. [DOI] [PubMed] [Google Scholar]
- Burke M, Buhrle C. BOLD response during uncoupling of neuronal activity and CBF. Neuroimage. 2006;32:1– 8. doi: 10.1016/j.neuroimage.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Pouratian N, Forage J, Bookheimer SY, Martin NA, Toga AW. Functional magnetic resonance imaging and optical imaging for dominant-hemisphere perisylvian arteriovenous malformations. Neurosurgery. 2004;55:804–12. doi: 10.1227/01.neu.0000137654.27826.71. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Diehl B, Piao Z, Tkach J, Busch RM, LaPresto E, Najm I, Bingaman B, Duncan J, Lüders H. Cortical stimulation for language mapping in focal epilepsy: correlations with tractography of the arcuate fasciculus. Epilepsia. 2010;51:639–46. doi: 10.1111/j.1528-1167.2009.02421.x. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. Multiple Comparisons Among Means. Journal of the American Statistical Association. 1961;56:52–64. [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–44. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci. 2009;13:175–81. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–5. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, Conry JA, Pearl PL, Ritter FF, Sato S, Vezina LG, Vaidya CJ, Wiggs E, Fratalli C, Risse G, Ratner NB, Gioia G, Theodore WH. Atypical Language in Lesional and Non-lesional Complex Partial Epilepsy. Neurology. 2007;69:1761–71. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Duke ES, Ritzl E, Miranda S, Liew C, Finegersh A, Martinez A, Dustin I, Sato S, Theodore WH. fMRI language dominance and FDG- PET hypometabolism. Neurology. 2011;76:1322–9. doi: 10.1212/WNL.0b013e31821527b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovacchini G, Bonwetsch R, Herscovitch P, Carson RE, Theodore WH. Cerebral blood flow in temporal lobe epilepsy: a partial volume correction study. Eur J Nucl Med Mol Imaging. 2007;34:2066–72. doi: 10.1007/s00259-007-0499-x. [DOI] [PubMed] [Google Scholar]
- Giussani C, Roux F-E, Ojemann J, Sganzerla EP, Pirillo D, Papagno C. Is Preoperative Functional Magnetic Resonance Imaging Reliable for Language Areas Mapping in Brain Tumor Surgery? Review of Language Functional Magnetic Resonance Imaging and Direct Cortical Stimulation Correlation Studies. Neurosurgery. 2010;66:113–120. doi: 10.1227/01.NEU.0000360392.15450.C9. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Hou BL, Bradbury M, Peck KK, Petrovich NM, Gutin PH, Holodny AI. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage. 2006;32:489–97. doi: 10.1016/j.neuroimage.2006.04.188. [DOI] [PubMed] [Google Scholar]
- Jayakar P, Bernal B, Santiago Medina L, Altman N. False lateralization of language cortex on functional MRI after a cluster of focal seizures. Neurology. 2002;58:490–2. doi: 10.1212/wnl.58.3.490. [DOI] [PubMed] [Google Scholar]
- Krainik A, Hund-Georgiadis M, Zysset S, von Cramon DY. Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke. 2005;36:1146–52. doi: 10.1161/01.STR.0000166178.40973.a7. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Pouratian N, Bookheimer SY, Martin NA. Factors predicting language lateralization in patients with perisylvian vascular malformations. J Neurosurg. 2010;113:723–30. doi: 10.3171/2010.2.JNS091595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Biondi A, Sourour N, Vlaicu M, du Montcel ST, Cohen L, Vivas E, Capelle L, Faillot T, Casasco A, Le Bihan D, Marsault C. Arteriovenous Brain Malformations: Is Functional MR Imaging Reliable for Studying Language Reorganization in Patients? Initial Observations. Radiology. 2002;223:672–682. doi: 10.1148/radiol.2233010792. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Lüders HO. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127:2316–30. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- Mbwana J, Berl MM, Ritzl EK, Rosenberger L, Mayo J, Weinstein S, Conry JA, Pearl PL, Shamim S, Moore EN, Sato S, Vezina LG, Theodore WH, Gaillard WD. Limitations to plasticity of language network reorganization in localization-related epilepsy. Brain. 2009;132:347–56. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Hermann B. How localized is localization-related epilepsy? Neurology. 2010;75:386–7. doi: 10.1212/WNL.0b013e3181eb58f8. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. J Neurosurg. 2002;97:21–32. doi: 10.3171/jns.2002.97.1.0021. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med. 1983;24:790–8. [PubMed] [Google Scholar]
- Ruff IM, Petrovich Brennan NM, Peck KK, Hou BL, Tabar V, Brennan CW, Holodny AI. Assessment of the language laterality index in patients with brain tumor using functional MR imaging: effects of thresholding, task selection, and prior surgery. AJNR Am J Neuroradiol. 2008;29 :528–35. doi: 10.3174/ajnr.A0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–84. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Stippich C, Rapps N, Dreyhaupt J, Durst A, Kress B, Nennig E, Tronnier VM, Sartor K. Localizing and lateralizing language in patients with brain tumors: feasibility of routine preoperative functional MR imaging in 81 consecutive patients. Radiology. 2007;243:828–36. doi: 10.1148/radiol.2433060068. [DOI] [PubMed] [Google Scholar]
- Tanriverdi T, Klein D, Mok K, Milot S, Al-Hashel J, Poulin N, Olivier A. Atypical speech activations: PET results of 92 patients with left-hemispheric epilepsy. Acta Neurochir (Wien) 2009;151:1175–90. doi: 10.1007/s00701-009-0373-7. [DOI] [PubMed] [Google Scholar]
- Thiel A, Herholz K, von Stockhausen HM, van Leyen-Pilgram K, Pietrzyk U, Kessler J, Wienhard K, Klug N, Heiss WD. Localization of Language-Related Cortex with 15O-Labeled Water PET in Patients with Gliomas. Neuroimage. 1998;7:284–295. doi: 10.1006/nimg.1998.0334. [DOI] [PubMed] [Google Scholar]
- Ulmer JL, Hacein-Bey L, Mathews VP, Mueller WM, DeYoe EA, Prost RW, Meyer GA, Krouwer HG, Schmainda KM. Lesion-induced pseudodominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery. 2004;55:569–581. doi: 10.1227/01.neu.0000134384.94749.b2. [DOI] [PubMed] [Google Scholar]
- Weber B, Wellmer J, Reuber M, Mormann F, Weis S, Urbach H, Ruhlmann J, Elger CE, Fernández G. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;129:346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- Wellmer J, Weber B, Urbach H, Reul J, Fernandez G, Elger CE. Cerebral lesions can impair fMRI-based language lateralization. Epilepsia. 2009;50:2213–24. doi: 10.1111/j.1528-1167.2009.02102.x. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33:522–30. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, Okujava M, Wolf P, Tuxhorn I, Ebner A. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]