Abstract
Hedgehog (Hh) signaling, via the key signal transducer Smoothened (SMO) and Gli transcription factors, is essential for embryonic development and carcinogenesis. While the biological relevance of hedgehog signaling to cancer is well established, very little is known about the molecular mechanisms by which signaling transduction of this pathway occurs. Rab23 was discovered as a negative regulator of the Hh pathway through a mouse genetic study. Here we report that Rab23 directly associates with Su(Fu) and inhibits Gli1 function in a Su(Fu)-dependent manner. By confocal microscope and immunoprecipitation, we detected interaction between Rab23 and Su(Fu). Using Gli1-mediated reporter gene analysis, we found that Rab23 can suppress Gli1 transcriptional activity in wild type but not Su(Fu) null fibroblasts. Similarly, Rab23 expression reduced the nuclear localization of Gli1 in wild type but not Su(Fu) null fibroblast cells. Consistent with the GTPase motif in the protein, we showed that Rab23 has GTPase activity. The dominant negative form of Rab23 was unable to suppress Gli1-mediated transcriptional activity. Taken together, these data provide evidence to support that Rab23 negatively regulates Gli1 activity in a Su(Fu)-dependent manner.
INTRODUCTION
The hedgehog pathway plays an important role in cell differentiation, tissue polarity, cell proliferation and carcinogenesis (1–3). The seven transmembrane domain containing protein smoothened (SMO) serves as the key player for signal transduction of this pathway, whose function is inhibited by another transmembrane protein Patched (PTC) in the absence of Hh ligands. Binding of Hh to its receptor PTC releases this inhibition, allowing SMO to signal downstream, leading to formation of active forms of Gli transcription factors. As transcription factors, Gli molecules can regulate target gene expression by direct association with a specific consensus sequence (4,5). In addition to the Drosophila homologues, mammalian cells have several novel cytoplasmic regulators of Hh signaling, including Rab23 (6) and tectonic (7).
Rab23 was initially identified through a forward genetic screening to be a negative regulator of hedgehog signaling by the mutant phenotype in mouse spinal cord. Shh signaling is necessary for the specification of ventral neural cell fates. The Rab23 mutants (open brain) have too many neural progenitors with ventral fates, which are accompanied by elevated Hh target gene expression (6). Since most Rab GTPases are involved in vesicle trafficking, Rab23 is suggested to transport molecules in the hedgehog pathway but the exact molecule with which Rab23 interacts is not known (8–10). Genetic evidence indicate that Rab23 regulates downstream of Sonic hedgehog (Shh), SMO and PTCH1, and possibly upstream or at the level of Gli transcriptional factors (11). At present, it is not clear the exact function of Rab23 in the hedgehog signaling pathway. Some even suggest that Rab23 may function beyond the Hh signaling pathway. Unlike many Rab proteins, Rab23 protein can be observed in the nucleus and in cytoplasm (12), suggesting that Rab23 may have other uncharacterized functions apart from membrane trafficking (13).
The aim of this study is to examine whether Rab23 physically interacts with components of the Hh signaling pathway. By co-localization studies, we studied interactions between Rab23 and hedgehog signaling molecules [Smoothened, Su(Fu) and Gli1]. Co-localization between Rab23 and Su(Fu) was further confirmed by immunoprecipitation (IP) and GST pull-down. The functional relevance of these interactions were demonstrated by the effects of wild type and a dominant negative form of Rab23 on Gli1-mediated reporter gene activity as well as Gli1 cellular localization. Our studies have extended the genetic evidence for a role of Rab23 in Hh signaling to indicate that Rab23 (via its GTPase activity) negatively regulate Gli1 activity through interaction with Su(Fu).
MATERIALS AND METHODS
Constructs
Rab23 cDNAs was generated from a discarded human lung tissue, and cloned into pEF6/V5-His TOPO® TA cloning vector (Invitrogen). SMO expression plasmids were derived from our previous reported study (14) by sub-cloning SMO into a pLNCX retrovirus vector with a MYC tag at the N-terminus. Su(Fu) expression plasmid was constructed by cloning human Su(Fu) into a pCDNA3-based vector with a MYC tag at the N-terminus. Gli1 constructs have been reported previously (15). Expression plasmids for Su(Fu) fragments (with Flag tag) were kindly provided by Dr. Fred de Sauvage from Genentech (16).
Cell culture, transfection and immunofluorescent staining
COS7 cells and NIH 3T3 fibroblasts were purchased from the American Type Culture Collection (Manassas, VA), and were maintained in Dulbecco’s Modified Essential Medium supplemented with 10% fetal bovine serum (Invitrogen). Su(Fu) null mouse embryonic fibroblasts (MEFs) and the matched MEFs were generated from mouse embryos with or without Su(Fu) alleles (17). Transfection was performed using LipofectAmine 2000 (Invitrogen) according to the manufacturer’s instructions [the ratio of plasmid (μg) to lipid (μl) was 1:2.5].
Immunofluorescent detection of MYC-tagged Gli1, SMO, Su(Fu) and V5-tagged Rab23 was performed as previously described (15) with Cy3-conjugated MYC antibody 9e10 (Sigma) (1: 100 dilution) (18,19) and FITC-conjugated V5 antibodies (Bethyl Laboratories). Gli1 localization was detected under a fluorescent microscope in Gli1 expressing cells, the percentage of Gli1 in the nucleus or the cytoplasm was calculated from each experiment and the experiment was repeated for three times with similar results. Confocal microscope was used to study co-localization between proteins as described previously (15).
GTPase assay
was performed according to Downward et al (20). Briefly, Rab23 protein was first purified from 293 cells (48 hrs) after ectopic expression of Rab23 (with V5 tag) using specific antibody to the V5 tag (4°C). The mixture was then washed 3 times with 1 ml of ice-cold wash buffer [HEPES, PH7.5 (50mM). NaCl (500mM), Triton X-100 (0.1% v/v), MgCl2 (5mM) and Sodium dodecyl sulfate (SDS), 0.005% (w/v)], one time with 1ml of ice-cold exchange buffer [HEPES, PH7.5 (50mM), MgCl2 (1mM), DTT (1mM), KCl (100mM), BSA(0.1mg/ml)]. Each sample was divided into four aliquots for nucleotide exchange assay. To each aliquot, 10μl of exchange buffer plus 1 mCi of [α-32]P and 3.33 pmol of unlabeled GTP was added, which was incubated at 20°C for 0, 20, 60 and 120 min with vigorous and continuous shaking and then put on ice. The mixture was washed 4 times with 1ml ice-cold wash buffer, and spotted onto thin layer chromatothin-layer chromatography plates (PEI cellulose, Cat# 15675; Merck, Rahway, NJ) until they run 2/3 of the way in 0.75M KH2PO4 pH 3.5. The TLC plate was dried before phosphor-imaging. The experiments were repeated four times with similar results.
Immunoprecipitation
Cells were lysed using NP-40 cell lysis buffer 48 hours after transfection. MYC-tagged Gli1 or Su(Fu) proteins were immunoprecipitated with anti-MYC antibody (Cell Signaling Inc.) for 3 hours, followed by incubation with A/G plus beads (Bethyl Laboratories, Inc.) for 1 hour. Rab23 protein was immunoprecipitated with agarose beads-conjugated anti-V5 antibodies (Bethly Laboratories Inc.). The immunocomplexes were separated by 8 (Gli1 and SuFu) or 12% (Rab23) SDS-PAGE and analyzed by Western blotting using appropriate antibodies according to previous studies (see Figures for details) (19).
Statistical analyses
were performed using the Student’s t-test to compare the results, with P values of <0.05 indicating statistically significant differences. Co-localization between proteins was estimated using the Pearson’s coefficient correlation (Pearson’s r value) generated by ImageJ analyses of the co-focal images, with +1 as the perfect co-localization, −1 as mutually exclusive and 0 as no association. In this study, we define any value >+0.2 as positively correlated, and any value below −0.2 will be viewed as negatively correlated (or mutually exclusive).
RESULTS
Co-localization of Rab23 with Su(Fu) and Gli1
Rab23 was initially cloned in 1994 (21), but its link to Hh signaling was made in 2001 in a forward genetic screening for genes responsible for neural tube developmental defects (6). Genetic studies predict that Rab23 acts downstream of Ptch1 and upstream or at the level of Gli transcriptional factors, but no physical interaction between Rab23 and other Hh signaling components have been reported. To investigate the role of Rab23 in Hh signaling, we first tested if Rab23 co-localizes with any of the signaling protein. Rab23 was cloned into an expression vector, and co-transfected with expression plasmids for SMO, Su(Fu) or Gli1.
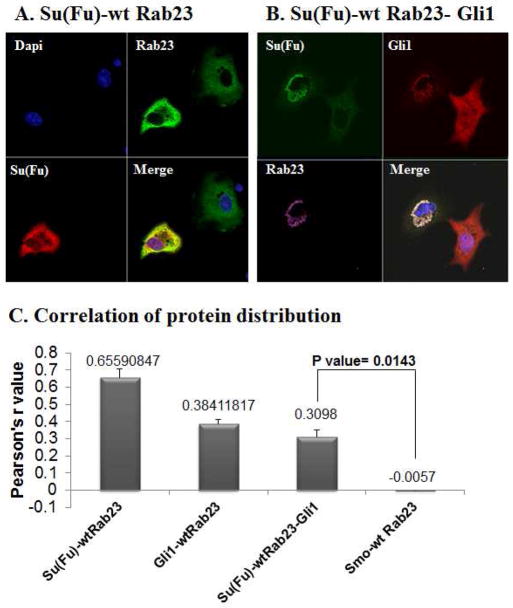
Following immunofluorescent staining, we examined protein co-localization between Rab23 and other Hh signaling proteins using confocal microscope. Consistent with genetic prediction (10), we did not detect any co-localization between SMO and Rab23 [see Fig. S1 and Fig. 1C with Pearson’s r value around 0 (from −0.0056 to 0.003), indicating no association]. On the other hand, we found significant co-localization between Su(Fu) and Rab23 (Fig. 1A and 1C, with Pearson’s r at 0.69, suggestive of being highly correlated in protein distribution). It is known that Su(Fu) associates with Gli1 and suppresses its function through at least two mechanisms: requesting Gli proteins in the cytoplasm and inhibiting Gli transcriptional activity (11,17,22). To examine if Rab23 is co-localized with Gli1, we expressed Rab23 and Gli1 with or without Su(Fu) in COS7 cells. As shown in Fig. 1, we detected co-localization of Rab23 with Gli1 in the presence of Su(Fu) [Fig. 1B and Fig. 1C, with Pearson’s r value between 0.25 for 3 proteins, 0.38 for Gli1 and Rab23, and 0.5 for Gli1 and Su(Fu) indicative of correlated protein distribution)]. We found the Pearson’s r value in cells transfected with wild type Rab23, Su(Fu) and Gli1 is statistically different from that in cells transfected with Smoothened and wild type Rab23 (p value= 0.0143, Fig. 1C), indicating that Rab23 protein mainly interacts with molecules downstream of Smo. We have evidence to believe that Rab23 mainly interacts with Su(Fu) because no co-localization between Rab23 and Gli1 was observed in Su(Fu) null MEF cells (Fig. S2). In Su(Fu) null MEFs, all cells expressing Gli1 have nuclear Gli1 expression whereas Rab23 is all in the cytoplasm (Fig. S2). Taken together, these studies indicate that Rab23 primarily interacts with Su(Fu) to regulate Hh signaling.
Fig. 1. Co-localization of Rab23 with Su(Fu) and Gli1.
Expressing plasmids for Rab23, Su(Fu) and Gli1 were transfected into COS7 or fibroblast cells. Immunofluorescent staining with specific antibodies was used to detect localization of these proteins. Co-localization of Rab23 (V5 tag) and Su(Fu) (MYC tag) was detected in cells with expression of both proteins (A). Similarly, co-localization of Rab23 (V5 tag), Su(Fu) (MYC tag) and Gli1 (Glu-Glu) was seen in cells expressing the three proteins (B). In contrast, no co-localization between Rab23 and SMO was detectable in all cells tested (Fig. S1). The co-localization between Rab23 and Su(Fu) was seen in COS7, NIH 3T3 fibroblast cells, C3H10T1/2 cells and mouse embryonic fibroblasts. Data from COS7 cells were shown in Fig. 1. Co-localization between proteins was analyzed using Imagej program (C, also see Methods). We defined the Pearson’s r values above +0.2 as co-localized, and above +0.5 was regarded as strongly co-localized. The Pearson’s r value in cells transfected with Rab23, Gli1 and Su(Fu) was statistically different from that in cells transfected with Smoothened (Smo) and Rab23 (p value= 0.0143).
The molecular domain of Su(Fu) responsible for interaction with Rab23
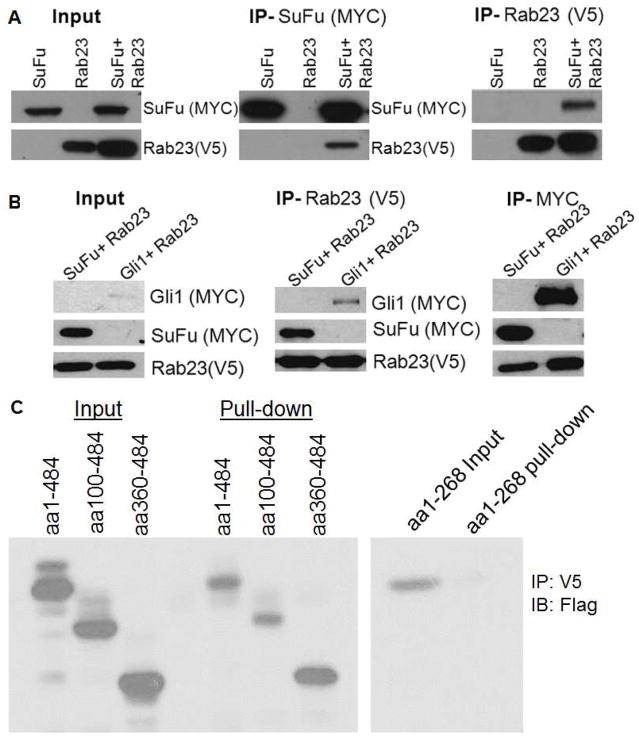
To substantiate the co-localization study, we performed Co-IP experiments. After ectopic expression of Rab23 and Su(Fu) in COS7 cells, we immunoprecipitated Rab23 protein (with V5 tag) with the specific tag antibodies, and detected Su(Fu) protein (MYC tag, Fig. 2A, 2B), suggesting that Rab23 protein interacts with Su(Fu). Conversely, when MYC antibody was used [against Su(Fu) tag], we detected Rab23 protein. We also detected interaction between Gli1 and Rab23 after ectopic expression of these two proteins in COS 7 cells (Fig. 2B). These Co-IP results are consistent with co-localization data.
Fig. 2. Co-IP between Rab23 and Su(Fu).
Following expression of Rab23 (V5 tag) and Su(Fu) (MYC tag) or Gli1 (MYC tag) in COS7 cells, Co-IP was performed using antibody-conjugated agarose beads against MYC or V5 following by detection with V5 or MYC antibodies (A and B). Interaction of Rab23 with different fragments of Su(Fu) (Flag tag) was assessed by Co-IP with V5 antibodies and immunoblotted with Flag antibodies (indicated as IB in Fig. 2C). While all the fragments with the c-terminus were able to interact with Rab23, interaction between Rab23 and aa1-268 was very weak (C).
To further identify the site of Su(Fu) protein responsible for Rab23 interaction, we examined the interaction between Rab23 and several fragments of Su(Fu) (16) by Co-IP. As shown in Fig. 2D, while all the fragments (with a Flag tag) containing the c-terminal domain of Su(Fu) were able to interact with Rab23, the fragment containing amino acid 1–268 had very weak interaction, suggesting that the site of Su(Fu) responsible for Rab23 interaction is within the c-terminal region of Su(Fu) possibly between aa 268 to 484. These analyses further confirm that Rab23 specifically interacts with Su(Fu) in regulation of Hh signaling.
Inhibition of Gli1 function by Rab23
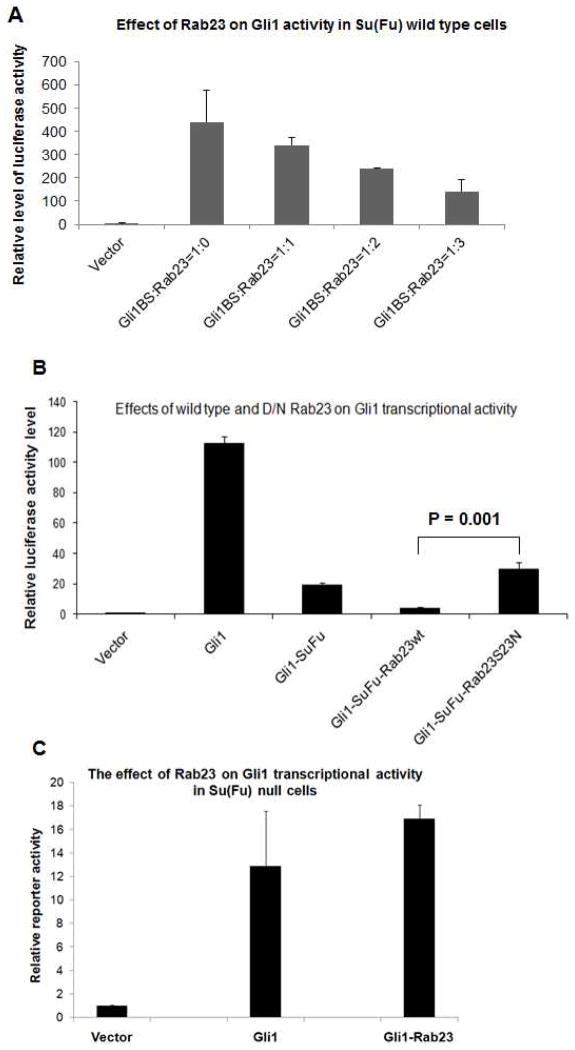
Next, we assessed the effect of Rab23 on Gli1 functions. It is known that Gli1 expression mediates Gli binding sites-driven expression of luciferase reporter gene. As shown in Fig. 3A and B, we found Rab23 suppressed Gli1-mediated luciferase activity in a dose-dependent manner. When Su(Fu) was co-expressed with Rab23, we detected further significant inhibition of Gli1 transcriptional activity (p value= 0.02). These results indicate that Rab23 not only interacts with Su(Fu) and Gli1, but also functionally inhibits Gli1 functions.
Fig. 3. Regulation of Rab23 on Gli1-mediated transcriptional activity in wild type and Su(Fu) null cells.
The luciferase reporter activity of Gli1 was assessed as previously reported in either wild type fibroblasts (A, B) or Su(Fu) null fibroblasts (C). Fig. 3A shows that Rab23 negatively regulates Gli1-mediated transcriptional activity in a dose-dependent manner. The luciferase reporter activity of Gli1 was assessed as previously reported in wild type fibroblasts. Three ratios of Gli1: Rab23 were used (1:1; 1:2; 1:3). The dominant negative (D/N) form of Rab23, Rab23S23N, was also used in the study (B). Statistical analysis was performed between Gli1-Su(Fu)-wtRab23 cells and Gli1-Su(Fu)-Rab23S23N cells using Student’s t test, and the p value was 0.001. The difference between Gli1 and Gli1-Rab23 in Su(Fu) null cells was not statistically significant (C, p value= 0.6).
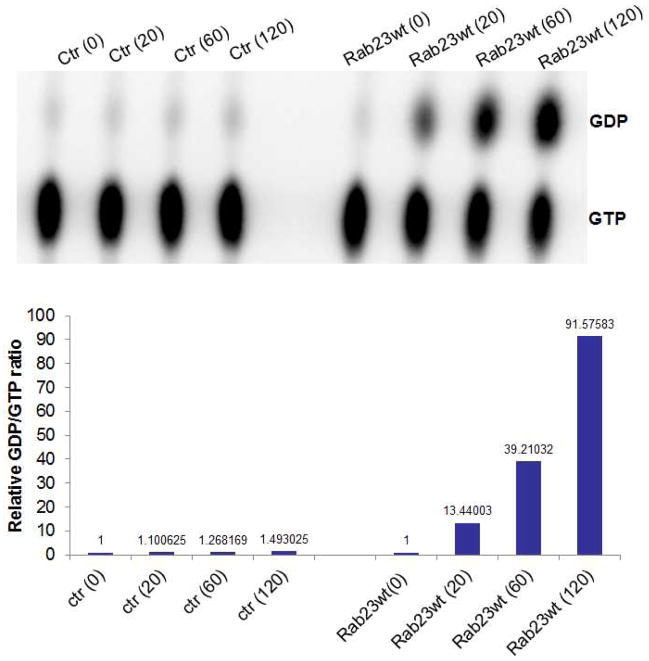
To demonstrate whether GTPase activity of Rab23 is required for this inhibition, we first examined if Rab23 has any GTPase activity. Rab23 was cloned into an expression vector and the protein was immunoprecipitated for GTP hydrolysis assay. As shown in Fig. 4, at different time points after incubating immuno-precipitated Rab23 protein with [α-32]P- GTP, we detected a stable increase in the ratio of GDP/GTP, indicating a GTP hydrolysis activity of Rab23.
Fig. 4.
GTP hydrolysis by Rab23. Ectopic expressed Rab23 protein was enriched by immunoprecipitation, and used for GTPase hydrolysis as described in the Methods. We found a steady increase of GDP/GTP ratio after incubation of [α-32]-GTP with Rab23 protein, indicating that Rab23 has the GTPase activity (to hydrolyze GTP to GDP and to lead a high GTP/GDP ratio). A shows an image of labeled GTP and GDP, and B shows the quantitative analyses of GDP/GTP ratio, indicative of GTP hydrolysis.
Next, we examined whether the GTPase activity is required for Rab23 activity. As indicated in Fig. 3B, we found that the dominant negative form of Rab23, Rab23S23N, failed to suppress Gli1 transcriptional activity. The difference between wild type Rab23 and Rab23S23N was statistically significant (P value is 0.001). This experiment indicates that Rab23 GTPase activity is required for its inhibition on Hh signaling.
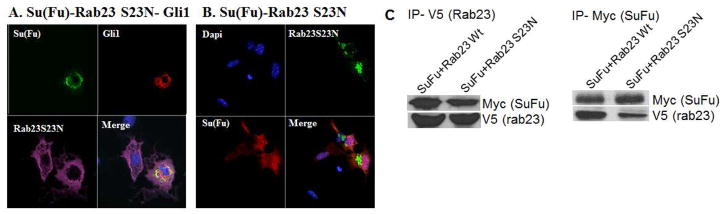
Furthermore, we found that Rab23-S23N was not co-localized Su(Fu) or Gli1 (Fig. 5A/B with Pearson’s r values below −0.2 indicative of no co-localization or even mutually exclusive). These data indicate that Rab23 can inhibit Gli1-mediated transcriptional activity, which is GTPase-dependent process. We generated a dominant negative mutant Rab23 construct with no predicted GTP hydrolysis activity, Rab23-S23N, based on the sequence comparison with other GTPases (13), and examined whether the GTPase activity is required for Rab23-Su(Fu) interaction. Cells were transfected with Su(Fu) with wild type or GTPase-dead Rab23. In comparison with the wild type Rab23 protein, the interaction between Rab23S23N and Su(Fu) was significantly reduced (Fig. 5C), suggesting the importance of GTPase activity for Rab23 functions.
Fig. 5. Immunofluorescent staining of Rab23 S23N with Su(Fu) and Gli1.
Fig. 4A and 4B were performed as described in Fig. 1 but Rab23 S23N was used instead of wild type Rab23. In cells expressing all three proteins, Rab23S23N had no co-localization with Su(Fu) and Gli1 (Pearson’s r= −0.23, indicative of no localization and mutually exclusive) (A). Similarly, in cells expressing Su(Fu) and Rab23S23N, the Pearson’s r value was below −0.2, suggestive of mutually exclusive in protein distribution (B). The results indicate that the GTPase activity of Rab23 is required for its association with Su(Fu). The co-localization was analyzed by ImageJ with multiple confocal images (n>5) from each group, and the Pearson’s r values were all below 0, indicative of no association between Rab23S23N and Su(Fu) or Gli1. In addition, a dominant negative form of Rab23 was used in the Co-IP experiment to examine whether the GTPase activity of Rab23 is involved in Su(Fu) association (C).
Su(Fu)-dependency of Rab23 effects on Gli1-mediated transcriptional activity
There are several possibilities for the inhibitory effects of Rab23 on Gli transcriptional factors like Gli1. One of which is Su(Fu)-dependent. It is also possible that Su(Fu) and Rab23 act independently on Gli1. To distinguish between the two, we tested the effect of Rab23 on Gli1-mediated luciferase reporter gene activity in wild type mouse embryonic fibroblast (MEF) cells as well as in Su(Fu) null MEF cells (23). We found that while Rab23 inhibited Gli1-mediated transcription in wild type MEF cells, Rab23 has no apparent effects on Gli1 transcriptional activity (Fig. 3C, p value= 0.6), indicating that the function of Rab23 on Gli1 transcription is Su(Fu)-dependent.
Rab23 inhibits Gli1 nuclear translocation through Su(Fu)
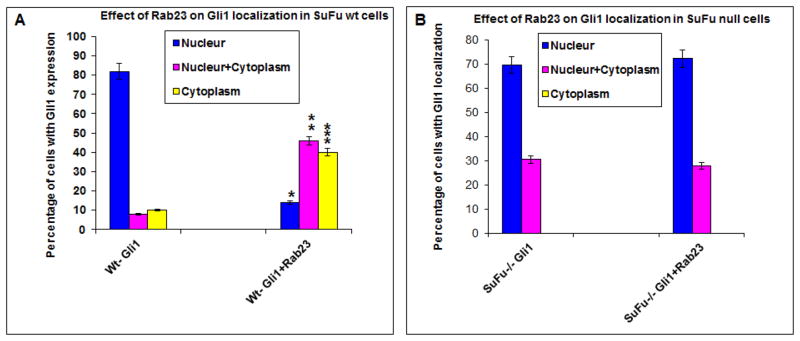
It is known that Su(Fu) can sequester Gli1 in the cytoplasm (22). If Rab23 exerts its inhibitory effect of Gli1 through Su(Fu), we predicted that ectopic expression of Rab23 should increase cytoplasmic localization of Gli1. We compared Gli1 location between the cells expressing Rab23 and Gli1 with those cells expressing only Gli1. As shown in Fig. 6A, we found that cells transfected with Gli1 have mostly (~80%) nuclear Gli1. With Rab23 co-expression, nuclear Gli1 protein was reduced to 20% whereas cytoplasmic Gli1 protein was increased to more than 40%, indicating that ectopic expression of Rab23 decreases the level of nuclear Gli1 protein. The difference between Gli1 transfected cells and Gli1+Rab23 transfected cells was statistically significant (p values are 0.0001 for nuclear localization, 0.012 for nuclear and cytoplasmic localization and 0.023 for cytoplasmic localization).
Fig. 6. Regulation of Gli1 nuclear localization.
Gli1 nuclear localization was detected by immunofluorescent staining after transfection with Gli1 and Rab23 in either wild type cells (A) or Su(Fu) null cells (B, Fig. S2). The detailed procedure was described in Methods and in previously published paper (15). The percentage of cells with nuclear (N), both nuclear and cytoplasm (N/C), and cytoplasm (C) Gli1 staining was calculated from the positively stained cells (n>50), and the experiments were repeated three times with similar results. The differences in Gli1 cellular localization between Gli1 transfected cells and Gli1+Rab23 transfected cells were analyzed by student’s t test in each category (N, N/C and C separately), and the p values are 0.0001 (indicated as * for nuclear localization), 0.012 (indicated as ** for nuclear and cytoplasmic localization) and 0.023 (indicated as *** for cytoplasmic localization). P values below 0.05 were regarded as statistically significant. In Su(Fu) null cells, the p values are 0.8 for both nuclear Gli1 and nuclear/cytoplasmic Gli1.
Next, we tested whether Su(Fu) is required for Rab23-mediated suppression of Gli1 nuclear localization. We co-transfected Gli1 with or without Rab23 into Su(Fu) null MEF cells and detected Gli1 localization. In consistent with the negative role of Su(Fu) on Gli1 nuclear translocation, we found all cells expressing Gli1 in the nucleus (Fig. S2). They are either nuclear (N) or both in nucleus and cytoplasm (N/C). If Rab23 can inhibit Gli1 independently of Su(Fu), we should see a shift of Gli1 protein localization to cytoplasm. If Rab23 functions in a Su(Fu)-dependent manner, Rab23 expression should not affect Gli1 protein localization. Our results showed that ectopic expression of Rab23 had no effects on Gli1 protein localization (Fig. 6B, p value= 0.8) in Su(Fu) null cells, indicating that Rab23 inhibits Gli1 localization through Su(Fu).
DISCUSSION
The role of Rab23 in Hh signaling
In this report, we showed for the first time that Rab23 exerts its inhibitory functions to Gli1 function through interaction with Su(Fu). Although we did not find any co-localization between Rab23 and SMO in cell lines we tested, a recent study suggested that loss of Rab23 affects ciliary translocation of SMO (24). Since cilium does not exist in our experimental conditions, our study did not rule out the possibility that Rab23 knockout may affect SMO. Our results indicate, however, the major role of Rab23 in our experimental systems is to inhibit Gli transcriptional factors through interaction with Su(Fu). Since the initial link of Rab23 to Hh signaling, direct protein-protein interactions between Rab23 and other Hh signaling molecules have not been reported. Our results show that Rab23 is capable of interacting with Gli transcriptional factors such as Gli1.
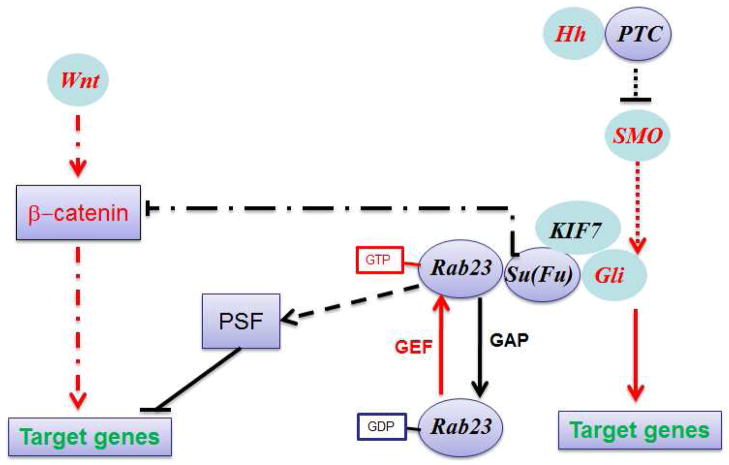
Based on Su(Fu) function, we predict that Rab23 can interact with all Gli1 molecules including Gli1, Gli2 and Gli3, and inhibit their transcriptional activities and nuclear localization. As a GTPase, Rab23 exist in two forms, the GTP bound form and the GDP bound form. Regulators of these forms include GTPase activating proteins (GAPs) for promoting GTP to GDP transition, and GTPase exchange factors (GEFs) for transition to the GTP bound form of Rab23. The GAPs and GEFs for Rab23 remain to be identified. Fig. S7 shows our model of Rab23-mediated inhibition of Gli transcriptional activity.
Is Rab23 a positive or a negative regulator in the Hh pathway?
Genetic analyses of neural tube development indicate a negative role of Rab23 in Hh signaling (6,10). Rab23 expression analyses in mouse and chicken embryonic brains (25,26) suggest a role in dorsalizing the neural tube, which is opposite to Shh function (ventralizing the neural tube)(27). In a study on chondrocyte differentiation, down-regulation of Rab23 was shown to decrease the level of Gli1 in chondrocytes, suggestive of a positive role of Rab23 in Gli1 regulation (28). Similarly, ectopic expression of Rab23 was shown to increase invasion of gastric cancer cells (8), and ectopic expression of Rab23 suppressed cell proliferation in liver cancer cells (23). Since Hh signaling is frequently activated in gastric and liver cancers (29,30), these results imply that Rab23 may play a positive role in Hh signaling in malignant cells.
The differential roles of Rab23 in normal and malignant cells are not unprecedented. For example, Ras is a well-known proto-oncogene, and mutation of K-Ras is a major driver for development of pancreatic cancer. However, ectopic expression of Ras can lead to senescence and cell cycle arrest through regulation of p16 and p53 (31). Thus, further studies will be needed to identify the molecular mechanisms by which Rab23 function differently in different cellular context.
Is Rab23 expression and its functions regulated by Hh signaling?
Due to lack of Rab23 GAPS and GEFs, it is not clear if Rab23 expression and its function are regulated by Hh signaling. Our gene expression profiling studies (32) suggested that Rab23 expression in keratinocytes and fibroblasts is not regulated by Hh signaling. There is evidence to indicate that Rab23 may be involved in other signaling pathway. For example, it was reported that Su(Fu) is also involved in Wnt/β-catenin pathway (33). In addition, published studies indicated that Rab23 is over-expressed in human cancers (8,12,23) with no clear evidence of Hh signaling activation. Furthermore, mis-sense mutations of Rab23 was identified in Carpenter Syndrome (34,35) also with no clear link to Hh signaling. It will be interesting to reveal the molecular mechanisms by which Rab23 expression and its functions are regulated. A recent study indicates that Rab23 may regulate functions of transcription repressor PSF (36). In Fig. 7, we proposed different mechanisms by which Rab23 functions in a cell.
Fig. 7. A model for Rab23 effects in a cell.
Our results indicate that Rab23 negatively regulates Hh signaling through interaction with Su(Fu). This effect requires the GTPase activity as D/N Rab23 was unable to inhibit Gli1-mediated transcriptional activity (Fig. 3) or associate with Gli1 (Fig. S5). Furthermore, Rab23 was not able to affect Gli1 functions without Su(Fu) (Fig. 6). The dash lines indicate potential multiple step processes. In addition, there is evidence to suggest that Rab23 regulates other signaling pathways such as wnt signaling and transcriptional repressor PSF. There are several questions remain to be answered. How Rab23 GTPase activity is regulated? What are the physiological GAPs and GEFs of Rab23? Is Rab23 GTPase activity regulated differently in different cell types (malignant vs. normal cells)?
CONCLUSION
In summary of our study, Rab23 and Su(Fu) co-localize and co-immunoprecipitate with each other to inhibit function and nuclear localization of Gli transcription factor Gli1. We identified the region of Su(Fu) responsible for Rab23 interaction, and found that the inhibitory effects of Rab23 on Gli1 is Su(Fu)-dependent. We thus conclude that the major effect of Rab23 is to inhibit Gli proteins through interaction with Su(Fu).
Supplementary Material
Highlights.
Rab23 was discovered as a negative regulator of the hedgehog (Hh) pathway through a mouse genetic study, but its physical interactions with Hh signaling molecules have not been reported. Here we report that Rab23 directly associates with Su(Fu) to inhibits transcription factor Gli1 function, but have no effects on Gli1 functions in Su(Fu) knockout cells. We have identified the region in Su(Fu) protein for Rab23 interaction, and have demonstrated the functional effects on Gli1 activity. Consistent with the GTPase motif in the protein, we showed that Rab23 has GTPase activity. The dominant negative form of Rab23 was unable to Gli1-mediated transcriptional activity. These results provide evidence to support that Rab23 negatively regulates Gli1 activity through physical interactions with Su(Fu).
Acknowledgments
This research was supported by a grant from the NIH (R01-CA94160) and Wells Center for Pediatric Research of Indiana University. We want to thank Drs. Fred de Sauvage and Pao-Tien Chuang for providing reagents for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jiang J, Hui CC. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahon AP, Ingham PW, Tabin CJ. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Xie G, Fan Q, Xie J. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 4.Kinzler KW, Vogelstein B. Mol Cell Biol. 1990;10:634–642. doi: 10.1128/mcb.10.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki H, Hui C, Nakafuku M, Kondoh H. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- 6.Eggenschwiler JT, Espinoza E, Anderson KV. Nature. 2001;412:194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- 7.Reiter JF, Skarnes WC. Genes Dev. 2006;20:22–27. doi: 10.1101/gad.1363606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou Q, Wu YH, Grabsch H, Zhu Y, Leong SH, Ganesan K, Cross D, Tan LK, Tao J, Gopalakrishnan V, Tang BL, Kon OL, Tan P. Cancer Res. 2008;68:4623–4630. doi: 10.1158/0008-5472.CAN-07-5870. [DOI] [PubMed] [Google Scholar]
- 9.Incardona JP, Gruenberg J, Roelink H. Curr Biol. 2002;12:983–995. doi: 10.1016/s0960-9822(02)00895-3. [DOI] [PubMed] [Google Scholar]
- 10.Eggenschwiler JT, Bulgakov OV, Qin J, Li T, Anderson KV. Dev Biol. 2006;290:1–12. doi: 10.1016/j.ydbio.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Svard J, Henricson KH, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Huang S, Yang L, An Y, Ma X, Zhang C, Xie G, Chen ZY, Xie J, Zhang H. Acta Histochem. 2010 doi: 10.1016/j.acthis.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Evans TM, Ferguson C, Wainwright BJ, Parton RG, Wicking C. Traffic. 2003;4:869–884. doi: 10.1046/j.1600-0854.2003.00141.x. [DOI] [PubMed] [Google Scholar]
- 14.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, Jr, de Sauvage FJ. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 15.Sheng T, Chi S, Zhang X, Xie J. J Biol Chem. 2006;281:9–12. doi: 10.1074/jbc.C500300200. [DOI] [PubMed] [Google Scholar]
- 16.Merchant M, Vajdos FF, Ultsch M, Maun HR, Wendt U, Cannon J, Desmarais W, Lazarus RA, de Vos AM, de Sauvage FJ. Mol Cell Biol. 2004;24:8627–8641. doi: 10.1128/MCB.24.19.8627-8641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MH, Wilson CW, Li YJ, Law KK, Lu CS, Gacayan R, Zhang X, Hui CC, Chuang PT. Genes Dev. 2009;23:1910–1928. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Athar M, Li C, Tang X, Chi S, Zhang X, Kim AL, Tyring SK, Kopelovich L, Hebert J, Epstein EH, Jr, Bickers DR, Xie J. Cancer research. 2004;64:7545–7552. doi: 10.1158/0008-5472.CAN-04-1393. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Chi S, He N, Zhang X, Guicherit O, Wagner R, Tyring S, Xie J. Oncogene. 2004;23:1608–1617. doi: 10.1038/sj.onc.1207273. [DOI] [PubMed] [Google Scholar]
- 20.Downward J. Methods Enzymol. 1995;255:110–117. doi: 10.1016/s0076-6879(95)55013-5. [DOI] [PubMed] [Google Scholar]
- 21.Olkkonen VM, Peterson JR, Dupree P, Lutcke A, Zerial M, Simons K. Gene. 1994;138:207–211. doi: 10.1016/0378-1119(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 22.Barnfield PC, Zhang X, Thanabalasingham V, Yoshida M, Hui CC. Differentiation. 2005;73:397–405. doi: 10.1111/j.1432-0436.2005.00042.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu YJ, Wang Q, Li W, Huang XH, Zhen MC, Huang SH, Chen LZ, Xue L, Zhang HW. World J Gastroenterol. 2007;13:1010–1017. doi: 10.3748/wjg.v13.i7.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boehlke C, Bashkurov M, Buescher A, Krick T, John AK, Nitschke R, Walz G, Kuehn EW. J Cell Sci. 2010;123:1460–1467. doi: 10.1242/jcs.058883. [DOI] [PubMed] [Google Scholar]
- 25.Guo A, Wang T, Ng EL, Aulia S, Chong KH, Teng FY, Wang Y, Tang BL. J Neurosci Res. 2006;83:1118–1127. doi: 10.1002/jnr.20788. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Volff JN, Wizenmann A. Dev Dyn. 2007;236:2993–3006. doi: 10.1002/dvdy.21331. [DOI] [PubMed] [Google Scholar]
- 27.Ribes V, Balaskas N, Sasai N, Cruz C, Dessaud E, Cayuso J, Tozer S, Yang LL, Novitch B, Marti E, Briscoe J. Genes Dev. 2010;24:1186–1200. doi: 10.1101/gad.559910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Clinton JM, Blackburn ML, Zhang Q, Zou J, Zielinska-Kwiatkowska A, Tang BL, Chansky HA. J Biol Chem. 2008;283:10649–10657. doi: 10.1074/jbc.M706795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma X, Chen K, Huang S, Zhang X, Adegboyega PA, Evers BM, Zhang H, Xie J. Carcinogenesis. 2005;26:1698–1705. doi: 10.1093/carcin/bgi130. [DOI] [PubMed] [Google Scholar]
- 30.Huang S, He J, Zhang X, Bian Y, Yang L, Xie G, Zhang K, Tang W, Stelter AA, Wang Q, Zhang H, Xie J. Carcinogenesis. 2006;27:1334–1340. doi: 10.1093/carcin/bgi378. [DOI] [PubMed] [Google Scholar]
- 31.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 32.Fan Q, He M, Sheng T, Zhang X, Sinha M, Luxon B, Zhao X, Xie J. J Biol Chem. 2010;285:36570–36576. doi: 10.1074/jbc.C110.164442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng X, Poon R, Zhang X, Cheah A, Ding Q, Hui CC, Alman B. J Biol Chem. 2001;276:40113–40119. doi: 10.1074/jbc.M105317200. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins D, Seelow D, Jehee FS, Perlyn CA, Alonso LG, Bueno DF, Donnai D, Josifova D, Mathijssen IM, Morton JE, Orstavik KH, Sweeney E, Wall SA, Marsh JL, Nurnberg P, Passos-Bueno MR, Wilkie AO. Am J Hum Genet. 2007;80:1162–1170. doi: 10.1086/518047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alessandri JL, Dagoneau N, Laville JM, Baruteau J, Hebert JC, Cormier-Daire V. Am J Med Genet A. 2010;152A:982–986. doi: 10.1002/ajmg.a.33327. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Cui Y, Zhang G, Garen A, Song X. Proc Natl Acad Sci U S A. 2009;106:16794–16798. doi: 10.1073/pnas.0909022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.