Abstract
Maintenance of cation homeostasis is essential for survival of all living organisms in their biological niches. It is also important for the survival of human pathogenic fungi in the host, where cation concentrations and pH will vary depending on different anatomical sites. However, the exact role of diverse cation transporters and ion channels in virulence of fungal pathogens remains elusive. In this study we functionally characterized ENA1 and NHA1, encoding a putative Na+/ATPase and Na+/H+ antiporter, respectively, in Cryptococcus neoformans, a basidiomycete fungal pathogen which causes fatal meningoencephalitis. Expression of NHA1 and ENA1 is induced in response to salt and osmotic shock mainly in a Hog1-dependent manner. Phenotypic analysis of the ena1, nha1, and ena1 nha1 mutants revealed that Ena1 controls cellular levels of toxic cations, such as Na+ and Li+ whereas both Ena1 and Nha1 are important for controlling less toxic K+ ions. Under alkaline conditions, Ena1 was highly induced and required for growth in the presence of low levels of Na+ or K+ salt and Nha1 played a role in survival under K+ stress. In contrast, Nha1, but not Ena1, was essential for survival at acidic conditions (pH 4.5) under high K+ stress. In addition, Ena1 and Nha1 were required for maintenance of plasma membrane potential and stability, which appeared to modulate antifungal drug susceptibility. Perturbation of ENA1 and NHA1 enhanced capsule production and melanin synthesis. However, Nha1 was dispensable for virulence of C. neoformans although Ena1 was essential. In conclusion, Ena1 and Nha1 play redundant and discrete roles in cation homeostasis, pH regulation, membrane potential, and virulence in C. neoformans, suggesting that these transporters could be novel antifungal drug targets for treatment of cryptococcosis.
Keywords: C. neoformans, Ena1, Nha1, cation transporters, antifungal drug
1. Introduction
Cation influx and efflux systems play critical roles in maintenance of plasma membrane potential, cellular ion homeostasis, and intracellular pH and nutrient uptake in all living organisms (Serrano et al., 1986). Potassium ion (K+) exists at higher concentration inside the cell (mM ranges) than in most natural environments (μM ranges) using diverse types of transporters to electrically neutralize negative charges generated from various cellular proteins and inorganic/organic acids and to regulate many physiological functions, such as enzyme activation and regulation of cell volume and intracellular pH (Rodriguez-Navarro, 2000). When intracellular K+ is limited, proton (H+) can be a substitute for electrical neutrality, which may increase intracellular pH and affect normal cell physiology. Alternatively, the sodium ion (Na+) could replace K+ under K+-deficiency and yet is generally more toxic for cells than K+(Arino, 2010; Sychrova, 2004).
To maintain optimal cation homeostasis, cells employ diverse high affinity cation transporters and ion channels (Sychrova, 2004). Saccharomyces cerevisiae has been used as a model organism to understand the regulatory mechanism of cation homeostasis in fungi. For K+-transport, two active transporters, Trk1p and Trk2p, and two channel proteins, Tok1p and Nsc1p, are involved in uptake of K+ into the cells (Bihler et al., 1998; Fairman et al., 1999; Gaber et al., 1988; Ko et al., 1990). For Na+-transport, two active sodium efflux pumps, Ena1 and Nha1, exist. Ena1, a putative Na+/ATPase plasma membrane transporter, mediates an efflux of toxic cations, such as Na+ and Li+, by hydrolyzing ATP (Benito et al., 2002). Ena1 is also involved in K+ transport. Nha1, a Na+/H+ antiporter, not only controls toxic Na+ and Li+ levels, but also modulates K+ and Rb+ levels (Banuelos et al., 1998; Kinclova et al., 2001; Prior et al., 1996). The proton motive force required for activating Nha1 is generated through the action of the Pma1 H+-ATPase. Both ion transporters appear to play complementary roles in regulating cation homeostasis because the ena1 nha1 mutant exhibits more severe cation susceptibility than each single mutant (Banuelos et al., 1998).
Regardless of the redundant roles played by the two Na+/K+ efflux pumps, the regulatory mechanisms of Ena1 and Nha1 are different. Nha1 is required for short-term adaptation to high salt shock and Ena1 for long-term adaptation (Proft and Struhl, 2004). Nha1 is constitutively expressed at the plasma membrane and relieves initial osmotic shock by extruding Na+ upon direct activation by the Hog1 mitogen activated protein kinase (MAPK), which results in re-assembling of transcription factors and transcription complex. Next, ENA1 is transcriptionally induced, which allows cells to achieve long-term adaptation to external osmotic shock. Expression of ENA1 is induced by high pH as well as Na+ and Li+ whereas expression of NHA1 is not modulated by low pH, salts or osmotic shock (Banuelos et al., 1998; Platara et al., 2006).
The ability to sense and adapt to changes in alkali metal ion concentration with in the host is important for survival of human pathogenic fungi, as cation concentration and pH levels vary depending on anatomical site. Particularly, Na+/K+-transport is closely related to maintenance of intracellular pH and establishment of the membrane potential, which appear to be key cellular factors for growth and survival of fungal pathogens. However, knowledge about the role of Na+/K+-transporters in virulence of human fungal pathogens is limited. In Cryptococcus neoformans, which causes fungal pneumonia and fatal meningoencephalitis in both immunocompromised and immunocompetent individuals (Hoang et al., 2004; Mitchell and Perfect, 1995), a cation transporter was found to be critical in virulence of the pathogen. The C. neoformans Ena1 ortholog has been identified as a key virulence determinant through signature-tagged insertional mutagenesis (Idnurm et al., 2009). Deletion of the ENA1 gene abolishes virulence of C. neoformans, indicating the importance of cation homeostasis for fungal pathogens. The ena1 mutant was also found to be less fit in strain competition experiments in the lungs of mice and unable to grow in human cerebral spinal fluid (Lee et al., 2010; Liu et al., 2008). In contrast to the S. cerevisiae ena1 mutant, Ena1 is required for sodium and potassium salt stress response under glucose-starvation conditions, but not under glucose-rich conditions (Idnurm et al., 2009; Ko et al., 2009). Furthermore, Ena1 was found to be essential in survival under high pH condition (Idnurm et al., 2009). However, the regulatory mechanism of the cation transporter remains elusive. Furthermore, other active cation transporters, such as Nha1, have not been functionally characterized in C. neoformans.
Our recent transcriptome analysis of the environmental stress response in C. neoformans revealed that the HOG pathway controls basal and osmotic stress-induced expression levels of the ENA1 and NHA1 genes. To further address the roles of the two cation transpoters in growth, differentiation, stress response, and virulence of C. neoformans, here we employed reverse genetics approaches to functionally characterize the role of Nha1 in comparison with Ena1 in the pathogen. In this study we found that Ena1 and Nha1 played discrete and overlapping roles in controlling intracellular concentrations of cations, such as Na+ and K+, potentially affecting membrane polarization. Intriguingly, inhibition of both cation transporters dramatically enhanced susceptibility to antifungal drugs, such as amphotericin B. Therefore the present study not only helps us to understand roles of cation transporters in C. neoformans, but also provides a unique opportunity to develop novel antifungal drug targets for treatment of fatal fungal meningitis.
2. Materials and Methods
2.1. Strains and media
Strains and primers used in this study are listed in Table 1 and S1 in the supplementary material. Yeast extract-peptone-dextrose (YPD) medium was used for culturing C. neoformans strains unless indicated otherwise. The Niger seed medium for melanin production, agar-based Dulbecco modified Eagle (DME) medium for capsule production, and V8 medium (adjusted to pH 5) for mating response were prepared as previously described (Alspaugh et al., 1997; Bahn et al., 2004; Granger et al., 1985; Hicks et al., 2004).
Table 1.
Strains used in this study
| Strain | Genotype | Parent | Reference |
|---|---|---|---|
| C. neoformans | |||
| H99 | MAT | (Perfect et al., 1993) | |
| KN99a | MATa | (Nielsen et al., 2003) | |
| YSB64 | MA hog1 ::NAT-STM#177 | H99 | (Bahn et al., 2005) |
| YSB586 | MA nha1 ::NEO | H99 | This study |
| YSB1161 | MA ena1 ::NAT-STM#58 | H99 | This study |
| YSB1169 | MAT ena1 ::NAT-STM#58 nha1 ::NEO | YSB586 | This study |
| YSB1201 | MAT nha1 ::NEO NHA1-NAT | YSB586 | This study |
| YSB1171 | MATanha1 ::NAT | KN99 | This study |
| YSB1371 | MATaena1 ::NEO | KN99 | This study |
| YSB1374 | MATaena1 ::NEO nha1 ::NAT | YSB1171 | This study |
| YSB1548 | MAT nha1 ::NEO NHA1-NAT-GFP | YSB586 | This study |
| KN99 | MAT | (Nielsen et al., 2003) | |
| AI167 | MAT ena1 ::NAT | FJW18 and KN99a | (Idnurm et al., 2009) |
| YSB590 | MAT ena1 ::NAT nha1 ::NEO | AI167 | This study |
| YSB1111 | MA nha1 ::NEO | KN99 | This study |
| YSB1283 | MA nha1 ::NEO NHA1-NAT | YSB1111 | This study |
|
| |||
| S. cerevisiae | |||
| MATahis3 1 leu2 0 met15 0 ura 0 NHA1-GFP::His3MX6 | BY4741 | (Huh et al., 2003) | |
Each NAT-STM# indicates the Natr marker with a unique signature tag.
2.2. Disruption of the NHA1 and ENA1 genes
For disruption of the NHA1 gene (CNAG_01678.2), information regarding the NHA1 genomic structure was obtained from the C. neoformans genomic database (Broad Institute). The NHA1 gene-disruption cassette was generated by using overlap PCR with primers listed in Table S1 as previously described (Davidson et al., 2002). Briefly, primers B1673 and B1674 for the 5′-flanking region of the NHA1 gene, primers B1675 and B1676 for the 3′-flanking region of the NHA1 gene, and primers M13Fe (M13 forward extended) and M13Re (M13 reverse extended) for the dominant selectable NEO marker (neomycin/G418-resistant marker) were used in the first round of PCR. Next, the NEO-marked NHA1-deletion cassette was amplified by overlap PCR with primers B1673 and B1676. The nha1 mutant and the ena1 nha1 double mutant were generated by introducing the NHA1-deletion cassette into the background of the C. neoformans serotype A strains H99, KN99, and the ena1 mutant [AI167; KN99 background; (Idnurm et al., 2009)] by biolistic transformation as previously described (Davidson et al., 2002). Stable transformants selected on YPD medium containing G418 were initially screened by diagnostic PCR for the 5′-junction with primers B1677 and B79, and their correct genotypes were subsequently verified by Southern blot analysis as described before (Jung et al., 2011).
To verify any phenotypes observed in the nha1 mutant, we constructed the nha1/NHA1 complemented strains as follows. First, a genomic fragment of the NHA1 gene containing promoter, the whole open reading frame (ORF), and terminator, was amplified via PCR by using primers B2081 and B2082, that include Xhol restriction sites, with the wild-type (WT) genomic DNA as template. The 4.5 kb gene product was cloned into a plasmid pTOP-V2 (Enzynomics) to produce a plasmid pTOP-NHA1. After sequencing to identify a clone with no errors, the NHA1 gene insert was subcloned with Xhol into a plasmid pJAF13 containing nourseothricin-resistance marker, NHT (nourseothricin acetyltransferase), to produce a plasmid pJAF13-NHA1. The Pacl-digested linearized pJAF13-NHA1 was biolistically transformed into the nha1 mutants (YSB586 and YSB1111). Targeted re-integration of the NHA1 gene into its native locus was confirmed by diagnostic PCR using primers B1677 and B1698.
To perform comparative phenotypic analysis of the nha1 mutant with the ena1 mutant, we independently generated the ena1 mutant in the H99 strain background. For disruption of ENA1, information regarding the ENA1 genomic structure was obtained from C. neoformans genomic database (Broad Institute). We constructed the ENA1 gene-disruption cassette by using double joint PCR (DJ- PCR) with primers described in Table S1. The ena1 mutants were generated by introduction of the ENA1 deletion cassette marked with NAT or NEO into the strains H99, KN99 (MATa), and nha1 mutant strains (MATa, YSB1171). Stable transformants selected on YPD medium containing nourseothricin or G418 were screened by diagnostic PCR and their correct genotypes were confirmed by Southern blot analysis.
2.3. Melanin and capsule assay
All C. neoformans strains for melanin and capsule assay were cultured on YPD liquid medium at 30°C for 16 hours. Three microliters of cells were spotted on agar-based DME medium for capsule assay and on agar-based niger seed medium, which contains indicated concentration of glucose (0.1%, 0.5%, or 1%). The plates were incubated at 30°C or 37°C, monitored daily, and photographed by using SPOT insight digital camera (Diagnostic Instrument Inc.) for up to 4 days. For quantitation of LAC1 transcript levels, WT and ena1 nha1 (YSB1169) double mutant were cultured in YPD liquid medium at 30°C for 16 hours. The overnight culture was inoculated 300 ml of fresh YPD liquid medium adjusted optical density at 600 nm (OD600) of the culture medium to approximately 0.4. Then culture medium was incubated until 1.0 of OD600 at 30°C. The 50 ml out of 300 ml culture was sampled and the remaining 250 ml culture was spun down. The supernatant was removed and added YNB liquid medium without glucose. During incubation, a 50 ml culture was sampled at 10 min, 30 min, 60 min, 90 min, and 120 min. Total RNAs from each sample for Northern blot analysis were prepared as previously described (Ko et al., 2009).
For measurement and visualization of capsule production, cells scraped from the DME medium were resuspended in phosphate buffer saline (PBS) buffer and stained with India ink. For quantitative measurement of capsule production, relative packed cell volume by using hematocrit capillary tubes was measured as previously described (Alspaugh et al., 2002; Jung et al., 2011).
2.4. Mating assay
Mating assay was performed as previously described (Bahn et al., 2004; Hicks et al., 2004). Each strain of opposite mating type (MAT and MATa) for mating assay were grown in YPD liquid medium at 30°C for 16 hours. Both mating type cells (107 cells/ml) were mixed with the same concentration and 5 μl of the mixture was spotted onto V8 medium. Then the plates were incubated in the dark at room temperature for 3 weeks. All colonial and cellular images were monitored and photograped by using microscope equiped with Olympus BX51 microscope (Diagnostic Instrument Inc.).
2.5. Stress and antifungal sensitivity test
Each strain was cultured in YPD liquid medium at 30°C for 16 hours, washed, serially diluted (1 to 104 dilutions), and spotted in 3 μl of volume onto solid YP or YPD medium containing indicated stress-inducing agents. For cation stress test, YP and YPD media containing indicated concentration of NaCl and KCl (0.5 to 1.5 M), LiCl (0.05 to 0.07 M), or CaCl2 (0.5 to 0.7 M) were used. For pH sensitivity test, cells were spotted onto YPD medium adjusted to pH 4.5 with succinic acid buffer or adjusted to pH 8.5 with Tris-HCl buffer. For measuring antifungal drug susceptibility, YPD media containing fluconazole (FCZ, Sigma), ketoconazole (KCZ, Sigma), and amphotericin B (AMB, Sigma) were used. For measuring thermotolerance, cells were spotted onto solid YPD medium and then incubated at 37°C or 39°C.
2.6. Northern blot analysis
For expression analysis of ENA1 and NHA1 under osmotic or salt stress, cells were cultured in 50ml YPD liquid medium for 16 hr at 30°C. Then 15 ml of overnight culture was inoculated into 135 ml of fresh YPD medium and further incubated at 30°C until optical density at 600 nm (OD600) of the culture medium reached approximately 1.0. For the zero time sample, 50 ml out of the 150 ml culture was sampled and the remaining 100 ml culture was added with 100 ml of YPD containing 2 M NaCl, 2 M KCl, or 2 M sorbitol. During incubation, a 50 ml culture was sampled at 10 min, 30 min, and 60 min. Total RNA from each sample for Northern blot analysis was prepared as previously described (Ko et al., 2009). For expression analysis of ENA1 and NHA1 in response to pH shock, cells were cultured in 50 ml of YPD liquid medium for 16 hr at 30°C. Then the overnight culture was inoculated into fresh YPD medium, of which volume was adjusted to 200 ml and incubated at 30°C until OD600 of the culture medium reached approximately 1.0. For the zero time sample, 50 ml out of the 200 ml culture was sampled. The remaining 150 ml culture was pelleted by centrifugation, added with 150 ml of fresh YPD medium adjusted to pH 8.5 with Tris-HCl buffer or pH 4.5 with 50mM succinic acid-NaOH buffer, and incubated at 30°C. During incubation, a 50 ml culture was sampled at 30 min and 60 min and total RNA was isolated.
2.7. Nha1 localization study
For the Nha1 localization study, the nha1/NHA1-GFP strain was constructed as follows. The terminator region of NHA1 (NHA1t) was amplified via PCR by using B3361 and B3362 containing Xbal restriction site with pTOP-NHA1 as template. The B3358, containing glycine-serine linker (5′-GGCGGTGGCTCT-3′) and B3360 primers were used to amplify the GFP gene with pACT-HOG1fGFP as a template. These PCR products were combined and overlap PCR was performed by using B3359 and B3362 containing Sacll and Xbal restriction sites and Sacll restriction site, respectively. The GFP-NHA1t product was cloned into a plasmid pTOP-V2 (Enzynomics) to produce a plasmid pTOP-GFPNHA1T. After sequencing, the Sacll-digested GFP-NHA1t was subcloned into a plasmid pJAF13 containing nourseothricin resistance marker to construct pJAF13-GFPNHA1T. The promoter and ORF region of NHA1 (NHA1PE) were amplified via PCR by using B3356 and B3357 including Apal or Xbal restriction sites, respectively, with pTOP-NHA1 as a template and cloned into a plasmid pTOP-V2 (Enzynomics) to produce a pTOP-NHA1PE. After sequencing, the Apal-and Xbal digested insert was subcloned into the Apal-and Xbaldigested pJAF13-GFPNHA1T to produce a plasmid pJAF13-NHA1GFP. The Pacl-digested linearized pJAF13-NHA1GFP was transformed into the nha1 Δ mutant (YSB586) and targeted re-integration of the NHA1-GFP gene into its native locus was confirmed by diagnostic PCR using primers B1677 and B1698. Functionality of the NHA1-GFP fusion gene was confirmed by comparative phenotypic analysis of the nha1/NHA-GFP and nha1/NHA1 strains.
For visualization of Nha1, we cultured the nha1Δ/NHA1-GFP strain in SC liquid media for 16 hr at 30°C. Then 5ml of overnight culture was inoculated into 45ml of fresh SC liquid media and incubated at 30°C until OD600 of the culture medium was approximately 1.0. For the zero time sample, 1 ml out of the 50 ml culture was sampled and the remaining culture was pelleted and added with fresh 50 ml of SC liquid media containing 1 M NaCl or 1 M KCl. During incubation, 1 ml of culture was sampled at 30 min and 60 min. Cells from each sample for GFP visualization were fixed by following the method recommended by Koshland (http://mcb.berkeley.edu/labs/koshland/Protocols/MICROSCOPY/gfpfix.html). Briefly, cells were spun down and supernatant removed. One hundred micro liters of 4% paraformaldehyde containing 3.4% sucrose were added to the sample and incubated at room temperature for 15 min. After incubation, cells were pelleted and washed in 500 μl 0.1 M potassium phosphate buffer adjusted to pH 7.5 containing 1.2 M sorbitol. Next, cells were resuspended in potassium phosphate buffer and stored in refrigerator. Fixed cells were visualized by confocal microscope (Carl Zeiss).
2.8. Virulence study
Each strain [WT (H99), ena1 (YSB1161), ena1 nha1 (YSB1169), nha1 (YSB586), nha1/NHA1(YSB1201) strains] was inoculated into 2 ml of YPD and grown overnight at 30°C. After incubation, cultures were pelleted by centrifugation and washed three times in 1ml sterile PBS. Cell suspensions were counted by hemocytometer and concentrations adjusted to 1×106 CFU/ml. Seven week old A/J female mice (Jackson Labs, Bar Harbor, MA) were anesthetized with an intraperitoneal dose of pentobarbital and 50 μ l of the prepared inocula (5×104 CFUs) were administered to the nares of each animal. Ten animals per group were infected with each strain. The concentration of each initial inoculum was verified by viable cell count with serial dilution on YPD agar plates. Mice were checked visually for the first 13 days of infection, and were weighed and checked for signs of infection beginning on day 14 post infection. Signs of morbidity included weight loss (1g/day for 2 days, or 2g loss in two days), abnormal gait, and extension of the cerebral portion of the cranium. Animals exhibiting these signs were sacrificed by administration of CO2. Animals who did not exhibit signs of infection by 43 days post infection were euthanized and target tissues (lungs, spleen, and brain) were harvested to determine whether or not the infection had been cleared (data not shown). All animal experiments were done at the University of Minnesota in strict accordance with good animal practice as defined by the National Institutes of Health Office of Animal Welfare (OLAW), and the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All experiments were reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC) under protocol number 1010A91133.
3. Results
3.1. Identification of the NHA1 gene in C. neoformans
Our prior transcriptome analysis of the stress-activated HOG signaling pathway in C. neoformans revealed that expression of two cation transporter genes, ENA1 (CNAG_00531.2) and NHA1 (CNAG_01678.2), appear to be regulated by the Hog1 MAPK under high salt stress (Ko et al., 2009). In S. cerevisiae, NHA1 is constitutively expressed but rapidly activated by Hog1-mediated phosphorylation in response to salt (Na+) shock for very early adaptation, whereas ENA1 is transcriptionally activated in a Hog1-dependent manner (Proft and Struhl, 2004). Therefore, the potentially discrete regulatory mechanism and a lack of any study for NHA1 in C. neoformans prompted us to investigate the role of the putative Na+/H+ antiporter with connection to the Ena1 Na+/ATPase efflux pump.
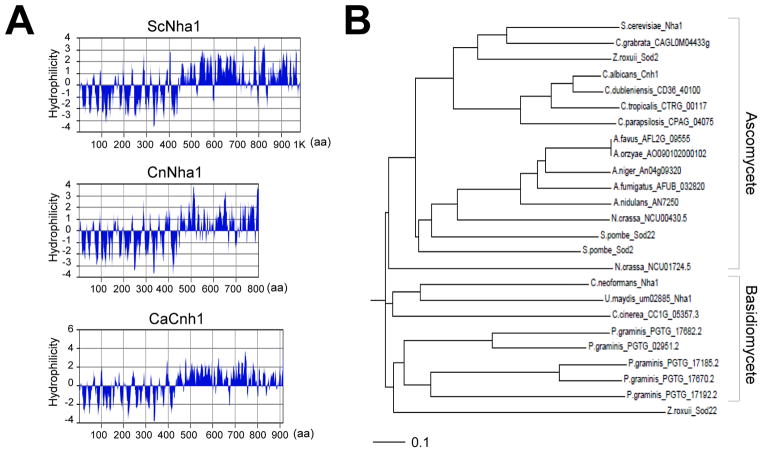
C. neoformans NHA1 is predicted to encode a 916 amino acid protein (CnNha1). Based on the hydrophilicity plot, CnNha1 exhibits a typical transporter domain structure, which is widely conserved in Nha1 orthologs in other fungi, such as C. albicans (CaCnh1) and S. cerevisiae (ScNha1; Fig. 1A). CnNha1 and other Nha1 orthologs contain the hydrophobic membrane spanning regions (11 or 12 membrane spanning regions) in the amino terminus and the hydrophilic cytoplasmic tail in the carboxy terminus (Fig. 1A). Furthermore, CnNha1 showed 42% identity in overall protein sequence to ScNha1 and CaCnh1. Based on the phylogenetic analysis, CnNha1 are grouped with Na+/H+ antiporter orthologs in other basidiomycete fungi (Fig. 1B). Taken together, CnNha1 is likely to be an ortholog for the Nha1 Na+/H+ antiporter in C. neoformans.
Fig. 1.
Identification of the NHA1 gene in C. neoformans. (A) The hydrophilicity plot of C. neoformans Nha1 (CnNha1), S. cerevisiae Nha1 (ScNha1), and C. albicans Cnh1 (CaCnh1). The hydrophilicity plots were depicted by protein analysis tool from MacVector software (version 7.2.3, Accelrys). (B) Phylogenetic tree of Nha1 proteins in C. neoformans and other fungi. The phylogenetic tree was generated by philodendron phylogenetic tree printer (http://iubio.bio.indiana.edu/treeapp/treeprint-form.html). The scale bar line represents an evolutionary distance of 0.1.
3.2. Differential expression of ENA1 and NHA1 in response to osmotic and salt stresses
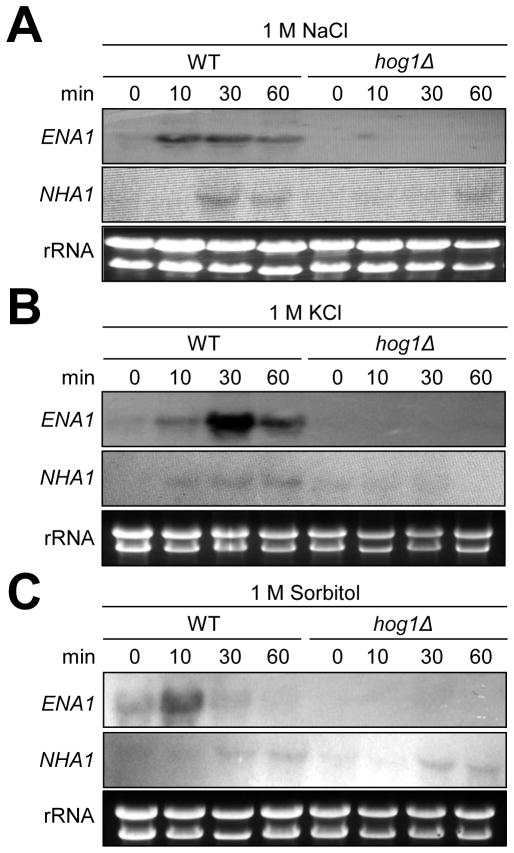
We monitored the expression pattern of NHA1 and ENA1 by Northern blot analysis under high salt shock. In agreement with the previous microarray data, both ENA1 and NHA1 were induced in response to 1 M NaCl. ENA1 was more rapidly (with in 10 min) and strongly induced than NHA1 (Fig. 2A). In response to 1 M KCl, ENA1 expression was also induced with in 10 min and peaked at 30 min whereas NHA1 was weakly induced (Fig. 2B). Extended running of Northern blot gel revealed two alternatively spliced ENA1 transcriptsin WT, but not in the ena1 mutant (Fig. 2 and 4A). These data indicate that both Ena1 and Nha1 transporters may be involved in Na+/K+ efflux in C. neoformans. The salt stress-induced expression of ENA1 and NHA1 was significantly blocked by a loss of Hog1 (Fig. 2A and 2B), indicating that the HOG pathway is the major controller of the two cation transporters.
Fig. 2.
Expression analysis of ENA1 and NHA1 under cation and osmotic stress conditions. Northern blot analysis was performed with total RNA isolated from WTH99 strain and hog1 mutants grown in YPD medium containing 1 M NaCl (A), 1 M KCl (B), or 1 M sorbitol (C) at different time points (0, 10, 30 and 60 min). Each membrane was hybridized with the radioactively labeled ENA1 specific probe, washed, and developed. Subsequently the same membrane was deprobed, re-hybridized with the NHA1 specific probe, washed, and developed.
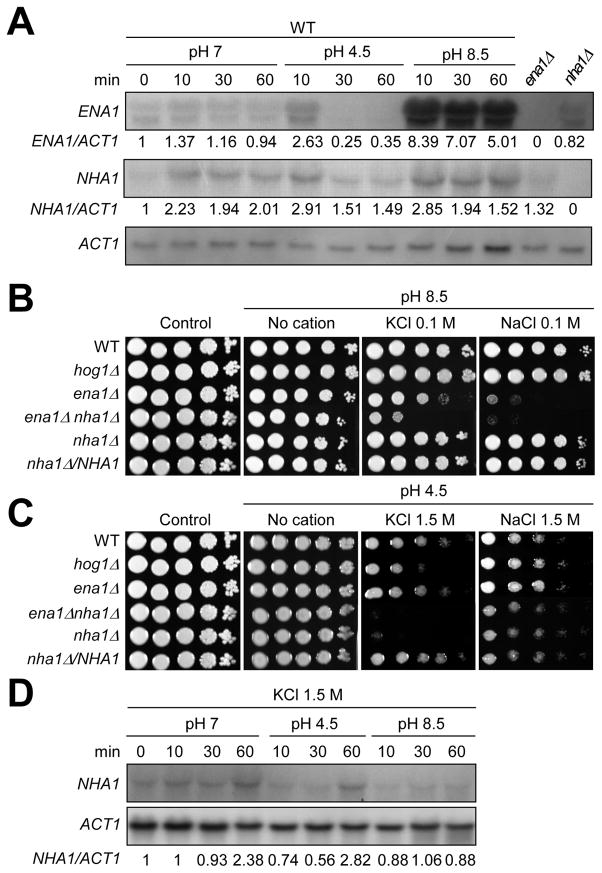
Fig. 4.
The role of Ena1 and Nha1 in growth under diverse pH conditions. (A) Northern blot analysis for monitoring ENA1 or NHA1 expression in response to different pH conditions. Total RNA was isolated from WT strain H99 grown in different pH conditions as described in Materials and Methods. The relative expression level of ENA1 or NHA1 was quantitatively measured with phosphoimager analysis by normalization with ACT1 expression levels. (B and C) Cells (WT, hog1, ena1, ena1 nha1, nha1 mutants and nha1/NHA1 complemented strain) were spotted on YPD agar medium that was adjusted to acidic (pH 4.5) or alkaline pH (pH 8.5) YPD and contained indicated concentration of KCl or NaCl. (D) Expression levels of NHA1 were monitored under different pH condition (pH 7, pH 4.5, and pH 8.5) in the presence of 1.5 M KCl. The relative expression level of NHA1 was quantitatively measured as described above in (A).
In order to address whether induction of ENA1 and NHA1 is triggered by salt stress or osmotic shock, we examined expression patterns of the two cation transporter genes in response to 1 M sorbitol. Interestingly, the expression patterns of NHA1 and ENA1 in response to 1 M sorbitol seemed to be different from those in response to 1 M NaCl or KCl. Upon exposure to 1 M sorbitol, ENA1 expression was transiently induced at 10 min and then rapidly returned to the basal levels in a Hog1-dependent manner (Fig. 2C). In contrast, NHA1 expression was weakly induced at later time points (30 to 60 min). However, the pattern of NHA1 expression in WT is indistinguishable from that of hog1Δ mutant (Fig. 2C). These data indicated that Ena1 is modulated by HOG pathway in response to cation and osmotic shock whereas Nha1 is mainly governed by Hog1 under cation shock, but not under osmotic shock.
3.3. Ena1 and Nha1 play discrete and redundant roles in maintenance of cation homeostasis in C. neoformans
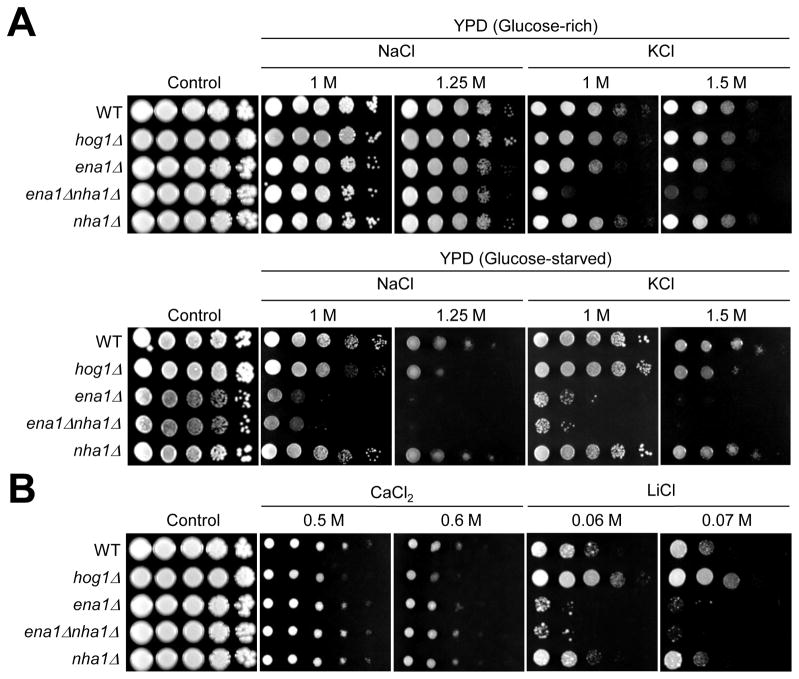
Deletion of the ENA1 gene renders C. neoformans cells less viable under high pH condition (pH 8.5) and attenuates virulence in mice (Idnurm et al., 2009). To characterize the role of Nha1 and its relationship with Ena1, we constructed the nha1 and ena1 nha1 double mutants in the H99 and KN99 strain background as described in Materials and Methods. The mutant set generated in these two strain backgrounds exhibited similar phenotypes and did not show any growth defects at a range of temperatures (25 to 39°C) (data not shown). The ena1 mutant exhibited reduced survival in the presence of high salt (1 M NaCl or KCl) only under glucose-starved condition, but not under glucose-rich condition (Fig. 3A), as described before (Idnurm et al., 2009; Ko et al., 2009), which is in contrast to the S. cerevisiae ena1 mutant showing high salt susceptibility under glucose-rich conditions (Prior et al., 1996). In contrast, deletion of the NHA1 gene alone did not affect susceptibility to salt stress under neutral pH condition (Fig. 3A). However, double deletion of both ENA1 and NHA1 genes dramatically enhances K+ salt stress susceptibility. The ena1 nha1 double mutant became extremely susceptible to K+-stress even under glucose rich conditions unlike each single mutant (Fig. 3A), suggesting that both Ena1 and Nha1 are involved in K+ homeostasis. In contrast, additional deletion of NHA1 did not further increase Na+-stress susceptibility of the ena1 mutant, indicating that Nha1 is largely dispensable for Na+ homeostasis.
Fig. 3.
The role of Ena1 and Nha1 in cation homeostasis. (A) WT H99 strain and hog1, ena1, ena1 nha1, and nha1 mutants were grown for 16hr at 30°C in liquid YPD medium, 10-fold serially diluted and spotted on YPD (glucose-rich) or YP (glucose-starved) media containing indicated concentrations of KCl or NaCl. (B) Cells were incubated for 16hr at 30°C in liquid YPD medium, 10-fold serially diluted and spotted on YPD medium containing indicated concentrations of CaCl2 or LiCl.
In S. cerevisiae, Ena1 and Nha1 control the intracellular concentration of toxic lithium ion (Li+) as well as that of Na+ and K+ (Haro et al., 1991; Prior et al., 1996). The C. neoformans ena1 mutant showed increased susceptibility to Li+-stress compared to wild-type (WT) whereas the nha1 mutant exhibited WT levels of Li+ susceptibility (Fig. 3B). Deletion of NHA1 did not further increase Li+ susceptibility of the ena1 mutant, indicating that Ena1, but Nha1, is mainly involved in Li+ homeostasis. However, ena1, nha1, and ena1 nha1 double mutants showed WT levels of resistance to CaCl2 (Fig. 3B). In conclusion, Ena1 is mainly involved in controlling cellular levels of toxic cations, such as Na+ and Li+, whereas both Ena1 and Nha1 are important for influx or efflux of less toxic K+ ions.
3.4. Both Ena1 and Nha1 play unique roles in survival of C. neoformans under alkaline and acidic pH
Functions of Ena1 and Nha1 are dependent on the electrochemical gradient of protons across the plasma membrane in S. cerevisiae (Catty et al., 1997; Sychrova et al., 1999). In yeast, Ena1 is mainly activated in alkaline condition, which has low concentrations of extracellular protons whereas Nha1 is activated in acidic condition, which has higher concentrations of extracellular protons (Sychrova et al., 1999). Moreover, Sod22, an ortholog of Nha1 in Schizosaccharomyces pombe, is activated in acidic pH conditions (Papouskova and Sychrova, 2007). In agreement with this finding, Ena1 was also found to be essential in survival of C. neoformans under high pH (8.5) conditions (Idnurm et al., 2009).
To further elucidate the role of Ena1 and Nha1 in survival of C. neoformans under different pH conditions, we first measured the expression patterns of ENA1 and NHA1 in response to high- and low-pH conditions. Supporting the previous finding by Idnurm et al., ENA1 expression was strongly increased in response to the alkaline condition (pH 8.5) (Fig 4A). Accordingly, the ena1 mutant, but not the nha1 mutant, exhibited severe growth defects even in the presence of very low concentration (0.1 M) of Na+ or K+ salt under the alkaline condition (Fig 4B). Interestingly, however, the ena1 nha1 double mutant displayed even higher susceptibility to 0.1 M KCl under high pH condition than the ena1 mutant. These data show that Ena1 plays a major role in survival at the alkaline pH condition containing low concentration of K+ or Na+ whereas Nha1 plays a minor role at the same condition.
In contrast to ENA1, Nha1 appears to play a role in survival of C. neoformans at the acidic pH condition. ENA1 expression was transiently induced after 10 min, but rapidly repressed and abolished after 30 min under the acidic condition (pH 4.5). In contrast, NHA1 expression was weakly induced after any pH changes (Fig. 4A). C. neoformans Nha1 was not required for cell survival under low pH (pH 4.5) per se. Notably, however, the nha1 mutant, but not the ena1 mutant, showed extreme susceptibility to 1.5 M KCl under acidic pH condition compared to WT (Fig. 4C), which was in stark contrast to the finding that the nha1 mutant was as resistant to 1.5 M KCl as WT under neutral pH (Fig. 3A). This phenomenon was not observed under high Na+ conditions (Fig. 4C), further indicating that Nha1 mainly controls cellular levels of K+, not Na+. Interestingly, the ena1 mutant did not exhibit increased susceptibility to 1.5 M NaCl under acidic pH condition (Fig. 4C). These data indicate that Nha1 plays a role in survival at acidic pH conditions under high K+ stress and Ena1 is dispensable for Na+ or K+-stress response under acidic conditions. Regardless of the essential role of Nha1, NHA1 expression was not strongly induced in response to acidic pH and high K+ stress (Fig. 4D). In conclusion, Ena1 and Nha1 play discrete and overlapping roles in controlling proton homeostasis across the plasma membrane of C. neoformans under different pH conditions.
3.5. Ena1 and Nha1 are required for maintenance of plasma membrane potential and stability
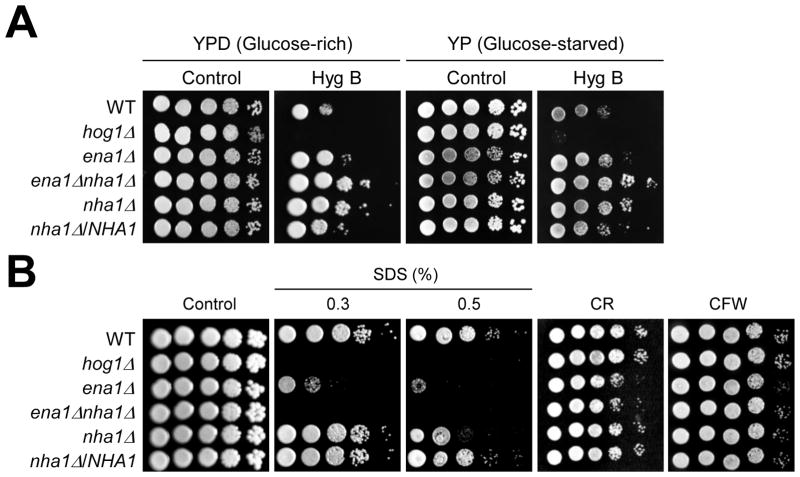
The role of Ena1 and Nha1 in intracellular proton homeostasis prompted us to investigate their function in maintenance of plasma membrane potential. For this purpose, we used achemical whose activity is affected by membrane potential. Hygromycin B (HygB), a positively charged aminoglycosidic antibiotic, is known to be incorporated into the cells in proportion to membrane potential and used as a hyper- or hypo-polarization indicator in yeast (Mulet et al., 1999; Perlin et al., 1988). For example, the HygB-resistant pma1 mutant shows the reduced membrane potential (Perlin et al., 1988) and deletion of Vnx1, a type of vacuolar monovalent cation/H+ antiporter in S. cerevisiae, confers resistance to HygB (Cagnac et al., 2007). To address whether Ena1 and Nha1 control membrane potential in C. neoformans, each strain was exposed to HygB in the glucose-rich and -starved conditions. Each nha1 and ena1 mutant exhibited slightly increased levels of resistance to HygB in both glucose-rich and -starved conditions. Interestingly, however, the ena1Δnha1Δ double mutant was even more resistant to HygB than the nha1Δ and ena1Δ mutant (Fig. 5A), which is accordance with the previous report showing that overexpression of NHA1 increases HygB susceptibility of S. cerevisiae (Kinclova-Zimmermannova et al., 2006). Therefore Ena1 and Nha1 could modulate membrane potential cooperatively although more direct measurement of membrane potential in the mutants will be needed in future studies.
Fig. 5.
The role of Ena1 and Nha1 in maintenance of membrane potential and stability. (A) Cells were spotted onto the YPD or YP medium containing the indicated concentration of Hyg B (Hygromycin B 60 μg/ml and 40 μg/ml in glucose-rich and-starved conditions, respectively). Strains were incubated at 30°C for 3 to 4 days and were photographed. (B) Cells were spotted onto the YPD medium including the indicated concentration of SDS, CR (Congored, 1%) or CFW (Calcofluor white, 2 mg/ml).
Related to this finding, inhibition of either Ena1 or Nha1 greatly exacerbated membrane stability. Both ena1 and nha1 mutants showed a greatly increased susceptibility to SDS, a membrane destabilizes. The ena1 nha1 double mutant was even more susceptible to SDS than each single mutant (Fig. 5B). However, the ena1 nha1 mutant was as resistant to cell wall biosynthesis inhibitors, such as congo red (CR) and calcofluor white (CFW), as WT, indicating that Ena1 and Nha1 affect cell membrane stability, but not cell wall integrity (Fig. 5B). Probably, perturbed membrane potential caused membrane instability in the ena1 and nha1 mutants, although other reason(s) might exist.
3.6. Inhibition of Ena1 and Nha1 increases antifungal drug susceptibility in C. neoformans
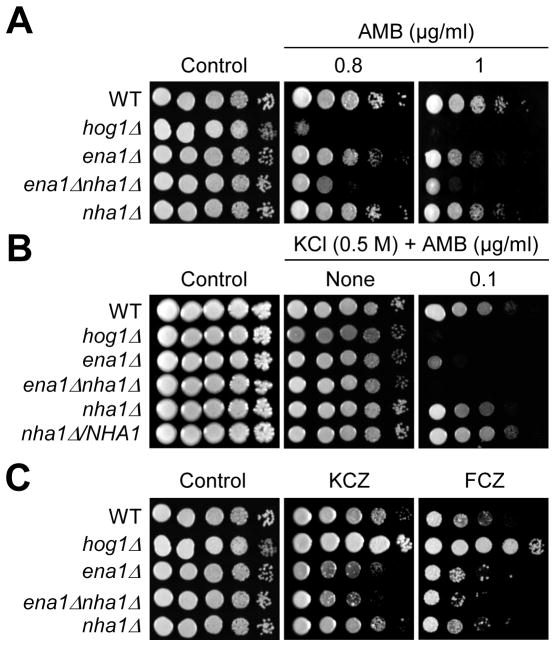
Based on the role of Ena1 and Nha1 in maintenance of membrane potential and stability, we hypothesized that inhibition of Ena1 and Nha1 may affect the capability of antifungal drugs to bind to the cell membrane or to be incorporated into the cells through the cell membrane. Polyene drugs, such as amphotericin B (AMB), bind to ergosterol in the cell membrane and form a transmembrane channel, which results in a leakage of monovalent ion (Na+, K+, H+, and Cl−). Azole drugs, such as fluconazole (FCZ) and ketoconazole (KCZ), inhibit the synthesis of ergosterol and destabilize the cell membrane stability and fluidity. The ena1 mutant exhibited a slightly increased susceptibility to AMB than WT. In contrast, the ena1 nha1 double mutant was highly susceptible to AMB, which is similar to the hog1 mutant (Fig. 6A). In the presence of mild K+ stress (0.5 M KCl), the ena1, nha1, or ena1 nha1 mutant exhibited even higher susceptibility to AMB (Fig. 6B). Unlike the hog1 mutant, however, the ena1 nha1 double mutant also exhibited increased susceptibility to the azole drugs (Fig. 6C). Hogl is known to repress ergosterol biosynthesis in C. neoformans and thereby its inhibition leads to increased expression of genes involved in ergosterol biosynthesis and contents of cellular ergosterol, which subsequently enhances azole resistance but reduces polyene resistance (Ko et al., 2009). Therefore, increased polyene and azole susceptibility observed in the ena1 nha1 double mutant appears to be independent of ergosterol biosynthesis, and instead results from defective membrane stability and cation imbalance. These data indicate that Ena1 and Nha1 play redundant roles in protecting C. neoformans from polyene and azole drugs by regulating membrane stability and cation homeostasis.
Fig. 6.
The role of Ena1 and Nha1 in antifungal drug resistance. (A and C) Strains (WT H99 strain and hog1, ena1, ena1 nha1, and nha1 mutants) were spotted onto YPD medium including the indicated concentration of polyene [AMB (amphotericin B)] or azole [FCZ (fluconazole, 14 μg/ml), KCZ (ketoconazole, 0.3 μg/ml)] drugs. (B) Each C. neoformans strain was grown for 16hr at 30°C in YPD liquid medium, 10-fold serially diluted and spotted YPD (0.5 M KCl) agar containing indicated concentrations of AMB. Cells were incubated at 30°C for 72hr and photographed.
3.7. Diverse signaling pathways are involved in ENA1 and NHA1 expressions in alkaline- and cation stress
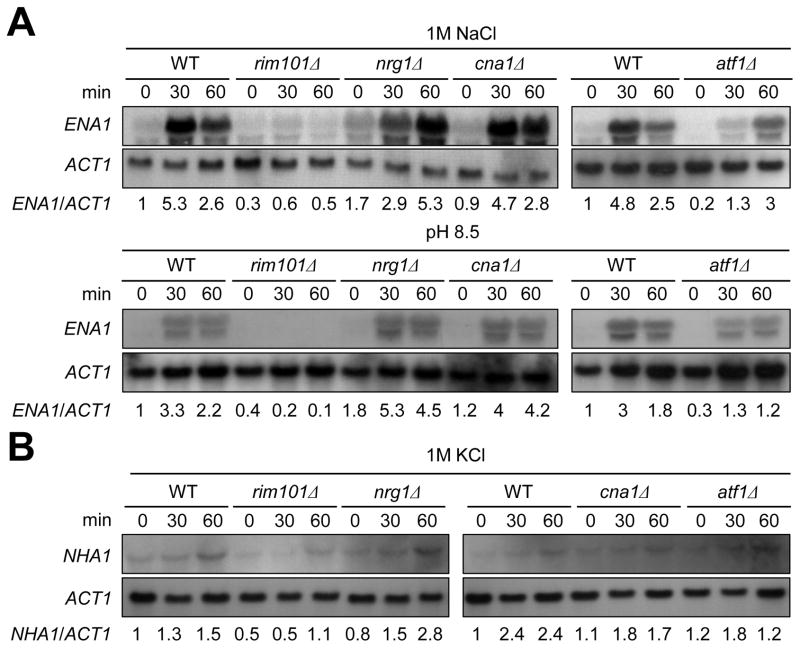
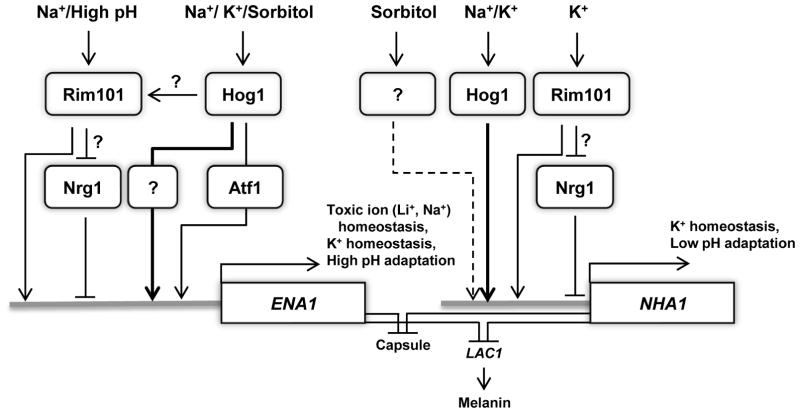
In the budding yeast, ENA1 expression is under control of diverse signaling cascades besides the HOG pathway. The calcium/calcineurin signaling pathway controls ENA1 expression through the Crz1 transcription factor (Mendizabal et al., 2001). Under high pH, the Rim101 pathway controls ENA1expression through an Nrg1 repressor protein (Lamb and Mitchell, 2003). In C. neoformans, however, ENA1expressionis not modulated by the calcineurin pathway under high pH condition but is regulated by Rim101 in capsule inducing conditions (Idnurm et al., 2009; O’Meara et al., 2010). To further identify signaling pathways, besides the HOG pathway, that control ENA1 and NHA1 expression in response to cation shock and high pH condition, we examined expression patterns of the two cation transporters in rim101, nrg1, cna1, and atf1 mutants. Atf1 is a transcription factor that is positively regulated by the Hog1 MAPK (our unpublished results). Northern blot analysis showed that both basal and induced levels of ENA1 were highly reduced in the rim101 mutant in both alkaline (pH 8.5) and salt stress conditions in C. neoformans (Fig. 7A). In contrast, basal expression levels of ENA1 expression were increased in the nrg1 mutant in both conditions. Under high pH conditions, ENA1 induction was greater in the nrg1 mutant than WT (Fig. 7A). These data indicate that the Rim101-signaling pathway may control ENA1 expression through the Nrg1 repressor in C. neoformans. In the atf1 mutant, basal ENA1 expression was decreased and its induction appeared to be decreased or delayed at both alkaline and salt stress conditions (Fig. 7A), indicating that the HOG pathway controls ENA1 partly via the Atf1 transcription factor. The calcineurin pathway appeared to be largely dispensable for regulation of ENA1 in the presence of 1 M NaCl, although down regulation of ENA1 seemed to be delayed at pH 8.5. In contrast to ENA1, Cna1 and Atf1 appeared to be dispensable for induction of NHA1 (Fig. 7B). Similar to ENA1 expression, however, induction of NHA1 was decreased in the rim101Δ mutant and slightly increased in the nrg1Δ mutant (Fig. 7B), implying that the Rim101-Nrg1 pathway could be involved in regulation of NHA1. Taken together, ENA1 expression is regulated at least by the two signaling pathways, the Hog1-Atf1 and Rim101-Nrg1 pathways, under cation stress and high pH conditions. Nha1 is mainly regulated by the Hog1 pathway in the Atf1-independent manner and the Rim101-Nrg1 pathway.
Fig. 7.
Expression patterns of ENA1 and NHA1 in diverse signaling pathways. Total RNA was isolated from WT H99 strain and rim101, nrg1, cna1 and atf1 mutants grown in YPD liquid medium containing 1 M NaCl (A, upper panel) or 1 M KCl (B), or YPD medium adjusted to high pH (8.5) (A, lower panel) at different time points (0, 30, 60 minutes). Each membrane was hybridized with the ENA1 (A) or NHA1 (B) specific probes, washed, and developed. Subsequently the same membrane was deprobed, re-hybridized with the ACT1 specific probe, washed, and developed. Expression levels of ENA1 or NHA1 relative to ACT1 were measured by phosphoimager analysis.
3.8 Nha1 cellular localization is similar in C. neoformans and S. cerevisiae
We examined the cellular location of Nha1 protein in C. neoformans. It has been reported that Nha1 proteins in other fungi including S. cerevisiae and C. albicans are preferentially localized in the plasma membrane (Kinclova-Zimmermannova and Sychrova, 2007; Kinclova et al., 2001). In order to find cellular localization of CnNha1, we constructed the nha1/NHA1-GFP strain that is generated by transforming the nha1 mutant with the NHA1 gene fused with the GFP gene at the C-terminal region. Phenotypes of the nha1/NHA1-GFP strain were equivalent to those of WT and nha1/NHA1 complemented strains (data not shown), indicating that the Nha1-GFP protein is functional as native Nha1. We examined cellular localization of CnNha1-GFP in comparison with ScNha1 that is similarly fused to GFP at the C-terminal region. Reflecting low RNA expression levels of NHA1, GFP-fluorescence signals were weakly detected throughout the cell in both S. cerevisiae and C. neoformans (Fig. 8). However, both ScNha1 and CnNha1 appeared to be more enriched in the periphery of cells than other parts of cells, although they were not evenly distributed. Therefore, cellular localization of C. neoformans Nha1 was similar to yeast Nha1.
Fig. 8.
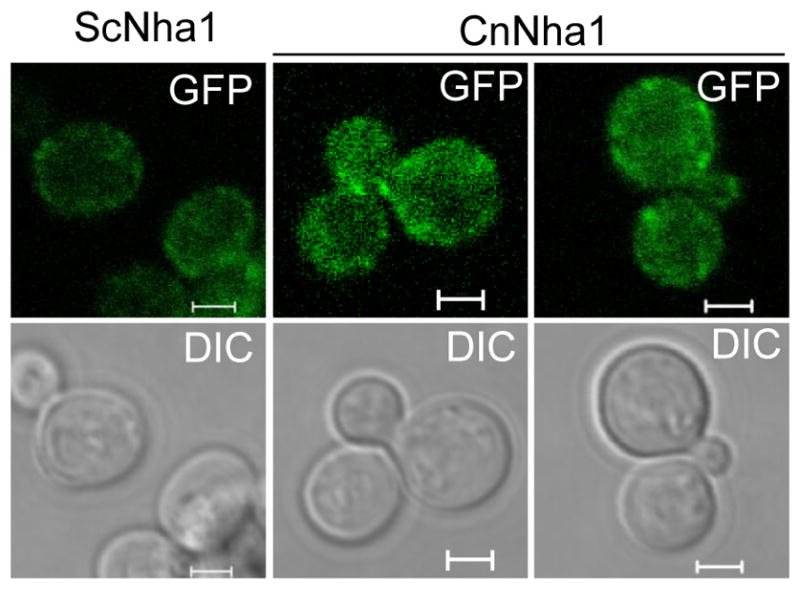
Localization of Nha1 in C. neoformans. C. neoformans and S. cerevisiae cells expressing Nha1-Gfpfusion proteins (CnNha1 and ScNha1, respectively) were cultured in SC liquid media at 30°C for 16 hr and were subcultured in SC liquid containing 1 M KCl for 1 h. Cellular localization of Nha1-Gfp proteins were visualized by confocal microscope (Carl Zeiss). The scale bar represents 2 μm.
3.9. Roles of two cation transporters in regulation of virulence factor production and sexual differentiation in C. neoformans
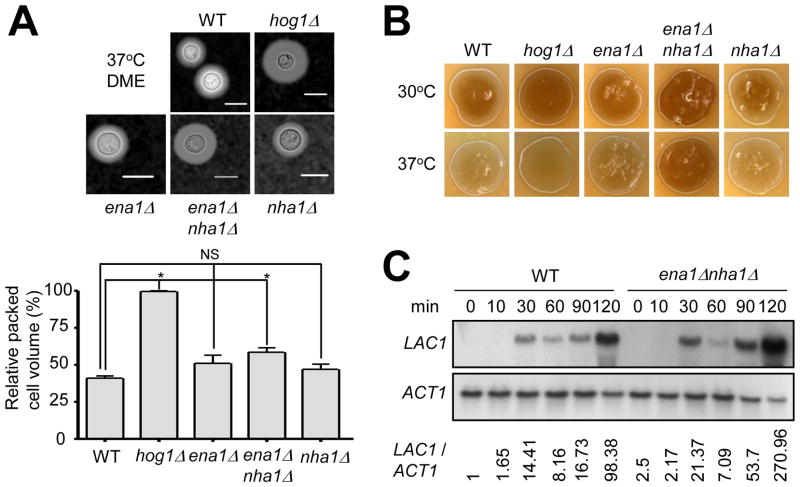
The HOG pathway plays a major role in production of virulence factors such as capsule and melanin (Bahn et al., 2005). The perturbation of HOG1 enhances capsule synthesis and melanin production (Bahn et al., 2005). The fact that expression levels of ENA1 and NHA1 are positively regulated by Hog1 led us to examine the role of the two cation transporters in capsule and melanin production. The ena1 and nha1 single mutants produced WT-levels of capsule and melanin. However, the ena1 nha1 double mutant showed slightly enhanced capsule production than WT based on both qualitative and quantitative observations (Fig. 9A). Similarly, the ena1 nha1 double mutant also displayed increased melanin production (Fig. 9B). In C. neoformans, melanin biosynthesis is catalyzed by laccase, encoded by the LAC1 gene, whose expression is induced by glucose starvation (Pukkila-Worley et al., 2005). In WT, LAC1 started to be strongly induced after 30 min and peaked at 120 min upon glucose starvation (Fig. 9C). Compared to WT, LAC1 induction was found to be much increased in the ena1 nha1 mutant (Fig. 9C). These data indicate that intracellular cation balance contributes to biosynthesis of capsule and melanin in C. neoformans.
Fig. 9.
The role of Ena1 and Nha1 in capsule and melanin production. (A) For capsule production measurement, each strain (WT H99 strain and hog1, ena1, ena1 nha1, and nha1 mutants) was spotted and cultured on DME agar medium at 37°C for 2 days. Capsules were visualized by India ink staining (upper panel) and the relative capsule volume was measured by calculating the ratio of the length of packed cell volume phase per length of total volume phase (lower panel). Three independent experiments with technical triplicates were performed. Statistical analysis was performed by using Bonferroni multiple comparison test. *, P < 0.01 and NS, not significant (P > 0.05). (B) Each C. neoformans strain was spotted, cultured on Niger seed medium containing concentration of glucose 0.1% at 30°C or 37°C for 1 day, and photographed. (C) The role of Ena1 and Nha1 in controlling LAC1 expression. WT H99 strain and ena1 nha1 mutants grown to a logarithmic phase (OD600nm = 1.0) in YPD liquid medium (zero time control) were shifted to YNB liquid medium (without glucose), and further incubated at 30°C. Northern blot analysis was performed with total RNA isolated from each cell grown at the indicated time points. Each membrane was hybridized with the LAC1 specific probe, washed, and developed. Subsequently the same membrane was deprobed, re-hybridized with the ACT1 specific probe, washed, and developed. The expression levels of LAC1 relative to ACT1 (LAC1/ACT1) were measured via phosphoimager analysis.
The Hog1 MAPK pathway is involved in sexual differentiation as well as virulence factor production. The deletion of HOG1 enhances mating response via increasing pheromone expression (Bahn et al., 2005). To address the role of Ena1 or Nha1 in sexual differentiation, a mating assay was performed with MAT and MATa cells of ena1 and nha1 mutants. Disruption of ENA1 and NHA1 did not affect sexual differentiation of C. neoformans (data not shown). In summary, the cation transporters Ena1 and Nha1 cooperatively regulate melanin and capsule production, but are dispensable for sexual differentiation.
3.10. Ena1 and Nha1 play different roles in the virulence of C. neoformans
As Ena1 plays an essential role in virulence of C. neoformans (Idnurm et al., 2009; Lee et al., 2010; Liu et al., 2008), we examined the role of Nha1 in virulence by using a murine model of systemic cryptococcosis. In this virulence assay, we used WT strain and ena1, nha1, ena1 nha1, and nha1/NHA1 strains that were constructed in the H99 strain background. In agreement with the previous study (Idnurm et al., 2009), the ena1 mutant constructed in the H99 strain background was a virulent in a mouse model as expected (Fig. 10). In contrast, the nha1 mutant was as virulent as WT, indicating that Nha1 is not involved in virulence of C. neoformans (Fig. 10). The ena1 nha1 mutant was as a virulent as the ena1 mutant (Fig. 10). The virulence attenuation observed in both ena1 and ena1 nha1 mutant was not due to clearance of the infection, as it was still possible to detect low levels of CFUs in the lungs of ena1Δ- or ena1Δnha1Δ- infected animals at day 43 post infection (data not shown). In addition, there was little or no dissemination of these mutants from the lungs, as few (only 1) or no CFUs were detected in the brains of infected animals (data not shown). We therefore concluded that Ena1 is essential and Nha1 is dispensable for virulence of C. neoformans.
Fig. 10.
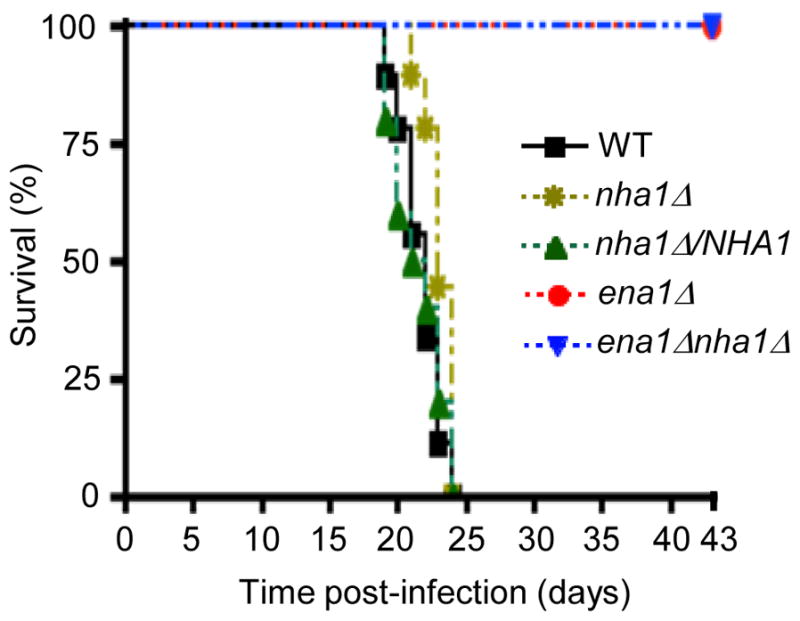
Nha1 is not required for virulence in C. neoformans. For virulence assays, seven week old A/J female mice were infected with 5 × 104 cells of WT, ena1, ena1 nha1, nha1, and nha1/NHA1 strains by intranasal inhalation. Survival (%) was monitored daily for 43 days after infection.
4. Discussion
In this study we aimed to elucidate the role of two cation transporters, Ena1 and Nha1, in the human pathogenic fungus C. neoformans, as key downstream effectors of the HOG signaling pathway. Our previous transcriptome analysis revealed that expression of ENA1 and NHA1 is differentially regulated in response to osmotic and oxidative stresses in a Hog1-dependent manner (Ko et al., 2009). The function of Ena1, a P-type ATPase, has been recently investigated in C. neoformans (Idnurm et al., 2009; Ko et al., 2009). Unlike other fungal Ena1 orthologs, however, cryptococcal Ena1 appears to play a limited role in counter acting salt stress, important only under glucose-starved conditions (ldnurm et al., 2009; Ko et al., 2009), indicating that another cation transporter may compensate for the loss of Ena1 for maintenance of cation homeostasis and salt stress response.
Here we provide several lines of evidence showing that Nha1, Na+/H+ antiporter, plays redundant and discrete roles with Ena1 in controlling cation homeostasis via the HOG pathway in C. neoformans. First, both ENA1 and NHA1 expression were induced by either Na+ or K+ salt stress in WT, but not in the hog1 mutant, although ENA1 was much more strongly induced than NHA1. Second, regardless of the expression patterns, Ena1, but not Nha1, appears to be a key player for counter acting salt stress conferred by toxic cations, such as Na+ or Li+. The nha1 mutant did not show any increased susceptibility to Na+ stress compared to WT under either glucose-rich or –starved conditions. Moreover, deletion of NHA1 did not further increase Na+ or Li+ susceptibility of the ena1 mutant. Third, both Ena1 and Nha1 play redundant roles in responding and adapting to the less toxic K+ cation. Disruption of NHA1 further increased K+ susceptibility of the ena1 mutant, particularly under glucose-rich condition. Fourth, Ena1 and Nha1 work oppositely in response to changes of extracellular pH. Under alkaline condition, Ena1 was highly induced in transcript levels and was required for survival even in the presence of low levels of Na+ or K+ salt. For survival under the high pH and low K+ salt, Nha1 is also required. In contrast, Nha1, but not Ena1, was essential for survival at acidic condition (pH 4.5) under high K+ stress. Indeed, this (pH 4.5, 1.5 M KCl) was the only condition where the nha1 mutant showed obvious growth defects. To sum up, Ena1 and Nha1 are two major cation transporters for controlling cation homeostasis in C. neoformans in both cooperative and opposite manners.
The Nha1 cation transporter is structurally conserved but functionally divergent in C. neoformans. Most yeast alkali metal cation/H+ antiporters, except SpSod2, consist of N-terminal hydrophobic region and C-terminal hydrophilic region. About 10 to 12 transmembrane regions appear to be present in the N-terminus of the Nha1 orthologs. CnNha1 also has 11 or 12 hydrophobic segments at its N-terminus based on the hydrophilicity plot. Although fungal Nha1 orthologs show high sequence homology to each other, they are divided into two groups depending on substrate specificity. One group, such as Na+/H+ antiporter in S. pombe, has a substrate specificity for Na+ and Li+ (Jia et al., 1992). The other group has a broad substrate specificity to regulate cation homeostasis in cells (Pribylova et al., 2006). Interestingly, unlike other Nha1, CnNha1 is involved in potassium homeostasis, but not sodium and lithium homeostasis. Even though the nha1Δ mutant displayed WT levels of resistance to NaCl or KCl, the ena1 nha1 double mutant was significantly more susceptible to KCl, but not to NaCl, than the ena1 mutant in the glucose-rich condition (Fig 3A). Furthermore, the ena1 nha1 double mutant was as resistant to K+ stress, but not to Na+ stress, as the nha1Δ mutant under low pH condition (pH 4.5), indicating that CnNha1 has substrate preference to potassium, but not to sodium.
One notable finding made in this study is that disruption of ENA1 and NHA1 enhances susceptibility to antifungal drugs, particularly azoles, in C. neoformans. The primary mode of action of azole drugs is to reduce sterol biosynthesis by inhibiting P450 cytochrome activity. However, a recent study showed that these azole drugs, including ketoconazole and miconazole, alter potassium homestasis in S. cerevisiae (Calahorra et al., 2011). The present study also showed that cation transporters play critical roles in conferring resistance against polyene (amphotericin B) as well as azoles (fluconazole and ketoconazole), indicating that cation transporters regulate antifungal drug susceptibility through cation balance between outside and inside membrane regardless of ergosterol biosynthesis. The finding could apply to therapeutic target of cryptococcosis with azole or polyene drugs with inhibitors of cation transporters.
In S. cerevisiae, the HOG pathway is also known to control ENA1 induction through the Sko1-Ssn6-Tup6 corepressor complex, which is recruited as a repressor to the ENA1 promoter (Marquez et al., 1998; Proft and Serrano, 1999). Upon incoming cation stress, activated Hog1 phosphorylates Sko1, which is subsequently dissociated from the Ssn6-Tup6 complex and activates ENA1 expression. Although none of the Sko1-like bZIP transcription factors have been discovered in the C. neoformans genome database, the Atf1 transcription factor, which is a distant ortholog of Sko1 in C. neoformans, is likely to be a good candidate because ENA1 expression is partly defective in the atf1 mutant. Connection between Hog1 and Atf1 for regulation of ENA1 expression should be further investigated in future.
Besides the HOG pathway, diverse signaling pathways are known to be involved in ENA1 expression in the budding yeast. The calcineurin pathway regulates ENA1 expression by Crz1 transcription factor under high pH and salt stress (Mendizabal et al., 2001). In response to high pH, the Rim101-Nrg1 and Snf1-Mig1/2 signaling pathways control ENA1 expression. Compared to expression patterns of ENA1 in S. cerevisiae, ENA1 expression in C. neoformans exhibited similar and different patterns. First, this study revealed that the calcineurin pathway did not affect the expression level of ENA1 salt stress condition. Previously, Idnurm and co-workers also reported similar results (ldnurm et al., 2009). Compared to ENA1 in S. cerevisiae, Crzl binding sites (5′-GAATGGCTG-3′ and 5′-GGGTGGCTG-3′) are not found in the promoter region of ENA1 in C. neoformans. Second, the nrg1 mutant in C. neoformans shows increased susceptibility to NaCl whereas the nrg1 mutant in S. cerevisiae confers tolerance to NaCl compared to WT (Cramer et al., 2006; Lamb and Mitchell, 2003). Nevertheless, the expression levels of ENA1 in the nrg1 mutant are enhanced in both S. cerevisiae and C. neoformans and the promoter region of cryptococcal ENA1 also has conserved Nrg1 binding sequences (5′-CCCCT-3′). Third, we found that ENA1 expression is blocked in the rim101 mutant in alkaline and cation stress (1 M NaCl) conditions. According to the study by O’Meara, the promoter of ENA1 has conserved predicted Rim101 binding sites (5′-GCCAAG-3′) (O’Meara et al., 2010). The one notable finding is that ENA1 expression was abolished in the hog1 mutant whereas ENA1 was slightly induced in rim101 mutant in response to 1 M NaCl shock, implying that the Rim101 signaling pathway could be modulated by the HOG pathway (Fig. 11). According to our previous transcriptome analysis, expression of RIM101 appears to be delayed in the hog1 mutant under 1 M NaCl shock (Ko et al., 2009). It indicates that the HOG1 pathway could cross talk with the Rim101 pathway. The connection between the Hog1 and Rim101 pathways is a topic of future research.
Fig. 11.
The proposed model for regulation and function of ENA1 and NHA1 in C. neoformans. Under cation shock and high pH, ENA1 expression is controlled by diverse signaling proteins. ENA1 is positively and negatively regulated by Rim101 and Nrg1 proteins, respectively. However, it is unclear whether Rim101 controls expression of ENA1 through the Nrg1 repressor protein. Hog1 appears to positively regulate ENA1 expression, partly through the Atf1 transcription factor. It is possible that Hog1 controls the Rim101 pathway directly or indirectly to regulate ENA1 expression. Upon Na+/K+ shock, NHA1 expression is induced in a Hog1-dependent manner. Under osmotic shock, such as high sorbitol addition, NHA1 is induced in a Hog1-independent manner. Under K+ shock, Rim101 positively regulated mRNA levels of NHA1. However, it remains unclear whether the Ena1-dependent regulation of NHA1 is mediated via the Nrg1 repressor. In C. neoformans, Ena1 plays a major role in toxic ion (Li+ and Na+) and K+ homeostasis as well as high pH adaptation whereas CnNha1 is involved in K+ homeostasis and low pH adaptation. Furthermore, Ena1 and Nha1 play redundant roles in negatively regulating production of melanin and capsule.
In contrast to ENA1, the regulatory mechanism of NHA1 remains elusive. In Candida species, expression levels of NHA1 do not appear to change significantly in response to salt stresses, similar to S. cerevisiae (Krauke and Sychrova, 2011; Soong et al., 2000). Upon osmotic shock in S. cerevisiae, however, the Hog1 kinase rapidly phosphorylates Nha1 at its C-terminal region through an unknown mechanism (Proft and Struhl, 2004). Phosphorylation of Nha1 via Hog1 results in reassociation of the general transcriptional machinery. In contrast, our study revealed that expression levels of NHA1 were induced under salt stress in a Hog1-dependent manner. Furthermore, NHA1 mRNA abundance was also regulated by the Rim101-Nrg1 pathway. The promoter region of Nha1 also has both Nrg1 binding site (5′-CCCCT-3′) and Rim101 binding site (5′-GCCAAG-3′) similar to that of Ena1. However, it is unclear whether Rim101 controls expression levels of NHA1 via Nrg1 repressor under K+ shock. Taken together, the HOG pathway and Rim101-Nrg1 pathway are key signaling cascades to control expression and activity of Nha1, although its regulatory mechanism is diverse among fungi.
An additional interesting finding of this study is that Ena1 and Nha1 play redundant roles in negatively regulating production of capsule and melanin in C. neoformans. Antioxidant melanin and antiphagocytic capsule are well established virulence factors in C. neoformans. Lac1 (laccase 1) mainly governs melanin production, whereas Lac2 is partially contributes to melanin biosynthesis (Pukkila-Worley et al., 2005; Williamson, 1994). Furthermore, cations such as Fe3+, Cu2+, and K+, are involved in phenoloxidase (laccase) activity in C. neoformans (Palumbo et al., 1985; Vidotto et al., 2002). This study showed that deletions of both ENA1 and NHA1 induce melanin biosynthesis through enhanced expression levels of LAC1. This result may result from imbalance of intracellular cations, which induces to increase expression of laccase. At this point, it is not clear whether perturbed cation homeostasis also affects activity of laccases. Metal ions, such as iron, are also known to be required for capsule production (Lian et al., 2005). In conclusion, cation homeostasis mediated by Ena1 and Nha1 is required for tight regulation of melanin and capsule biosynthesis.
Intracellular cation homeostasis is essential for survival and virulence in fungi. The perturbation of ENA1 or VPH1, encoding a vesicular (H+)-ATPase proton pump, results in severe virulence defects in C. neoformans (Erickson et al., 2001; Idnurm et al., 2009). Furthermore, CN/H1, a Na+/H+ antiporter in C. albicans, is involved in morphological change and shows attenuated virulence in deletion mutants (Soong et al., 2000). However, our animal study exhibited that Nha1 is dispensable for virulence in C. neoformans. In fact this finding is not surprising because Nha1 is only involved in less toxic K+ stress, but not in toxic Na+ stress like Ena1. The only growth condition (acidic pH and high K+) where the nha1 mutant exhibited severe growth defects may not be experienced by C. neoformans during host infection and disease progressions contrast, the role of Ena1 in counteracting high concentration of toxic ions, such as sodium ion, and growth at alkaline pH appears to be required for the pathogen to survive in the host. Although the exact reason is not clear at this point, Idnurm and colleagues proposed that Ena1 might be essential for survival in the localized alkaline microenvironments generated by the virulence factor urease in C. neoformans within the host (Erickson et al., 2001; Idnurm et al., 2009). However, other explanation may exist for such a dramatic role of Ena1 in virulence of C. neoformans.
In summary, we demonstrated that cation transporters, Ena1 and Nha1, have redundant and discrete roles in cation homeostasis, pH regulation, membrane potential, and virulence in C. neoformans as summarized in Figure 11. Based on our data, we suggest that cation transporters could be good targets for combination antifungal drug therapy for treatment of cryptococcosis.
Supplementary Material
Highlights.
Cation transporters Ena1 and Nha1 play redundant and discrete roles in cation homeostasis of Cryptococcus.
Ena1 and Nha1 affect antifungal drug susceptibility in Cryptococcus.
Both Ena1 and Nha1 regulate production of capsule and melanin in a cooperative manner.
The HOG and Rim101 pathways control expression of ENA1 and NHA1 in Cryptococcus
Acknowledgments
We thank Alex Idnurm and Andrew Alspaugh for kindly providing Cryptococcus strains. We thank Min-Kyung Sung and Won-Ki Huh for assistance with microscope and providing a strain, and Ah Reum Choi for technical assistance. This work was supported by the National Research Foundation of Korea grants (No. 2008-0061963, No. 2010-0029117) from MEST (to Y.S.B). This work was also supported in part by RO1 grant AI080275, AI070152, and AI089244 from the NIH/NIAID (to K.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alspaugh JA, Perfect JR, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–17. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh JA, Pukkila-Worley R, Harashima T, Cavallo LM, Funnell D, Cox GM, Perfect JR, Kronstad JW, Heitman J. Adenylyl cyclase functions downstream of the Gα protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell. 2002;1:75–84. doi: 10.1128/EC.1.1.75-84.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arino J. Integrative responses to high pH stress in S. cerevisiae. OMICS. 2010;14:517–23. doi: 10.1089/omi.2010.0044. [DOI] [PubMed] [Google Scholar]
- Bahn YS, Hicks JK, Giles SS, Cox GM, Heitman J. Adenylyl cyclase-associated protein Aca1 regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase A cascade. Eukaryot. Cell. 2004;3:1476–91. doi: 10.1128/EC.3.6.1476-1491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn YS, Kojima K, Cox GM, Heitman J. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol. Biol. Cell. 2005;16:2285–300. doi: 10.1091/mbc.E04-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuelos MA, Sychrova H, Bleykasten-Grosshans C, Souciet JL, Potier S. The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology. 1998;144 (Pt 10):2749–58. doi: 10.1099/00221287-144-10-2749. [DOI] [PubMed] [Google Scholar]
- Benito B, Garciadeblas B, Rodriguez-Navarro A. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology. 2002;148:933–41. doi: 10.1099/00221287-148-4-933. [DOI] [PubMed] [Google Scholar]
- Bihler H, Slayman CL, Bertl A. NSC1: a novel high-current inward rectifier for cations in the plasma membrane of Saccharomyces cerevisiae. FEBS Lett. 1998;432:59–64. doi: 10.1016/s0014-5793(98)00832-1. [DOI] [PubMed] [Google Scholar]
- Cagnac O, Leterrier M, Yeager M, Blumwald E. Identification and characterization of Vnx1p, a novel type of vacuolar monovalent cation/H+ antiporter of Saccharomyces cerevisiae. J Biol Chem. 2007;282:24284–93. doi: 10.1074/jbc.M703116200. [DOI] [PubMed] [Google Scholar]
- Calahorra M, Lozano C, Sanchez NS, Pena A. Ketoconazole and miconazole alter potassium homeostasis in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2011;1808:433–45. doi: 10.1016/j.bbamem.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Catty P, de Kerchove d’Exaerde A, Goffeau A. The complete inventory of the yeast Saccharomyces cerevisiae P-type transport ATPases. FEBS Lett. 1997;409:325–32. doi: 10.1016/s0014-5793(97)00446-8. [DOI] [PubMed] [Google Scholar]
- Cramer KL, Gerrald QD, Nichols CB, Price MS, Alspaugh JA. Transcription factor Nrg1 mediates capsule formation, stress response, and pathogenesis in Cryptococcus neoformans. Eukaryot. Cell. 2006;5:1147–56. doi: 10.1128/EC.00145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RC, Blankenship JR, Kraus PR, de Jesus Berrios M, Hull CM, D’Souza C, Wang P, Heitman J. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology. 2002;148:2607–15. doi: 10.1099/00221287-148-8-2607. [DOI] [PubMed] [Google Scholar]
- Erickson T, Liu L, Gueyikian A, Zhu X, Gibbons J, Williamson PR. Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Mol Microbiol. 2001;42:1121–31. doi: 10.1046/j.1365-2958.2001.02712.x. [DOI] [PubMed] [Google Scholar]
- Fairman C, Zhou X, Kung C. Potassium uptake through the TOK1K+ channel in the budding yeast. J Membr Biol. 1999;168:149–57. doi: 10.1007/s002329900505. [DOI] [PubMed] [Google Scholar]
- Gaber RF, Styles CA, Fink GR. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:2848–59. doi: 10.1128/mcb.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DL, Perfect JR, Durack DT. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985;76:508–16. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro R, Garciadeblas B, Rodriguez-Navarro A. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 1991;291:189–91. doi: 10.1016/0014-5793(91)81280-l. [DOI] [PubMed] [Google Scholar]
- Hicks JK, D’Souza CA, Cox GM, Heitman J. Cyclic AMP-dependent protein kinase catalytic subunits have divergent roles in virulence factor production in two varieties of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell. 2004;3:14–26. doi: 10.1128/EC.3.1.14-26.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang LM, Maguire JA, Doyle P, Fyfe M, Roscoe DL. Cryptococcus neoformans infections at Vancouver Hospital and Health Sciences Centre (1997–2002): epidemiology, microbiology and histopathology. J Med Microbiol. 2004;53:935–40. doi: 10.1099/jmm.0.05427-0. [DOI] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–91. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Idnurm A, Walton FJ, Floyd A, Reedy JL, Heitman J. Identification of ENA1 as a virulence gene of the human pathogenic fungus Cryptococcus neoformans through signature-tagged insertional mutagenesis. Eukaryot. Cell. 2009;8:315–326. doi: 10.1128/EC.00375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia ZP, McCullough N, Martel R, Hemmingsen S, Young PG. Gene amplification at a locus encoding a putative Na+/H+ antiporter confers sodium and lithium tolerance in fission yeast. EMBO J. 1992;11:1631–40. doi: 10.1002/j.1460-2075.1992.tb05209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KW, Kim SY, Okagaki LH, Nielsen K, Bahn YS. Ste50 adaptor protein governs sexual differentiation of Cryptococcus neoformans via the pheromone-response MAPK signaling pathway. Fungal Genet Biol. 2011;48:154–65. doi: 10.1016/j.fgb.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinclova-Zimmermannova O, Gaskova D, Sychrova H. The Na+, K+/H+ -antiporter Nha1 influences the plasma membrane potential of Saccharomyces cerevisiae. FEMS Yeast Res. 2006;6:792–800. doi: 10.1111/j.1567-1364.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- Kinclova-Zimmermannova O, Sychrova H. Plasma-membrane Cnh1 Na+/H+ antiporter regulates potassium homeostasis in Candida albicans. Microbiology. 2007;153:2603–12. doi: 10.1099/mic.0.2007/008011-0. [DOI] [PubMed] [Google Scholar]
- Kinclova O, Ramos J, Potier S, Sychrova H. Functional study of the Saccharomyces cerevisiae Nha1p C-terminus. Mol Microbiol. 2001;40:656–68. doi: 10.1046/j.1365-2958.2001.02412.x. [DOI] [PubMed] [Google Scholar]
- Ko CH, Buckley AM, Gaber RF. TRK2 is required for low affinity K+ transport in Saccharomyces cerevisiae. Genetics. 1990;125:305–12. doi: 10.1093/genetics/125.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YJ, Yu YM, Kim GB, Lee GW, Maeng PJ, Kim SS, Floyd A, Heitman J, Bahn YS. Remodeling of global transcription patterns of Cryptococcus neoformans genes mediated by the stress-activated HOG signaling pathways. Eukaryot. Cell. 2009;8:1197–1217. doi: 10.1128/EC.00120-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauke Y, Sychrova H. Cnh1 Na+/H+ antiporter and Ena1 Na+ -ATPase play different roles in cation homeostasis and cell physiology of Candida glabrata. FEMS Yeast Res. 2011;11:29–41. doi: 10.1111/j.1567-1364.2010.00686.x. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Mitchell AP. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol Cell Biol. 2003;23:677–86. doi: 10.1128/MCB.23.2.677-686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Toffaletti DL, Tenor J, Soderblom EJ, Thompson JW, Moseley MA, Price M, Perfect JR. Survival defects of Cryptococcus neoformans mutants exposed to human cerebrospinal fluid result in attenuated virulence in an experimental model of meningitis. Infect Immun. 2010;78:4213–25. doi: 10.1128/IAI.00551-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian T, Simmer MI, D’Souza CA, Steen BR, Zuyderduyn SD, Jones SJ, Marra MA, Kronstad JW. Iron-regulated transcription and capsule formation in the fungal pathogen Cryptococcus neoformans. Mol Microbiol. 2005;55:1452–72. doi: 10.1111/j.1365-2958.2004.04474.x. [DOI] [PubMed] [Google Scholar]
- Liu OW, Chun CD, Chow ED, Chen C, Madhani HD, Noble SM. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell. 2008;135:174–88. doi: 10.1016/j.cell.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez JA, Pascual-Ahuir A, Proft M, Serrano R. The Ssn6-Tup1 repressor complex of Saccharomyces cerevisiae is involved in the osmotic induction of HOG-dependent and -independent genes. EMBO J. 1998;17:2543–53. doi: 10.1093/emboj/17.9.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizabal I, Pascual-Ahuir A, Serrano R, de Larrinoa IF. Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol. Genet. Genomics. 2001;265:801–11. doi: 10.1007/s004380100474. [DOI] [PubMed] [Google Scholar]
- Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS--100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol Rev. 1995;8:515–48. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulet JM, Leube MP, Kron SJ, Rios G, Fink GR, Serrano R. A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol Cell Biol. 1999;19:3328–37. doi: 10.1128/mcb.19.5.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K, Cox GM, Wang P, Toffaletti DL, Perfect JR, Heitman J. Sexual cycle of Cryptococcus neoformans var grubii and virulence of congenic a and α isolates. Infect Immun. 2003;71:4831–41. doi: 10.1128/IAI.71.9.4831-4841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara TR, Norton D, Price MS, Hay C, Clements MF, Nichols CB, Alspaugh JA. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog. 2010;6:e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A, Misuraca G, D’Ischia M, Prota G. Effect of metal ions on the kinetics of tyrosine oxidation catalysed by tyrosinase. Biochem J. 1985;228:647–51. doi: 10.1042/bj2280647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouskova K, Sychrova H. Schizosaccharomyces pombe possesses two plasma membrane alkali metal cation/H+ antiporters differing in their substrate specificity. FEMS Yeast Res. 2007;7:188–95. doi: 10.1111/j.1567-1364.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- Perfect JR, Ketabchi N, Cox GM, Ingram CW, Beiser CL. Karyotyping of Cryptococcus neoformans as an epidemiological tool. J Clin Microbiol. 1993;31:3305–9. doi: 10.1128/jcm.31.12.3305-3309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin DS, Brown CL, Haber JE. Membrane potential defect in hygromycin B-resistant pma1 mutants of Saccharomyces cerevisiae. J Biol Chem. 1988;263:18118–22. [PubMed] [Google Scholar]
- Platara M, Ruiz A, Serrano R, Palomino A, Moreno F, Arino J. The transcriptional response of the yeast Na+-ATPase ENA1 gene to alkaline stress involves three main signaling pathways. J Biol Chem. 2006;281:36632–42. doi: 10.1074/jbc.M606483200. [DOI] [PubMed] [Google Scholar]
- Pribylova L, Papouskova K, Zavrel M, Souciet JL, Sychrova H. Exploration of yeast alkali metal cation/H+ antiporters: sequence and structure comparison. Folia Microbiol (Praha) 2006;51:413–24. doi: 10.1007/BF02931585. [DOI] [PubMed] [Google Scholar]
- Prior C, Potier S, Souciet JL, Sychrova H. Characterization of the NHA1 gene encoding a Na+/H+-antiporter of the yeast Saccharomyces cerevisiae. FEBS Lett. 1996;387:89–93. doi: 10.1016/0014-5793(96)00470-x. [DOI] [PubMed] [Google Scholar]
- Proft M, Serrano R. Repressors and upstream repressing sequences of the stress-regulated ENA1 gene in Saccharomyces cerevisiae: bZIP protein Sko1p confers HOG-dependent osmotic regulation. Mol Cell Biol. 1999;19:537–46. doi: 10.1128/mcb.19.1.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proft M, Struhl K. MAP kinase-mediated stress relief that precedes and regualtes the timing of transcriptional induction. Cell. 2004;118:351–361. doi: 10.1016/j.cell.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Pukkila-Worley R, Gerrald QD, Kraus PR, Boily MJ, Davis MJ, Giles SS, Cox GM, Heitman J, Alspaugh JA. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot. Cell. 2005;4:190–201. doi: 10.1128/EC.4.1.190-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro A. Potassium transport in fungi and plants. Biochim Biophys Acta. 2000;1469:1–30. doi: 10.1016/s0304-4157(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Serrano R, Kielland-Brandt MC, Fink GR. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986;319:689–93. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Soong TW, Yong TF, Ramanan N, Wang Y. The Candida albicans antiporter gene CNH1 has a role in Na+ and H+ transport, salt tolerance, and morphogenesis. Microbiology. 2000;146 (Pt 5):1035–44. doi: 10.1099/00221287-146-5-1035. [DOI] [PubMed] [Google Scholar]
- Sychrova H. Yeast as a model organism to study transport and homeostasis of alkali metal cations. Physiol Res. 2004;53(Suppl 1):S91–8. [PubMed] [Google Scholar]
- Sychrova H, Ramirez J, Pena A. Involvement of Nha1 antiporter in regulation of intracellular pH in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1999;171:167–72. doi: 10.1111/j.1574-6968.1999.tb13428.x. [DOI] [PubMed] [Google Scholar]
- Vidotto V, Defina N, Pugliese A, Aoki S, Nakamura K, Takeo K. Effect of different K+ concentrations on Cryptococcus neoformans phenoloxidase activity. Mycopathologia. 2002;156:171–6. doi: 10.1023/a:1023376324422. [DOI] [PubMed] [Google Scholar]
- Williamson PR. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol. 1994;176:656–64. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.